Simian Immunodeficiency Virus-Derived Extracellular Vesicles Induce a Chronic Inflammatory Phenotype in Healthy Astrocytes Unresolved by Anti-Retroviral Therapy
Abstract
1. Introduction
2. Methods
2.1. Isolation and Characterization of EVs
2.2. Affinity Purification of Extracellular Vesicles
2.3. Nanoparticle Tracking Analysis
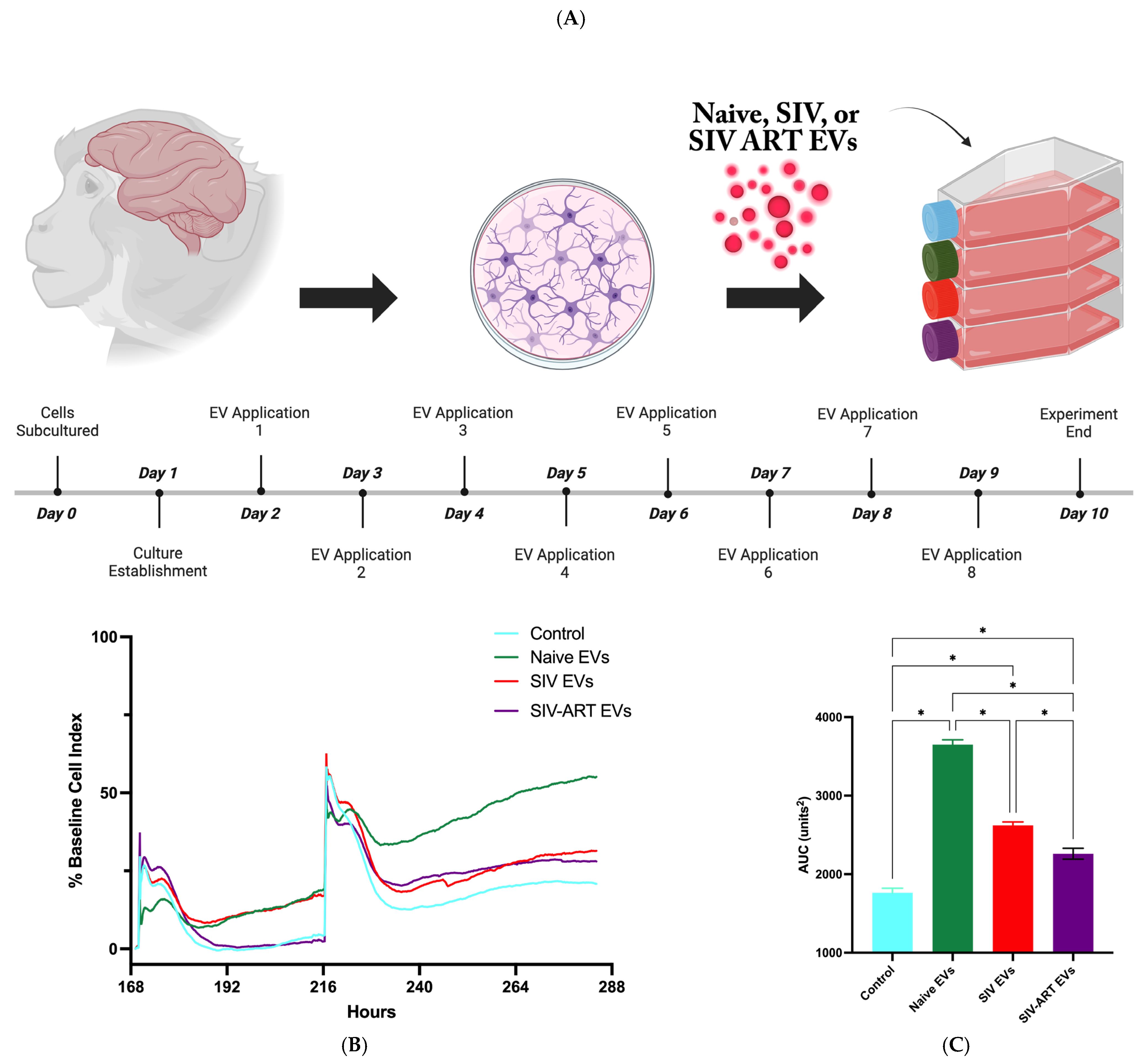
2.4. Mixed Glial Culture Establishment
- Group A: control group (astrocyte media containing exosome-depleted fetal bovine serum).
