Estimated Glomerular Filtration Rate Variability in Patients with Diabetes Receiving SGLT2 Inhibitors Versus DPP4 Inhibitors
Abstract
1. Introduction
2. Patients, Materials, and Methods
2.1. Database
2.2. Study Design
2.3. Study Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. The Baseline Characteristics of Patients Receiving SGLT2i and DPP4i Treatment
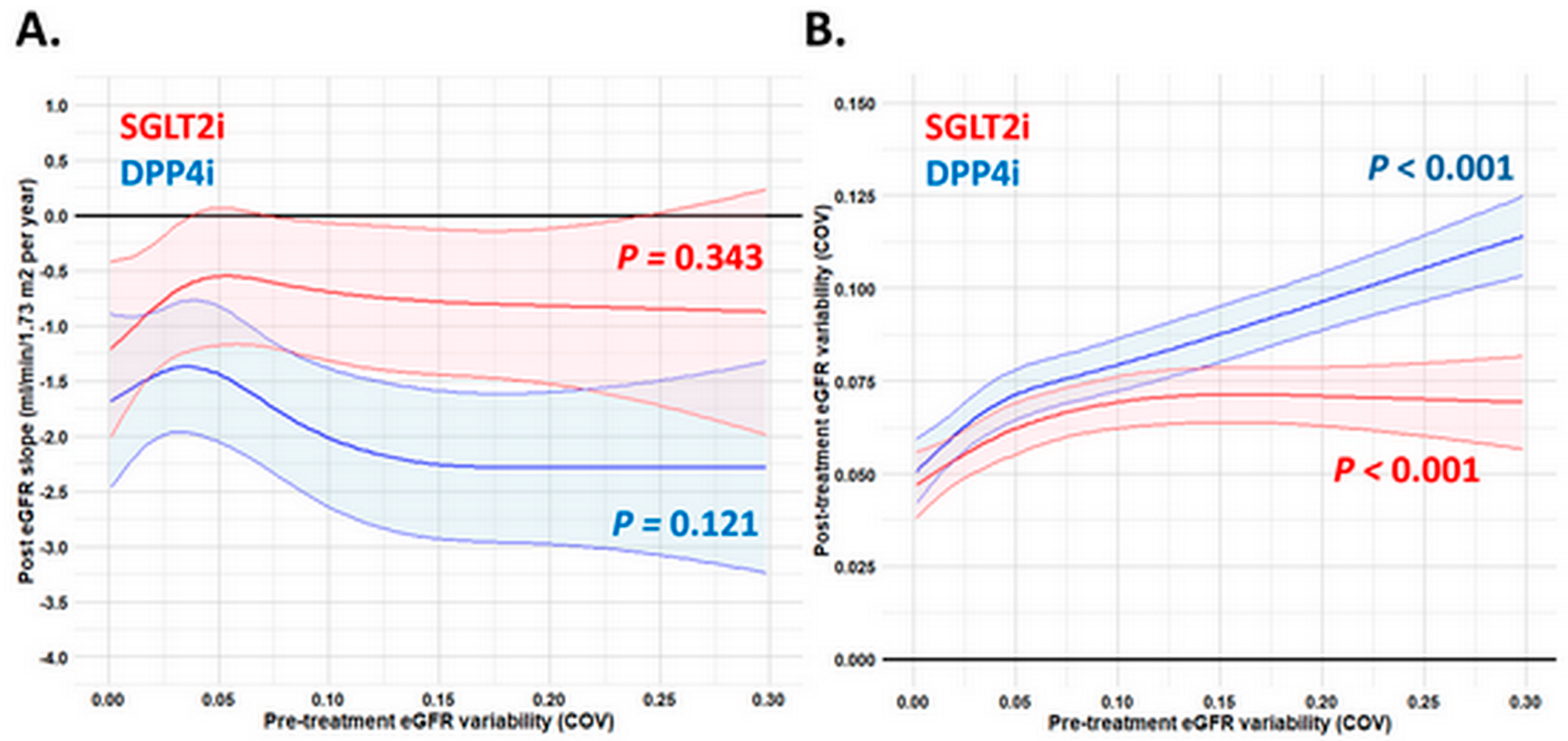
3.2. Pre-Treatment and Post-Treatment eGFR Slope and Variability in Patients Receiving SGLT2i and DPP4i Treatment
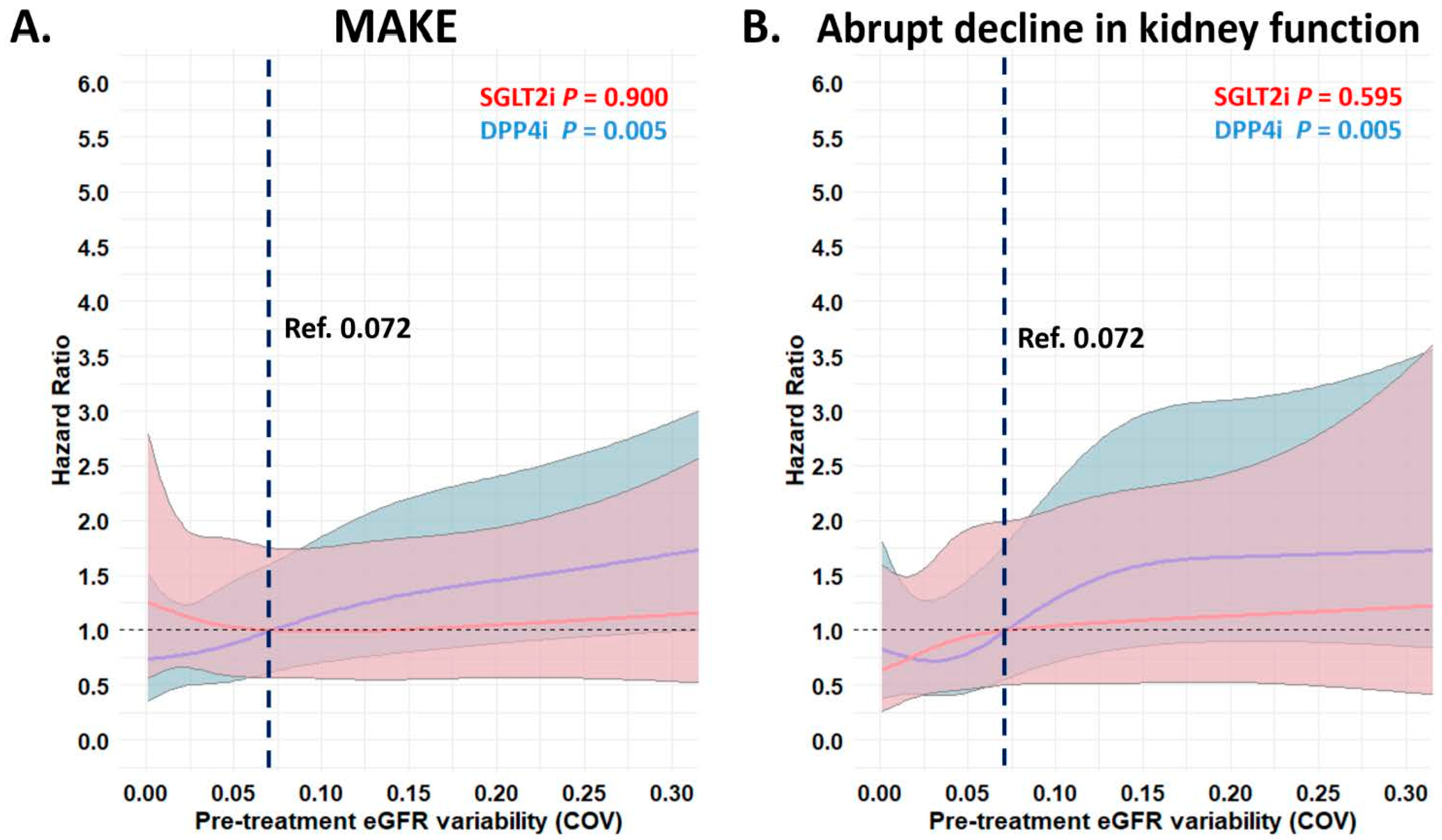
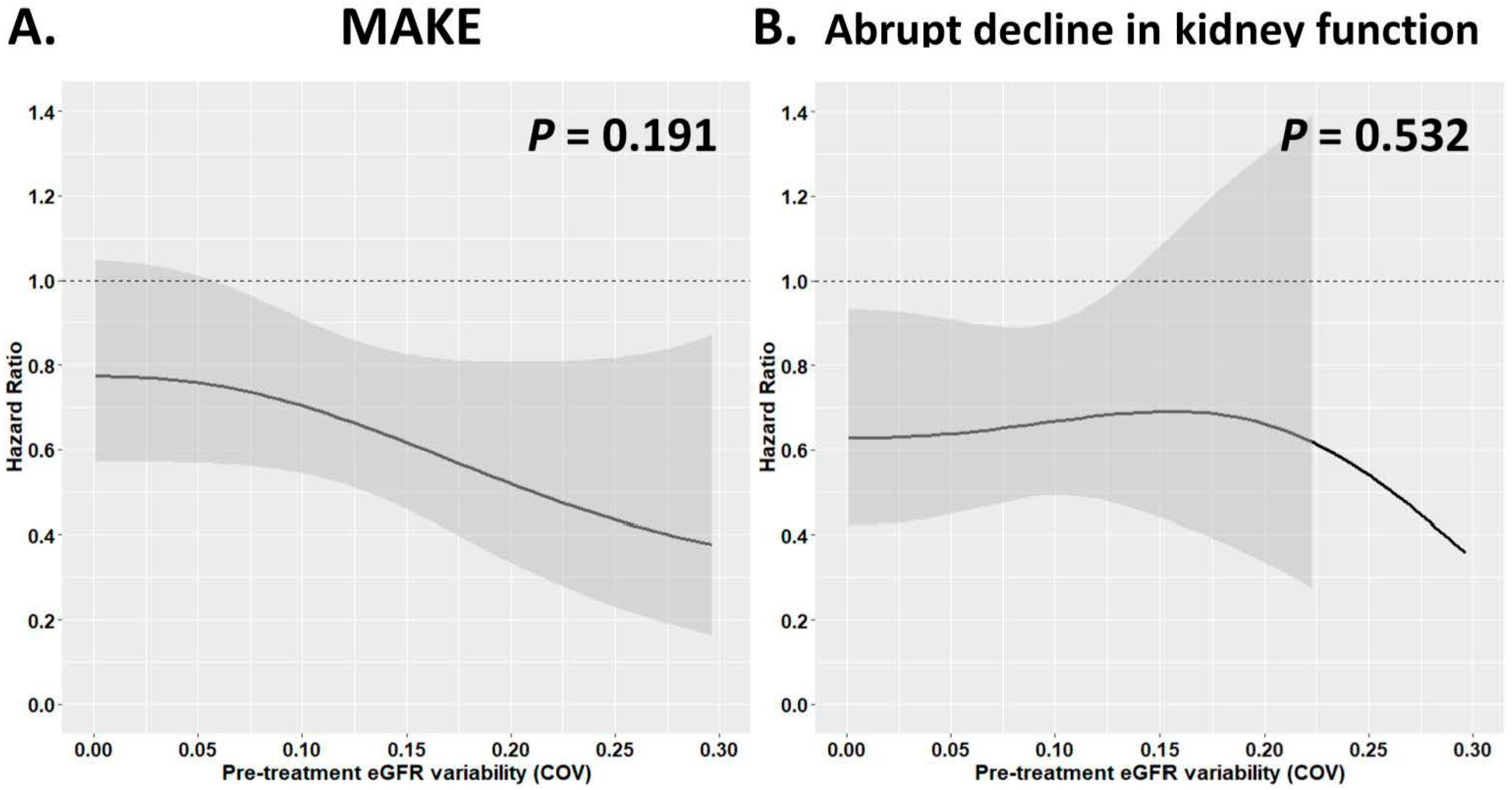
3.3. Risk of Adverse Kidney Outcomes with Different Pre-Treatment eGFR Variability in Patients Receiving SGLT2i vs. DPP4i Treatment
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| BMI | body mass index |
| BP | blood pressure |
| BW | body weight |
| CI | confidence interval |
| CKD | chronic kidney disease |
| COV | coefficient of variation |
| DPP4i | dipeptidyl peptidase-4 inhibitor |
| eGFR | estimated glomerular filtration rate |
| ESKD | end-stage kidney disease |
| HbA1c | glycated hemoglobin |
| HDL | high-density lipoprotein |
| HR | hazard ratio |
| LDL | low-density lipoprotein |
| MAKE | major adverse kidney event |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| RAAS | renin–angiotensin system |
| SGLT2i | sodium–glucose cotransporter-2 inhibitor |
| SD | standard deviation |
| T2D | type 2 diabetes |
| UACR | urine albumin-to-creatinine ratio |
References
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Am. J. Kidney Dis. 2014, 64, 510–533. [Google Scholar] [CrossRef] [PubMed]
- Warren, B.; Rebholz, C.M.; Sang, Y.; Lee, A.K.; Coresh, J.; Selvin, E.; Grams, M.E. Diabetes and Trajectories of Estimated Glomerular Filtration Rate: A Prospective Cohort Analysis of the Atherosclerosis Risk in Communities Study. Diabetes Care 2018, 41, 1646–1653. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Ballew, S.H.; Matsushita, K.; Astor, B.C.; Carrero, J.J.; Chang, A.R.; Inker, L.A.; Kenealy, T.; Kovesdy, C.P.; et al. Evaluating Glomerular Filtration Rate Slope as a Surrogate End Point for ESKD in Clinical Trials: An Individual Participant Meta-Analysis of Observational Data. J. Am. Soc. Nephrol. 