Sex-Specific Differences in Antidepressant and Antipsychotic Treatment Outcomes and Serum Levels in Children and Adolescents
Abstract
1. Introduction
2. Methods
2.1. Setting and Participants
2.2. Study Medication
2.3. Therapeutic Drug Monitoring and Pharmacokinetic Analyses
2.4. Assessment of Patient’s Characteristics, Clinical Response, and ADEs
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Drug Specific Analyses
3.3. Pharmacokinetic Analyses
3.4. Pharmacodynamic Analyses
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2016, 18, 447–457. [Google Scholar] [CrossRef]
- Rademaker, M. Do women have more adverse drug reactions? Am. J. Clin. Dermatol. 2001, 2, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Castberg, I.; Westin, A.A.; Skogvoll, E.; Spigset, O. Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta. Psychiatr. Scand. 2017, 136, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U.; Marksteiner, J.; Kemmler, G.; Saria, A.; Aichhorn, W. Effects of age and sex on olanzapine plasma concentrations. J. Clin. Psychopharmacol. 2005, 25, 570–574. [Google Scholar] [CrossRef]
- Fekete, S.; Scherf-Clavel, M.; Gerlach, M.; Romanos, M.; Kittel-Schneider, S.; Unterecker, S.; Egberts, K. Dose-Corrected Serum Concentrations and Metabolite to Parent Compound Ratios of Venlafaxine and Risperidone from Childhood to Old Age. Pharmacopsychiatry 2021, 54, 117–125. [Google Scholar] [CrossRef]
- Hansen, M.R.; Kuhlmann, I.B.; Pottegard, A.; Damkier, P. Therapeutic Drug Monitoring of Venlafaxine in an Everyday Clinical Setting: Analysis of Age, Sex and Dose Concentration Relationships. Basic. Clin. Pharmacol. Toxicol. 2017, 121, 298–302. [Google Scholar] [CrossRef]
- Unterecker, S.; Riederer, P.; Proft, F.; Maloney, J.; Deckert, J.; Pfuhlmann, B. Effects of gender and age on serum concentrations of antidepressants under naturalistic conditions. J. Neural Transm. 2013, 120, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Hiemke, C.; Bergemann, N.; Clement, H.W.; Conca, A.; Deckert, J.; Domschke, K.; Eckermann, G.; Egberts, K.; Gerlach, M.; Greiner, C.; et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: Update 2017. Pharmacopsychiatry 2018, 51, 9–62. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.; Mehler-Wex, C.; Walitza, S.; Warnke, A.; Wewetzer, C. Neuro-/Psychopharmaka im Kindes- und Jugendalter: Grundlagen und Therapie; Springer: New York, NY, USA, 2016; ISBN 978-3211792759. [Google Scholar]
- Egberts, K.; Gerlach, M.; Correll, C.U.; Plener, P.L.; Malzahn, U.; Heuschmann, P.; Unterecker, S.; Scherf-Clavel, M.; Rock, H.; Antony, G.; et al. Serious Adverse Drug Reactions in Children and Adolescents Treated On- and Off-Label with Antidepressants and Antipsychotics in Clinical Practice. Pharmacopsychiatry 2022, 55, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.; Mas, S.; Plana, M.T.; Gasso, P.; Mendez, I.; Torra, M.; Arnaiz, J.A.; Lafuente, A.; Lazaro, L. Plasma fluoxetine concentrations and clinical improvement in an adolescent sample diagnosed with major depressive disorder, obsessive-compulsive disorder, or generalized anxiety disorder. J. Clin. Psychopharmacol. 2014, 34, 318–326. [Google Scholar] [CrossRef]
- Frey, M.; Smigielski, L.; Tini, E.; Fekete, S.; Fleischhaker, C.; Wewetzer, C.; Karwautz, A.; Correll, C.U.; Gerlach, M.; Taurines, R.; et al. Therapeutic Drug Monitoring in Children and Adolescents: Findings on Fluoxetine from the TDM-VIGIL Trial. Pharmaceutics 2023, 15, 2202. [Google Scholar] [CrossRef]
- Koelch, M.; Pfalzer, A.K.; Kliegl, K.; Rothenhofer, S.; Ludolph, A.G.; Fegert, J.M.; Burger, R.; Mehler-Wex, C.; Stingl, J.; Taurines, R.; et al. Therapeutic drug monitoring of children and adolescents treated with fluoxetine. Pharmacopsychiatry 2012, 45, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Wohkittel, C.; Gerlach, M.; Taurines, R.; Wewetzer, C.; Unterecker, S.; Burger, R.; Schreck, D.; Mehler-Wex, C.; Romanos, M.; Egberts, K. Relationship between clozapine dose, serum concentration, and clinical outcome in children and adolescents in clinical practice. J. Neural Transm. 