Glycosaminoglycans Targeted by Colchicine in MCF-7 Cells
Abstract
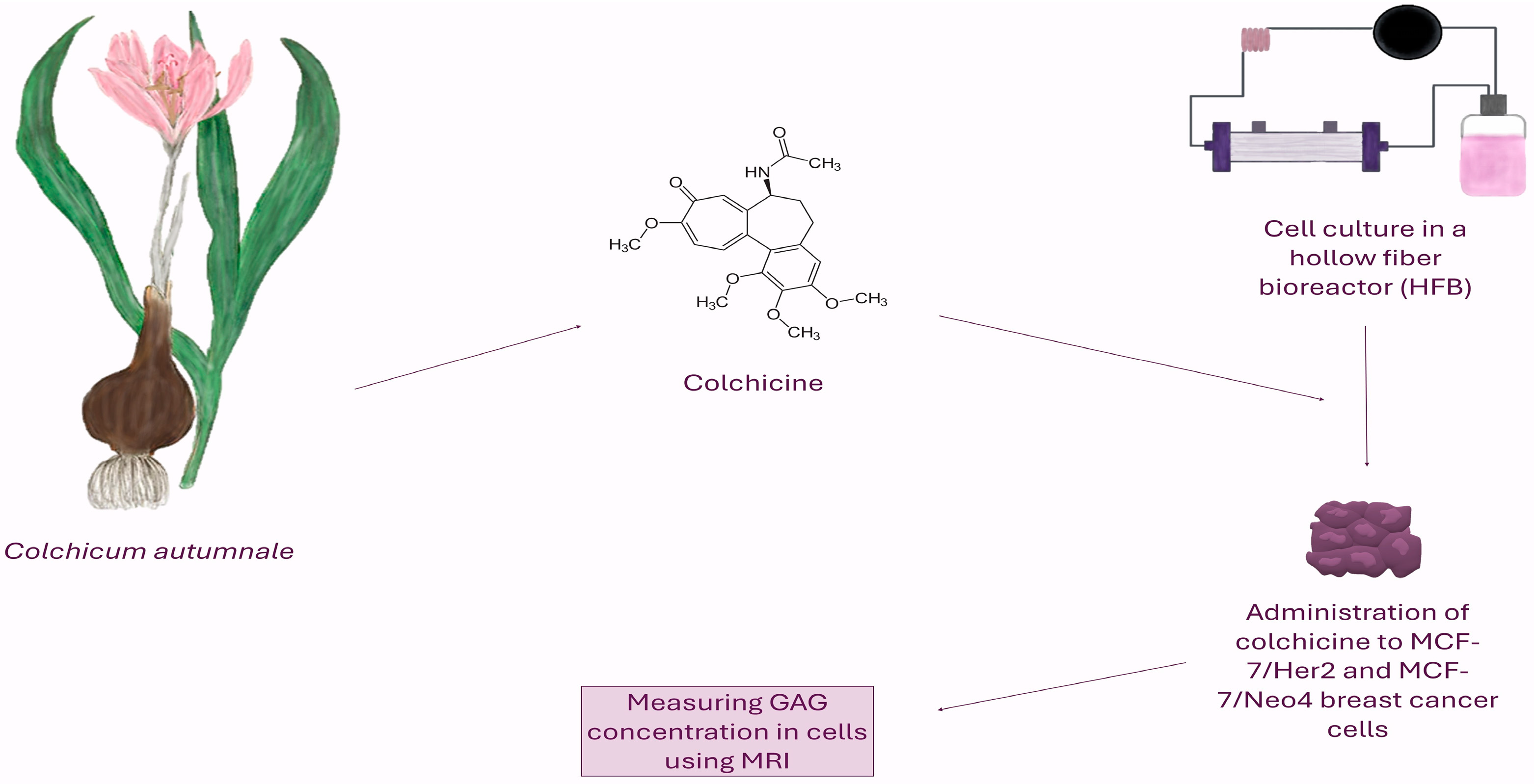
1. Introduction
2. Materials and Methods
2.1. Chemicals and Aparature
2.2. Cell Culture Methods
2.3. Cells Treatment
2.4. Methods for Counting Cells in a Bioreactor
2.5. Magnetic Resonance Imaging
2.6. Measurement of Glycosaminoglycans
- bath—medium around the breast cancer cells;
- R—relaxivity (mmol/L/s);
- tissue—breast cancer cell tissues;
- —concentration of Na+ ions in bath, 154 (mmol/L);
- —T1 relaxation time of the breast cancer cells after administration of Gd(DTPA)2− solution in sec;
- —T1 relaxation time of the breast cancer cells before administration of Gd(DTPA)2− solution in sec;
- —T1 relaxation time of the bath after administration of Gd(DTPA)2− solution in sec;
- —T1 relaxation time of bath before administration of Gd(DTPA)2− solution in sec.
