Extended Stability of Ascorbic Acid in Pediatric TPN Admixtures: The Role of Storage Temperature and Emulsion Integrity
Abstract
1. Introduction
- The nutrient amounts required to sustain life must fulfill the patient’s metabolic and energetic needs.
- The ratio between the nitrogen content and the energy provided by non-protein components (where 1 g of nitrogen should equate to 130–200 kcal) must be appropriate.
- The ratio between carbohydrates and lipids (50–75 kcal from carbohydrates for every 25–50 kcal from lipids) must be balanced to ensure the proper utilization of these components in the body’s biochemical processes.
2. Materials and Methods
2.1. Preparation of Parenteral Nutrition Admixtures
- Carbohydrate source: Glucose 50% solution.
- Protein source: Aminoven Infant®.
- Lipid emulsion: Smoflipid® or an equivalent product.
- Electrolytes: Glycophos®, 10% NaCl, 15% KCl, 20% MgSO4, and calcium gluconate. Ascorbic acid: Soluvit® N, Cernevit® are multivitamin products (1 mL of Soluvit® N is equivalent to 12 mg of Vitamin C and 1 mL of Cernevit® is equivalent to 10 mg of Vitamin C).
- Trace elements: Peditrace®.
- Diluent: Water for injection (WFI).
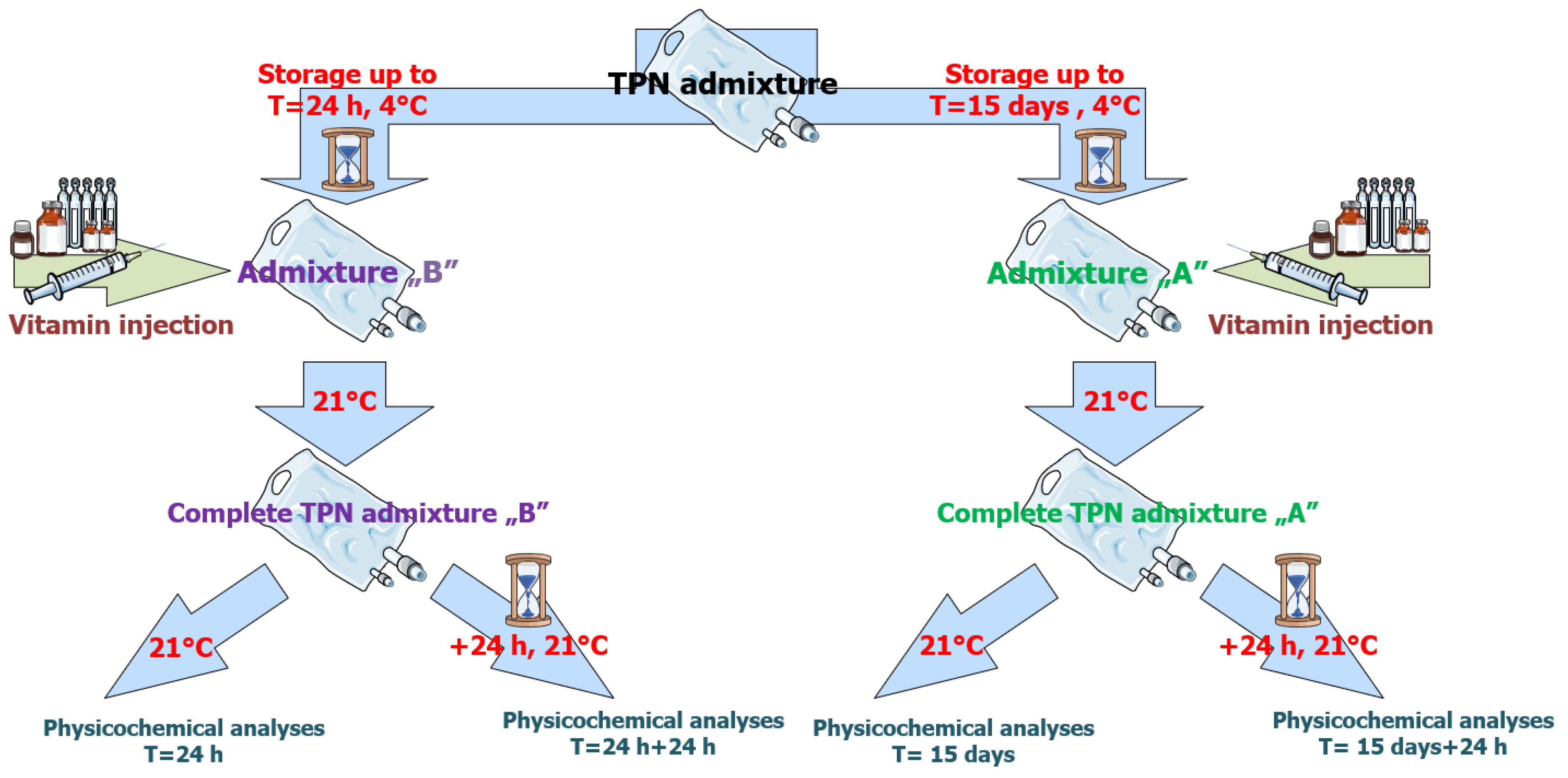
2.2. Storage and Sampling Protocol
- Group A: 15 days of storage at 4 ± 2 °C, followed by vitamin addition and additional 24 h of storage at 21 ± 2 °C with light protection (t = 15 days, and t = 15 days + 24 h).
- Group B: Vitamins added at 24 h post-preparation, followed by an additional 24 h of storage at 21 ± 2 °C (t = 24 h, and t = 24 h + 24 h).
2.3. Visual Inspection and Organoleptic Evaluation
2.4. Optical Microscopy for Lipid Droplet Analysis
2.5. Particle Size Distribution Analysis
- Dynamic Light Scattering (DLS): Samples were diluted 1:100 with water for injection (WFI) and measured using a Zetasizer Nano ZS (Malvern Panalytical Ltd., Malvern, UK). Z-average size and polydispersity index (PDI) were recorded. The PDI threshold for acceptable stability was <0.2.
- Laser Diffraction (LD): Droplet volume distribution was determined using a Mastersizer 3000 (Malvern Panalytical Ltd., Malvern, UK). Approximately 2 mL of admixture was diluted into 500 mL of WFI under stirring. D10, D50, and D90 values were calculated, reflecting the droplet diameter at which 10%, 50%, and 90% of the total volume is composed of smaller particles.
- Optical Microscopy: As described above, used as a qualitative check.