- Group B: EVs derived from healthy SIV-negative rhesus macaques (RMs).
- Group C: EVs derived from SIV-infected untreated RMs.
- Group D: EVs derived from SIV-infected ART-treated RMs.
2.5. xCELLigence Assay
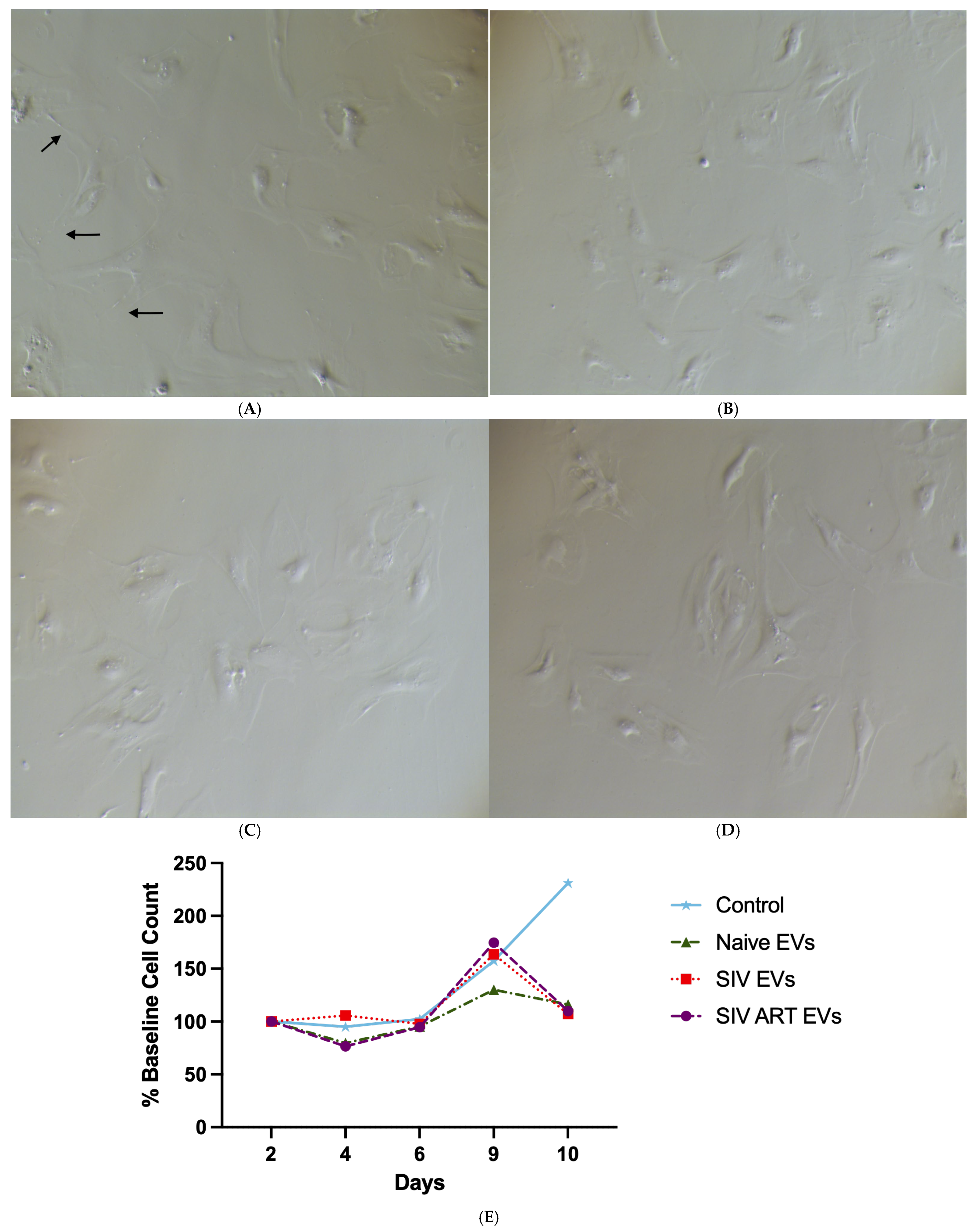
2.6. Imaging and Analysis of Cell Complexity
2.7. Cell Survival and Assessments of Cell Growth
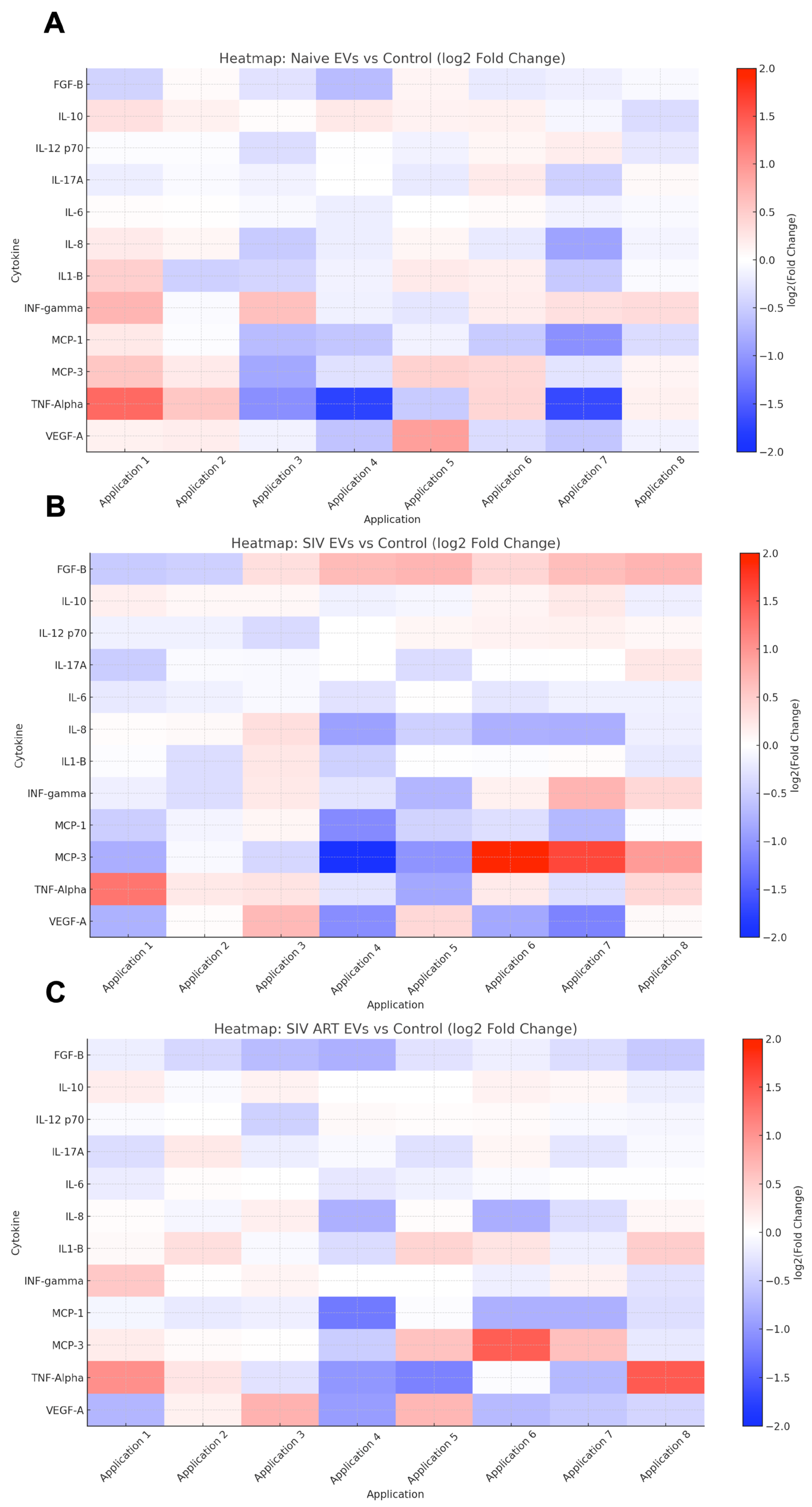
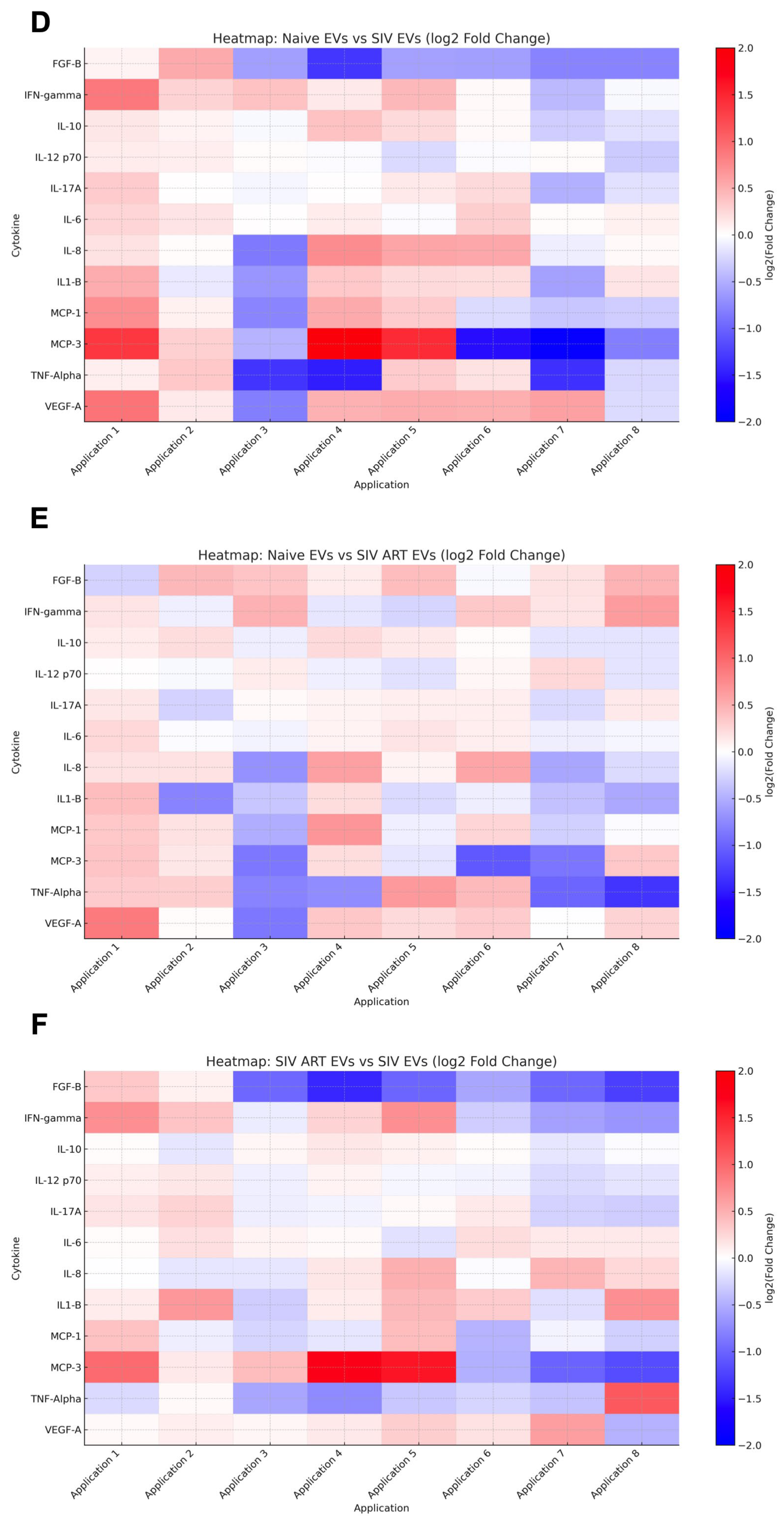
2.8. Quantification of Senescence-Associated Secretory Phenotype Proteins
2.9. Statistical Analysis
3. Results
3.1. EVs Acutely Enhance Glial Adhesion and Monolayer Impedance
3.2. Repeated SIV EV Exposure Impairs Glial Proliferation and Survival
3.3. Repeated SIV EV Exposure Disrupts Astrocyte Morphological Complexity and Process Elaboration
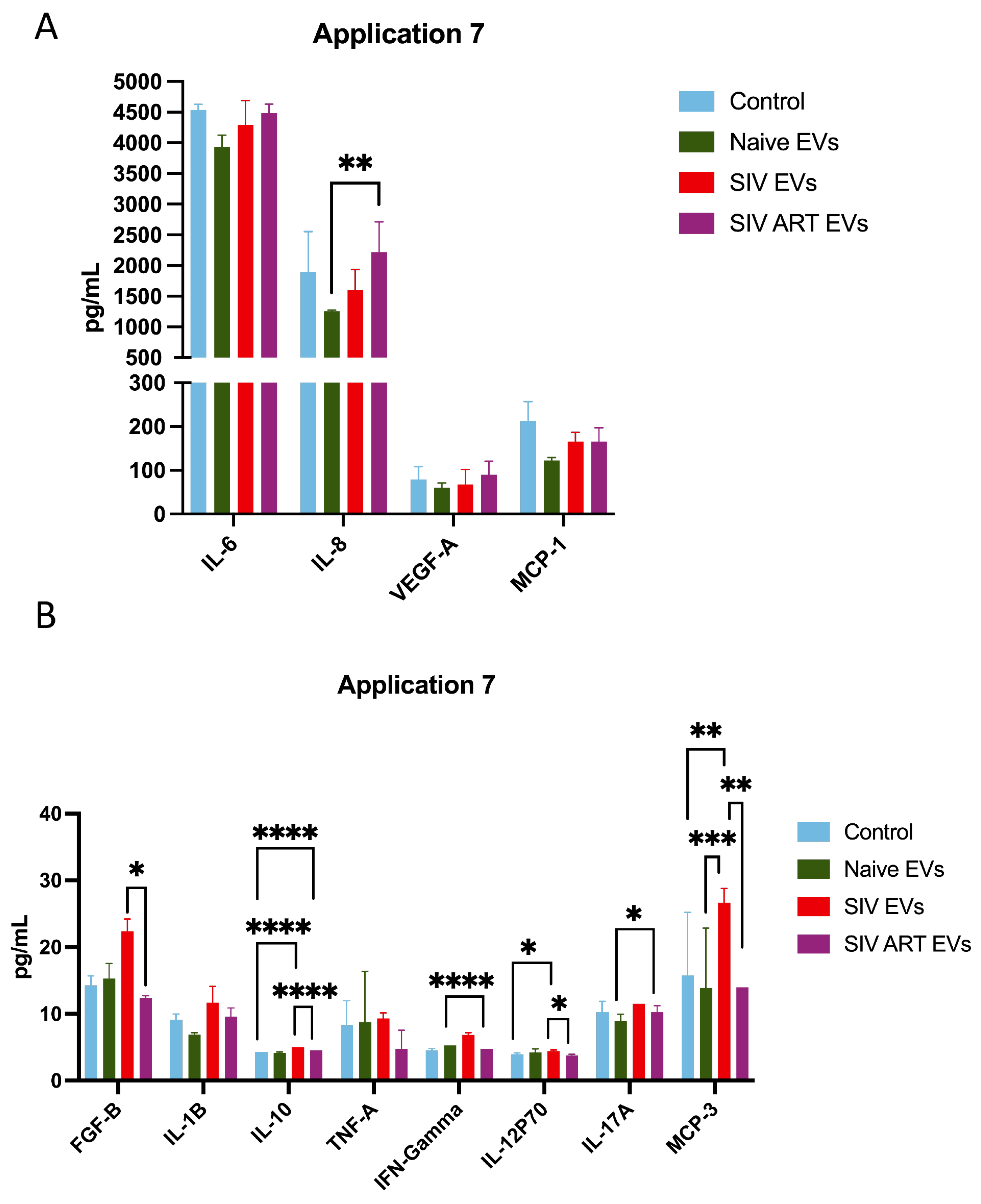
3.4. Repeated SIV EV Treatment Induces Immediate Secretion of Inflammatory Cytokines: Limited Attenuation by ART
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn, M.D.; MacLean, A.G. Extracellular Vesicles as a Means of Viral Immune Evasion, CNS Invasion, and Glia-Induced Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 695899. [Google Scholar] [CrossRef]
- McNamara, R.P.; Costantini, L.M.; Myers, T.A.; Schouest, B.; Maness, N.J.; Griffith, J.D.; Damania, B.A.; MacLean, A.G.; Dittmer, D.P. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. mBio 2018, 9, e02344-17. [Google Scholar] [CrossRef] [PubMed]
- Marques de Menezes, E.G.; Jang, K.; George, A.F.; Nyegaard, M.; Neidleman, J.; Inglis, H.C.; Danesh, A.; Deng, X.; Afshari, A.; Kim, Y.H.; et al. Seminal Plasma-Derived Extracellular-Vesicle Fractions from HIV-Infected Men Exhibit Unique MicroRNA Signatures and Induce a Proinflammatory Response in Cells Isolated from the Female Reproductive Tract. J. Virol. 2020, 94, e00525-20. [Google Scholar] [CrossRef]