2019, 30, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Gansevoort, R.T.; Coresh, J.; Inker, L.A.; Heerspink, H.L.; Grams, M.E.; Greene, T.; Tighiouart, H.; Matsushita, K.; Ballew, S.H.; et al. Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD: A Scientific Workshop Sponsored by the National Kidney Foundation in Collaboration with the US Food and Drug Administration and European Medicines Agency. Am. J. Kidney Dis. 2020, 75, 84–104. [Google Scholar] [CrossRef]
- Inker, L.A.; Collier, W.; Greene, T.; Miao, S.; Chaudhari, J.; Appel, G.B.; Badve, S.V.; Caravaca-Fontan, F.; Del Vecchio, L.; Floege, J.; et al. A meta-analysis of GFR slope as a surrogate endpoint for kidney failure. Nat. Med. 2023, 29, 1867–1876. [Google Scholar] [CrossRef]
- Tseng, C.L.; Lafrance, J.P.; Lu, S.E.; Soroka, O.; Miller, D.R.; Maney, M.; Pogach, L.M. Variability in estimated glomerular filtration rate values is a risk factor in chronic kidney disease progression among patients with diabetes. BMC Nephrol. 2015, 16, 34. [Google Scholar] [CrossRef]
- Malhotra, R.; Katz, R.; Jotwani, V.; Agarwal, A.; Cohen, D.L.; Cushman, W.C.; Ishani, A.; Killeen, A.A.; Kitzman, D.W.; Oparil, S.; et al. Estimated GFR Variability and Risk of Cardiovascular Events and Mortality in SPRINT (Systolic Blood Pressure Intervention Trial). Am. J. Kidney Dis. 2021, 78, 48–56. [Google Scholar] [CrossRef]
- Fravel, M.A.; Ernst, M.E.; Webb, K.L.; Wetmore, J.B.; Wolfe, R.; Woods, R.L.; Reid, C.M.; Chowdhury, E.; Murray, A.M.; Polkinghorne, K.R.; et al. GFR Variability, Survival, and Cardiovascular Events in Older Adults. Kidney Med. 2023, 5, 100583. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Missikpode, C.; Ricardo, A.C.; Yang, W.; Anderson, A.H.; Lash, J.P.; Kelly, T.N.; Investigators, C.S. Time-Updated Estimated Glomerular Filtration Rate Variability is Associated With Mortality, Cardiovascular Disease and End-Stage Kidney Disease in Patients With CKD: The CRIC Study. Am. J. Kidney Dis. 2025, 85, 695–703.e1. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Obi, Y.; Hayashi, T.; Kotani, N.; Uemura, Y.; Imai, E.; Makino, H.; Hishida, A. Visit-to-visit variability in estimated glomerular filtration rate predicts hospitalization and death due to cardiovascular events. Clin. Exp. Nephrol. 2019, 23, 661–668. [Google Scholar] [CrossRef]
- Hein, A.M.; Scialla, J.J.; Sun, J.L.; Greene, S.J.; Shaw, L.K.; Chiswell, K.; Pun, P.H.; Mentz, R.J. Estimated Glomerular Filtration Rate Variability in Patients With Heart Failure and Chronic Kidney Disease. J. Card. Fail. 2021, 27, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Harris, K.; Heerspink, H.J.L.; Badve, S.V.; Jardine, M.J.; Harrap, S.; Hamet, P.; Marre, M.; Poulter, N.; Kotwal, S.; et al. Variability in estimated glomerular filtration rate and the risk of major clinical outcomes in diabetes: Post hoc analysis from the ADVANCE trial. Diabetes Obes. Metab. 2021, 23, 1420–1425. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The, E.-K.C.G.; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Au, P.C.M.; Tan, K.C.B.; Cheung, B.M.Y.; Wong, I.C.K.; Li, H.L.; Cheung, C.L. Association Between SGLT2 Inhibitors vs DPP4 Inhibitors and Renal Outcomes Among Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, e2962–e2970. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kaneko, H.; Okada, A.; Komuro, J.; Ko, T.; Fujiu, K.; Takeda, N.; Morita, H.; Nishiyama, A.; Ieda, M.; et al. Kidney outcomes with SGLT2 inhibitor vs. DPP4 inhibitor use in older adults with diabetes. Nephrol. Dial. Transplant. 2024, 40, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Melzer Cohen, C.; Schechter, M.; Rozenberg, A.; Yanuv, I.; Sehtman-Shachar, D.R.; Fishkin, A.; Rosenzweig, D.; Chodick, G.; Karasik, A.; Mosenzon, O. Long-Term, Real-World Kidney Outcomes with SGLT2i versus DPP4i in Type 2 Diabetes without Cardiovascular or Kidney Disease. Clin. J. Am. Soc. Nephrol. 2023, 18, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Chanawong, A.; Uitrakul, S.; Incomenoy, S.; Poonchuay, N. Renoprotective Effect of Thai Patients with Type 2 Diabetes Mellitus Treated with SGLT-2 Inhibitors versus DPP-4 Inhibitors: A Real-World Observational Study. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 5581417. [Google Scholar] [CrossRef]