2016, 123, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Taurines, R.; Fekete, S.; Preuss-Wiedenhoff, A.; Warnke, A.; Wewetzer, C.; Plener, P.; Burger, R.; Gerlach, M.; Romanos, M.; Egberts, K.M. Therapeutic drug monitoring in children and adolescents with schizophrenia and other psychotic disorders using risperidone. J. Neural Transm. 2022, 129, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, K.; Taurines, R.; Preuss, A.; Burger, R.; Rothenhofer, S.; Wewetzer, C.; Pfuhlmann, B.; Fegert, J.; Gerlach, M.; Mehler-Wex, C. Serum concentrations, therapeutic response and side effects in children and adolescents with impulsive-aggressive symptoms during risperidone therapy. Pharmacopsychiatry 2010, 43, 58–65. [Google Scholar] [CrossRef]
- Fekete, S.; Wewetzer, C.; Mehler-Wex, C.; Holtkamp, K.; Burger, R.; Reichert, S.; Taurines, R.; Romanos, M.; Gerlach, M.; Egberts, K. Therapeutic Drug Monitoring in Children and Adolescents Under Pharmacotherapy with Olanzapine in Daily Clinical Practice. Ther. Drug Monit. 2017, 39, 273–281. [Google Scholar] [CrossRef]
- Fekete, S.; Egberts, K.; Preissler, T.; Wewetzer, C.; Mehler-Wex, C.; Romanos, M.; Gerlach, M. Estimation of a preliminary therapeutic reference range for children and adolescents with tic disorders treated with tiapride. Eur. J. Clin. Pharmacol. 2021, 77, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Ringeling, L.T.; Hermans, R.A.; Bayraktar, I.; Bosch, T.M.; Egberts, K.M.; Kloosterboer, S.M.; de Winter, B.; Dierckx, B.; Koch, B.C.P. Clinical pharmacokinetics of antipsychotics in pediatric populations: A scoping review focusing on dosing regimen. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 501–509. [Google Scholar] [CrossRef]
- Weller, E.B.; Kloos, A.; Kang, J.; Weller, R.A. Depression in children and adolescents: Does gender make a difference? Curr. Psychiatry Rep. 2006, 8, 108–114. [Google Scholar] [CrossRef]
- Kennedy, S.H. Treating each and every depressed patient. J. Psychopharmacol. 2008, 22, 19–23. [Google Scholar] [CrossRef]
- Smith, S. Gender differences in antipsychotic prescribing. Int. Rev. Psychiatry 2010, 22, 472–484. [Google Scholar] [CrossRef]
- Storosum, B.W.C.; Mattila, T.; Wohlfarth, T.D.; Gispen-de Wied, C.C.; Roes, K.C.B.; den Brink, W.V.; de Haan, L.; Denys, D.; Zantvoord, J.B. Gender differences in the response to antipsychotic medication in patients with schizophrenia: An individual patient data meta-analysis of placebo-controlled studies. Psychiatry Res. 2023, 320, 114997. [Google Scholar] [CrossRef]
- Strawn, J.R.; Mills, J.A.; Suresh, V.; Mayes, T.; Gentry, M.T.; Trivedi, M.; Croarkin, P.E. The impact of age on antidepressant response: A mega-analysis of individuals with major depressive disorder. J. Psychiatr. Res. 2023, 159, 266–273. [Google Scholar] [CrossRef]
- Taurines, R.; Kunkel, G.; Fekete, S.; Fegert, J.M.; Wewetzer, C.; Correll, C.U.; Holtkamp, K.; Boege, I.; Renner, T.J.; Imgart, H.; et al. Serum Concentration-Dose Relationship and Modulation Factors in Children and Adolescents Treated with Fluvoxamine. Pharmaceutics 2024, 16, 772. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Egberts, K.M.; Clement, H.W.; Schneider-Momm, K.; Taurines, R.; Fekete, S.; Wewetzer, C.; Karwautz, A.; Correll, C.U.; Plener, P.L.; et al. Therapeutic drug monitoring in children and adolescents with schizophrenia-spectrum, affective, behavioural, tic and other psychiatric disorders treated with aripiprazole: Results of the TDM-VIGIL pharmacovigilance study. J. Neural Transm. 2024, 132, 295–312. [Google Scholar] [CrossRef]
- Tini, E.; Smigielski, L.; Romanos, M.; Wewetzer, C.; Karwautz, A.; Reitzle, K.; Correll, C.U.; Plener, P.L.; Malzahn, U.; Heuschmann, P.; et al. Therapeutic drug monitoring of sertraline in children and adolescents: A naturalistic study with insights into the clinical response and treatment of obsessive-compulsive disorder. Compr. Psychiatry 2022, 115, 152301. [Google Scholar] [CrossRef]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; National Institute of Mental Health: Rockville, MD, USA, 1976. [Google Scholar]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: https://www.R-project.org/ (accessed on 25 July 2025).
