Advances in Oral Drug Delivery Systems for Natural Polyunsaturated Fatty Acids: Enhancing Bioavailability and Therapeutic Potential
Abstract
1. Introduction
2. Sources, Extraction, and Purification of Omega-3 and Omega-6
3. Challenges in the Absorption and Stability of Fatty Acids
4. Oral Delivery Systems Applied to Omega-3 and -6
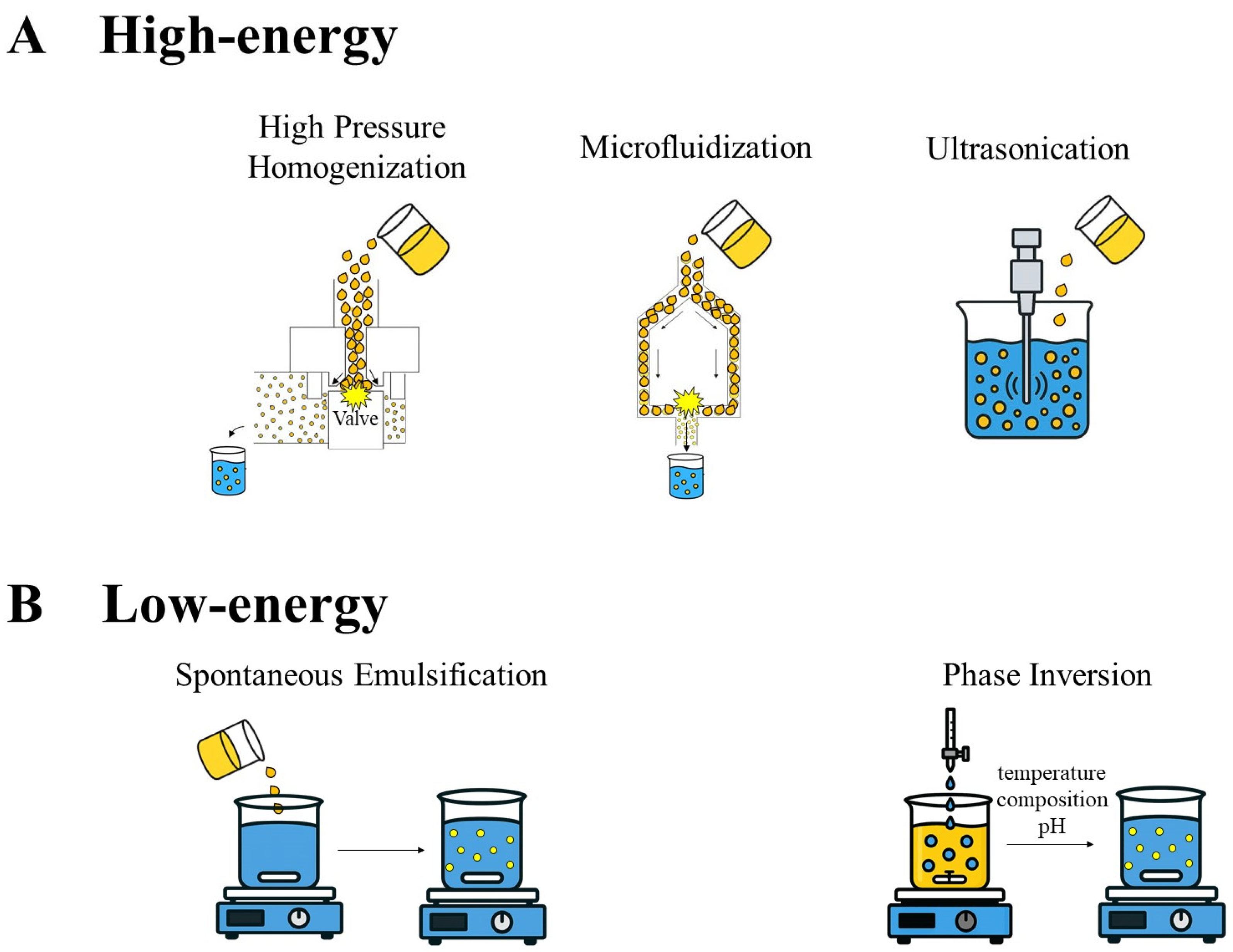
4.1. Nanoemulsions
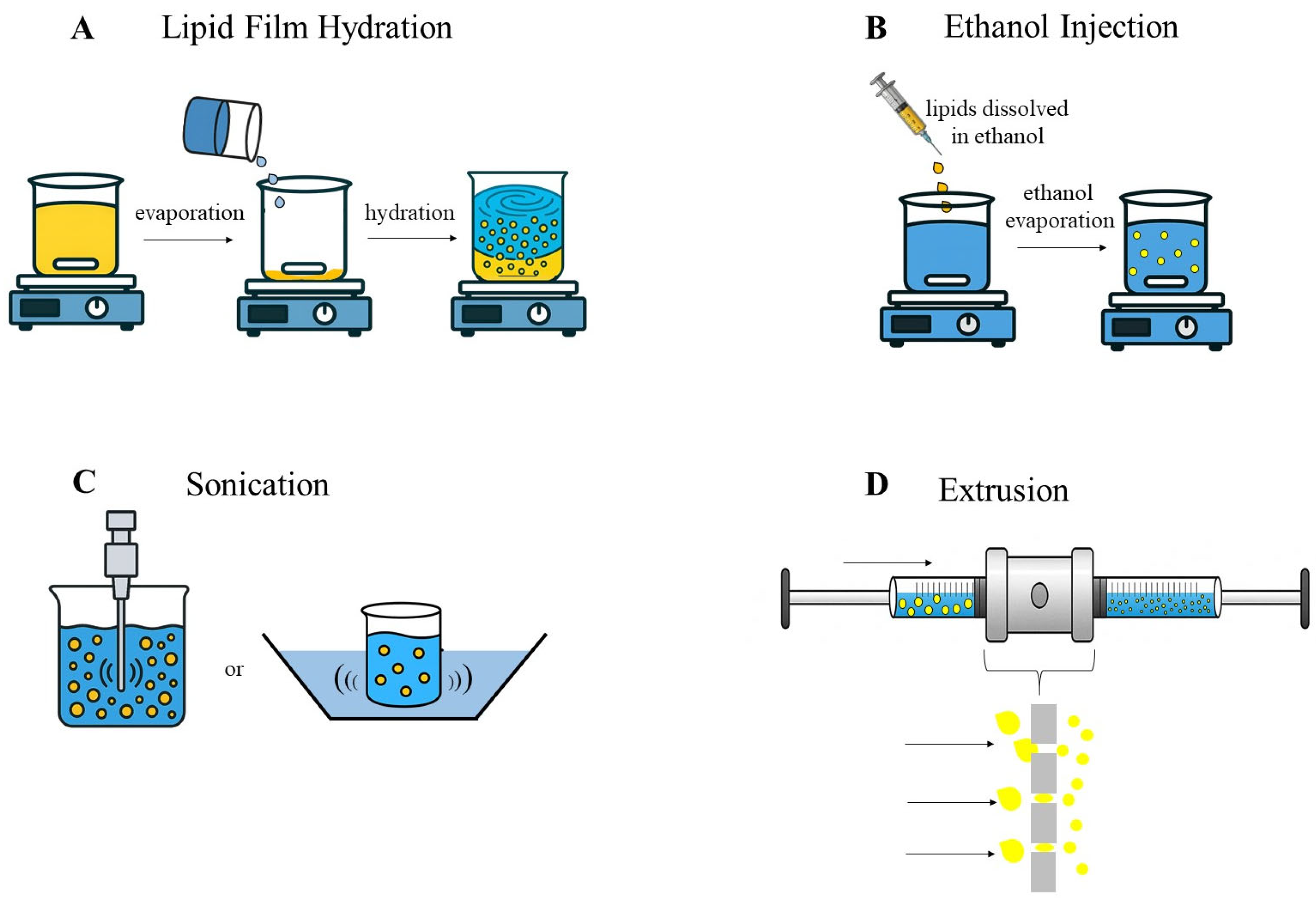
4.2. Liposomes
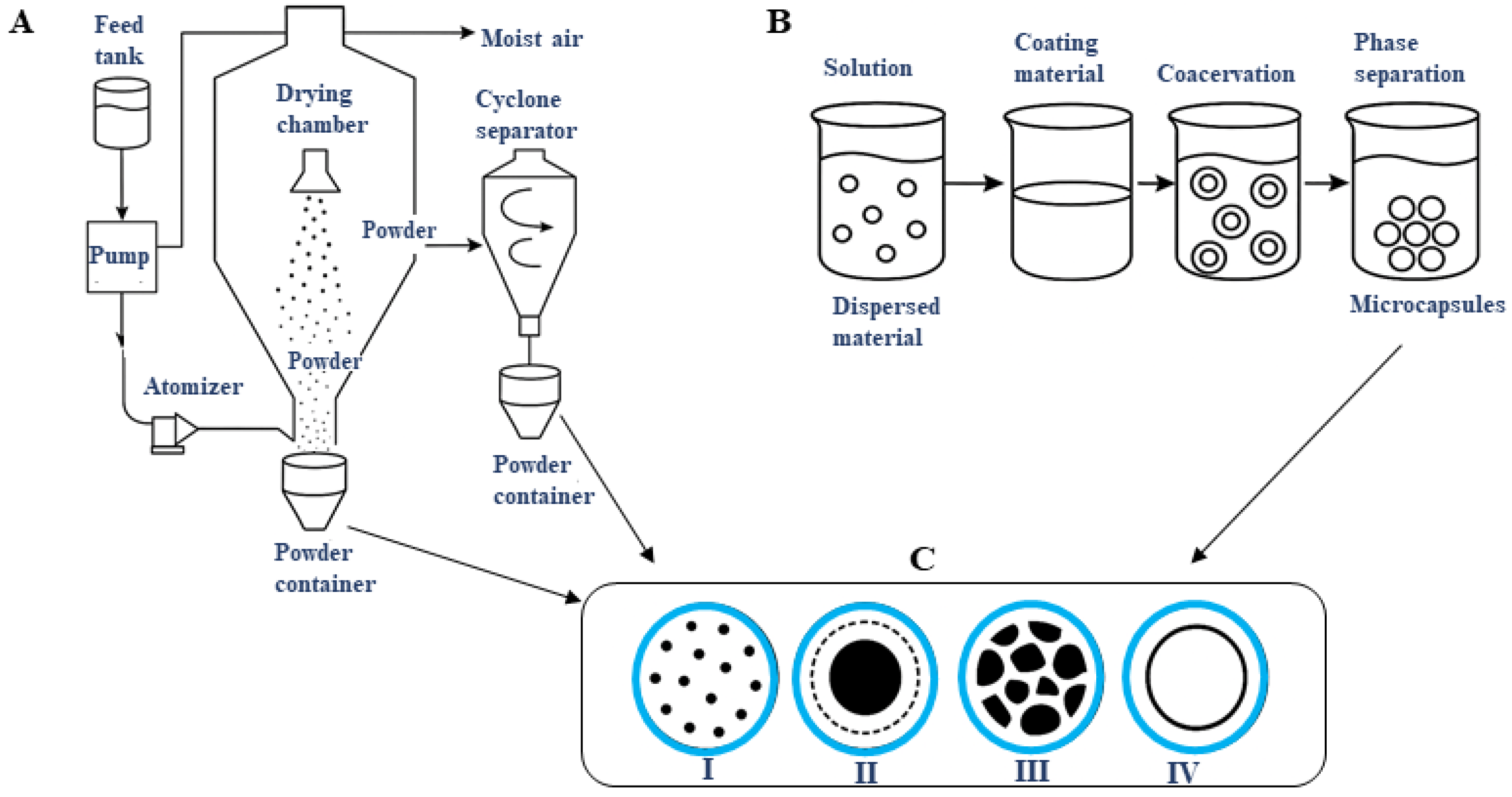
4.3. Microencapsulation
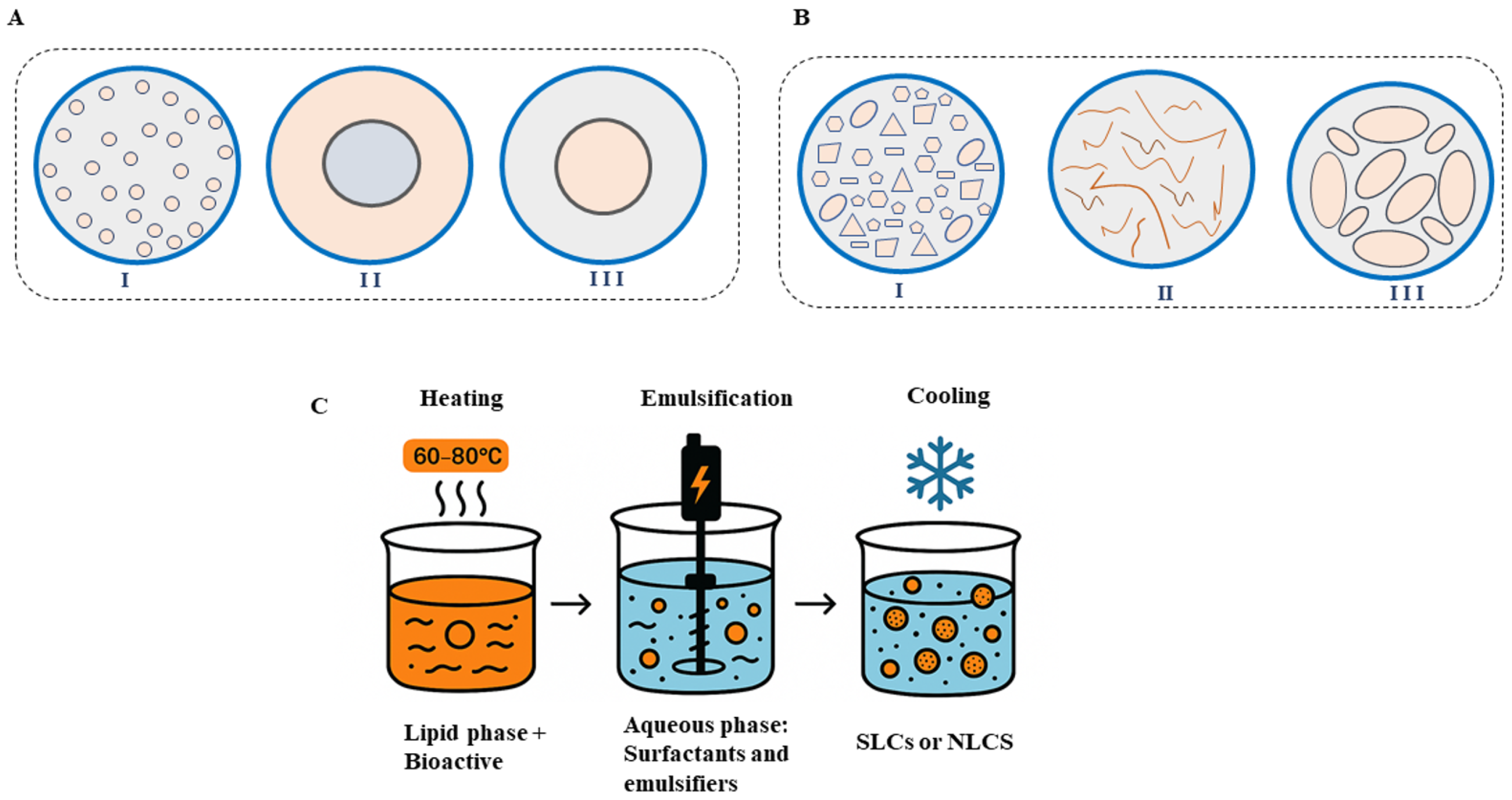
4.4. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
5. Therapeutic Applications, Clinical Benefits, and Possible Adverse Effects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| -COOH | Carboxyl |
| SFA | Saturated fatty acids |
| MUFA | Monounsaturated fatty acids |
| PUFA | Polyunsaturated fatty acids |
| n-3 | Ômega 3 |
| n-6 | Ômega 6 |
| n-9 | Ômega 9 |
| LC-PUFA | Long-chain polyunsaturated fatty acids |
| ALA | Alpha-linolenic acid |
| EPA | Eicosapentanoic acid |
| DHA | Docosahexaenoic acid |
| LA | Linoleic acid |
| ARA | Arachidonic acid |
| ROS | Reactive oxygen species |
| TAG | Triacylglycerols |
| MAGs | Monoacylglycerols |
| FFAs | Free fatty acids |
| Ees | Ethyl esters |
| O/W | Oil-in-water |
| W/O | Water-in-oil |
| W/O/W | Multiple emulsions |
| O/W/O | |
| PDI | Polydispersity index |
| ULVs | Unilamellar vesicles |
| MLVs | Multilamellar vesicles |
| DLS | Dynamic light scattering |
| GC-HS | Headspace gas chromatography |
| FTIR | Fourier-transform infrared |
| UV-Vis | Ultraviolet and visible light |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| SD | Spray drying |
| SFD | Spray freeze-drying |
| FD | Freeze-drying |
| MFD | Microwave freeze-drying |
| SLNs | Solid nanolipids |
| NLCs | Nanostructured lipid carriers |
| kDa | Kilodalton |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| COX-2 | Cyclooxygenase-2 |
| oxLDL | Oxidized low-density lipoprotein |
| PPARs | Peroxisome proliferator-activated receptors |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| ICH | International Council for Harmonization |
| HPH | High-pressure homogenization |
| MKO | Krill oil |
| MLO | Flaxseed oil |
| WPC | Whey protein concentrate |
| MD | Maltodextrin |
| GA | Arabic gum |
| HCO-40 | Hydrogenated castor oil |
| SNEDDS | Self-nanoemulsifying drug delivery system |
| WPI | Whey protein isolate |
| EPC | Egg phosphatidylcholine |
| CHOL | Cholesterol |
| PBS | Phosphate-buffered saline |
| SMEDS | Self-micro-emulsifying delivery system |
| IL-4 | Interleukin-4 |
| IL-5 | Interleukin-5 |
| IL-10 | Interleukin-10 |
| PASI | Psoriasis Area and Severity Index |
| HBL | Hydrophilic–Lipophilic Balance |
| SDS | Sodium dodecyl sulfate |
| US | Ultrasound |
| MF | Microfluidization |
| PTX | Paclitaxel |
| LN | Lipid nanoemulsion |
| FA | Folic acid |
| FO | Fish oil |
| FPH | Fish protein hydrolysate |
| CH | Chitosan |
| MMP-9 | Matrix Metalloproteinase-9 |
| NL | Nanoliposomes |
| PI | Phosphatidylinositol |
| PA | Phosphatidic acid |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PC | Phosphatidylcholine |
| Lyso-PC | Lysophosphatidyl-choline |
References
- Tinkov, A.A.; Bogdański, P.; Skrypnik, D.; Skrypnik, K.; Skalny, A.V.; Aaseth, J.; Skalnaya, M.G.; Suliburska, J. Trace element and mineral levels in serum, hair, and urine of obese women in relation to body composition, blood pressure, lipid profile, and insulin resistance. Biomolecules 2021, 11, 689. [Google Scholar] [CrossRef]
- Easton, Z.J.W.; Regnault, T.R.H. Regnault the Impact of Maternal Body Composition and Dietary Fat Consumption upon Placental Lipid Processing and Offspring Metabolic Health. Nutrients 2020, 12, 3031. [Google Scholar] [CrossRef]
- Roccisano, D.; Kumaratilake, J.; Saniotis, A.; Henneberg, M. Dietary Fats and Oils: Some Evolutionary and Historical Perspectives Concerning Edible Lipids for Human Consumption. Food Nutr. Sci. 2019, 10, 689–702. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, Y.; Wang, H.; Jiang, H. A dose-response meta-analysis of the association between the maternal omega-3 long-chain polyunsaturated fatty acids supplement and risk of asthma/wheeze in offspring. BMC Pediatr. 2022, 22, 422. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Calder. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Hussain, M.; Jiang, B.; Zheng, L.; Pan, Y.; Hu, J.; Khan, A.; Ashraf, A.; Zou, X. Omega-3 long-chain polyunsaturated fatty acids: Metabolism and health implications. Prog. Lipid. Res. 2023, 92, 101255. [Google Scholar] [CrossRef]
- Castro, L.F.C.; Tocher, D.R.; Monroig, O. Long-chain polyunsaturated fatty acid biosynthesis in chordates: Insights into the evolution of Fads and Elovl gene repertoire. Prog. Lipid Res. 2016, 62, 25–40. [Google Scholar] [CrossRef]
- Chen, H.; Leng, X.; Liu, S.; Zeng, Z.; Huang, F.; Huang, R.; Zou, Y.; Xue, Y. Association between dietary intake of omega-3 polyunsaturated fatty acids and all-cause and cardiovascular mortality among hypertensive adults: Results from NHANES 1999–2018. Clin. Nutr. 2023, 1446, 2669. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Chen, G.; Qian, Z.M.; Zhang, J.; Zhang, S.; Zhang, Z.; Vaughn, M.G.; Aaron, H.E.; Wang, C.; Lip, G.Y.H.; Lin, H. Regular use of fish oil supplements and course of cardiovascular diseases: Prospective cohort study. BMJ Med. 2024, 3, e000451. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6 and omega-3 fatty acids: Endocannabinoids, genetics and obesity. OCL 2020, 27, 7. [Google Scholar] [CrossRef]
- Calder, P.C. Dietary factors and low-grade inflammation in relation to overweight and obesity revisted. Br. J. Nutr. 2022, 127, 1455–1457. [Google Scholar] [CrossRef]
- Strobel, C.; Jahreis, G.; Kuhnt, K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Redruello-Requejo, M.; Samaniego-Vaesken, M.d.L.; Puga, A.M.; Montero-Bravo, A.; Ruperto, M.; Rodríguez-Alonso, P.; Partearroyo, T.; Varela-Moreiras, G. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2023, 15, 562. [Google Scholar] [CrossRef]
- Uriho, A.; Yang, S.; Tang, X.; Liu, C.-S.; Wang, S.; Cong, Y.; Zhang, J.; Zhou, P. Benefits of blended oil consumption over other sources of lipids on the cardiovascular system in obese rats. Food Funct. 2019, 10, 5290–5301. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Dietary Changes and Their Influence in the Development of Kidney Disease. Kidney Dial. 2022, 2, 131–137. [Google Scholar] [CrossRef]