- —Glycosaminoglycan concentration (mg/L);
- —Fixed charge density (mmol/L);
- —Molecular weight of GAG in (mg/mmol).
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deftereos, S.; Giannopoulos, G.; Angelidis, C.; Alexopoulos, N.; Filippatos, G.; Papoutsidakis, N.; Sianos, G.; Goudevenos, J.; Alexopoulos, D.; Pyrgakis, V.; et al. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation 2015, 132, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Vitry, J.; Marceau, F.; Vaillancourt, M.; Winter, P.; Bachelard, H.; Naccache, P.H.; Tuszynski, J.A.; Fernandes, M.J. The Development of a Targeted and More Potent, Anti-Inflammatory Derivative of Colchicine: Implications for Gout. Biochem. Pharmacol. 2020, 180, 114125. [Google Scholar] [CrossRef]
- Kurek, J.; Myszkowski, K.; Okulicz-Kozaryn, I.; Kurant, A.; Kamińska, E.; Szulc, M.; Rubiś, B.; Kaczmarek, M.; Mikołajczak, P.Ł.; Murias, M. Cytotoxic, Analgesic and Anti-Inflammatory Activity of Colchicine and Its C-10 Sulfur Containing Derivatives. Sci. Rep. 2021, 11, 9034. [Google Scholar] [CrossRef]
- Cocco, G.; Chu, D.C.C.; Pandolfi, S. Colchicine in Clinical Medicine. A Guide for Internists. Eur. J. Intern. Med. 2010, 21, 503–508. [Google Scholar] [CrossRef]
- Malichová, V.; Potĕsilová, H.; Preininger, V.; Santavý, F. Alkaloids from Leaves and Flowers of Colchicum autumnale L. Planta Med. 1979, 36, 119–127. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, X.; Chang, H. Proliferation inhibition and apoptosis of breast cancer MCF-7 cells under the influence of colchicine. J. BUON 2016, 21, 570–575. [Google Scholar]
- PubChem Colchicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6167 (accessed on 17 July 2025).
- Nett, R.S.; Lau, W.; Sattely, E.S. Discovery and Engineering of Colchicine Alkaloid Biosynthesis. Nature 2020, 584, 148–153. [Google Scholar] [CrossRef]
- Wertheimer, A.I.; Davis, M.W.; Lauterio, T.J. A New Perspective on the Pharmacoeconomics of Colchicine. Curr. Med. Res. Opin. 2011, 27, 931–937. [Google Scholar] [CrossRef]
- Slobodnick, A.; Shah, B.; Pillinger, M.H.; Krasnokutsky, S. Colchicine: Old and New. Am. J. Med. 2015, 128, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Majcher, U.; Klejborowska, G.; Kaik, M.; Maj, E.; Wietrzyk, J.; Moshari, M.; Preto, J.; Tuszynski, J.A.; Huczyński, A. Synthesis and Biological Evaluation of Novel Triple-Modified Colchicine Derivatives as Potent Tubulin-Targeting Anticancer Agents. Cells 2018, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer Potential of Alkaloids: A Key Emphasis to Colchicine, Vinblastine, Vincristine, Vindesine, Vinorelbine and Vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, P.R.; Mondhe, D.M. Potential Anticancer Role of Colchicine-Based Derivatives: An Overview. Anticancer. Drugs 2017, 28, 250–262. [Google Scholar] [CrossRef]
- Dubey, K.K.; Kumar, P.; Labrou, N.E.; Shukla, P. Biotherapeutic Potential and Mechanisms of Action of Colchicine. Crit. Rev. Biotechnol. 2017, 37, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs That Target Dynamic Microtubules: A New Molecular Perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, C.; Kotsialou, Z.; Kossyvakis, C.; Vrettou, A.R.; Zacharoulis, A.; Kolokathis, F.; Kekeris, V.; Giannopoulos, G. Colchicine Pharmacokinetics and Mechanism of Action. Curr. Pharm. Des. 2018, 24, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-Mitotic Activity of Colchicine and the Structural Basis for Its Interaction with Tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef]
- Nogales, E. Structural Insights into Microtubule Function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef]
- Jordan, M.A. Mechanism of Action of Antitumor Drugs That Interact with Microtubules and Tubulin. Curr. Med. Chem. Anticancer Agents 2002, 2, 1–17. [Google Scholar] [CrossRef]
- Karahalil, B.; Yardım-Akaydin, S.; Nacak Baytas, S. An Overview of Microtubule Targeting Agents for Cancer Therapy. Arh. Hig. Rada Toksikol. 2019, 70, 160–172. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, X.; Feng, B. A Review of Research Progress of Antitumor Drugs Based on Tubulin Targets. Transl. Cancer Res. 2020, 9, 4020–4027. [Google Scholar] [CrossRef]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on Mechanisms of Action and Therapeutic Uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef]
- Sivakumar, G. Colchicine Semisynthetics: Chemotherapeutics for Cancer? Curr. Med. Chem. 2013, 20, 892–898. [Google Scholar] [PubMed]