- Yoshida, N.; Hanai, K.; Babazono, T. Comparative effects of sodium-glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors on kidney function decline in Japanese individuals with type 2 diabetes. Clin. Exp. Nephrol. 2024, 28, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Cherney, D.; Postmus, D.; Stefansson, B.V.; Chertow, G.M.; Dwyer, J.P.; Greene, T.; Kosiborod, M.; Langkilde, A.M.; McMurray, J.J.V.; et al. A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022, 101, 174–184. [Google Scholar] [CrossRef]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Krolewski, A.S.; Skupien, J.; Rossing, P.; Warram, J.H. Fast renal decline to end-stage renal disease: An unrecognized feature of nephropathy in diabetes. Kidney Int. 2017, 91, 1300–1311. [Google Scholar] [CrossRef]
- Misra, P.S.; Szeto, S.G.; Krizova, A.; Gilbert, R.E.; Yuen, D.A. Renal histology in diabetic nephropathy predicts progression to end-stage kidney disease but not the rate of renal function decline. BMC Nephrol. 2020, 21, 285. [Google Scholar] [CrossRef]
- Malan, L.; Smuts, C.M.; Baumgartner, J.; Ricci, C. Missing data imputation via the expectation-maximization algorithm can improve principal component analysis aimed at deriving biomarker profiles and dietary patterns. Nutr. Res. 2020, 75, 67–76. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Ma, R.; He, J.; Zhang, X.; Rui, D.; Ding, Y.; Li, Y.; Jian, L.; Cheng, J.; et al. Comparison of the effects of imputation methods for missing data in predictive modelling of cohort study datasets. BMC Med. Res. Methodol. 2024, 24, 41. [Google Scholar] [CrossRef]
- Inker, L.A.; Heerspink, H.J.L.; Tighiouart, H.; Levey, A.S.; Coresh, J.; Gansevoort, R.T.; Simon, A.L.; Ying, J.; Beck, G.J.; Wanner, C.; et al. GFR Slope as a Surrogate End Point for Kidney Disease Progression in Clinical Trials: A Meta-Analysis of Treatment Effects of Randomized Controlled Trials. J. Am. Soc. Nephrol. 2019, 30, 1735–1745. [Google Scholar] [CrossRef]