- Twining, C.W.; Bernhardt, J.R.; Derry, A.; Hudson, C.M.; Ishikawa, A.; Kabeya, N.; Kainz, M.J.; Kitano, J.; Kowarik, C.; Ladd, S.N.; et al. The evolutionary ecology of fatty-acid variation: Implications for consumer adaptation and diversification. Ecol. Lett. 2021, 24, 1709–1731. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Grujić-Milanović, J.D.; Miloradović, Z.Z.; Mihailović-Stanojević, N.D.; Banjac, V.V.; Vidosavljević, S.; Ivanov, M.S.; Karanović, D.J.; Vajić, U.V.; Jovović, D.M. Excesive consumption of unsaturated fatty acids leads to oxidative and inflammatory instability in Wistar rats. Biomed. Pharmacother. 2021, 139, 111691. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V.; Yau, S.Y.; McAinch, A.J.; Hryciw, D.H. Role of omega-6 and omega-3 fatty acids in fetal programming. Clin. Exp. Pharmacol. Physiol. 2020, 47, 907–915. [Google Scholar] [CrossRef]
- Madore, C.; Leyrolle, Q.; Morel, L.; Rossitto, M.; Greenhalgh, A.D.; Delpech, J.C.; Martinat, M.; Bosch-Bouju, C.; Bourel, J.; Rani, B.; et al. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat. Commun. 2020, 11, 6133. [Google Scholar] [CrossRef]
- Rahimi, V.; Tavanai, E.; Falahzadeh, S.; Ranjbar, A.R.; Farahani, S. Omega-3 fatty acids and health of auditory and vestibular systems: A comprehensive review. Eur. J. Nutr. 2024, 63, 1453–1469. [Google Scholar] [CrossRef] [PubMed]
- Isesele, P.O.; Mazurak, V.C. Regulation of Skeletal Muscle Satellite Cell Differentiation by Omega-3 Polyunsaturated Fatty Acids: A Critical Review. Front. Physiol. 2021, 12, 682091. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of dietary n–3 and n–6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Bayram, S.Ş.; Kızıltan, G. The Role of Omega- 3 Polyunsaturated Fatty Acids in Diabetes Mellitus Management: A Narrative Review. Curr. Nutr. Rep. 2024, 13, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Tachtsis, B.; Whitfield, J.; JHawley, A.; Hoffman, N.J. Omega-3 Polyunsaturated Fatty Acids Mitigate Palmitate-Induced Impairments in Skeletal Muscle Cell Viability and Differentiation. Front. Physiol. 2020, 11, 563. [Google Scholar] [CrossRef]
- Stoffel, W.; Schmidt-Soltau, I.; Binczek, E.; Thomas, A.; Thevis, M.; Wegner, I. Dietary ω3-and ω6-Polyunsaturated fatty acids reconstitute fertility of Juvenile and adult Fads2-Deficient mice. Mol. Metab. 2020, 36, 100974. [Google Scholar] [CrossRef]
- Zhang, X.; JRitonja, A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef]
- Floros, S.; Toskas, A.; Pasidi, E.; Vareltzis, P. Bioaccessibility and Oxidative Stability of Omega-3 Fatty Acids in Supplements, Sardines and Enriched Eggs Studied Using a Static In Vitro Gastrointestinal Model. Molecules 2022, 27, 415. [Google Scholar] [CrossRef]
- Beltrame, G.; Ahonen, E.; Damerau, A.; Gudmundsson, H.G.; Haraldsson, G.G.; Linderborg, K.M. Lipid Structure Influences the Digestion and Oxidation Behavior of Docosahexaenoic and Eicosapentaenoic Acids in the Simulated Digestion System. J. Agric. Food Chem. 2023, 71, 10087–10096. [Google Scholar] [CrossRef]
- Afroze, S.; Janakiraman, A.K.; Gunasekaran, B.; Djearamane, S.; Wong, L.S. Potentials of omega-3 fatty acids as therapeutic drugs and its obstacles in the pathway: A critical review. J. Pharm. Pharmacogn. Res. 2024, 12, 120–145. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S. Advances in nanoencapsulated phytomedicines (phytochemicals and their extracts) for the treatment of obesity, diabetes, and their associated complications. In Natural Products in Obesity and Diabetes: Therapeutic Potential and Role in Prevention and Treatment; Springer International Publishing: Cham, Switzerland, 2022; pp. 507–532. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Kurt, E.; LBassi, T.; Sa, L.; Xie, D. Biotechnological production of omega-3 fatty acids: Current status and future perspectives. Front. Microbiol. 2023, 14, 1280296. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef]
- Dewapriya, P.; Kim, S.K. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous production of DHA and squalene from Aurantiochytrium sp. grown on forest biomass hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.S. Omega−3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—A review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; O’Keefe, J.H. Omega-6 vegetable oils as a driver of coronary heart disease: The oxidized linoleic acid hypothesis. Open Heart. 2018, 5, e000898. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Sinclair, A.J.; Holman, B.W.B. The sources, synthesis and biological actions of omega-3 and omega-6 fatty acids in red meat: An overview. Foods 2021, 10, 1358. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Ying, R.-F.; Lv, B.-F.; Yang, L.-H.; Xu, Z.; Yan, L.-Q.; Bu, J.-Z.; Wei, Y.-S. Flaxseed oil: Extraction, Health benefits and products. Qual. Assur. Saf. Crops Foods 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, L.; Jin, J.; Li, X.; Fu, J.; Wang, M.; Guan, Y.; Song, X. Phytochemical and biological characteristics of Mexican chia seed oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [PubMed]
- Omidi, H.; Tahmasebi, Z.; Badi, H.A.N.; Torabi, H.; Miransari, M. Fatty acid composition of canola (Brassica napus L.), as affected by agronomical, genotypic and environmental parameters. Comptes Rendus Biol. 2010, 333, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al Juhaimi, F.; Uslu, N.; Ghafoor, K.; Ahmed, I.A.M.; Babiker, E.E. The effect of olive varieties on fatty acid composition and tocopherol contents of cold pressed virgin olive oils. J. Oleo Sci. 2019, 68, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Szalóki-Dorkó, L.; Kumar, P.; Székely, D.; Végvári, G.; Ficzek, G.; Simon, G.; Abrankó, L.; Tormási, J.; Bujdosó, G.; Máté, M. Comparative Study of Different Walnut (Juglans regia L.) Varieties Based on Their Nutritional Values. Plants 2024, 13, 2097. [Google Scholar] [CrossRef]
- Henriques, J.; Dick, J.R.; Tocher, D.R.; Bell, J.G. Nutritional quality of salmon products available from major retailers in the UK: Content and composition of n-3 long-chain PUFA. Br. J. Nutr. 2014, 112, 964–975. [Google Scholar] [CrossRef]
- Mkadem, H.; Kaanane, A. Seasonal changes in chemical composition and fatty acids of sardines (Sardina pilchardus) from the Dakhla coast (Morocco). Moroc. J. Agric. Sci. 2020, 1, 3. Available online: https://agromaroc.net/index.php/MJAS/article/view/853/900 (accessed on 11 September 2025).