- Springer-Verlag GmbH, Heidelberg. PSIAC. Available online: https://www.psiac.de/ (accessed on 18 March 2019).
- Merikangas, K.R.; He, J.P.; Burstein, M.; Swanson, S.A.; Avenevoli, S.; Cui, L.; Benjet, C.; Georgiades, K.; Swendsen, J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J. Am. Acad. Child. Adolesc. Psychiatry 2010, 49, 980–989. [Google Scholar] [CrossRef]
- Galmiche, M.; Dechelotte, P.; Lambert, G.; Tavolacci, M.P. Prevalence of eating disorders over the 2000–2018 period: A systematic literature review. Am. J. Clin. Nutr. 2019, 109, 1402–1413. [Google Scholar] [CrossRef]
- Klipker, K.; Baumgarten, F.; Gobel, K.; Lampert, T.; Holling, H. Mental health problems in children and adolescents in Germany. Results of the cross-sectional KiGGS Wave 2 study and trends. J. Heal. Monit. 2018, 3, 34–41. [Google Scholar]
- Bachmann, C.J.; Lempp, T.; Glaeske, G.; Hoffmann, F. Antipsychotic prescription in children and adolescents: An analysis of data from a German statutory health insurance company from 2005 to 2012. Dtsch. Arztebl. Int. 2014, 111, 25–34. [Google Scholar] [PubMed][Green Version]
- Bachmann, C.J.; Aagaard, L.; Burcu, M.; Glaeske, G.; Kalverdijk, L.J.; Petersen, I.; Schuiling-Veninga, C.C.; Wijlaars, L.; Zito, J.M.; Hoffmann, F. Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005–2012. Eur. Neuropsychopharmacol. 2016, 26, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Moldauer, L.; Hahn, R.D.; Wise, A.; Bertzos, K.; Eisenberg, B.; Greenberg, E.; Liu, C.; Gopalkrishnan, M.; McVoy, M.; et al. A Multicenter Double-Blind, Placebo-Controlled Trial of Escitalopram in Children and Adolescents with Generalized Anxiety Disorder. J. Child. Adolesc. Psychopharmacol. 2023, 33, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ilzarbe, L.; Ilzarbe, D.; Gutierrez-Arango, F.; Baeza, I. Sex Differences in Serum Prolactin Levels in Children and Adolescents on Antipsychotics: A Systematic Review and Meta-Analysis. Curr. Neuropharmacol. 2023, 21, 1319–1328. [Google Scholar] [CrossRef]
- Molden, E.; Lunde, H.; Lunder, N.; Refsum, H. Pharmacokinetic variability of aripiprazole and the active metabolite dehydroaripiprazole in psychiatric patients. Ther. Drug Monit. 2006, 28, 744–749. [Google Scholar] [CrossRef]