2.6. Zeta Potential Measurement
2.7. pH Measurement
2.8. Quantification of Ascorbic Acid via HPLC
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microscopic Observations
3.2. Dynamic Light Scattering (DLS) and Laser Diffraction (LD)
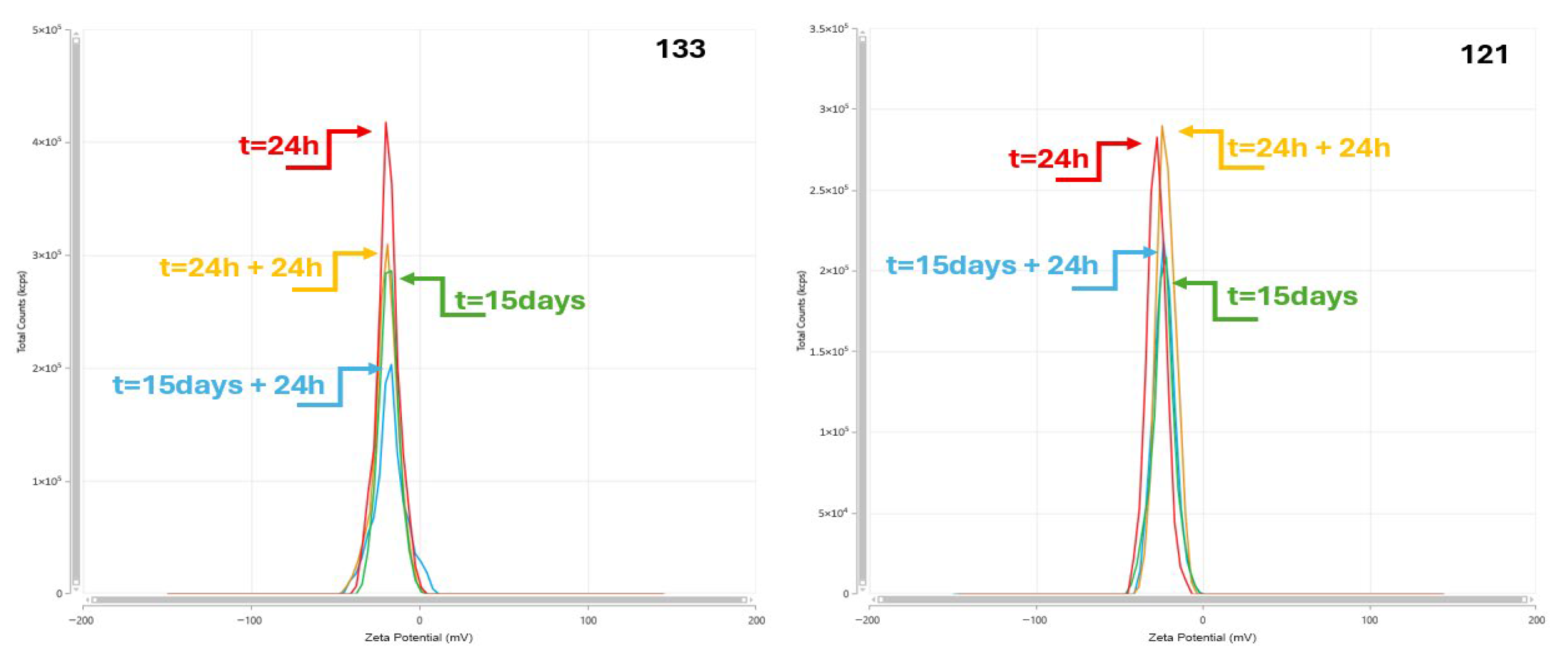
3.3. Zeta Potential
3.4. pH Measurement
3.5. Ascorbic Acid Stability (HPLC Results)
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnett, M.I.; Pertkiewicz, M.; Cosslett, A.G.; Mühlebach, S. Basics in clinical nutrition: Parenteral nutrition admixtures, how to prepare parenteral nutrition (PN) admixtures. e-Spen Eur. e-J. Clin. Nutr. Metab. 2009, 4, e114–e116. [Google Scholar] [CrossRef]
- Stawny, M.; Olijarczyk, R.; Jaroszkiewicz, E.; Jelińska, A. Pharmaceutical point of view on parenteral nutrition. Sci. World J. 2013, 2013, 415310. [Google Scholar] [CrossRef]
- De Cloet, J.; Van Biervliet, S.; Van Winckel, M. Physicochemical stable standard all-in-one parenteral nutrition admixtures for infants and children in accordance with the ESPGHAN/ESPEN guidelines. Nutrition 2018, 49, 41–47. [Google Scholar] [CrossRef]
- Watrobska-Swietlikowska, D.; MacLoughlin, R. The effect of UV-protected ethylene vinyl acetate (EVA) bags on the physicochemical stability of pediatric parenteral nutrition admixtures. DARU J. Pharm. Sci. 2019, 27, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Berlana, D. Parenteral Nutrition Overview. Nutrients 2022, 14, 4480. [Google Scholar] [CrossRef]
- Hartman, C.; Shamir, R.; Simchowitz, V.; Lohner, S.; Cai, W.; Decsi, T.; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Complications. Clin. Nutr. 2018, 37, 2418–2429. [Google Scholar] [CrossRef] [PubMed]
- Sobol, Ż.; Chiczewski, R.; Wątróbska-Świetlikowska, D. The Modern Approach to Total Parenteral Nutrition: Multidirectional Therapy Perspectives with a Focus on the Physicochemical Stability of the Lipid Fraction. Nutrients 2025, 17, 846. [Google Scholar] [CrossRef] [PubMed]
- Dupertuis, Y.M.; Morch, A.; Fathi, M.; Sierro, C. Physical Characteristics of Total Parenteral Nutrition Bags Significantly Affect the Stability of Vitamins C and B 1: A Controlled Prospective Study. J. Parenter. Enter. Nutr. 2002, 26, 310–316. [Google Scholar] [CrossRef]
- Ferguson, T.I.; Emery, S.; Price-Davies, R.; Cosslett, A.G. A review of stability issues associated with vitamins in parenteral nutrition. E-SPEN J. 2014, 9, e49–e53. [Google Scholar] [CrossRef]
- Bouchoud, L.; Sadeghipour, F.; Klingmüller, M.; Fonzo-Christe, C.; Bonnabry, P. Long-term physico-chemical stability of standard parenteral nutritions for neonates. Clin. Nutr. 2010, 29, 808–812. [Google Scholar] [CrossRef]
- Pironi, L.; Guidetti, M.; Zolezzi, C.; Fasano, M.C.; Paganelli, F.; Merli, C.; Bersani, G.; Pizzoferrato, A.; Miglioli, M. Peroxidation potential of lipid emulsions after compounding in all-in-one solutions. Nutrition 2003, 19, 784–788. [Google Scholar] [CrossRef]
- Allwood, M.C.; Kearney, M.C.J. Compatibility and stability of additives in parenteral nutrition admixtures. Nutrition 1998, 14, 697–706. [Google Scholar] [CrossRef]
- Steger, P.J.; Mühlebach, S.F. Lipid Peroxidation of Intravenous Lipid Emulsions and All-in-One Admixtures in Total Parenteral Nutrition Bags: The Influence of Trace Elements. JPEN J. Parenter. Enteral. Nutr. 2000, 24, 37–41. [Google Scholar] [CrossRef]
- Spínola, V.; Llorent-Martínez, E.J.; Castilho, P.C. Determination of vitamin C in foods: Current state of method validation. Foods 2021, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Dettlaff, K.; Jelińska, A.; Ogrodowczyk, M. Stability studies of parenteral nutrition with a high dose of vitamin C. J. Oncol. Pharm. Pract. 2020, 26, 1894–1902. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef]
- Dong, Y.; Busatto, N.; Roth, P.J.; Martin-Fabiani, I. Colloidal assembly of polydisperse particle blends during drying. Soft Matter 2020, 16, 8453–8461. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.M.; Bardhan, A.; Mandal, T.; Chakraborty, M.; Khatun, N.; Layek, M.; Sharma, S.; Chakravarty, M.; Saha, R.; Saha, B. An effect of hydrophobicity of cosurfactant on the growth of cerium tetrafluoride hexagonal nanorods in water-in-oil microemulsion template. J. Mol. Liq. 2023, 391, 123333. [Google Scholar] [CrossRef]
- Rahaman, S.M.; Chakraborty, M.; Kundu, S.; Dhibar, S.; Kumar, D.K.U.A.; Ibrahim, S.M.; Chakravarty, M.; Saha, B. Controlled synthesis of samarium trifluoride nanoparticles in a water-in-oil microemulsion: Effects of water-to-surfactant ratio on particles and phosphate removal. J. Hazard. Mater. Adv. 2023, 11, 100348. [Google Scholar] [CrossRef]