- Yang, L.; Niu, F.; Yao, H.; Liao, K.; Chen, X.; Kook, Y.; Ma, R.; Hu, G.; Buch, S. Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J. Neuroimmune Pharmacol. 2018, 13, 330–344. [Google Scholar] [CrossRef]
- Campbell, L.A.; Mocchetti, I. Extracellular Vesicles and HIV-Associated Neurocognitive Disorders: Implications in Neuropathogenesis and Disease Diagnosis. Neurotox. Res. 2021, 39, 2098–2107. [Google Scholar] [CrossRef]
- Chemparathy, D.T.; Ray, S.; Ochs, C.; Ferguson, N.; Gawande, D.Y.; Dravid, S.M.; Callen, S.; Sil, S.; Buch, S. Neuropathogenic role of astrocyte-derived extracellular vesicles in HIV-associated neurocognitive disorders. J. Extracell. Vesicles 2024, 13, e12439. [Google Scholar] [CrossRef]
- Hu, G.; Yang, L.; Cai, Y.; Niu, F.; Mezzacappa, F.; Callen, S.; Fox, H.S.; Buch, S. Emerging roles of extracellular vesicles in neurodegenerative disorders: Focus on HIV-associated neurological complications. Cell Death Dis. 2016, 7, e2481. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, K.E.; Chand, S.; Wheeler, S.; Tiwari, S.; Flores, A.; Hernandez, J.; Savine, M.; Gowen, A.; Pendyala, G.; Yelamanchili, S.V. Role of Extracellular Vesicles in Substance Abuse and HIV-Related Neurological Pathologies. Int. J. Mol. Sci. 2020, 21, 6765. [Google Scholar] [CrossRef]
- Guha, D.; Lorenz, D.R.; Misra, V.; Chettimada, S.; Morgello, S.; Gabuzda, D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J. Neuroinflamm. 2019, 16, 254. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Wolinsky, S.M.; Gabuzda, D. Small RNA sequencing of extracellular vesicles identifies circulating miRNAs related to inflammation and oxidative stress in HIV patients. BMC Immunol. 