- Roy, V.C.; Park, J.S.; Ho, T.C.; Chun, B.S. Lipid Indexes and Quality Evaluation of Omega-3 Rich Oil from the Waste of Japanese Spanish Mackerel Extracted by Supercritical CO2. Mar. Drugs 2022, 20, 70. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Zhang, Y.; Li, L.; Wang, X. Regiospecific Analysis of Fatty Acids and Calculation of Triglyceride Molecular Species in Marine Fish Oils. Biomed Res. Int. 2018, 2018, 9016840. [Google Scholar] [CrossRef]
- Torno, C.; Staats, S.; De Pascual-Teresa, S.; Rimbach, G.; Schulz, C. Fatty acid profile is modulated by dietary resveratrol in rainbow trout (Oncorhynchus mykiss). Mar. Drugs 2017, 15, 252. [Google Scholar] [CrossRef]
- Zhang, A.-H.; Ji, X.-J.; Wu, W.-J.; Ren, L.-J.; Yu, Y.-D.; Huang, H. Lipid fraction and intracellular metabolite analysis reveal the mechanism of arachidonic acid-rich oil accumulation in the aging process of Mortierella alpina. J. Agric. Food Chem. 2015, 63, 9812–9819. [Google Scholar] [CrossRef]
- Baskar, G.; Kalavathy, G.; Aiswarya, R.; Selvakumari, I.A. Advances in bio-oil extraction from nonedible oil seeds and algal biomass. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Kidlington, UK, 2019; pp. 187–210. [Google Scholar] [CrossRef]
- Adepoju, T.F.; Olawale, O. Optimization and Predictive Capability of Rsm Using Controllable Variables in Azadiracha Indica Oilseeds Extraction Process. Int. J. Chem. Mater. Res. 2015, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Nde, D.B.; Anuanwen, C.F. Optimization methods for the extraction of vegetable oils: A review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of pigments from microalgae and cyanobacteria-a review on current methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Kabutey, A.; Herák, D.; Mizera, Č. Assessment of Quality and Efficiency of Cold-Pressed Oil from Selected Oilseeds. Foods 2023, 12, 3636. [Google Scholar] [CrossRef]
- Lee, S.Y.; Weingarten, M.; Ottenheim, C. Current upstream and downstream process strategies for sustainable yeast lipid production. Bioresour. Technol. 2024, 414, 131601. [Google Scholar] [CrossRef]
- Sheikh, S.M.; Kazi, Z.S. Technologies for Oil Extraction: A Review. Int. J. Environ. Agric. Biotechnol. (IJEAB) 2016, 1, 238506. [Google Scholar]
- Wenwei, C.; Guangrong, H.; Zhenbao, J.; Yao, H. Optimization of aqueous enzymatic extraction of oil from shrimp processing by-products using response surface methodology. Food Sci. Technol. 2019, 39, 231–236. [Google Scholar] [CrossRef]
- Calvo, A.; Morante, J.; Plánder, S.; Székely, E. Fractionation of biologically active components of grape seed (Vitis vinifera) by supercritical fluid extraction. Acta Aliment. 2017, 46, 27–34. [Google Scholar] [CrossRef]
- Rosas-Mendoza, M.E.; Coria-Hernández, J.; Meléndez-Pérez, R.; Arjona-Román, J.L. Characteristics of chia (Salvia hispanica L.) seed oil extracted by ultrasound assistance. J. Mex. Chem. Soc. 2017, 61, 326–335. [Google Scholar] [CrossRef]
- Creencia, E.C.; Nillama, J.A.P.; Librando, I.L. Microwave-assisted extraction and physicochemical evaluation of oil from Hevea brasiliensis seeds. Resources 2018, 7, 28. [Google Scholar] [CrossRef]
- Crexi, V.T.; Monte, M.L.; de S, L.A.; Pinto, L.A.A. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem. 2010, 119, 945–950. [Google Scholar] [CrossRef]
- Bonilla-Mendez, J.R.; Hoyos-Concha, J.L. Methods of extraction, refining and concentration of fish oil as a source of omega-3 fatty acids. Corpoica Cienc. Tecnol. Agropecu. 2018, 19, 645–668. [Google Scholar] [CrossRef]
- Noriega-Rodríguez, J.A.; Ortega-García, J.; Angulo-Guerrero, O.; García, H.S.; Medina-Juárez, L.A.; Gámez-Meza, N. Oil production from sardine (Sardinops sagax caerulea) Producción de aceite a partir de sardina (Sardinops sagax caerulea). CYTA—J. Food 2009, 7, 173–179. [Google Scholar] [CrossRef]
- Alfio, V.G.; Manzo, C.; Micillo, R. From fishwaste to value: An overview of the sustainable recovery of omega-3 for food supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Klooster, S.T.; Schroën, K.; Berton-Carabin, C. Lipid oxidation products in model food emulsions: Do they stay in or leave droplets, that’s the question. Food Chem. 2023, 405, 134992. [Google Scholar] [CrossRef]
- Dellarosa, N.; Laghi, L.; Martinsdóttir, E.; Jónsdóttir, R.; Sveinsdóttir, K. Enrichment of convenience seafood with omega-3 and seaweed extracts: Effect on lipid oxidation. Lebensm. Wiss. Technol. 2015, 62, 746–752. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation products from omega-3 and omega-6 fatty acids during a simulated shelf life of edible oils. Lebensm. Wiss. Technol. 2019, 101, 113–122. [Google Scholar] [CrossRef]
- Demets, R.; Gheysen, L.; Van Loey, A.; Foubert, I. Oxidative stability differences of aqueous model systems of photoautotrophic n–3 LC–PUFA rich microalgae: The antioxidative role of endogenous carotenoids. Food Res. Int. 2023, 172, 113055. [Google Scholar] [CrossRef]
- Migliaccio, V.; Sica, R.; Di Gregorio, I.; Putti, R.; Lionetti, L. High-fish oil and high-lard diets differently affect testicular antioxidant defense and mitochondrial fusion/fission balance in male wistar rats: Potential protective effect of ω3 polyunsaturated fatty acids targeting mitochondria dynamics. Int. J. Mol. Sci. 2019, 20, 3110. [Google Scholar] [CrossRef]
- Qiu, B.; Zandkarimi, F.; Bezjian, C.T.; Reznik, E.; Soni, R.K.; Gu, W.; Jiang, X.; Stockwell, B.R. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell 2024, 187, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Dimauro, C.; Cesarani, A.; Dal Bosco, A.; Bartolini, D.; Galli, F.; Migni, A.; Sebastiani, B.; Signorini, C.; Oger, C.; et al. A Dynamic Model for Estimating the Interaction of ROS–PUFA–Antioxidants in Rabbit. Antioxidants 2022, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Islam, M.A.; Othman, N.H.; Noor, A.M.; Ibrahim, M. Effect of rice bran oil addition on the oxidative degradation and fatty acid composition of soybean oil during heating. Acta Sci. Pol. Technol. Aliment. 2019, 18, 427–438. [Google Scholar] [CrossRef]
- Runeberg, P.; Ryabukhin, D.; Lagerquist, L.; Rahkila, J.; Eklund, P. Transformations and antioxidative activities of lignans and stilbenes at high temperatures. Food Chem. 2023, 404, 134641. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Cao, C.; Kong, B.; Wang, H.; Zhang, H.; Liu, Q. Effects of different pH conditions on interfacial composition and protein-lipid co-oxidation of whey protein isolate-stabilised O/W emulsions. Food Hydrocoll. 2022, 131, 107752. [Google Scholar] [CrossRef]