- Boullata, J.I.; Mirtallo, J.M.; Sacks, G.S.; Salman, G.; Gura, K.; Canada, T.; Maguire, A.; ASPEN Parenteral Nutrition Safety Committee. Parenteral nutrition compatibility and stability: A comprehensive review. J. Parenter. Enter. Nutr. 2022, 46, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Turmezei, J.; Javorszky, E.; Szabo, E.; Dredan, J.; Kallai-Szabo, B.; Zelko, R. Effect of storage temperature on the stability of total parenteral nutrition admixtures prepared for infants. Acta Pol. Pharm. 2015, 72, 843–849. [Google Scholar] [PubMed]
- Peverini, M.; Barberini, G. Management of COVID-19 and clinical nutrition. In Management, Body Systems, and Case Studies in COVID-19; Elsevier: Amsterdam, The Netherlands, 2024; pp. 77–87. [Google Scholar] [CrossRef]
- Öztürk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]

| TPN | Glucose 50% | Aminoacids (Aminoven Infant) | Lipid Emulsion | Water for Injection | Glycophos® | 10% NaCl | 15% KCl | Peditrace® | 20% MgSO4 | Calcium Gluconicum | Soluvit® | Vitalipid® | Cernevit® | Supliven® |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | 58.7 | 58.7 | 18.3 | 91.6 | 1.6 | 5.3 | 4.4 | 0 | 1.8 | 7 | 1.8 | 0 | 0.7 | |
| 102 | 66.3 | 66.3 | 26.3 | 15.6 | 2 | 4.5 | 1.8 | 2.9 | 0.4 | 5.5 | 2 | 0 | 0 | |
| 103 | 59.1 | 53.2 | 32.5 | 88.7 | 0.7 | 4.4 | 3.3 | 0 | 1.8 | 4.4 | 1.5 | 0 | 0.4 | |
| 104 | 69.5 | 65.5 | 40.1 | 45.4 | 0.8 | 10.7 | 9.6 | 0 | 1.1 | 5.3 | 0 | 0 | 0.7 | 1.3 |
| 105 | 88.4 | 68.7 | 33.7 | 36.5 | 0.6 | 4.2 | 3.5 | 2.1 | 1.1 | 7 | 2.8 | 1.4 | 0 | 0 |
| 106 | 58.4 | 44.9 | 24.7 | 95.4 | 0.8 | 4.9 | 6.7 | 0 | 2.8 | 9.7 | 0 | 0 | 0.6 | 1.1 |
| 107 | 75.1 | 44.7 | 26.8 | 89.4 | 0.4 | 2 | 1.6 | 2.7 | 0.5 | 3.2 | 1.8 | 1.8 | 0 | 0 |
| 108 | 88.9 | 86.9 | 46.8 | 10.7 | 0.9 | 2.9 | 3.3 | 0 | 1.3 | 7 | 0 | 0 | 0.7 | 0.5 |
| 109 | 81.2 | 43.8 | 10.3 | 72.2 | 1 | 4.1 | 2.1 | 2.3 | 0.3 | 5.9 | 2.3 | 2.6 | 0 | 0 |
| 110 | 94 | 82.9 | 49.8 | 2.8 | 0.8 | 3 | 3.3 | 0 | 1.1 | 7.6 | 1.4 | 2.8 | 0 | 0.6 |
| 111 | 60.2 | 40.1 | 10 | 103.6 | 1.3 | 2.7 | 2 | 2 | 0.3 | 5.7 | 2 | 3.3 | 0 | 0 |
| 112 | 68.4 | 63.5 | 28.2 | 63.5 | 2.1 | 3.7 | 6.5 | 1.4 | 1.4 | 8.5 | 1.4 | 1.4 | 0 | 0 |
| 113 | 56.9 | 59.4 | 33.4 | 78.6 | 1 | 6.3 | 2 | 0 | 1.7 | 7.8 | 1.2 | 1.2 | 0 | 0.5 |
| 114 | 89.9 | 62.1 | 42.8 | 40.7 | 1.1 | 1.5 | 1.5 | 3.2 | 0.9 | 4.3 | 2.1 | 0 | 0 | |
| 115 | 114 | 67.3 | 47.8 | 4.3 | 0.4 | 4.3 | 0.9 | 3.3 | 0.7 | 4.8 | 2.2 | 0 | 0 | |
| 116 | 70.8 | 43.6 | 28.6 | 89.9 | 0.5 | 3.3 | 1.6 | 2.2 | 0.5 | 4.1 | 2.2 | 2.7 | 0 | 0 |
| 117 | 95.6 | 82.2 | 41.1 | 10.1 | 0.3 | 3.4 | 1.7 | 2.5 | 1 | 8.7 | 1.7 | 1.7 | 0 | 0 |
| 118 | 56.5 | 64.5 | 35.6 | 72.6 | 0.1 | 5.4 | 3.4 | 0 | 2.4 | 7.5 | 1.3 | 0 | 0.7 | |
| 119 | 58.7 | 58.7 | 18.3 | 91.6 | 1.6 | 5.3 | 4.4 | 0 | 1.8 | 7 | 1.8 | 0 | 0.7 | |
| 120 | 103.4 | 66.3 | 26.3 | 15.6 | 2 | 4.5 | 1.8 | 2.9 | 0.4 | 5.5 | 2 | 0 | 0 | |
| 121 | 59.1 | 53.2 | 32.5 | 88.7 | 0.7 | 4.4 | 3.3 | 0 | 1.8 | 4.4 | 1.5 | 0 | 0.4 | |
| 122 | 69.5 | 65.5 | 40.1 | 45.4 | 0.8 | 10.7 | 9.6 | 0 | 1.1 | 5.3 | 0 | 0 | 0.7 | 1.3 |
| 123 | 88.4 | 68.7 | 33.7 | 36.5 | 0.6 | 4.2 | 3.5 | 2.1 | 1.1 | 7 | 1.4 | 2.8 | 0 | 0 |
| 124 | 58.4 | 44.9 | 24.7 | 95.4 | 0.8 | 4.9 | 6.7 | 0 | 2.8 | 9.7 | 0 | 0 | 0.6 | 1.1 |
| 125 | 75.1 | 44.7 | 26.8 | 89.4 | 0.4 | 2 | 1.6 | 2.7 | 0.5 | 3.2 | 1.8 | 1.8 | 0 | 0 |
| 126 | 88.9 | 86.9 | 46.8 | 10.7 | 0.9 | 2.9 | 3.3 | 0 | 1.3 | 7 | 0 | 0 | 0.7 | 0.5 |
| 127 | 81.2 | 43.8 | 10.3 | 72.2 | 1 | 4.1 | 2.1 | 2.3 | 0.3 | 5.9 | 2.3 | 2.6 | 0 | 0 |
| 128 | 94 | 82.9 | 49.8 | 2.8 | 0.8 | 3 | 3.3 | 0 | 1.1 | 7.6 | 1.4 | 2.8 | 0 | 0.6 |
| 129 | 60.2 | 40.1 | 10 | 103.6 | 1.3 | 2.7 | 2 | 2 | 0.3 | 5.7 | 2 | 3.3 | 0 | 0 |
| 130 | 68.4 | 63.5 | 28.2 | 63.5 | 2.1 | 3.7 | 6.5 | 1.4 | 1.4 | 8.5 | 1.4 | 1.4 | 0 | 0 |
| 131 | 56.9 | 59.4 | 33.4 | 78.6 | 1 | 6.3 | 2 | 0 | 1.7 | 7.8 | 1.2 | 1.2 | 0 | 0.5 |
| 132 | 89.9 | 62.1 | 42.8 | 40.7 | 1.1 | 1.5 | 1.5 | 3.2 | 0.9 | 4.3 | 2.1 | 0 | 0 | |
| 133 | 114 | 67.3 | 47.8 | 4.3 | 0.4 | 4.3 | 0.9 | 3.3 | 0.7 | 4.8 | 2.2 | 0 | 0 | |
| 134 | 70.8 | 43.6 | 28.6 | 89.9 | 0.5 | 3.3 | 1.6 | 2.2 | 0.5 | 4.1 | 2.2 | 2.7 | 0 | 0 |
| 135 | 95.6 | 82.2 | 41.1 | 10.1 | 0.3 | 3.4 | 1.7 | 2.5 | 1 | 8.7 | 1.7 | 1.7 | 0 | 0 |
| 136 | 56.5 | 64.5 | 35.6 | 72.6 | 0.1 | 5.4 | 3.4 | 0 | 2.4 | 7.5 | 1.3 | 0 | 0.7 | |