- Bachmann, C.J.; Rieger-Gies, A.; Heinzel-Gutenbrunner, M.; Hiemke, C.; Remschmidt, H.; Theisen, F. Large variability of aripiprazole and dehydroaripiprazole serum concentrations in adolescent patients with schizophrenia. Ther. Drug Monit. 2008, 30, 462–466. [Google Scholar] [CrossRef]
- Brill, M.J.; Diepstraten, J.; van Rongen, A.; van Kralingen, S.; van den Anker, J.N.; Knibbe, C.A. Impact of obesity on drug metabolism and elimination in adults and children. Clin. Pharmacokinet. 2012, 51, 277–304. [Google Scholar] [CrossRef]
- Warrings, B.; Samanski, L.; Deckert, J.; Unterecker, S.; Scherf-Clavel, M. Impact of Body Mass Index on Serum Concentrations of Antidepressants and Antipsychotics. Ther. Drug Monit. 2021, 43, 286–291. [Google Scholar] [CrossRef]
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.L.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
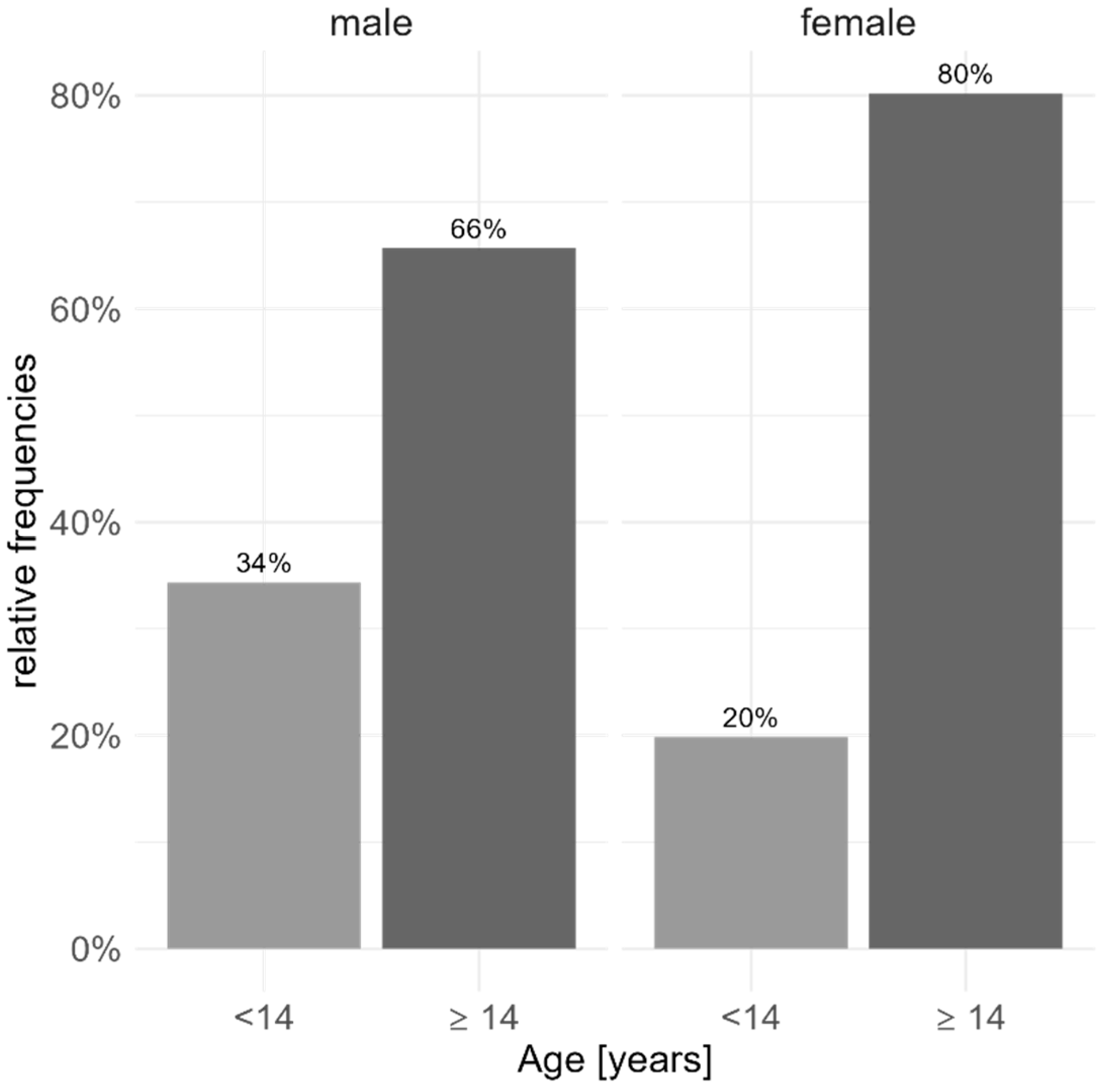
| All Patients | Male | Female | p Value (Effect Size) | |
|---|---|---|---|---|
| Number of patients | 705 | 236 | 469 | |
| Age (mean; SD (min, max)) (Baseline) | 14.6, 2.2 (6, 18) | 14.1, 2.9 (6, 18) | 14.9, 1.7 (8, 18) | 0.020 (r: 0.06) |
| number < 14 years | 174 | 81 | 93 | <0.001 (Cohen‘s ω: 0.159) |
| number ≥ 14 years | 531 | 155 | 376 | |
| Weight [kg] (Baseline) | 55.7, 16.5 (19.9, 135) | 58.6, 20.7 (19.9, 135) | 54.3, 13.7 (21.4, 114.0) | 0.004 (r: 0.07) |
| BMI (Baseline) | 20.4, 4.6 (10.8, 40.3) | 20.7, 4.8 (12.6, 40.3) | 20.3, 4.5 (10.8, 37.4) | 0.424 |
| Most common diagnoses according to the ICD 10 (N (relative number) (Baseline) | <0.001 (Cohen’s ω: 0.569) | |||
| Moderate depressive episode (F32.1) | 265 (0.229) | 61 (0.158) | 204 (0.265) | |
| Anorexia nervosa (F50.0) | 111 (0.096) | 1 (0.003) | 110 (0.194) | |
| Severe depressive episode without psychotic symptoms (F32.2) | 85 (0.073) | 13 (0.040) | 72 (0.158) | |
| Social phobias (F40.1) | 49 (0.042) | 25 (0.080) | 24 (0.062) | |
| Mixed obsessional thoughts and acts (F42.2) | 49 (0.042) | 18 (0.063) | 31 (0.086) | |
| Disturbance of activity and attention (F90.0) | 41 (0.035) | 26 (0.097) | 15 (0.045) | |
| Hyperkinetic conduct disorder (F90.1) | 33 (0.028) | 31 (0.128) | 2 (0.006) | |