2020, 21, 57. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A. Extracellular Vesicles as Mediators of Neuroinflammation in Intercellular and Inter-Organ Crosstalk. Int. J. Mol. Sci. 2024, 25, 7041. [Google Scholar] [CrossRef]
- Caobi, A.; Nair, M.; Raymond, A.D. Extracellular Vesicles in the Pathogenesis of Viral Infections in Humans. Viruses 2020, 12, 1200. [Google Scholar] [CrossRef]
- Chelvanambi, S.; Bogatcheva, N.V.; Bednorz, M.; Agarwal, S.; Maier, B.; Alves, N.J.; Li, W.; Syed, F.; Saber, M.M.; Dahl, N.; et al. HIV-Nef Protein Persists in the Lungs of Aviremic Patients with HIV and Induces Endothelial Cell Death. Am. J. Respir. Cell Mol. Biol. 2019, 60, 357–366. [Google Scholar] [CrossRef]
- Chelvanambi, S.; Gupta, S.K.; Chen, X.; Ellis, B.W.; Maier, B.F.; Colbert, T.M.; Kuriakose, J.; Zorlutuna, P.; Jolicoeur, P.; Obukhov, A.G.; et al. HIV-Nef Protein Transfer to Endothelial Cells Requires Rac1 Activation and Leads to Endothelial Dysfunction Implications for Statin Treatment in HIV Patients. Circ. Res. 2019, 125, 805–820. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.P.; Chugh, P.E.; Bailey, A.; Costantini, L.M.; Ma, Z.; Bigi, R.; Cheves, A.; Eason, A.B.; Landis, J.T.; Host, K.M.; et al. Extracellular vesicles from Kaposi Sarcoma-associated herpesvirus lymphoma induce long-term endothelial cell reprogramming. PLoS Pathog. 2019, 15, e1007536. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Ferdin, J.; Goricar, K.; Dolzan, V.; Plemenitas, A.; Martin, J.N.; Peterlin, B.M.; Deeks, S.G.; Lenassi, M. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS ONE 2018, 13, e0191613. [Google Scholar] [CrossRef]