| 137 | 61.2 | 35.8 | 14.9 | 121.2 | 1.2 | 3.6 | 1.2 | 0.7 | 0.4 | 5.4 | 1.5 | 3 | 0 | 0 |
| 138 | 61.2 | 35.8 | 7.8 | 113.4 | 1.2 | 3.6 | 1.2 | 0.7 | 0.4 | 5.4 | 1.5 | 3 | 0 | 0 |
| 139 | 90.1 | 68.4 | 34.2 | 34.2 | 2 | 6.5 | 5 | 1.6 | 0.8 | 6.5 | 0 | 0 | 0.8 | 0 |
| 140 | 79.2 | 69.7 | 57 | 14.3 | 1.7 | 13.1 | 3.2 | 1.6 | 0.6 | 7.9 | 1.6 | 0 | 0 | |
| 141 | 69.1 | 68.6 | 38.4 | 54.9 | 0.5 | 2.3 | 3.1 | 1.1 | 1.1 | 9.2 | 0 | 0 | 0.5 | 0 |
| 142 | 44.9 | 23.5 | 6.8 | 141.1 | 1.1 | 4.2 | 2.3 | 1 | 1 | 2.6 | 1 | 1 | 0 | 0 |
| 143 | 81.4 | 48.1 | 15.7 | 53.6 | 1.7 | 5.7 | 2.8 | 1.4 | 0.2 | 8 | 1.8 | 1.8 | 0 | 0 |
| 144 | 100.5 | 68.6 | 43.4 | 13.7 | 1.8 | 3.4 | 2.1 | 1.8 | 1.8 | 8.2 | 2.3 | 2.3 | 0 | 0 |
| 145 | 60.4 | 40.9 | 5.6 | 130 | 2 | 0.9 | 3.3 | 1.3 | 0.4 | 1.5 | 1.9 | 1.9 | 0 | 0 |
| 146 | 102.8 | 58.6 | 22.6 | 26.7 | 2.9 | 2.5 | 2.1 | 1.4 | 0.7 | 7 | 2.1 | 0 | 0 | |
| 147 | 43.2 | 37.8 | 4.8 | 131.9 | 1.2 | 4.6 | 1.3 | 0.9 | 0.7 | 2.2 | 1.2 | 1.2 | 0 | 0 |
| 148 | 87.2 | 89 | 51.3 | 5.4 | 1.4 | 2.3 | 2.5 | 1.8 | 0.9 | 6.3 | 1.8 | 0 | 0 | |
| 149 | 55.1 | 42.5 | 22 | 110.2 | 1.6 | 1.3 | 1.6 | 1.9 | 0.3 | 7.2 | 3.1 | 3.1 | 0 | 0 |
| 150 | 70.9 | 59.1 | 25.9 | 34 | 1.2 | 7.1 | 3.7 | 1.5 | 0.9 | 5.9 | 1.5 | 1.5 | 0 | 0 |
| Sample Number | SAMPLES “B” | SAMPLES “A” | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t = 24 h | t = 24 h + 24 h | t = 15 days | t = 15 days + 24 h | |||||||||
| Dx(10) | Dx(50) | Dx(90) | Dx(10) | Dx(50) | Dx(90) | Dx(10) | Dx(50) | Dx(90) | Dx(10) | Dx(50) | Dx(90) | |
| 101 | 0.067 | 0.198 | 0.474 | 0.084 | 0.222 | 0.474 | 0.071 | 0.205 | 0.480 | 0.072 | 0.206 | 0.485 |
| 102 | 0.158 | 0.290 | 0.462 | 0.157 | 0.283 | 0.449 | 0.174 | 0.295 | 0.447 | 0.171 | 0.294 | 0.452 |
| 103 | 0.066 | 0.207 | 0.538 | 0.113 | 0.252 | 0.467 | 0.066 | 0.196 | 0.462 | 0.090 | 0.233 | 0.488 |
| 104 | 0.144 | 0.277 | 0.458 | 0.142 | 0.275 | 0.458 | 0.109 | 0.250 | 0.477 | 0.101 | 0.244 | 0.480 |
| 105 | 0.100 | 0.241 | 0.476 | 0.130 | 0.276 | 0.491 | 0.091 | 0.233 | 0.487 | 0.097 | 0.246 | 0.502 |
| 106 | 0.065 | 0.192 | 0.453 | 0.077 | 0.208 | 0.448 | 0.077 | 0.217 | 0.501 | 0.072 | 0.211 | 0.500 |
| 107 | 0.161 | 0.290 | 0.459 | 0.157 | 0.285 | 0.454 | 0.158 | 0.286 | 0.453 | 0.156 | 0.285 | 0.455 |
| 108 | 0.145 | 0.278 | 0.461 | 0.128 | 0.263 | 0.458 | 0.116 | 0.256 | 0.470 | 0.125 | 0.268 | 0.486 |
| 109 | 0.161 | 0.288 | 0.454 | 0.164 | 0.287 | 0.448 | 0.179 | 0.298 | 0.445 | 0.173 | 0.295 | 0.448 |
| 110 | 0.138 | 0.277 | 0.474 | 0.105 | 0.244 | 0.460 | 0.145 | 0.285 | 0.485 | 0.083 | 0.223 | 0.482 |
| 111 | 0.161 | 0.289 | 0.457 | 0.167 | 0.291 | 0.451 | 0.177 | 0.296 | 0.447 | 0.171 | 0.293 | 0.449 |
| 112 | 0.109 | 0.248 | 0.462 | 0.121 | 0.264 | 0.480 | 0.092 | 0.234 | 0.485 | 0.100 | 0.248 | 0.501 |
| 113 | 0.058 | 0.180 | 0.447 | 0.061 | 0.190 | 0.476 | 0.084 | 0.227 | 0.499 | 0.084 | 0.229 | 0.502 |
| 114 | 0.137 | 0.274 | 0.469 | 0.111 | 0.258 | 0.497 | 0.078 | 0.215 | 0.480 | 0.102 | 0.254 | 0.516 |
| 115 | 0.157 | 0.287 | 0.458 | 0.138 | 0.275 | 0.465 | 0.170 | 0.294 | 0.454 | 0.162 | 0.289 | 0.455 |
| 116 | 0.158 | 0.288 | 0.459 | 0.113 | 0.251 | 0.460 | 0.172 | 0.296 | 0.455 | 0.149 | 0.279 | 0.453 |
| 117 | 0.142 | 0.276 | 0.459 | 0.116 | 0.258 | 0.476 | 0.106 | 0.246 | 0.466 | 0.122 | 0.265 | 0.479 |
| 118 | 0.074 | 0.209 | 0.473 | 0.117 | 0.258 | 0.476 | 0.058 | 0.180 | 0.450 | 0.087 | 0.226 | 0.470 |
| 119 | 0.096 | 0.227 | 0.436 | 0.103 | 0.230 | 0.424 | 0.089 | 0.216 | 0.426 | 0.092 | 0.219 | 0.425 |
| 120 | 0.133 | 0.255 | 0.418 | 0.152 | 0.269 | 0.421 | 0.151 | 0.268 | 0.420 | 0.150 | 0.267 | 0.419 |
| 121 | 0.094 | 0.225 | 0.437 | 0.101 | 0.227 | 0.422 | 0.072 | 0.194 | 0.437 | 0.080 | 0.207 | 0.424 |
| 122 | 0.130 | 0.247 | 0.404 | 0.128 | 0.245 | 0.403 | 0.090 | 0.212 | 0.406 | 0.106 | 0.229 | 0.411 |
| 123 | 0.116 | 0.245 | 0.436 | 0.128 | 0.252 | 0.426 | 0.113 | 0.237 | 0.420 | 0.114 | 0.239 | 0.422 |
| 124 | 0.102 | 0.229 | 0.423 | 0.106 | 0.228 | 0.408 | 0.074 | 0.199 | 0.417 | 0.069 | 0.192 | 0.417 |
| 125 | 0.145 | 0.262 | 0.420 | 0.137 | 0.253 | 0.407 | 0.121 | 0.241 | 0.408 | 0.115 | 0.234 | 0.403 |
| 126 | 0.137 | 0.253 | 0.406 | 0.137 | 0.252 | 0.403 | 0.127 | 0.247 | 0.414 | 0.128 | 0.247 | 0.411 |
| 127 | 0.159 | 0.280 | 0.439 | 0.162 | 0.279 | 0.430 | 0.127 | 0.287 | 0.433 | 0.167 | 0.282 | 0.429 |
| 128 | 0.120 | 0.245 | 0.426 | 0.134 | 0.254 | 0.416 | 0.129 | 0.248 | 0.411 | 0.133 | 0.254 | 0.418 |
| 129 | 0.153 | 0.274 | 0.435 | 0.156 | 0.276 | 0.430 | 0.167 | 0.283 | 0.432 | 0.156 | 0.274 | 0.425 |