- Ganguly, A.; Yang, H.; Zhang, H.; Cabral, F.; Patel, K.D. Microtubule Dynamics Control Tail Retraction in Migrating Vascular Endothelial Cells. Mol. Cancer Ther. 2013, 12, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Correction: McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8, Erratum in Pharmaceuticals 2020, 13, 72. https://doi.org/10.3390/ph13040072. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Patnaik, J.; Mullins, M.R.; Lemasters, J.J. Free Tubulin Modulates Mitochondrial Membrane Potential in Cancer Cells. Cancer Res. 2010, 70, 10192–10201. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental Regulation of Metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef]
- Xu, X.; Farach-Carson, M.C.; Jia, X. Three-Dimensional in Vitro Tumor Models for Cancer Research and Drug Evaluation. Biotechnol. Adv. 2014, 32, 1256–1268. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, F.; Guo, M.; Tang, Y.; Xu, Q. Bridging the gap: The role of 3D cell cultures in mimicking tumor microenvironment for enhanced drug testing accuracy. Front. Bioeng. Biotechnol. 2025, 13, 1498141. [Google Scholar] [CrossRef] [PubMed]
- Chitcholtan, K.; Asselin, E.; Parent, S.; Sykes, P.H.; Evans, J.J. Differences in Growth Properties of Endometrial Cancer in Three Dimensional (3D) Culture and 2D Cell Monolayer. Exp. Cell Res. 2013, 319, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The Role of Extracellular Matrix in Biomechanics and Its Impact on Bioengineering of Cells and 3D Tissues. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef]
- Harunaga, J.S.; Yamada, K.M. Cell-Matrix Adhesions in 3D. Matrix Biol. 2011, 30, 363–368. [Google Scholar] [CrossRef]
- Wang, K.; Kievit, F.M.; Erickson, A.E.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Culture on 3D Chitosan-Hyaluronic Acid Scaffolds Enhances Stem Cell Marker Expression and Drug Resistance in Human Glioblastoma Cancer Stem Cells. Adv. Healthc. Mater. 2016, 5, 3173–3181. [Google Scholar] [CrossRef]
- Could 3D Models of Cancer Enhance Drug Screening?—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0142961219308622?via%3Dihub (accessed on 14 July 2025).
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-Dimensional Cell Culture: A Powerful Tool in Tumor Research and Drug Discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Fontana, F.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. In Vitro 3D Cultures to Model the Tumor Microenvironment. Cancers 2021, 13, 2970. [Google Scholar] [CrossRef]
- Tomás-Bort, E.; Kieler, M.; Sharma, S.; Candido, J.B.; Loessner, D. 3D Approaches to Model the Tumor Microenvironment of Pancreatic Cancer. Theranostics 2020, 10, 5074–5089. [Google Scholar] [CrossRef]
- Debnath, J.; Muthuswamy, S.K.; Brugge, J.S. Morphogenesis and Oncogenesis of MCF-10A Mammary Epithelial Acini Grown in Three-Dimensional Basement Membrane Cultures. Methods 2003, 30, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, G.; Li, M.; Wang, L.; Elson, E.L.; Lu, T.J.; Genin, G.M.; Xu, F. An Approach to Quantifying 3D Responses of Cells to Extreme Strain. Sci. Rep. 2016, 6, 19550. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, R.J.F.C.; Zayed, N.M.A.; Pascu, E.I.; Cavanagh, B.; Hobbs, C.; Santarella, F.; Simpson, C.R.; Murphy, C.M.; Sridharan, R.; González-Vázquez, A.; et al. Functionalising Collagen-Based Scaffolds With Platelet-Rich Plasma for Enhanced Skin Wound Healing Potential. Front. Bioeng. Biotechnol. 2019, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Fischer, S.C.; Mattheyer, C.; Pampaloni, F.; Stelzer, E.H.K. Multiscale Image Analysis Reveals Structural Heterogeneity of the Cell Microenvironment in Homotypic Spheroids. Sci. Rep. 2017, 7, 43693. [Google Scholar] [CrossRef]
- Trouard, T.P.; Harkins, K.D.; Divijak, J.L.; Gillies, R.J.; Galons, J.-P. Ischemia-Induced Changes of Intracellular Water Diffusion in Rat Glioma Cell Cultures. Magn. Reson. Med. 2008, 60, 258–264. [Google Scholar] [CrossRef]
- Preechapornkul, P.; Chotivanich, K.; Imwong, M.; Dondorp, A.; Lee, S.; Day, N.; White, N.; Pukrittayakamee, S. Optimizing the Culture of Plasmodium Falciparum in Hollow Fiber Bioreactors. Southeast Asian J. Trop. Med. Public Health 2010, 41, 761–769. [Google Scholar]
- Chapman, L.A.C.; Shipley, R.J.; Whiteley, J.P.; Ellis, M.J.; Byrne, H.M.; Waters, S.L. Optimising Cell Aggregate Expansion in a Perfused Hollow Fibre Bioreactor via Mathematical Modelling. PLoS ONE 2014, 9, e105813. [Google Scholar] [CrossRef]
- Global Superstructure Optimisation of Red Blood Cell Production in a Parallelised Hollow Fibre Bioreactor.—University of Surrey. Available online: https://openresearch.surrey.ac.uk/esploro/outputs/99516661302346?skipUsageReporting=true (accessed on 15 July 2025).