- Khan, M.B.; Lang, M.J.; Huang, M.B.; Raymond, A.; Bond, V.C.; Shiramizu, B.; Powell, M.D. Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Abeta(1-42) secretion in SH-SY5Y neural cells. J. Neurovirol. 2016, 22, 179–190. [Google Scholar] [CrossRef]
- McNamara, R.P.; Dittmer, D.P. Extracellular vesicles in virus infection and pathogenesis. Curr. Opin. Virol. 2020, 44, 129–138. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Hoang, A.; Dragoljevic, D.; Dubrovsky, L.; Pushkarsky, T.; Low, H.; Ditiatkovski, M.; Fu, Y.; Ohkawa, R.; Meikle, P.J.; et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019, 15, e1007907. [Google Scholar] [CrossRef]
- Raab-Traub, N.; Dittmer, D.P. Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Izquierdo-Altarejos, P.; Martinez-Garcia, M.; Atienza-Perez, I.; Hernandez, A.; Moreno-Manzano, V.; Llansola, M.; Felipo, V. Extracellular Vesicles from Mesenchymal Stem Cells Reverse Neuroinflammation and Restore Motor Coordination in Hyperammonemic Rats. J. Neuroimmune Pharmacol. 2024, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Volarevic, A.; Djonov, V.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes as New Remedy for the Treatment of Neurocognitive Disorders. Int. J. Mol. Sci. 2021, 22, 1433. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.P.; Caro-Vegas, C.P.; Costantini, L.M.; Landis, J.T.; Griffith, J.D.; Damania, B.A.; Dittmer, D.P. Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. J. Extracell Vesicles 2018, 7, 1541396. [Google Scholar] [CrossRef]