| 130 | 0.121 | 0.246 | 0.428 | 0.116 | 0.241 | 0.422 | 0.095 | 0.222 | 0.424 | 0.085 | 0.211 | 0.421 |
| 131 | 0.101 | 0.227 | 0.425 | 0.115 | 0.239 | 0.419 | 0.089 | 0.216 | 0.424 | 0.082 | 0.207 | 0.417 |
| 132 | 0.132 | 0.254 | 0.421 | 0.129 | 0.249 | 0.416 | 0.128 | 0.250 | 0.420 | 0.109 | 0.233 | 0.414 |
| 133 | 0.122 | 0.244 | 0.413 | 0.136 | 0.253 | 0.406 | 0.150 | 0.262 | 0.405 | 0.138 | 0.251 | 0.401 |
| 134 | 0.142 | 0.259 | 0.412 | 0.126 | 0.244 | 0.404 | 0.105 | 0.227 | 0.411 | 0.112 | 0.234 | 0.412 |
| 135 | 0.145 | 0.261 | 0.414 | 0.135 | 0.255 | 0.417 | 0.103 | 0.229 | 0.421 | 0.119 | 0.245 | 0.431 |
| 136 | 0.119 | 0.242 | 0.422 | 0.116 | 0.240 | 0.421 | 0.085 | 0.214 | 0.432 | 0.090 | 0.219 | 0.437 |
| 137 | 0.189 | 0.304 | 0.446 | 0.169 | 0.289 | 0.442 | 0.162 | 0.283 | 0.440 | 0.171 | 0.289 | 0.441 |
| 138 | 0.171 | 0.293 | 0.448 | 0.160 | 0.283 | 0.445 | 0.152 | 0.279 | 0.448 | 0.132 | 0.263 | 0.447 |
| 139 | 0.137 | 0.274 | 0.468 | 0.135 | 0.272 | 0.465 | 0.126 | 0.268 | 0.475 | 0.120 | 0.262 | 0.477 |
| 140 | 0.180 | 0.295 | 0.438 | 0.153 | 0.276 | 0.436 | 0.176 | 0.290 | 0.432 | 0.171 | 0.287 | 0.433 |
| 141 | 0.092 | 0.226 | 0.453 | 0.118 | 0.256 | 0.461 | 0.103 | 0.246 | 0.481 | 0.108 | 0.252 | 0.486 |
| 142 | 0.165 | 0.286 | 0.444 | 0.140 | 0.266 | 0.439 | 0.150 | 0.276 | 0.445 | 0.144 | 0.273 | 0.448 |
| 143 | 0.171 | 0.291 | 0.444 | 0.151 | 0.278 | 0.447 | 0.164 | 0.285 | 0.444 | 0.165 | 0.288 | 0.447 |
| 144 | 0.172 | 0.299 | 0.459 | 0.142 | 0.275 | 0.458 | 0.112 | 0.251 | 0.466 | 0.132 | 0.274 | 0.478 |
| 145 | 0.167 | 0.297 | 0.468 | 0.156 | 0.290 | 0.469 | 0.144 | 0.276 | 0.455 | 0.157 | 0.287 | 0.457 |
| 146 | 0.174 | 0.296 | 0.451 | 0.159 | 0.287 | 0.455 | 0.161 | 0.284 | 0.446 | 0.168 | 0.290 | 0.448 |
| 147 | 0.148 | 0.281 | 0.464 | 0.165 | 0.284 | 0.436 | 0.144 | 0.269 | 0.438 | 0.169 | 0.290 | 0.445 |
| 148 | 0.143 | 0.278 | 0.464 | 0.093 | 0.231 | 0.460 | 0.096 | 0.235 | 0.464 | 0.129 | 0.272 | 0.485 |
| 149 | 0.079 | 0.209 | 0.443 | 0.070 | 0.195 | 0.437 | 0.062 | 0.184 | 0.447 | 0.066 | 0.190 | 0.449 |
| 150 | 0.163 | 0.286 | 0.446 | 0.161 | 0.285 | 0.446 | 0.132 | 0.262 | 0.444 | 0.158 | 0.283 | 0.447 |
| Sample Number | SAMPLES “B” | SAMPLES “A” | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t = 24 h | t = 24 h + 24 h | t = 15 days | t = 15 days + 24 h | |||||||||||||
| Z-Average [µm] | PDI | ZETA Potential [mV] | Conductivity | Z-Average [µm] | PDI | ZETA Potential [mV] | Conductivity | Z-Average [µm] | PDI | ZETA Potential [mV] | Conductivity | Z-Average [µm] | PDI | ZETA Potential [mV] | Conductivity | |
| 101 | 240.900 | 0.208 | −21.240 | 0.224 | 249.500 | 0.122 | −26.360 | 0.097 | 233.750 | 0.114 | −39.745 | 0.028 | 249.800 | 0.206 | −31.410 | 0.032 |
| 102 | 245.650 | 0.135 | −20.410 | 0.207 | 250.150 | 0.124 | −23.670 | 0.087 | 234.000 | 0.123 | −32.675 | 0.022 | 244.750 | 0.095 | −32.055 | 0.022 |
| 103 | 241.300 | 0.132 | −20.050 | 0.216 | 248.400 | 0.099 | −25.130 | 0.090 | 239.400 | 0.119 | −31.580 | 0.023 | 232.300 | 0.126 | −31.760 | 0.024 |
| 104 | 239.900 | 0.110 | −23.100 | 0.141 | 251.750 | 0.103 | −23.360 | 0.114 | 238.050 | 0.142 | −33.645 | 0.045 | 236.050 | 0.123 | −34.305 | 0.047 |
| 105 | 245.750 | 0.125 | −22.310 | 0.119 | 253.300 | 0.106 | −22.650 | 0.106 | 240.650 | 0.119 | −30.855 | 0.035 | 236.100 | 0.148 | −33.190 | 0.029 |
| 106 | 241.400 | 0.103 | −20.250 | 0.129 | 268.900 | 0.147 | −22.970 | 0.116 | 234.900 | 0.103 | −31.680 | 0.032 | 235.800 | 0.158 | −28.335 | 0.035 |
| 107 | 240.800 | 0.132 | −21.095 | 0.089 | 245.200 | 0.122 | −23.630 | 0.098 | 238.900 | 0.128 | −32.765 | 0.014 | 235.200 | 0.106 | −33.985 | 0.014 |
| 108 | 233.600 | 0.092 | −20.570 | 0.102 | 251.650 | 0.169 | −22.560 | 0.107 | 235.900 | 0.071 | −29.835 | 0.023 | 238.700 | 0.124 | −32.840 | 0.024 |
| 109 | 252.500 | 0.086 | −21.520 | 0.095 | 250.250 | 0.112 | −21.490 | 0.106 | 248.400 | 0.152 | −31.930 | 0.019 | 239.650 | 0.108 | −31.015 | 0.019 |
| 110 | 249.400 | 0.123 | −21.010 | 0.101 | 250.250 | 0.111 | −21.320 | 0.108 | 240.800 | 0.110 | −30.395 | 0.021 | 236.250 | 0.122 | −33.110 | 0.024 |
| 111 | 244.050 | 0.109 | −23.705 | 0.095 | 248.300 | 0.115 | −23.680 | 0.103 | 247.400 | 0.136 | −31.950 | 0.017 | 239.400 | 0.111 | −32.780 | 0.017 |
| 112 | 240.850 | 0.118 | −24.305 | 0.116 | 251.000 | 0.111 | −24.100 | 0.115 | 237.600 | 0.123 | −28.950 | 0.034 | 233.600 | 0.134 | −31.670 | 0.034 |