| Recurrent depressive disorder, current episode moderate (F33.1) | 32 (0.028) | 6 (0.028) | 26 (0.083) | |
| Paranoid schizophrenia (F20.0) | 27 (0.023) | 16 (0.078) | 11 (0.038) | |
| Post-traumatic stress disorder (F43.1) | 26 (0.022) | 7 (0.037) | 19 (0.069) | |
| Bulimia nervosa (F50.2) | 22 (0.019) | 0 (0.000) | 22 (0.086) | |
| Severe depressive episode with psychotic symptoms (F32.3) | 20 (0.017) | 9 (0.049) | 11 (0.047) | |
| Emotionally unstable personality disorder (F60.3) | 20 (0.017) | 2 (0.012) | 18 (0.080) | |
| Depressive conduct disorder (F92.0) | 19 (0.016) | 7 (0.041) | 12 (0.058) | |
| Atypical anorexia nervosa (F50.1) | 14 (0.012) | 1 (0.006) | 13 (0.067) | |
| Mild depressive episode (F32.0) | 11 (0.009) | 5 (0.031) | 6 (0.033) | |
| Generalized anxiety disorder (F41.1) | 11 (0.009) | 3 (0.019) | 8 (0.046) | |
| Predominantly compulsive acts [obsessional rituals] (F42.1) | 11 (0.009) | 3 (0.019) | 8 (0.048) | |
| Atypical autism (F84.1) | 10 (0.009) | 9 (0.059) | 1 (0.006) | |
| Severity of illness (CGI-S) (Baseline) | 0.702 | |||
| Extremely ill | 29 | 6 | 23 | |
| Severely ill | 234 | 84 | 150 | |
| Markedly ill | 331 | 107 | 222 | |
| Moderately ill | 78 | 27 | 52 | |
| Mildly ill | 6 | 2 | 4 | |
| Treatment outcome CGI-I (last visit) | 0.227 | |||
| no improvement | 21 | 5 | 16 | |
| low response | 43 | 13 | 30 | |
| moderate response | 72 | 20 | 52 | |
| very good response | 35 | 16 | 19 | |
| NA | 4 | 1 | 3 | |
| Adverse drug effects (last visit) | 0.556 | |||
| none | 89 | 30 | 59 | |
| mild | 71 | 21 | 50 | |
| moderate | 1 | 1 | 0 | |
| serious | 9 | 3 | 6 | |
| Number of substances (mean, SD (min, max)) (last visit) | 1.3, 0.6 (1, 4) | 1.24, 0.48 (1, 3) | 1.34, 0.58 (1, 4) | 0.052 |
| TDM availability | ||||
| Antidepressants (first TDM per patient) | ||||
| Fluoxetine | 356 | 78 | 276 | |
| Sertraline | 177 | 64 | 109 | |
| Mirtazapine | 116 | 11 | 104 | |
| Escitalopram | 97 | 17 | 80 | |
| Citalopram | 74 | 10 | 64 | |
| Venlafaxine | 28 | 1 | 27 | |
| Fluvoxamine | 15 | 4 | 11 | |
| Doxepin | 7 | 0 | 7 | |
| Clomipramine | 5 | 5 | 0 | |
| Amitriptyline | 5 | 0 | 5 | |
| Trimipramine | 2 | 0 | 2 | |
| Antipsychotics | ||||
| Aripiprazole | 194 | 88 | 106 | |