- Renner, N.A.; Sansing, H.A.; Inglis, F.M.; Mehra, S.; Kaushal, D.; Lackner, A.A.; Maclean, A.G. Transient acidification and subsequent proinflammatory cytokine stimulation of astrocytes induce distinct activation phenotypes. J. Cell. Physiol. 2013, 228, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- MacLean, A.G.; Walker, E.; Sahu, G.K.; Skowron, G.; Marx, P.; von Laer, D.; Junghans, R.P.; Braun, S.E. A novel real-time CTL assay to measure designer T-cell function against HIV Env(+) cells. J. Med. Primatol. 2014, 43, 341–348. [Google Scholar] [CrossRef]
- Sansing, H.A.; Renner, N.A.; Maclean, A.G. An inverted blood-brain barrier model that permits interactions between glia and inflammatory stimuli. J. Neurosci. Methods 2012, 207, 91–96. [Google Scholar] [CrossRef]
- Csiszar, A.; Sosnowska, D.; Wang, M.; Lakatta, E.G.; Sonntag, W.E.; Ungvari, Z. Age-Associated Proinflammatory Secretory Phenotype in Vascular Smooth Muscle Cells From the Non-human Primate Macaca mulatta: Reversal by Resveratrol Treatment. J. Gerontol. Ser. A 2012, 67, 811–820. [Google Scholar] [CrossRef]
- Van Zandt, A.R.; Horn, M.D.; Peterson, T.A.; Dickinson, S.Y.; Frost, E.M.; MacLean, A.G. THC Reverses SIV-Induced Senescence in Astrocytes: Possible Compensatory Mechanism Against HIV Associated Brain Injury? Front. Cell. Neurosci. 2025, 19, 1642917. [Google Scholar] [CrossRef]
- Renner, N.A.; Ivey, N.S.; Redmann, R.K.; Lackner, A.A.; MacLean, A.G. MCP-3/CCL7 production by astrocytes: Implications for SIV neuroinvasion and AIDS encephalitis. J. Neurovirol. 2011, 17, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Crenshaw, B.J.; Gu, L.; Sims, B.; Matthews, Q.L. Exosome Biogenesis and Biological Function in Response to Viral Infections. Open Virol. J. 2018, 12, 134–148. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Toborek, M. Extracellular vesicles of the blood-brain barrier. Tissue Barriers 2016, 4, e1131804. [Google Scholar] [CrossRef]