| 113 | 253.600 | 0.158 | −21.430 | 0.101 | 256.650 | 0.179 | −22.805 | 0.112 | 236.900 | 0.123 | −28.455 | 0.028 | 250.200 | 0.139 | −31.385 | 0.029 |
| 114 | 248.700 | 0.124 | −22.620 | 0.092 | 256.550 | 0.183 | −23.220 | 0.101 | 235.350 | 0.098 | −32.915 | 0.015 | 243.350 | 0.108 | −33.360 | 0.013 |
| 115 | 250.400 | 0.116 | −22.240 | 0.095 | 243.450 | 0.106 | −23.240 | 0.103 | 234.450 | 0.077 | −31.330 | 0.018 | 238.000 | 0.119 | −32.825 | 0.175 |
| 116 | 246.900 | 0.139 | −22.360 | 0.092 | 247.450 | 0.143 | −25.410 | 0.102 | 228.450 | 0.114 | −32.155 | 0.016 | 241.550 | 0.119 | −31.695 | 0.016 |
| 117 | 230.200 | 0.130 | −17.250 | 0.188 | 236.300 | 0.142 | −21.660 | 0.083 | 238.250 | 0.091 | −29.145 | 0.021 | 238.950 | 0.127 | −29.825 | 0.023 |
| 118 | 251.950 | 0.181 | −19.220 | 0.206 | 233.400 | 0.107 | −22.450 | 0.095 | 237.350 | 0.113 | −30.025 | 0.029 | 247.950 | 0.105 | −30.820 | 0.028 |
| 119 | 221.150 | 0.991 | −16.830 | 0.206 | 215.600 | 0.074 | −20.680 | 0.096 | 226.400 | 0.101 | −26.960 | 0.033 | 225.400 | 0.092 | −30.195 | 0.032 |
| 120 | 225.400 | 1.000 | −17.860 | 0.198 | 222.450 | 0.035 | −21.460 | 0.099 | 226.100 | 0.017 | −31.005 | 0.023 | 228.500 | 0.048 | −28.755 | 0.026 |
| 121 | 211.650 | 0.777 | −16.145 | 0.203 | 208.300 | 0.065 | −18.880 | 0.108 | 223.600 | 0.079 | −26.930 | 0.027 | 227.350 | 0.058 | −30.345 | 0.027 |
| 122 | 220.400 | 0.133 | −16.720 | 0.221 | 209.150 | 0.044 | −19.400 | 0.106 | 218.700 | 0.062 | −25.325 | 0.048 | 221.700 | 0.047 | −26.275 | 0.056 |
| 123 | 221.750 | 0.121 | −12.730 | 0.199 | 210.800 | 0.077 | −21.840 | 0.079 | 221.200 | 0.097 | −27.305 | 0.026 | 223.950 | 0.057 | −27.230 | 0.031 |
| 124 | 215.950 | 0.116 | −18.180 | 0.215 | 215.650 | 0.062 | −30.650 | 0.091 | 220.000 | 0.074 | −23.120 | 0.034 | 229.550 | 0.057 | −23.985 | 0.037 |
| 125 | 214.700 | 0.078 | −15.120 | 0.191 | 209.700 | 0.071 | −20.740 | 0.069 | 225.550 | 0.119 | −28.045 | 0.016 | 234.050 | 0.150 | −30.340 | 0.017 |
| 126 | 217.450 | 0.085 | −15.170 | 0.201 | 212.850 | 0.039 | −20.800 | 0.078 | 218.300 | 0.077 | −27.840 | 0.027 | 221.600 | 0.065 | −29.290 | 0.029 |
| 127 | 227.050 | 0.049 | −16.980 | 0.198 | 222.550 | 0.094 | −22.180 | 0.075 | 234.300 | 0.094 | −29.565 | 0.021 | 232.900 | 0.105 | −30.105 | 0.022 |
| 128 | 221.050 | 0.085 | −15.110 | 0.202 | 219.750 | 0.118 | −22.980 | 0.081 | 224.250 | 0.074 | −27.110 | 0.024 | 217.550 | 0.087 | −28.450 | 0.026 |
| 129 | 226.100 | 0.144 | −17.680 | 0.197 | 225.400 | 0.125 | −23.440 | 0.074 | 235.650 | 0.115 | −29.805 | 0.019 | 233.250 | 0.079 | −28.940 | 0.020 |
| 130 | 219.250 | 0.063 | −17.220 | 0.210 | 219.200 | 0.143 | −16.790 | 0.155 | 226.050 | 0.073 | −25.740 | 0.037 | 224.450 | 0.073 | −25.765 | 0.040 |
| 131 | 216.550 | 0.077 | −13.930 | 0.205 | 212.850 | 0.055 | −14.490 | 0.146 | 226.450 | 0.089 | −27.905 | 0.026 | 221.000 | 0.096 | −29.345 | 0.029 |
| 132 | 235.350 | 0.183 | −16.300 | 0.195 | 221.150 | 0.111 | −22.680 | 0.085 | 227.150 | 0.035 | −28.300 | 0.016 | 224.550 | 0.063 | −28.940 | 0.019 |
| 133 | 210.800 | 0.080 | −14.140 | 0.198 | 209.050 | 0.050 | −16.070 | 0.136 | 219.750 | 0.073 | −28.665 | 0.019 | 225.550 | 0.077 | −26.145 | 0.020 |
| 134 | 217.800 | 0.144 | −15.370 | 0.173 | 208.500 | 0.057 | −15.485 | 0.113 | 212.000 | 0.087 | −29.350 | 0.021 | 220.200 | 0.073 | −29.255 | 0.020 |
| 135 | 209.550 | 0.098 | −15.380 | 0.177 | 208.650 | 0.067 | −15.390 | 0.116 | 220.500 | 0.085 | −28.450 | 0.022 | 219.100 | 0.079 | −30.640 | 0.022 |
| 136 | 212.700 | 0.030 | −12.940 | 0.184 | 214.800 | 0.074 | −17.650 | 0.110 | 216.400 | 0.071 | −28.370 | 0.035 | 219.550 | 0.061 | −32.450 | 0.003 |
| 137 | 228.200 | 0.075 | −17.520 | −0.167 | 232.800 | 0.133 | −21.220 | 0.099 | 239.950 | 0.131 | −33.300 | 0.018 | 238.000 | 0.138 | −31.165 | 0.020 |
| 138 | 228.050 | 0.115 | −17.220 | 0.171 | 229.000 | 0.090 | −20.740 | 0.100 | 252.500 | 0.176 | −31.740 | 0.018 | 243.700 | 0.124 | −31.045 | 0.019 |
| 139 | 223.300 | 0.110 | −17.015 | 0.186 | 224.150 | 0.079 | −20.050 | 0.104 | 237.250 | 0.138 | −33.835 | 0.035 | 236.800 | 0.131 | −31.725 | 0.032 |
| 140 | 228.550 | 0.097 | −16.105 | 0.188 | 234.150 | 0.090 | −20.015 | 0.107 | 235.250 | 0.113 | −32.850 | 0.039 | 236.000 | 0.106 | −35.295 | 0.045 |
| 141 | 227.050 | 0.103 | −15.250 | 0.174 | 227.300 | 0.121 | −21.095 | 0.088 | 247.750 | 0.109 | −29.510 | 0.023 | 241.150 | 0.126 | −32.190 | 0.028 |
| 142 | 229.500 | 0.138 | −15.740 | 0.170 | 229.350 | 0.090 | −18.900 | 0.087 | 249.600 | 0.141 | −31.835 | 0.020 | 239.700 | 0.102 | −33.690 | 0.021 |