- Rifkin, D.E.; Shlipak, M.G.; Katz, R.; Fried, L.F.; Siscovick, D.; Chonchol, M.; Newman, A.B.; Sarnak, M.J. Rapid kidney function decline and mortality risk in older adults. Arch. Intern. Med. 2008, 168, 2212–2218. [Google Scholar] [CrossRef]
- Zoppini, G.; Targher, G.; Chonchol, M.; Ortalda, V.; Negri, C.; Stoico, V.; Bonora, E. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin. J. Am. Soc. Nephrol. 2012, 7, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes, C.K.D.W.G. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Paradigm Shift in Hyperglycemic Glomerular Hyperfiltration: Blunted Tubuloglomerular Feedback or Preglomerular Vasodilation? Hypertension 2023, 80, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef]
- Scholtes, R.A.; van Baar, M.J.B.; Kok, M.D.; Bjornstad, P.; Cherney, D.Z.I.; Joles, J.A.; van Raalte, D.H. Renal haemodynamic and protective effects of renoactive drugs in type 2 diabetes: Interaction with SGLT2 inhibitors. Nephrology 2021, 26, 377–390. [Google Scholar] [CrossRef]
- Waikar, S.S.; Rebholz, C.M.; Zheng, Z.; Hurwitz, S.; Hsu, C.Y.; Feldman, H.I.; Xie, D.; Liu, K.D.; Mifflin, T.E.; Eckfeldt, J.H.; et al. Biological Variability of Estimated GFR and Albuminuria in CKD. Am. J. Kidney Dis. 2018, 72, 538–546. [Google Scholar] [CrossRef]
- Baaten, C.; Vondenhoff, S.; Noels, H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients With Chronic Kidney Disease. Circ. Res. 2023, 132, 970–992. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Rosen, C.J. Clinical Credence—SGLT2 Inhibitors, Diabetes, and Chronic Kidney Disease. N. Engl. J. Med. 2019, 380, 2371–2373. [Google Scholar] [CrossRef]
- Upadhyay, A. SGLT2 Inhibitors and Kidney Protection: Mechanisms Beyond Tubuloglomerular Feedback. Kidney360 2024, 5, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Tuersun, A.; Mohetaer, M.; Hou, G.; Cheng, G. Safety and Efficiency of Dipeptidyl Peptidase IV Inhibitors in Patients with Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Curr. Ther. Res. Clin. Exp. 2024, 101, 100763. [Google Scholar] [CrossRef]