- Suyuti, A.; Hendradi, E.; Purwanti, T. Physicochemical Characteristics, Entrapment Efficiency, and Stability of Nanostructured Lipid Carriers Loaded Coenzyme Q10 with Different Lipid Ratios. J. Res. Pharm. 2023, 27, 1134–1142. [Google Scholar] [CrossRef]
- Ucar, A.; Özgeriş, F.B.; Çilingir Yeltekin, A.; Parlak, V.; Alak, G.; Keleş, M.S.; Atamanalp, M. The effect of N-acetylcysteine supplementation on the oxidative stress levels, apoptosis, DNA damage, and hematopoietic effect in pesticide-exposed fish blood. J. Biochem. Mol. Toxicol. 2019, 33, e22311. [Google Scholar] [CrossRef]
- Biobaku, F.; Ghanim, H.; Batra, M.; Dandona, P. Macronutrient-Mediated Inflammation and Oxidative Stress: Relevance to Insulin Resistance, Obesity, and Atherogenesis. J. Clin. Endocrinol. Metab. 2019, 104, 6118–6128. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Gonzalez, A.; Huerta-Salgado, C.; Orozco-Aguilar, J.; Aguirre, F.; Tacchi, F.; Simon, F.; Cabello-Verrugio, C. Role of Oxidative Stress in Hepatic and Extrahepatic Dysfunctions during Nonalcoholic Fatty Liver Disease (NAFLD). Oxid. Med. Cell. Longev. 2020, 2020, 1617805. [Google Scholar] [CrossRef]
- Abdelhafez, A.; Khabir, Z.; Prestidge, C.A.; Garcia-Bennett, A.; Joyce, P. The impact of formulation design on the oral bioavailability of omega-3 polyunsaturated fatty acids. Food Res. Int. 2025, 208, 116171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Yao, X.; Wang, K.; Cao, Y.; Zhang, C.; Chang, J.; Ren, H. Oxidation stability of seed oils from four woody oil plant species. CYTA—J. Food 2024, 22, 2285839. [Google Scholar] [CrossRef]
- Xu, E.; Chen, C.; Fu, J.; Zhu, L.; Shu, J.; Jin, M.; Wang, Y.; Zong, X. Dietary fatty acids in gut health: Absorption, metabolism and function. Anim. Nutr. 2021, 7, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, L.; Vachon, A.; Plourde, M.D.S. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J. Nutr. 2021, 151, 1111–1118. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Ou, X.; Liu, X.; Xu, Z.; Xiang, X.; Yang, Y.; Wang, Q. In-depth multiomic characterization of the effects of obesity in high-fat diet-fed mice. FEBS Open Bio 2024, 14, 771–792. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, Z.; Chen, X.; Miao, R.; Nilsson, C.; Lin, Y. Pharmacokinetics of Single and Multiple Doses of Omega-3 Carboxylic Acids in Healthy Chinese Subjects: A Phase I, Open-Label Study. Clin. Pharmacol. Drug Dev. 2020, 9, 985–994. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.X. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Krishna, A.R.; Gurumoorthy, S.; Elayappan, P.; Sakthivadivel, P.; Kumaran, S.; Pushparaj, P. A Review on the Application of Nanotechnology in Food Industries. Curr. Res. Nutr. Food Sci. 2022, 10, 871–883. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Mohite, P.; Rajput, T.; Pandhare, R.; Sangale, A.; Singh, S.; Prajapati, B.G. Nanoemulsion in Management of Colorectal Cancer: Challenges and Future Prospects. Nanomanufacturing 2023, 3, 139–166. [Google Scholar] [CrossRef]
- Malode, M.G.P.; Chauhan, S.A.; Bartare, S.A.; Malode, L.M.; Manwar, J.V.; Bakal, R.L. A Critical Review on Nanoemulsion: Advantages, Techniques and Characterization. J. Appl. Pharm. Sci. Res. 2022, 4, 6–12. [Google Scholar] [CrossRef]
- Rao, S.; Radhakrishnan, P.; Valiathan, S.; M, S. Rosehip oil nanoemulsion as a stable delivery system for omega-3 fatty acids to enhance the nutritional value of yogurt. Food Chem. Adv. 2023, 3, 100545. [Google Scholar] [CrossRef]
- Shi, X.; Cao, Y.; Li, N.; Zhu, N.; Chen, Y.; Ma, B. Composition, physicochemical properties, preparation methods and application research status on Functional oils and fats of nanoemulsion: A comprehensive review. IOP Conf. Ser. Earth Environ. Sci. 2021, 792, 012021. [Google Scholar] [CrossRef]
- Alfaro-Rodríguez, M.C.; Prieto, P.; García, M.C.; Martín-Piñero, M.J.; Muñoz, J. Influence of nanoemulsion/gum ratio on droplet size distribution, rheology and physical stability of nanoemulgels containing inulin and omega-3 fatty acids. J. Sci. Food Agric. 2022, 102, 6397–6403. [Google Scholar] [CrossRef]
- Hamed, S.F.; Abo-Elwafa, G.A. Preparation of novel nanoemulsions from omega-3 rich oil. Grasas Aceites 2020, 71, e350. [Google Scholar] [CrossRef]
- Adena, S.K.R.; Herneisey, M.; Pierce, E.; Hartmeier, P.R.; Adlakha, S.; Hosfeld, M.A.I.; Drennen, J.K.; Janjic, J.M. Quality by design methodology applied to process optimization and scale up of curcumin nanoemulsions produced by catastrophic phase inversion. Pharmaceutics 2021, 13, 880. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation of Factors Influencing Formation of Nanoemulsion by Spontaneous Emulsification: Impact on Droplet Size, Polydispersity Index, and Stability. Bioengineering 2022, 9, 384. [Google Scholar] [CrossRef]
- Prakasha, R.; Vinay, G.M.; Srilatha, P.; Pandey, H. Nanoemulsions as Carriers of Bioactive Compounds in Functional Foods: Preparation and Application. Eur. J. Nutr. Food Saf. 2025, 17, 78–95. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Aldawsari, H.M.; Hosny, K.M.; Ahmad, J.; Akhter, S.; Kammoun, A.K.; Alghaith, A.F.; Asfour, H.Z.; Al-Rabia, M.W.; Md, S. Formulation design and pharmacokinetic evaluation of docosahexaenoic acid containing self-nanoemulsifying drug delivery system for oral administration. Nanomater. Nanotechnol. 2020, 10, 1847980420950988. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y.; et al. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hadian, Z. A Review of Nanoliposomal Delivery System for Stabilization of Bioactive Omega-3 Fatty Acids. Electron. Physician 2016, 8, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Vieira, M.d.C.; Vieira, S.A.G.; Skupien, J.A.; Boeck, C.R. Nanoencapsulation of unsaturated omega-3 fatty acids as protection against oxidation: A systematic review and data-mining. Crit. Rev. Food Sci. Nutr. 2022, 62, 4356–4370. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, L.; Li, M.; Lyu, F.; Liu, J.; Ding, Y. Omega-3 polyunsaturated fatty acid encapsulation system: Physical and oxidative stability, and medical applications. Food Front. 2022, 3, 239–255. [Google Scholar] [CrossRef]
- Jala, R.C.R.; Zhang, H.; Yang, M.; Guo, R.; Li, S.; Xu, X.; Yang, D.; Xu, X. Encapsulation of DHA oils for better bioavailability: A review from the practical aspect. J. Am. Oil Chem. Soc. 2025, 102, 1089–1112. [Google Scholar] [CrossRef]