| 143 | 231.700 | 0.128 | −17.655 | 0.174 | 227.800 | 0.134 | −20.665 | 0.092 | 249.750 | 0.130 | −34.000 | 0.026 | 237.850 | 0.074 | −31.815 | 0.027 |
| 144 | 229.600 | 0.086 | −15.925 | 0.170 | 227.800 | 0.102 | −20.845 | 0.091 | 237.900 | 0.125 | −29.580 | 0.024 | 239.100 | 0.119 | −27.390 | 0.022 |
| 145 | 225.700 | 0.137 | −16.890 | 0.169 | 240.750 | 0.161 | −16.465 | 0.154 | 233.750 | 0.131 | −35.915 | 0.017 | 136.750 | 0.219 | −36.660 | 0.021 |
| 146 | 229.950 | 0.123 | −16.465 | 0.177 | 231.400 | 0.131 | −15.995 | 0.160 | 232.150 | 0.099 | −31.905 | 0.022 | 239.800 | 0.124 | −29.850 | 0.032 |
| 147 | 228.300 | 0.104 | −16.310 | 0.170 | 233.500 | 0.113 | −16.455 | 0.159 | 255.950 | 0.184 | −34.925 | 0.020 | 236.400 | 0.078 | −35.115 | 0.043 |
| 148 | 229.200 | 0.097 | −16.040 | 0.177 | 233.000 | 0.116 | −15.420 | 0.160 | 234.300 | 0.141 | −31.455 | 0.022 | 249.850 | 0.165 | −29.940 | 0.021 |
| 149 | 218.250 | 0.131 | −16.030 | 0.170 | 229.650 | 0.122 | −15.915 | 0.156 | 240.700 | 0.108 | −36.150 | 0.015 | 253.200 | 0.189 | −31.695 | 0.023 |
| 150 | 230.100 | 0.099 | −16.555 | 0.184 | 234.450 | 0.078 | −17.205 | 0.171 | 240.750 | 0.130 | −31.875 | 0.030 | 239.350 | 0.115 | −32.995 | 0.031 |
| Sample Number | SAMPLES “B” | SAMPLES “A” | ||
|---|---|---|---|---|
| t = 24 h | t = 24 h + 24 h | t = 15 days | t = 15 days + 24 h | |
| 101 | 6.06 | 5.97 | 5.87 | 6.04 |
| 102 | 5.98 | 6.00 | 5.98 | 5.96 |
| 103 | 6.07 | 6.01 | 5.94 | 5.90 |
| 104 | 6.13 | 5.95 | 6.05 | 5.94 |
| 105 | 6.14 | 5.89 | 5.95 | 5.92 |
| 106 | 5.87 | 5.95 | 6.02 | 6.14 |
| 107 | 5.92 | 5.89 | 5.97 | 6.01 |
| 108 | 6.13 | 6.03 | 5.91 | 6.08 |
| 109 | 5.98 | 5.96 | 6.06 | 6.02 |
| 110 | 6.08 | 5.90 | 6.07 | 6.11 |
| 111 | 5.86 | 5.95 | 5.92 | 5.89 |
| 112 | 6.00 | 5.97 | 6.13 | 6.03 |
| 113 | 5.89 | 5.87 | 6.00 | 6.05 |
| 114 | 6.07 | 6.01 | 6.10 | 5.94 |
| 115 | 6.13 | 5.95 | 5.95 | 6.05 |
| 116 | 5.89 | 5.94 | 6.04 | 5.98 |
| 117 | 6.15 | 6.07 | 6.03 | 6.16 |
| 118 | 5.93 | 6.15 | 5.98 | 5.96 |
| 119 | 6.08 | 6.11 | 5.86 | 6.08 |
| 120 | 6.13 | 6.07 | 5.97 | 6.01 |
| 121 | 5.89 | 6.12 | 5.91 | 6.08 |
| 122 | 5.95 | 5.89 | 5.92 | 6.09 |
| 123 | 6.14 | 5.96 | 6.11 | 6.08 |
| 124 | 5.87 | 5.95 | 5.92 | 6.11 |
| 125 | 6.00 | 6.02 | 6.14 | 6.15 |
| 126 | 6.07 | 6.03 | 6.16 | 5.87 |
| 127 | 5.97 | 6.12 | 5.96 | 6.14 |
| 128 | 6.12 | 5.89 | 6.12 | 5.91 |
| 129 | 5.89 | 5.95 | 5.89 | 5.92 |
| 130 | 6.02 | 6.06 | 6.02 | 6.02 |
| 131 | 6.11 | 6.07 | 6.11 | 5.95 |
| 132 | 5.89 | 6.09 | 5.90 | 6.00 |
| 133 | 6.03 | 6.00 | 5.88 | 6.10 |
| 134 | 5.94 | 5.90 | 5.94 | 5.95 |
| 135 | 6.12 | 6.13 | 6.11 | 6.04 |
| 136 | 6.01 | 6.00 | 6.05 | 5.99 |
| 137 | 5.96 | 6.10 | 5.94 | 5.90 |
| 138 | 6.08 | 5.95 | 6.05 | 5.94 |
| 139 | 5.88 | 6.04 | 5.98 | 6.05 |
| 140 | 5.89 | 6.12 | 6.02 | 6.09 |
| 141 | 6.17 | 5.89 | 6.12 | 6.06 |
| 142 | 5.89 | 5.89 | 6.08 | 5.95 |
| 143 | 5.88 | 5.96 | 6.12 | 5.94 |
| 144 | 6.06 | 5.97 | 6.04 | 6.03 |
| 145 | 6.00 | 6.01 | 5.96 | 6.14 |
| 146 | 5.89 | 5.95 | 5.99 | 5.87 |
| 147 | 6.17 | 5.89 | 6.12 | 6.05 |
| 148 | 5.89 | 5.89 | 6.00 | 5.88 |
| 149 | 5.99 | 6.01 | 5.92 | 5.90 |
| 150 | 5.91 | 6.02 | 6.12 | 5.86 |
| Sample Number | T = 0 | T = 24 h | T = 24 + 24 h | T = 15 days | T = 15 days + 24 h |
|---|---|---|---|---|---|
| 101 | 0.0720 | 0.0691 (96.00%) | 0.0691 (96.00%) | 0.0677 (94.12%) | 0.0670 (93.18%) |
| 102 | 0.0850 | 0.0816 (98.17%) | 0.0816 (94.17%) | 0.0800 (93.50%) | 0.0792 (91.17%) |
| 103 | 0.0600 | 0.0589 (95.29%) | 0.0565 (93.57%) | 0.0561 (91.00%) | 0.0547 (94.29%) |
| 104 | 0.0700 | 0.0667 (97.68%) | 0.0655 (99.64%) | 0.0637 (98.04%) | 0.0660 (93.21%) |
| 105 | 0.0560 | 0.0547 (98.00%) | 0.0558 (96.17%) | 0.0549 (96.67%) | 0.0522 (95.83%) |
| 106 | 0.0600 | 0.0588 (97.22%) | 0.0577 (95.97%) | 0.0580 (97.50%) | 0.0575 (93.19%) |
| 107 | 0.0720 | 0.0700 (99.57%) | 0.0691 (98.57%) | 0.0702 (96.00%) | 0.0671 (92.43%) |
| 108 | 0.0700 | 0.0697 (95.98%) | 0.0690 (95.98%) | 0.0672 (94.13%) | 0.0647 (93.15%) |
| 109 | 0.0920 | 0.0883 (96.00%) | 0.0883 (96.00%) | 0.0866 (94.00%) | 0.0857 (93.17%) |
| 110 | 0.0600 | 0.0576 (96.00%) | 0.0576 (96.00%) | 0.0564 (94.13%) | 0.0559 (93.13%) |
| 111 | 0.0800 | 0.0768 (96.07%) | 0.0768 (96.07%) | 0.0753 (94.11%) | 0.0745 (93.04%) |
| 112 | 0.0560 | 0.0538 (96.04%) | 0.0538 (96.04%) | 0.0527 (94.17%) | 0.0521 (93.13%) |
| 113 | 0.0480 | 0.0461 (96.07%) | 0.0461 (96.07%) | 0.0452 (94.05%) | 0.0447 (93.10%) |