- Lovshin, J.A.; Rajasekeran, H.; Lytvyn, Y.; Lovblom, L.E.; Khan, S.; Alemu, R.; Locke, A.; Lai, V.; He, H.; Hittle, L.; et al. Dipeptidyl Peptidase 4 Inhibition Stimulates Distal Tubular Natriuresis and Increases in Circulating SDF-1alpha(1-67) in Patients With Type 2 Diabetes. Diabetes Care 2017, 40, 1073–1081. [Google Scholar] [CrossRef]
- Sward, P.; Rippe, B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol. 2012, 204, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Fujita, H.; Fujishima, H.; Shimizu, T.; Sato, T.; Morii, T.; Tsukiyama, K.; Narita, T.; Takahashi, T.; Drucker, D.J.; et al. Stromal cell-derived factor-1 is upregulated by dipeptidyl peptidase-4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int. 2016, 90, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Is the Popularity of Dipeptidyl-Peptidase-4 Inhibitors Justified? Insights From Mechanistic Studies and Clinical Trials. Am. J. Med. 2018, 131, e287–e289. [Google Scholar] [CrossRef] [PubMed]


| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| SGLT2i (n = 6155) | DPP4i (n = 6175) | ASMD | SGLT2i (n = 3777) | DPP4i (n = 3777) | ASMD | |
| Baseline characteristics | ||||||
| Diabetes duration | 8.2 ± 5.3 | 8.0 ± 5.7 | 0.035 | 8.1 ± 5.3 | 8.0 ± 5.7 | 0.011 |
| Age (mean ± SD) | 62.4 ± 11.4 | 68.5 ± 11.4 | 0.537 | 65.0 ± 10.6 | 65.6 ± 11.3 | 0.048 |
| Male | 3732 (61) | 3280 (53) | 0.152 | 2124 (56) | 2116 (56) | 0.004 |
| Ischemic heart etiology | 683 (11) | 397 (6) | 0.166 | 312 (8) | 299 (8) | 0.013 |
| Cerebral vascular accidents | 100 (2) | 161 (3) | 0.068 | 80 (2) | 83 (2) | 0.005 |
| Congestive heart failure | 306 (5) | 226 (4) | 0.065 | 145 (4) | 144 (4) | 0.001 |
| Chronic lung disease | 198 (3) | 262 (4) | 0.054 | 136 (4) | 138 (4) | 0.003 |
| Chronic liver disease | 2112 (34) | 2075 (34) | 0.015 | 1277 (34) | 1236 (33) | 0.023 |
| Peripheral artery disease | 58 (1) | 73 (1) | 0.023 | 39 (1) | 45 (1) | 0.015 |
| Gout | 865 (14) | 972 (16) | 0.047 | 529 (14) | 532 (14) | 0.002 |
| Malignancy | 671 (11) | 1216 (20) | 0.246 | 518 (14) | 553 (15) | 0.027 |
| Baseline vital signs | ||||||