- Ahari, H.; Nasiri, M. Ultrasonic technique for production of nanoemulsions for food packaging purposes: A review study. Coatings 2021, 11, 847. [Google Scholar] [CrossRef]
- Abdel-Razek, A.G.; Hassanein, M.M.; Ozçelik, B.; Baranenko, D.A.; El-Messery, T.M. Omega fatty acid-balanced oil formula and enhancing its oxidative stability by encapsulation with whey protein concentrate. Food Biosci. 2022, 50, 101975. [Google Scholar] [CrossRef]
- Jagtap, A.A.; Badhe, Y.S.; Hegde, M.V.; Zanwar, A.A. Development and characterization of stabilized omega-3 fatty acid and micronutrient emulsion formulation for food fortification. J. Food Sci. Technol. 2021, 58, 996–1004. [Google Scholar] [CrossRef]
- Mittal, S.; Ali, J.; Baboota, S. Enhanced anti-psoriatic activity of tacrolimus loaded nanoemulsion gel via omega 3—Fatty acid (EPA and DHA) rich oils-fish oil and linseed oil. J. Drug Deliv. Sci. Technol. 2021, 63, 102458. [Google Scholar] [CrossRef]
- Almasi, K.; Esnaashari, S.S.; Khosravani, M.; Adabi, M. Yogurt fortified with omega-3 using nanoemulsion containing flaxseed oil: Investigation of physicochemical properties. Food Sci. Nutr. 2021, 9, 6186–6193. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, Z. Food-grade systems for delivery of DHA and EPA: Opportunities, fabrication, characterization and future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2348–2365. [Google Scholar] [CrossRef] [PubMed]
- Inapurapu, S.P.; Ibrahim, A.; Kona, S.R.; Pawar, S.C.; Bodiga, S.; Bodiga, V.L. Development and characterization of ω-3 fatty acid nanoemulsions with improved physicochemical stability and bioaccessibility. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125515. [Google Scholar] [CrossRef]
- Zhang, L.; Han, C.; Liu, M.; Yang, H.; Zhang, F.; Liu, B.; Meng, X. The formation, stability of DHA/EPA nanoemulsion prepared by emulsion phase inversion method and its application in apple juice. Food Res. Int. 2020, 133, 109132. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tan, T.; Chu, W.; Zhang, Y.; Ye, Y.; Wang, S.; Qin, Y.; Tang, J.; Cao, X. Co-delivery of paclitaxel (PTX) and docosahexaenoic acid (DHA) by targeting lipid nanoemulsions for cancer therapy. Drug Deliv. 2022, 29, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.E.; Zhou, Q.; Robinson, S.; Li, W. The composition and oxidative stability of vegetarian omega-3 algal oil nanoemulsions suitable for functional food enrichment. J. Sci. Food Agric. 2020, 100, 695–704. [Google Scholar] [CrossRef]
- Riquelme, N.; Robert, P.; Arancibia, C. Desserts Enriched with a Nanoemulsion Loaded with Vitamin D3 and Omega-3 Fatty Acids for Older People. Foods 2024, 13, 2073. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Zhou, Q.; Lane, K.E.; Li, W. Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model. Foods 2024, 13, 2407. [Google Scholar] [CrossRef]
- Yousefpoor, Y.; Esnaashari, S.S.; Baharifar, H.; Mehrabi, M.; Amani, A. Current challenges ahead in preparation, characterization, and pharmaceutical applications of nanoemulsions. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1920. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Xu, D. Docosahexaenoic Acid Delivery Systems, Bioavailability, Functionality, and Applications: A Review. Foods 2022, 11, 2685. [Google Scholar] [CrossRef]
- Jensen, G.M.; Hodgson, D.F. Opportunities and challenges in commercial pharmaceutical liposome applications. Adv. Drug Deliv. Rev. 2020, 154–155, 2–12. [Google Scholar] [CrossRef]
- Choudhury, A.; Sonowal, K.; Laskar, R.E.; Deka, D.; Dey, B.K. Liposome: A carrier for effective drug delivery. J. Appl. Pharm. Res. 2020, 8, 22–28. [Google Scholar] [CrossRef]
- Rahim, M.A.; Zahran, H.A.; Jaffar, H.M.; Ambreen, S.; Ramadan, M.F.; Al-Asmari, F.; Castro-Muñoz, R.; Zongo, E. Liposomal Encapsulation in Food Systems: A Review of Formulation, Processing, and Applications. Food Sci. Nutr. 2025, 13, e70587. [Google Scholar] [CrossRef]
- Bhat, R.S.; Alsuhaibani, A.S.; Albugami, F.S.; Aldawsari, F.S. Omega 3 Fatty Acid as A Health Supplement: An Overview of its Manufacture and Regulatory Aspects. Curr. Res. Nutr. Food Sci. 2024, 12, 70–90. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]
- Romero-Arrieta, M.R.; Uria-Canseco, E.; Perez-Casas, S. Simultaneous encapsulation of hydrophilic and lipophilic molecules in liposomes of DSPC. Thermochim. Acta 2020, 687, 178462. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef]
- Mousavipour, N.; Babaei, S.; Moghimipour, E.; Moosavi-Nasab, M.; Ceylan, Z. A novel perspective with characterized nanoliposomes: Limitation of lipid oxidation in fish oil. Lebensm. Wiss. Technol. 2021, 152, 112387. [Google Scholar] [CrossRef]
- Amrei, S.M.H.G.; Ahmadi, M.; Shahidi, S.A.; Ariaii, P.; Golestan, L. Preparation, characterization, and antioxidant activity of nanoliposomes-encapsulated turmeric and omega-3. J. Food Meas. Charact. 2023, 17, 2697–2707. [Google Scholar] [CrossRef]
- Choudhary, P.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Liposomal encapsulation of omega-3 fatty acid and α-lipoic acid conjugate for cow milk fortification. J. Food Process. Preserv. 2022, 46, e16082. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, J.; Zafar, S.; Warsi, M.H.; Abdel-Wahab, B.A.; Akhter, S.; Alam, M.A. Omega-3 fatty acids as adjunctive therapeutics: Prospective of nanoparticles in its formulation development. Ther. Deliv. 2019, 11, 851–868. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Dogra, A.; Nagpal, T.; Sharma, C.; Singh, S.; Shaiva, N.; Saini, G.; Luhach, K. Liposome-like encapsulation of fish oil-based self-nano emulsifying formulation for improved bioavailability. Appl. Food Res. 2025, 5, 100745. [Google Scholar] [CrossRef]
- Shabana, S.; Hamouda, H.I.; Hamadou, A.H.; Ahmed, B.; Chi, Z.; Liu, C. Marine phospholipid nanoliposomes: A promising therapeutic approach for inflammatory bowel disease: Preparation, safety, and efficacy evaluation. Colloids Surf. B Biointerfaces 2024, 234, 113702. [Google Scholar] [CrossRef]
- Amiri, H.; Shabanpour, B.; Pourashouri, P.; Kashiri, M. Preparation of functional supplement powder using nanoliposome-containing marine bioactive compounds. J. Food Sci. 2024, 89, 8658–8672. [Google Scholar] [CrossRef]
- Shariat, S.; Hakimzadeh, V.; Pardakhty, A. The physicochemical and organoleptic evaluation of the nano/ micro encapsulation of Omega-3 fatty acids in lipid vesicular systems. Nanomed. J. 2020, 7, 80–86. Available online: https://nmj.mums.ac.ir/article_14024_4194cc67ada64bbebc5d54d1d3147665.pdf (accessed on 11 September 2025).