- Guillemin, G.; Boussin, F.D.; Croitoru, J.; Franck-Duchenne, M.; Le Grand, R.; Lazarini, F.; Dormont, D. Obtention and characterization of primary astrocyte and microglial cultures from adult monkey brains. J. Neurosci. Res. 1997, 49, 576–591. [Google Scholar] [CrossRef]
- Ramesh, G.; Philipp, M.T. Pathogenesis of Lyme neuroborreliosis: Mitogen-activated protein kinases Erk1, Erk2, and p38 in the response of astrocytes to Borrelia burgdorferi lipoproteins. Neurosci. Lett. 2005, 384, 112–116. [Google Scholar] [CrossRef]
- Butchi, N.B.; Du, M.; Peterson, K.E. Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia. Glia 2010, 58, 650–664. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New molecular targets of diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef]
- Lee, K.M.; Chiu, K.B.; Renner, N.A.; Sansing, H.A.; Didier, P.J.; MacLean, A.G. Form follows function: Astrocyte morphology and immune dysfunction in SIV neuroAIDS. J. Neurovirol. 2014, 20, 474–484. [Google Scholar] [CrossRef][Green Version]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, L.; Ward, A.; Choi, S.-H.; Pushkarsky, T.; Brichacek, B.; Vanpouille, C.; Adzhubei, A.A.; Mukhamedova, N.; Sviridov, D.; Margolis, L.; et al. Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts. mBio 2020, 11, 10. [Google Scholar] [CrossRef]
- Habib, A.; Liang, Y.; Zhu, N. Exosomes multifunctional roles in HIV-1: Insight into the immune regulation, vaccine development and current progress in delivery system. Front. Immunol. 2023, 14, 1249133. [Google Scholar] [CrossRef] [PubMed]