| Baseline body weight (KG) | 74.9 ± 15.3 | 67.4 ± 12.9 | 0.532 | 70.8 ± 12.7 | 70.2 ± 13.4 | 0.044 |
| Baseline SBP (mmHg) | 139.7 ± 19.4 | 138.5 ± 19.8 | 0.058 | 139.1 ± 19.1 | 139.2 ± 19.4 | 0.002 |
| Baseline DBP (mmHg) | 78.1 ± 11.9 | 76.2 ± 12.4 | 0.163 | 77.1 ± 11.4 | 77.2 ± 12.0 | 0.007 |
| Baseline heart rate (bpm) | 83.8 ± 13.6 | 82.8 ± 13.9 | 0.073 | 83.0 ± 13.5 | 83.2 ± 13.9 | 0.014 |
| Baseline laboratory data | ||||||
|
Pre-treatment eGFR slope (mL/min/1.73 m2/year) (med, IQR) | −1.37 (−4.34, 1.28) | −2.18 (−5.78, 0.22) | 0.225 | −1.70 (−4.92, 0.95) | −1.78 (−4.72, 0.63) | 0.010 |
|
Pre-treatment eGFR COV (med, IQR) | 0.050 (0.022, 0.096) | 0.060 (0.027, 0.110) | 0.157 | 0.055 (0.025, 0.101) | 0.052 (0.023, 0.096) | 0.009 |
|
Pre-treatment eGFR SD (mL/min/1.73 m2/year) (med, IQR) | 4.04 (2.15, 7.18) | 4.12 (2.18, 7.23) | 0.011 | 4.17 (2.19, 7.23) | 3.95 (2.03, 6.90) | 0.065 |
|
Baseline eGFR (mL/min/1.73 m2/year) | 85.4 ± 21.3 | 75.0 ± 25.0 | 0.447 | 81.2 ± 21.1 | 80.7 ± 23.9 | 0.022 |
|
Baseline urine albumin-to-creatinine ratio (mg/g) (med, IQR) | 63.9 (12.0, 275.1) | 72.0 (12.0, 384.0) | 0.065 | 61.7 (11.3, 282.0) | 65.0 (11.0, 376.0) | 0.008 |
| Baseline HbA1c (%) | 8.3 ± 1.5 | 7.6 ± 1.4 | 0.478 | 8.0 ± 1.3 | 7.9 ± 1.6 | 0.047 |
| Baseline ALT (U/L) | 35.8 ± 33.6 | 31.6 ± 33.5 | 0.125 | 33.4 ± 29.7 | 33.5 ± 30.8 | 0.003 |
| Baseline triglycerides (mg/dL) | 184.8 ± 219.8 | 156.0 ± 122.6 | 0.162 | 163.0 ± 131.3 | 159.0 ± 127.4 | 0.031 |
| Baseline LDL (mg/dL) | 91.7 ± 29.1 | 97.3 ± 61.2 | 0.116 | 92.8 ± 29.3 | 92.6 ± 35.8 | 0.007 |
| Baseline HDL (mg/dL) | 44.3 ± 11.3 | 46.0 ± 12.3 | 0.145 | 45.3 ± 11.6 | 45.5 ± 12.2 | 0.014 |
| Baseline medications | ||||||
| Use of anti-platelet agent | 2009 (33) | 1763 (29) | 0.089 | 1144 (30) | 1129 (30) | 0.009 |
| Use of statin | 3808 (62) | 3484 (56) | 0.111 | 2281 (60) | 2280 (60) | 0.001 |
| Use of CCB | 1136 (18) | 1489 (24) | 0.139 | 770 (20) | 763 (20) | 0.005 |
| Use of beta-blocker | 2302 (37) | 1801 (29) | 0.175 | 1247 (33) | 1222 (32) | 0.014 |
| Use of RAAS inhibitor | 4031 (65) | 3674 (59) | 0.124 | 2379 (63) | 2349 (62) | 0.016 |