| Quetiapine | 178 | 43 | 131 | |
| Olanzapine | 154 | 40 | 114 | |
| Risperidone | 46 | 37 | 8 | |
| Clozapine | 36 | 18 | 18 | |
| Pipamperone | 20 | 14 | 6 | |
| Amisulpride | 11 | 4 | 7 | |
| Haloperidol | 7 | 2 | 5 | |
| Melperone | 6 | 0 | 6 | |
| Tiapride | 3 | 3 | 0 | |
| Sulpiride | 2 | 2 | 0 | |
| Paliperidone | 1 | 0 | 1 | |
| Trazodone | 1 | 0 | 1 | |
| Anticonvulsants | ||||
| Valproic acid | 13 | 10 | 3 | |
| Levetiracetam | 5 | 0 | 2 | |
| Lamotrigine | 2 | 0 | 1 | |
| Topiramate | 2 | 2 | 0 | |
| ADHD-medication | ||||
| Atomoxetine | 1 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherf-Clavel, M.; Fekete, S.; Gerlach, M.; Correll, C.U.; Plener, P.; Fegert, J.M.; Karwautz, A.; Heuschmann, P.; Banaschewski, T.; Briegel, W.; et al. Sex-Specific Differences in Antidepressant and Antipsychotic Treatment Outcomes and Serum Levels in Children and Adolescents. Pharmaceutics 2025, 17, 983. https://doi.org/10.3390/pharmaceutics17080983
Scherf-Clavel M, Fekete S, Gerlach M, Correll CU, Plener P, Fegert JM, Karwautz A, Heuschmann P, Banaschewski T, Briegel W, et al. Sex-Specific Differences in Antidepressant and Antipsychotic Treatment Outcomes and Serum Levels in Children and Adolescents. Pharmaceutics. 2025; 17(8):983. https://doi.org/10.3390/pharmaceutics17080983
Chicago/Turabian StyleScherf-Clavel, Maike, Stefanie Fekete, Manfred Gerlach, Christoph U. Correll, Paul Plener, Jörg M. Fegert, Andreas Karwautz, Peter Heuschmann, Tobias Banaschewski, Wolfgang Briegel, and et al. 2025. "Sex-Specific Differences in Antidepressant and Antipsychotic Treatment Outcomes and Serum Levels in Children and Adolescents" Pharmaceutics 17, no. 8: 983. https://doi.org/10.3390/pharmaceutics17080983
APA StyleScherf-Clavel, M., Fekete, S., Gerlach, M., Correll, C. U., Plener, P., Fegert, J. M., Karwautz, A., Heuschmann, P., Banaschewski, T., Briegel, W., Fleischhaker, C., Hellenschmidt, T., Imgart, H., Kaess, M., Kölch, M., Reitzle, K., Renner, T. J., Rexroth, C., Schulte-Körne, G., ... Taurines, R. (2025). Sex-Specific Differences in Antidepressant and Antipsychotic Treatment Outcomes and Serum Levels in Children and Adolescents. Pharmaceutics, 17(8), 983. https://doi.org/10.3390/pharmaceutics17080983