- MacLean, A.; Horn, M.; Midkiff, C.; Van Zandt, A.; Saied, A. Combination antiretroviral therapy prevents SIV- induced aging in the hippocampus and neurodegeneration throughout the brain. Res. Sq. 2024, rs.3.rs-4681317. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Zandt, A.R.; Horn, M.D.; McNamara, R.P.; Peterson, T.A.; Maness, N.J.; Schoest, B.; Frost, E.M.; Zhou, Y.; Moström, M.J.; Dittmer, D.P.; et al. Simian Immunodeficiency Virus-Derived Extracellular Vesicles Induce a Chronic Inflammatory Phenotype in Healthy Astrocytes Unresolved by Anti-Retroviral Therapy. Pharmaceutics 2025, 17, 1374. https://doi.org/10.3390/pharmaceutics17111374
Van Zandt AR, Horn MD, McNamara RP, Peterson TA, Maness NJ, Schoest B, Frost EM, Zhou Y, Moström MJ, Dittmer DP, et al. Simian Immunodeficiency Virus-Derived Extracellular Vesicles Induce a Chronic Inflammatory Phenotype in Healthy Astrocytes Unresolved by Anti-Retroviral Therapy. Pharmaceutics. 2025; 17(11):1374. https://doi.org/10.3390/pharmaceutics17111374
Chicago/Turabian StyleVan Zandt, Alison R., Miranda D. Horn, Ryan P. McNamara, Tiffany A. Peterson, Nicholas J. Maness, Blake Schoest, Elise M. Frost, Yijun Zhou, Matilda J. Moström, Dirk P. Dittmer, and et al. 2025. "Simian Immunodeficiency Virus-Derived Extracellular Vesicles Induce a Chronic Inflammatory Phenotype in Healthy Astrocytes Unresolved by Anti-Retroviral Therapy" Pharmaceutics 17, no. 11: 1374. https://doi.org/10.3390/pharmaceutics17111374
APA StyleVan Zandt, A. R., Horn, M. D., McNamara, R. P., Peterson, T. A., Maness, N. J., Schoest, B., Frost, E. M., Zhou, Y., Moström, M. J., Dittmer, D. P., & MacLean, A. G. (2025). Simian Immunodeficiency Virus-Derived Extracellular Vesicles Induce a Chronic Inflammatory Phenotype in Healthy Astrocytes Unresolved by Anti-Retroviral Therapy. Pharmaceutics, 17(11), 1374. https://doi.org/10.3390/pharmaceutics17111374