| Use of loop diuretics | 461 (7) | 667 (11) | 0.115 | 308 (8) | 316 (8) | 0.008 |
| Use of thiazide | 1114 (18) | 937 (15) | 0.079 | 631 (17) | 608 (16) | 0.016 |
| Use of MRA | 232 (4) | 244 (4) | 0.009 | 139 (4) | 131 (3) | 0.011 |
| Use of vasodilator | 333 (5) | 294 (5) | 0.030 | 182 (5) | 178 (5) | 0.005 |
| Use of NSAIDs | 776 (13) | 1051 (17) | 0.124 | 554 (15) | 542 (14) | 0.009 |
| Use of UA lowering agent | 656 (11) | 873 (14) | 0.106 | 428 (11) | 466 (12) | 0.031 |
| Use of anti-diabetic agent | ||||||
| Metformin | 5488 (89) | 5051 (82) | 0.210 | 3305 (88) | 3285 (87) | 0.016 |
| SU | 3211 (52) | 2564 (42) | 0.215 | 1785 (47) | 1760 (47) | 0.013 |
| Glinide | 176 (3) | 340 (6) | 0.132 | 140 (4) | 135 (4) | 0.007 |
| Glitazone | 1133 (18) | 373 (6) | 0.384 | 404 (11) | 352 (9) | 0.046 |
| Acarbose | 910 (15) | 719 (12) | 0.093 | 491 (13) | 498 (13) | 0.005 |
| Insulin | 942 (15) | 703 (11) | 0.115 | 488 (13) | 459 (12) | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, Y.-W.; Chao, T.-F.; Cheng, Y.-W.; Chen, S.-W.; Chan, Y.-H. Estimated Glomerular Filtration Rate Variability in Patients with Diabetes Receiving SGLT2 Inhibitors Versus DPP4 Inhibitors. Pharmaceutics 2025, 17, 1370. https://doi.org/10.3390/pharmaceutics17111370
Kao Y-W, Chao T-F, Cheng Y-W, Chen S-W, Chan Y-H. Estimated Glomerular Filtration Rate Variability in Patients with Diabetes Receiving SGLT2 Inhibitors Versus DPP4 Inhibitors. Pharmaceutics. 2025; 17(11):1370. https://doi.org/10.3390/pharmaceutics17111370
Chicago/Turabian StyleKao, Yi-Wei, Tze-Fan Chao, Yu-Wen Cheng, Shao-Wei Chen, and Yi-Hsin Chan. 2025. "Estimated Glomerular Filtration Rate Variability in Patients with Diabetes Receiving SGLT2 Inhibitors Versus DPP4 Inhibitors" Pharmaceutics 17, no. 11: 1370. https://doi.org/10.3390/pharmaceutics17111370
APA StyleKao, Y.-W., Chao, T.-F., Cheng, Y.-W., Chen, S.-W., & Chan, Y.-H. (2025). Estimated Glomerular Filtration Rate Variability in Patients with Diabetes Receiving SGLT2 Inhibitors Versus DPP4 Inhibitors. Pharmaceutics, 17(11), 1370. https://doi.org/10.3390/pharmaceutics17111370