- Lasoń, E. Nanoliposomes as a Smart Delivery System of Nutraceutical Supplements. Eng. Biomater. 2024, 27, 9–14. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Soofi, M.; Rezaei, M. Enhanced physicochemical stability of ω-3 PUFAs concentrates-loaded nanoliposomes decorated by chitosan/gelatin blend coatings. Food Chem. 2021, 345, 128865. [Google Scholar] [CrossRef]
- Zelikina, D.; Chebotarev, S.; Komarova, A.; Balakina, E.; Antipova, A.; Martirosova, E.; Anokhina, M.; Palmina, N.; Bogdanova, N.; Lysakova, E.; et al. Efficiency of an oral delivery system based on a liposomal form of a combination of curcumin with a balanced amount of n-3 and n-6 PUFAs encapsulated in an electrostatic complex of WPI with chitosan. Colloids Surf. A Physicochem Eng. Asp. 2022, 651, 129630. [Google Scholar] [CrossRef]
- Kurniawan, M.O.; Mittal, A.; Benjakul, S.; Singh, A. Ultrasonicated omega-3-enriched skipjack tuna eyeball oil nanoliposome: Preparation, characterisation, and fortification in milk. Int. J. Food Sci. Technol. 2024, 59, 6975–6986. [Google Scholar] [CrossRef]
- Kuznetcova, D.V.; Linder, M.; Jeandel, C.; Paris, C.; Desor, F.; Baranenko, D.A.; Nadtochii, L.A.; Arab-Tehrany, E.; Yen, F.T. Nanoliposomes and nanoemulsions based on chia seed lipids: Preparation and characterization. Int. J. Mol. Sci. 2020, 21, 9079. [Google Scholar] [CrossRef]
- Benjakul, S.; Saetang, J.; Mittal, A.; Seow, E.K.; Singh, A. Incorporation of omega-3 enriched shrimp oil nanoliposomes in threadfin bream surimi gel: Gel properties, oxidative stability, and bioavailability assessed via Caco-2 cells. Food Biosci. 2025, 71, 107120. [Google Scholar] [CrossRef]
- Hassanshahi, G.; Karimabad, M.N.; Jebali, A. The therapeutic effect of PEGlated nanoliposome of pistachio unsaturated oils and its efficacy to attenuate inflammation in multiple sclerosis: A randomized, double-blind, placebo-controlled clinical trial phase I. J. Neuroimmunol. 2022, 362, 577768. [Google Scholar] [CrossRef]
- Trucillo, P.; Zamparelli, R.; Iuorio, S.; De Stefanis, P.; Reverchon, E. Economic analysis of a new business for liposome manufacturing using a high-pressure system. Processes 2020, 8, 1604. [Google Scholar] [CrossRef]
- Homroy, S.; Chopra, R.; Singh, P.K.; Dhiman, A.; Chand, M.; Talwar, B. Role of encapsulation on the bioavailability of omega-3 fatty acids. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13272. [Google Scholar] [CrossRef] [PubMed]
- Azumah, J.; Smistad, G.; Hiorth, M. Preparation of stable polymer-liposome complexes by a novel approach employing a one-pot method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129924. [Google Scholar] [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Haghighi, F.H.; Chronopoulou, L.; Palocci, C. Liposome–Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10, 284. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Emon, D.D.; Islam, M.S.; Mazumder, M.A.R.; Aziz, M.G.; Rahman, M.S. Recent applications of microencapsulation techniques for delivery of functional ingredient in food products: A comprehensive review. Food Chem. Adv. 2025, 6, 100923. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.; Jaiswal, S.G.; Dave, J.; Wei, S.; Hailu, G.G. Recent trends in the encapsulation of functional lipids: Comprehensive review. Sustain. Food Technol. 2024, 2, 1610–1630. [Google Scholar] [CrossRef]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Sánchez-Osorno, D.M.; López-Jaramillo, M.C.; Paz, A.V.C.; Villa, A.L.; Peresin, M.S.; Martínez-Galán, J.P. Recent Advances in the Microencapsulation of Essential Oils, Lipids, and Compound Lipids through Spray Drying: A Review. Pharmaceutics 2023, 15, 1490. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and protection of omega-3-rich fish oils using food-grade delivery systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef]
- Akram, S.; Bao, Y.; Butt, M.S.; Shukat, R.; Afzal, A.; Huang, J.-Y. Fabrication and characterization of gum arabic- and maltodextrin-based microcapsules containing polyunsaturated oils. J. Sci. Food Agric. 2021, 101, 6384–6394. [Google Scholar] [CrossRef]
- Marfil, P.H.M.; Paulo, B.B.; Alvim, I.D.; Nicoletti, V.R. Production and characterization of palm oil microcapsules obtained by complex coacervation in gelatin/gum Arabic. J. Food Process Eng. 2018, 41, e12673. [Google Scholar] [CrossRef]
- Hamed, S.F.; Hashim, A.F.; Abdel Hamid, H.A.; Abd-Elsalam, K.A.; Golonka, I.; Musiał, W.; El-Sherbiny, I.M. Edible alginate/chitosan-based nanocomposite microspheres as delivery vehicles of omega-3 rich oils. Carbohydr. Polym. 2020, 239, 116201. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, C.; Ma, X.; Yan, W.; Wang, F. Preparation and characterization of walnut oil microcapsules by complex coacervation with sodium alginate and chitosan. Lebensm. Wiss. Technol. 2025, 222, 117630. [Google Scholar] [CrossRef]
- Kalkumbe, A.; Waghmare, P.S.; Kamble, P.H. Microencapsulation: A review. Int. Res. J. Mod. Eng. Technol. Sci. 2022, 3, 3844–3850. [Google Scholar]
- Muhoza, B.; Yuyang, H.; Uriho, A.; Harindintwali, J.D.; Liu, Q.; Li, Y. Spray-and freeze-drying of microcapsules prepared by complex coacervation method: A review. Food Hydrocoll. 2023, 140, 108650. [Google Scholar] [CrossRef]
- Yang, M.; Li, L.; Zhu, X.; Liang, L.; Chen, J.; Cao, W.; Liu, W.; Duan, X.; Ren, G.; Liu, Z. Microencapsulation of fish oil by spray drying, spray freeze-drying, freeze-drying, and microwave freeze-drying. J. Food Sci. 2024, 15, 37–48. [Google Scholar] [CrossRef]
- Bukke, S.P.N.; Venkatesh, C.; Bandenahalli Rajanna, S.; Shanmugam Saraswathi, T.; Kusuma, P.K.; Goruntla, N.; Balasuramanyam, N.; Munishamireddy, S. Solid lipid nanocarriers for drug delivery: Design innovations and characterization strategies—A comprehensive review. Discov. Appl. Sci. 2024, 6, 279. [Google Scholar] [CrossRef]
- Gokul, V.; Kothapalli, P.; Vasanthan, M. A Comprehensive Review on Solid Lipid Nanoparticles as a Carrier for Oral Absorption of Phyto-Bioactives. Cureus 2024, 16, e68339. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Mall, J.; Naseem, N.; Haider, M.F.; Rahman, M.A.; Khan, S.; Siddiqui, S.N. Nanostructured lipid carriers as a drug delivery system: A comprehensive review with therapeutic applications. Intell. Pharm. 2025, 3, 243–255. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Garg, J.; Pathania, K.; Sah, S.P.; Pawar, S.V. Nanostructured lipid carriers: A promising drug carrier for targeting brain tumours. Futur. J. Pharm. Sci. 2022, 8, 25. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Silva, T.J.; da Silva, M.G.; Hashimoto, J.C.; Ribeiro, A.P.B. Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols. Foods 2025, 14, 973. [Google Scholar] [CrossRef] [PubMed]
- Shahparast, Y.; Eskandani, M.; Rajaei, A.; Khosroushahi, A.Y. Preparation, physicochemical characterization and oxidative stability of omega-3 fish oil/α-tocopherol-co-loaded nanostructured lipidic carriers. Adv. Pharm. Bull. 2019, 9, 393–400. [Google Scholar] [CrossRef]
- Li, Z.; Yin, Z.; Li, B.; He, J.; Liu, Y.; Zhang, N.; Li, X.; Cai, Q.; Meng, W. Docosahexaenoic Acid-Loaded Nanostructured Lipid Carriers for the Treatment of Peri-Implantitis in Rats. Int. J. Mol. Sci. 2023, 24, 1872. [Google Scholar] [CrossRef] [PubMed]
- Korpak, K.; Rossi, M.; Van Meerhaeghe, A.; Boudjeltia, K.Z.; Compagnie, M. Omega-3 long-chain polyunsaturated fatty acids and their bioactive lipids: A strategy to improve resistance to respiratory tract infectious diseases in the elderly? Nutr. Healthy Aging 2024, 9, 55–76. [Google Scholar] [CrossRef]
- Duan, J.; Song, Y.; Zhang, X.; Wang, C. Effect of ω-3 Polyunsaturated Fatty Acids-Derived Bioactive Lipids on Metabolic Disorders. Front. Physiol. 2021, 12, 646491. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016, 785, 44–49. [Google Scholar] [CrossRef]
- Li, Q.; Cui, K.; Wu, M.; Xu, D.; Mai, K.; Ai, Q. Polyunsaturated Fatty Acids Influence LPS-Induced Inflammation of Fish Macrophages Through Differential Modulation of Pathogen Recognition and p38 MAPK/NF-κB Signaling. Front. Immunol. 2020, 11, 559332. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.; Yang, W.; Liu, J.; Gao, M.Q. Omega-3 polyunsaturated fatty acids ameliorated inflammatory response of mammary epithelial cells and mammary gland induced by lipopolysaccharide. Acta Biochim. Biophys. Sin. 2021, 53, 1142–1153. [Google Scholar] [CrossRef]
- Romacho, T.; Glosse, P.; Richter, I.; Elsen, M.; Schoemaker, M.H.; Van Tol, E.A.; Eckel, J. Nutritional ingredients modulate adipokine secretion and inflammation in human primary adipocytes. Nutrients 2015, 7, 865. [Google Scholar] [CrossRef]
- Fedullo, A.L.; Schiattarella, A.; Morlando, M.; Raguzzini, A.; Toti, E.; De Franciscis, P.; Peluso, I. Mediterranean diet for the prevention of gestational diabetes in the covid-19 era: Implications of ll-6 in diabesity. Int. J. Mol. Sci. 2021, 22, 1213. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Goonapienuwala, B.L.; Moustaid-Moussa, N. Omega-3 Fatty Acids and Adipose Tissue: Inflammation and Browning. Annu. Rev. Nutr. 2020, 40, 25–49. [Google Scholar] [CrossRef]
- Flachs, P.; Rossmeisl, M.; Kuda, O.; Kopecky, J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: A key to lean phenotype. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 986–1003. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. The FTO Gene, Browning of Adipose Tissue and Omega-3 Fatty Acids. Lifestyle Genom. 2016, 9, 123–126. [Google Scholar] [CrossRef]
- Baum, J.; Shouse, S.; Lassiter, K.; Bottje, W.; Dridi, S. Leucine and Omega—3 Fatty Acids Regulate Cell Bioenergetics via mTOR. FASEB J. 2015, 29, 742.16. [Google Scholar] [CrossRef]
- Pandurangan, S.B.; Al-Maiman, S.A.; Al-Harbi, L.N.; Alshatwi, A.A. Beneficial fatty acid ratio of Salvia hispanica L. (Chia Seed) potentially inhibits adipocyte hypertrophy, and decreases adipokines expression and inflammation in macrophage. Foods 2020, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.E.; Smesny, S.; Kim, S.-W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry 2017, 7, e1220. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, Y.; Mu, C.; Tang, P.; Liu, Y.; Huang, Y.; Luo, H.; Liu, J.-Y.; Li, X. The Biomarkers in Extreme Longevity: Insights Gained from Metabolomics and Proteomics. Int. J. Med Sci. 2024, 21, 2725–2744. [Google Scholar] [CrossRef]
- Azzolino, D.; Bertoni, C.; De Cosmi, V.; Spolidoro, G.C.I.; Agostoni, C.; Lucchi, T.; Mazzocchi, A. Omega-3 polyunsatured fatty acids and physical performance across the lifespan: A narrative review. Front. Nutr. 2024, 11, 1414132. [Google Scholar] [CrossRef]