| 114 | 0.0840 | 0.0807 (98.63%) | 0.0807 (95.91%) | 0.0790 (94.89%) | 0.0782 (97.73%) |
| 115 | 0.0880 | 0.0868 (95.57%) | 0.0844 (96.48%) | 0.0835 (96.02%) | 0.0860 (99.20%) |
| 116 | 0.0880 | 0.0841 (96.91%) | 0.0849 (95.15%) | 0.0845 (97.94%) | 0.0873 (94.41%) |
| 117 | 0.0680 | 0.0659 (97.88%) | 0.0647 (93.65%) | 0.0666 (97.69%) | 0.0642 (95.96%) |
| 118 | 0.0520 | 0.0509 (98.61%) | 0.0487 (97.50%) | 0.0508 (96.81%) | 0.0499 (98.06%) |
| 119 | 0.0720 | 0.0710 (99.00%) | 0.0702 (98.50%) | 0.0697 (98.38%) | 0.0706 (93.88%) |
| 120 | 0.0800 | 0.0792 (94.67%) | 0.0788 (94.50%) | 0.0787 (94.00%) | 0.0751 (96.50%) |
| 121 | 0.0600 | 0.0568 (98.14%) | 0.0567 (96.43%) | 0.0564 (95.00%) | 0.0579 (90.29%) |
| 122 | 0.0700 | 0.0687 (96.07%) | 0.0675 (96.07%) | 0.0665 (94.11%) | 0.0632 (93.04%) |
| 123 | 0.0560 | 0.0538 (96.00%) | 0.0538 (96.00%) | 0.0527 (94.00%) | 0.0521 (93.17%) |
| 124 | 0.0600 | 0.0576 (95.97%) | 0.0576 (95.97%) | 0.0564 (94.03%) | 0.0559 (93.06%) |
| 125 | 0.0720 | 0.0691 (96.00%) | 0.0691 (96.00%) | 0.0677 (94.14%) | 0.0670 (93.14%) |
| 126 | 0.0700 | 0.0672 (95.98%) | 0.0672 (95.98%) | 0.0659 (94.13%) | 0.0652 (93.15%) |
| 127 | 0.0920 | 0.0883 (96.00%) | 0.0883 (96.00%) | 0.0866 (94.00%) | 0.0857 (93.17%) |
| 128 | 0.0600 | 0.0576 (96.00%) | 0.0576 (96.00%) | 0.0564 (94.13%) | 0.0559 (93.13%) |
| 129 | 0.0800 | 0.0768 (96.07%) | 0.0768 (96.07%) | 0.0753 (94.11%) | 0.0745 (93.04%) |
| 130 | 0.0560 | 0.0538 (96.04%) | 0.0538 (96.04%) | 0.0527 (94.17%) | 0.0521 (93.13%) |
| 131 | 0.0480 | 0.0461 (96.67%) | 0.0461 (96.19%) | 0.0452 (96.67%) | 0.0447 (95.12%) |
| 132 | 0.0840 | 0.0812 (98.75%) | 0.0808 (98.75%) | 0.0812 (97.16%) | 0.0799 (96.48%) |
| 133 | 0.0880 | 0.0869 (93.52%) | 0.0869 (95.91%) | 0.0855 (91.70%) | 0.0849 (91.36%) |
| 134 | 0.0880 | 0.0823 (95.29%) | 0.0844 (93.09%) | 0.0807 (91.91%) | 0.0804 (92.50%) |
| 135 | 0.0680 | 0.0648 (98.46%) | 0.0633 (96.54%) | 0.0625 (96.73%) | 0.0629 (96.92%) |
| 136 | 0.0520 | 0.0512 (96.00%) | 0.0502 (96.00%) | 0.0503 (94.00%) | 0.0504 (93.17%) |
| 137 | 0.0600 | 0.0576 (97.50%) | 0.0576 (93.33%) | 0.0564 (92.00%) | 0.0559 (90.17%) |
| 138 | 0.0600 | 0.0585 (99.13%) | 0.0560 (93.13%) | 0.0552 (95.00%) | 0.0541 (97.75%) |
| 139 | 0.0800 | 0.0793 (97.34%) | 0.0745 (99.69%) | 0.0760 (95.94%) | 0.0782 (92.97%) |
| 140 | 0.0640 | 0.0623 (99.80%) | 0.0638 (97.20%) | 0.0614 (98.80%) | 0.0595 (99.20%) |
| 141 | 0.0500 | 0.0499 (96.25%) | 0.0486 (94.75%) | 0.0494 (93.25%) | 0.0496 (91.50%) |
| 142 | 0.0400 | 0.0385 (94.44%) | 0.0379 (95.83%) | 0.0373 (92.78%) | 0.0366 (94.03%) |
| 143 | 0.0720 | 0.0680 (94.89%) | 0.0690 (97.07%) | 0.0668 (91.74%) | 0.0677 (90.65%) |
| 144 | 0.0920 | 0.0873 (96.32%) | 0.0893 (95.79%) | 0.0844 (92.24%) | 0.0834 (90.92%) |
| 145 | 0.0760 | 0.0732 (95.12%) | 0.0728 (96.19%) | 0.0701 (96.67%) | 0.0691 (95.12%) |
| 146 | 0.0840 | 0.0799 (92.71%) | 0.0808 (98.13%) | 0.0812 (94.38%) | 0.0799 (95.42%) |
| 147 | 0.0480 | 0.0445 (95.56%) | 0.0471 (94.17%) | 0.0453 (93.33%) | 0.0458 (91.11%) |
| 148 | 0.0720 | 0.0688 (95.81%) | 0.0678 (93.79%) | 0.0672 (93.47%) | 0.0656 (92.34%) |
| 149 | 0.1240 | 0.1188 (95.00%) | 0.1163 (96.83%) | 0.1159 (93.67%) | 0.1145 (92.17%) |
| 150 | 0.0600 | 0.0570 (95.00%) | 0.0581 (96.83%) | 0.0562 (93.67%) | 0.0553 (92.17%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiczewski, R.; Sobol, Ż.; Pacholska, A.; Wątróbska-Świetlikowska, D. Extended Stability of Ascorbic Acid in Pediatric TPN Admixtures: The Role of Storage Temperature and Emulsion Integrity. Pharmaceutics 2025, 17, 1375. https://doi.org/10.3390/pharmaceutics17111375
Chiczewski R, Sobol Ż, Pacholska A, Wątróbska-Świetlikowska D. Extended Stability of Ascorbic Acid in Pediatric TPN Admixtures: The Role of Storage Temperature and Emulsion Integrity. Pharmaceutics. 2025; 17(11):1375. https://doi.org/10.3390/pharmaceutics17111375
Chicago/Turabian StyleChiczewski, Rafał, Żaneta Sobol, Alicja Pacholska, and Dorota Wątróbska-Świetlikowska. 2025. "Extended Stability of Ascorbic Acid in Pediatric TPN Admixtures: The Role of Storage Temperature and Emulsion Integrity" Pharmaceutics 17, no. 11: 1375. https://doi.org/10.3390/pharmaceutics17111375
APA StyleChiczewski, R., Sobol, Ż., Pacholska, A., & Wątróbska-Świetlikowska, D. (2025). Extended Stability of Ascorbic Acid in Pediatric TPN Admixtures: The Role of Storage Temperature and Emulsion Integrity. Pharmaceutics, 17(11), 1375. https://doi.org/10.3390/pharmaceutics17111375