- Crivelli, S.M.; Giovagnoni, C.; Visseren, L.; Scheithauer, A.-L.; de Wit, N.; den Hoedt, S.; Losen, M.; Mulder, M.T.; Walter, J.; de Vries, H.E.; et al. Sphingolipids in Alzheimer’s disease, how can we target them? Drug Deliv. Rev. 2020, 159, 214–231. [Google Scholar] [CrossRef]
- Bianconi, S.; Santillán, M.E.; del Rosario Solís, M.; Martini, A.C.; Ponzio, M.F.; Vincenti, L.M.; Schiöth, H.B.; Carlini, V.P.; Stutz, G. Effects of dietary omega-3 PUFAs on growth and development: Somatic, neurobiological and reproductive functions in a murine model. J. Nutr. Biochem. 2018, 61, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Church, M.W.; Jen, K.C.; Anumba, J.I.; Jackson, D.A.; Adams, B.R.; Hotra, J.W. Neurotoxicology and Teratology Excess omega-3 fatty acid consumption by mothers during pregnancy and lactation caused shorter life span and abnormal ABRs in old adult offspring. Neurotoxicol. Teratol. 2010, 32, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Fang, Z.; Zhang, T.; Chen, Y. Polyunsaturated fatty acid intake and incidence of type 2 diabetes in adults: A dose response meta-analysis of cohort studies. Diabetol. Metab. Syndr. 2022, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef]
- Hooper, L.; Abdelhamid, A.; Brainard, J.; Deane, K.H.O.; Song, F. Creation of a database to assess effects of omega-3, omega-6 and total polyunsaturated fats on health: Methodology for a set of systematic reviews. BMJ Open 2019, 9, e029554. [Google Scholar] [CrossRef]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C.; et al. Systematic Review on N-3 and N-6 Polyunsaturated Fatty Acid Intake in European Countries in Light of the Current Recommendations—Focus on Specific Population Groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef]
- Hajri, T. Effects of oxidized lipids and lipoproteins on cardiac function. Front. Biosci. 2018, 23, 1822–1847. [Google Scholar] [CrossRef]
- Findeisen, H.M.; Voges, V.C.; Braun, L.C.; Sonnenberg, J.; Schwarz, D.; Körner, H.; Reinecke, H.; Sohrabi, Y. LXRα Regulates oxLDL-Induced Trained Immunity in Macrophages. Int. J. Mol. Sci. 2022, 23, 6166. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Nikiforov, N.G.; Markin, A.M.; Kashirskikh, D.A.; Myasoedova, V.A.; Gerasimova, E.V.; Orekhov, A.N. Overview of OxLDL and Its Impact on Cardiovascular Health: Focus on Atherosclerosis. Front. Pharmacol. 2021, 11, 613780. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Zhang, B.; Li, D.; Yan, J.; Yang, J.; Sun, J.; Cao, H.; Wang, Y.; Zhang, F. Effects of different n-6/n-3 polyunsaturated fatty acids ratios on lipid metabolism in patients with hyperlipidemia: A randomized controlled clinical trial. Front. Nutr. 2023, 10, 1166702. [Google Scholar] [CrossRef]
- Ahmadi, A.R.; Shirani, F.; Abiri, B.; Siavash, M.; Haghighi, S.; Akbari, M. Impact of omega-3 fatty acids supplementation on the gene expression of peroxisome proliferator activated receptors-γ, α and fibroblast growth factor-21 serum levels in patients with various presentation of metabolic conditions: A GRADE assessed systematic review and dose–response meta-analysis of clinical trials. Front. Nutr. 2023, 10, 1202688. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Juliano, R.A.; Copland, C.; Bhatt, D.L.; Libby, P.; Mason, R.P. EPA and DHA containing phospholipids have contrasting effects on membrane structure. J. Lipid Res. 2021, 62, 100106. [Google Scholar] [CrossRef] [PubMed]
- Michaeloudes, C.; Christodoulides, S.; Christodoulou, P.; Kyriakou, T.-C.; Patrikios, I.; Stephanou, A. Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations. Nutrients 2023, 15, 4830. [Google Scholar] [CrossRef]
- Tseng, P.-T.; Zeng, B.-Y.; Hsu, C.-W.; Liang, C.-S.; Stubbs, B.; Chen, Y.-W.; Chen, T.-Y.; Lei, W.-T.; Chen, J.-J.; Shiue, Y.-L.; et al. The Optimal Dosage and Duration of ω-3 PUFA Supplementation in Heart Failure Management: Evidence from a Network Meta-Analysis. Adv. Nutr. 2025, 16, 100366. [Google Scholar] [CrossRef]
- Uti, D.E.; Alum, E.U.; Atangwho, I.J.; Ugwu, O.P.C.; Egbung, G.E.; Aja, P.M. Lipid-based nano-carriers for the delivery of anti-obesity natural compounds: Advances in targeted delivery and precision therapeutics. J. Nanobiotechnol. 2025, 23, 336. [Google Scholar] [CrossRef]
- Alfutaimani, A.S.; Alharbi, N.K.; Alahmari, A.S.; Alqabbani, A.A.; Aldayel, A.M. Exploring the landscape of Lipid Nanoparticles (LNPs): A comprehensive review of LNPs types and biological sources of lipids. Int. J. Pharm. X 2024, 8, 100305. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Farag, H.A.M.; Salih, T.H.; Awlqadr, F.H.; Al-Manhel, A.J.A.; Vieira, I.R.S.; Conte-Junior, C.A. Application of Nanoparticles in Human Nutrition: A Review. Nutrients 2024, 16, 636. [Google Scholar] [CrossRef]
- Fernandes, F.; Dias-Teixeira, M.; Delerue-Matos, C.; Grosso, C. Critical review of lipid-based nanoparticles as carriers of neuroprotective drugs and extracts. Nanomaterials 2021, 11, 563. [Google Scholar] [CrossRef]
- Cuenoud, B.; Rochat, I.; Gosoniu, M.L.; Dupuis, L.; Berk, E.; Jaudszus, A.; Mainz, J.G.; Hafen, G.; Beaumont, M.; Cruz-Hernandez, C. Monoacylglycerol form of omega-3s improves its bioavailability in humans compared to other forms. Nutrients 2020, 12, 1014. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Maeng, H.J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Lúcio, M.; Giannino, N.; Barreira, S.; Catita, J.; Gonçalves, H.; Ribeiro, A.; Fernandes, E.; Carvalho, I.; Pinho, H.; Cerqueira, F.; et al. Nanostructured Lipid Carriers Enriched Hydrogels for Skin Topical Administration of Quercetin and Omega-3 Fatty Acid. Pharmaceutics 2023, 15, 2078. [Google Scholar] [CrossRef]
- Gimondi, S.; Guimarães, C.F.; Vieira, S.F.; Gonçalves, V.M.F.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic mixing system for precise PLGA-PEG nanoparticles size control. Nanomedicine 2022, 40, 102482. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.; Gerogiorgis, D.I. Design space identification and visualization for continuous pharmaceutical manufacturing. Pharmaceutics 2020, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Winter, E.; Pizzol, C.D.; Locatelli, C.; Crezkynski-Pasa, T.B. Development and evaluation of lipid nanoparticles for drug delivery: Study of toxicity in vitro and in vivo. J. Nanosci. Nanotechnol. 2016, 16, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Madhunapantula, S.V.; Robertson, G.P. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin. Drug Metab. Toxicol. 2012, 8, 47–69. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Patel, M.; Bajwa, N.; Prasad, R.; Vora, L.K. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape. Small 2025, 21, 2502315. [Google Scholar] [CrossRef]
- Ashfaq, R.; Rasul, A.; Asghar, S.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Lipid Nanoparticles: An Effective Tool to Improve the Bioavailability of Nutraceuticals. Int. J. Mol. Sci. 2023, 24, 15764. [Google Scholar] [CrossRef]
- Xiao, S.; Tang, Y.; Lin, Y.; Lv, Z.; Chen, L. Tracking Osteoarthritis Progress through Cationic Nanoprobe-Enhanced Photoacoustic Imaging of Cartilage. Acta Biomater. 2020, 109, 153–162. [Google Scholar] [CrossRef]
- Rao, A.; Briskey, D.; Nalley, J.O.; Ganuza, E. Omega-3 eicosapentaenoic acid (Epa) rich extract from the microalga nannochloropsis decreases cholesterol in healthy individuals: A double-blind, randomized, placebo-controlled, three-month supplementation study. Nutrients 2020, 12, 1869. [Google Scholar] [CrossRef]
| Type | Source | Omega-3 (%) | Omega-6 (%) | References |
|---|---|---|---|---|
| Vegetable | Flaxseed oil | 65.84 (ALA) | 16.39 (LA) | [43] |
| Vegetable | Chia oil | 63.64 (ALA) | 19.84 (LA) | [44] |
| Vegetable | Canola oil | 11 (ALA) | 21 (LA) | [45] |
| Vegetable | Olive oil | 19.47 (ALA) | 17.93 (LA) | [46] |
| Vegetable | Walnuts | 17.9 (ALA) | 63.8 (LA) | [47] |
| Animal | Salmon | 3–4 (EPA) 9–12 (DHA) | - | [48] |
| Animal | Sardine | 17.3–23.7 (EPA) 5.82–13.5 (DHA) | - | [49] |
| Animal | Mackerel | 4.93–5.81 (EPA) 12.56–15.01 (DHA) | - | [50] |
| Animal | Tuna | 9.32–9.56 (EPA) 18.76–25.88 (DHA) | - | [51] |
| Animal | Trout | 3.65–5.54 (EPA) 13.53–32.81 (DHA) | - | [52] |
| Microbial | Schizochytrium sp. | 35–40% (DHA) | - | [37] |
| Microbial | Aurantiochytrium sp. | 25.98–35.76 (DHA) | - | [39] |
| Microbial | Mortierella alpina | - | 46.9–66.4 (ARA) | [53] |
| Type of Omega | Source of Omega | Stabilizer/Matrix | Production Method | Droplet Size | Additional Remarks | References |
|---|---|---|---|---|---|---|
| ALA | Soybean and walnut oil | Xanthan gum (in nanoemulgel) + inulin | Microfluidization | ~138 nm | Greater stability with lower oil/gum ratio (1:3); gel-like behavior; high viscosity | [98] |
| EPA and DHA | Fish and flaxseed oil | Tween 80 | Spontaneous emulsification + High-pressure homogenization | <130 nm | Development of topical gel for psoriasis treatment in mice; ↑ skin permeation (1.3–1.4×) and dermal retention; ↓ TNF-α and IL-6; ↓ PASI | [113] |
| ALA | Flaxseed oil (3%) | Tween 80 (28.97%) + Span 80 (7.03%) + ethanol (10%) | Low-energy method (HLB) | ~60 nm | Application in yogurt, maintaining pH, acidity, transparency, and functional potential; stability for 11 months | [114] |
| EPA and DHA | Fish oil | Surfactants: Tween 20 + SDS + lecithin; antioxidant: rosemary extract | High-pressure homogenization | ~175 nm | Oxidative stability increased up to 3× with rosemary extract; stability for 11 weeks at 25 °C | [99] |
| ALA | Flaxseed oil | Sucrose ester (emulsifier) + purified water (aqueous continuous phase) | High-energy homogenization | 674–799 nm | Good stability after freeze–thaw cycles; ↑ plasma EPA and DHA levels in rats; sensory acceptability enhanced by the nanostructured formulation | [112] |
| EPA, DHA, Omega-6, and balanced PUFA mixtures | Mixtures of vegetable oils (olive + palm olein) + krill oil (MKO) or flaxseed oil (MLO) | Whey protein concentrate (WPC) + maltodextrin (MD) + arabic gum (GA), at a ratio of 8:2:1 | Pre-homogenization with Ultra-Turrax + Microfluidization or Ultrasound | ~198.5 nm (MKO by US); ~201.3 nm (MKO by MF); ~824.9 nm (MLO by US); ~714.2 nm (MLO by MF) | Higher encapsulation efficiency with microfluidization + spray-drying (EE > 85%); improved oxidative stability and more spherical morphology with spray-drying | [111] |
| ALA, EPA and DHA | Fish/vegetable oil | Surfactant: Laureth-21; Co-surfactant: PEG-40 hydrogenated castor oil (HCO-40) | SNEDDS (self-nanoemulsifying drug delivery system) + pseudo-ternary phase diagram | 71–195 nm | High encapsulation (43–87%); greater release and permeation vs. tablet and suspension; ↓ ulceration in rats | [104] |
| DHA | Refined fish oil | Tween 80, Span 80 (surfactants) + whey protein isolate (WPI) | High-pressure homogenization | 120–180 nm | High encapsulation efficiency (~90%); stable under pH, salts, and temperature variations; ↑ bioavailability in rats; ↓ lipid peroxidation | [115] |
| ALA | Flaxseed oil | Different food-grade emulsifiers (Tween 80 was the most effective) | High-pressure homogenization | 70–150 nm | Nanoemulsions maintained stable characteristics under refrigerated storage, showing lower lipid oxidation compared with pure oil | [116] |
| ALA | Flaxseed oil | Food-grade surfactants (mainly Tween 80 and lecithin) | High-pressure homogenization and ultrasound | 50–150 nm | Nanoemulsions exhibited good physicochemical stability, higher oxidative resistance, and potential for application in functional beverages and nutritional supplements | [117] |
| DHA | DHA in triglyceride form (commercial) | Egg phosphatidylcholine (EPC), cholesterol (CHOL), DSPE-PEG2000-FA (when folate-decorated) | Thin-film solvent evaporation followed by microfluidizer processing | ~157.7 nm (PTX/DHA-LNs); ~186.6 nm (PTX/DHA-FA-LNs, folate-decorated) | High encapsulation efficiency (EE > 90%); stable in PBS and serum for 24 h; controlled release without burst effect (100% in 48 h); enhanced folate receptor-mediated internalization and improved antitumor efficacy in mice | [118] |
| Type of Omega | Source of Omega | Liposomal Composition | Production Method | Size/EE% | Additional Remarks | References |
|---|---|---|---|---|---|---|
| EPA and DHA | Fish oil | Liposome + nanoemulsion (SMEDS) | High-shear homogenization and softgel encapsulation | 100–300 nm | 13.2-fold (EPA) and 4.7-fold (DHA) increase in bioavailability in rats compared with conventional fish oil; low oxidation for 6 months | [137] |
| EPA and DHA | Cod liver oil and shrimp lipid extract, and carp FPH | Soy lecithin + FO (cod) + shrimp extract + FPH; coating with CS/WPC (mono-, bi-, or composite layer) | Ultrasonication, layer-by-layer coating (CS and WPC), and lyophilization | 38.1–100 nm/92.8–97.7% | Better oxidative stability in bilayer nanoliposomes (3 months); controlled release (low in stomach and high in intestine); 1.5 g of powder in 100 g of milk supplied daily PUFA and amino acid requirements with good sensory acceptance (fishy odor and taste masked) | [139] |
| EPA and DHA | Fish oil | Soy lecithin + curcumin (ethanolic extract) + omega-3 (1:4, 1:8, 1:12, 1:16 extract:lecithin ratio) | Dissolution of extract in ethanol, addition to acetate buffer, homogenization and ultrasonication | 100–170 nm/>50% | Controlled release in simulated gastrointestinal medium; ↑ antioxidant activity in formulations with higher curcumin + omega-3 content; ↑ antimicrobial activity | [134] |
| EPA and DHA | Shrimp oil | Soy phosphatidylcholine (2.5%) + cholesterol + enriched shrimp oil (2%) + glycerol (2% v/v) | Dissolution in heated ethanol, oil addition, hydration in water + glycerol, ultrasonication, solvent removal, and lyophilization | ~170–200 nm/97.6% | ↑ oxidative stability during 25 days of storage; good sensory acceptance; free fatty acid permeation in Caco-2 cells reduced from 85% to 75%, demonstrating modulation of absorption | [146] |
| ALA | Chia oil | Soy phosphatidylcholine + Tween 80 + chia oil + LA/hydroxypropyl-β-cyclodextrin inclusion complex | Thin-film hydration + probe sonication | ~52.2 nm/80.2% (LA); 76.4% (chia oil/ALA) | Applied in fortified cow’s milk, providing per serving (240 mL) 236 mg LA and 720 mg ALA; stable for 7 days at 4 °C; remained sensorially acceptable | [135] |
| EPA and DHA | Pistachio oil | Pistachio oil + lecithin + PEG (PEGylated nanoliposomes) | Oil + lecithin, sonication, and nanoliposomal suspension formation | 100–250 nm | ↑ in serum EPA and DHA levels in clinical trial patients, with consequent ↓ of inflammatory cytokines and MMP-9, ↑ IL-4, IL-5, and IL-10; no severe adverse events | [147] |
| EPA and DHA | Fish oil | Salmon lecithin + PUFAs + coating with chitosan/gelatin blend | Lecithin hydration (2%), oil addition (1:10, 1:5, 1:2), sonication, and coating with chitosan/gelatin (0.3:0.1 or 0.2:0.2) | Without coating: ~209–491 nm/62.9–74.5%; coated (SDNLs): ~420–454 nm/81.6% | Coating enhanced thermal and oxidative stability, acting as a physical and antioxidant barriers | [142] |
| EPA and DHA | Skipjack tuna eye oil | Soy lecithin (1–5%) + EPA/DHA-enriched oil (1–5%); addition of glycerol (2% v/v) as stabilizer | Hydration in ethanol, evaporation, nanoliposome formation, ultrasonication | Without ultrasonication: 22.8 nm/88%; with ultrasonication: 31–67 nm/98% | Ultrasonication ↑ encapsulation efficiency and the average particle size; fortification in pasteurized milk with 2.5% NL maintained sensory acceptance and ↑ PUFA content in milk; good oxidative stability | [144] |
| ALA | Chia oil | Phospholipid fraction rich in PI, PA, PE, PG, PC, and lyso-PC, obtained from the polar residue of chia oil | Folch extraction + spontaneous lipid hydration + sonication | ~118 nm | Transformation of phospholipid-rich byproducts (extraction residue) into functional nanocarriers | [145] |
| EPA and DHA | Fish oil | Soy lecithin + cholesterol + brown and green macroalgae extracts | Thin-film lipid hydration + sonication + lyophilization | 129–266 nm/99.9% | Nanoliposomes strongly ↓ lipid oxidation; good color stability and controlled release profile (<35%); comparable to or better than synthetic antioxidant (BHT) | [133] |
| EPA and DHA | Fish oil | Omega-3-rich phosphatidylcholine + cholesterol (PEGylated nanoliposome formation) | Lipid film hydration + sonication | 90–120 nm | Resistance to simulated gastric fluid; intestinal absorption confirmed in Caco-2 cells; anti-inflammatory effect in colitis model; safety demonstrated in cells, blood, and mice | [138] |
| EPA, DHA, and linoleic acid | Fish oil (EPA/DHA) + linoleic acid | Phosphatidylcholine + cholesterol, co-encapsulating PUFAs + curcumin; surface functionalized with chitosan and whey protein | Thin-film hydration + sonication, followed by CH/WPI coating | 150–200 nm/85% for curcumin and PUFAs | Chitosan and whey protein coating enhanced oxidative and thermal stability, improved water solubility and oral bioavailability, and enabled co-delivery of PUFAs + antioxidant (curcumin) | [143] |
| Types | Subtypes | Examples | Properties | Applications |
|---|---|---|---|---|
| Biopolymeric | Polysaccharides | Arabic Gum | High solubility, good emulsification | Supplements, drinks |
| Pectin | Pectins are natural polysaccharides, formed by galacturonic acid chains, and are widely used as gelling and encapsulating agents in the food and pharmaceutical industries | Gastrointestinal capsules | ||
| Sodium alginate | Forms ionic gels with Ca2+ | Gastrointestinal capsules | ||
| Modified starch | Improves acid resistance and enzymatic digestion, good oxidative stability and solubility | Gastrointestinal capsules | ||
| Carrageenan | Gel formation, compatible with controlled release | Gastrointestinal capsules | ||
| Maltodextrin | Spray-drying matrix former | Gastrointestinal capsules | ||
| Cellulosic | Ethylcellulose | Coating agent for tablets and capsules, providing controlled drug release | Encapsulating agent in supplement formulations | |
| Hydroxypropyl- methylcellulose | Cellulose derivative with methyl and hydroxypropyl group substitutions | Modified-release capsules | ||
| Proteins | Gelatin | Forms thermoreversible gels and is a good emulsifier | Softgels and capsules | |
| Casein | Good interaction with lipids | Electrostatic complexes between casein and anionic polysaccharides | ||
| Soy protein | Good oil retention | Functionality comparable to casein | ||
| Lipids | Lecithin | More effective encapsulating agents for the nanoencapsulation of omegas, due to their ability to stabilize oil/water interfaces and form self-organized nanometric structures. | Liquid supplements and softgels | |
| Mono/di-glycerides | Amphiphilic compounds are auxiliary agents in the encapsulation of omega family fatty acids | They help in the formation of dry microcapsules with proteins, polysaccharides, and modified starches, etc. | ||
| Synthetic biodegradable polymers | Poly-lactic-co-glycolic acid | Extended release and biodegradable, resulting in natural products (lactic acid and glycolic acid) | Microparticles and nanoparticles are used to encapsulate medicines and dietary supplements, promoting sustained release | |
| Polycaprolactone | Synthetic semicrystalline polyester, biodegradable and biocompatible | Targeted capsules and microcapsules | ||
| Polyethylene glycol | It is a hydrophilic, non-ionic polymer of the polyol family | Targeted capsules | ||
| Auxiliary functional compounds | Natural antioxidants (e.g., tocopherols, ascorbic acid) | Act by interrupting free radical chain reactions, stabilizing lipids during processing and storage | Reduce the oxidation of omegas during and after encapsulation | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazula, M.F.; Pozzan, R.; dos Reis, G.A.; Maciel, M.; Horlem, T.; Banckes, T.N.; Pereira, J.L.S.; Sales-Campos, C.; Fernandes, L.C.; Martinez-Burgos, W.J.; et al. Advances in Oral Drug Delivery Systems for Natural Polyunsaturated Fatty Acids: Enhancing Bioavailability and Therapeutic Potential. Pharmaceutics 2025, 17, 1377. https://doi.org/10.3390/pharmaceutics17111377
Zazula MF, Pozzan R, dos Reis GA, Maciel M, Horlem T, Banckes TN, Pereira JLS, Sales-Campos C, Fernandes LC, Martinez-Burgos WJ, et al. Advances in Oral Drug Delivery Systems for Natural Polyunsaturated Fatty Acids: Enhancing Bioavailability and Therapeutic Potential. Pharmaceutics. 2025; 17(11):1377. https://doi.org/10.3390/pharmaceutics17111377
Chicago/Turabian StyleZazula, Matheus Felipe, Roberta Pozzan, Guilherme Anacleto dos Reis, Mônica Maciel, Thomas Horlem, Tayná Nery Banckes, Josilene Lima Serra Pereira, Ceci Sales-Campos, Luiz Claudio Fernandes, Walter José Martinez-Burgos, and et al. 2025. "Advances in Oral Drug Delivery Systems for Natural Polyunsaturated Fatty Acids: Enhancing Bioavailability and Therapeutic Potential" Pharmaceutics 17, no. 11: 1377. https://doi.org/10.3390/pharmaceutics17111377
APA StyleZazula, M. F., Pozzan, R., dos Reis, G. A., Maciel, M., Horlem, T., Banckes, T. N., Pereira, J. L. S., Sales-Campos, C., Fernandes, L. C., Martinez-Burgos, W. J., & Naliwaiko, K. (2025). Advances in Oral Drug Delivery Systems for Natural Polyunsaturated Fatty Acids: Enhancing Bioavailability and Therapeutic Potential. Pharmaceutics, 17(11), 1377. https://doi.org/10.3390/pharmaceutics17111377