- Berth, A.; Lecouturier, D.; Loubiere, K.; Dhulster, P.; Delaplace, G. Modelling and Optimisation of Gas-Liquid Mass Transfer in a Microporous Hollow Fiber Membrane Aerated Bioreactor Used to Produce Surfactin. Biochem. Eng. J. 2019, 145, 109–119. [Google Scholar] [CrossRef]
- Gramer, M.J.; Poeschl, D.M. Comparison of Cell Growth in T-Flasks, in Micro Hollow Fiber Bioreactors, and in an Industrial Scale Hollow Fiber Bioreactor System. Cytotechnology 2000, 34, 111–119. [Google Scholar] [CrossRef]
- Roberts, I.; Baila, S.; Rice, R.B.; Janssens, M.E.; Nguyen, K.; Moens, N.; Ruban, L.; Hernandez, D.; Coffey, P.; Mason, C. Scale-up of Human Embryonic Stem Cell Culture Using a Hollow Fibre Bioreactor. Biotechnol. Lett. 2012, 34, 2307–2315. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ito, A.; Jitsunobu, H.; Yamaguchi, K.; Kawabe, Y.; Mizumoto, H.; Kamihira, M. Hollow Fiber Bioreactor Perfusion Culture System for Magnetic Force-Based Skeletal Muscle Tissue Engineering. J. Chem. Eng. Jpn. 2012, 45, 348–354. [Google Scholar]
- Interest in Hollow-Fiber Perfusion Bioreactors Is Growing. Available online: https://www.bioprocessintl.com/process-development/interest-in-hollow-fiber-perfusion-bioreactors-is-growing (accessed on 15 July 2025).
- Pihl, A.F.; Offersgaard, A.F.; Mathiesen, C.K.; Prentoe, J.; Fahnøe, U.; Krarup, H.; Bukh, J.; Gottwein, J.M. High Density Huh7.5 Cell Hollow Fiber Bioreactor Culture for High-Yield Production of Hepatitis C Virus and Studies of Antivirals. Sci. Rep. 2018, 8, 17505. [Google Scholar] [CrossRef]
- (PDF) New Developments in Hollow-Fiber Cell Culture. ResearchGate. Available online: https://www.researchgate.net/publication/242719331_New_Developments_in_Hollow-Fiber_Cell_Culture (accessed on 15 July 2025).
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Pomin, V.H.; Mulloy, B. Glycosaminoglycans and Proteoglycans. Pharmaceuticals 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Sasarman, F.; Maftei, C.; Campeau, P.M.; Brunel-Guitton, C.; Mitchell, G.A.; Allard, P. Biosynthesis of Glycosaminoglycans: Associated Disorders and Biochemical Tests. J. Inherit. Metab. Dis. 2016, 39, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.; Presto, J.; Spillmann, D.; Lindahl, U.; Kjellén, L. Heparin/Heparan Sulfate Biosynthesis: Processive Formation of N-Sulfated Domains. J. Biol. Chem. 2008, 283, 20008–20014. [Google Scholar] [CrossRef]
- Berdiaki, A.; Neagu, M.; Tzanakakis, P.; Spyridaki, I.; Pérez, S.; Nikitovic, D. Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease. Biomolecules. 2024, 14, 1186. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Biosynthesis and Function of Chondroitin Sulfate. Biochim. Biophys. Acta 2013, 1830, 4719–4733. [Google Scholar] [CrossRef]
- Pomin, V.H. Keratan Sulfate: An up-to-Date Review. Int. J. Biol. Macromol. 2015, 72, 282–289. [Google Scholar] [CrossRef]
- Nikitovic, D.; Papoutsidakis, A.; Karamanos, N.K.; Tzanakakis, G.N. Lumican Affects Tumor Cell Functions, Tumor-ECM Interactions, Angiogenesis and Inflammatory Response. Matrix Biol. 2014, 35, 206–214. [Google Scholar] [CrossRef]
- Kouvidi, K.; Berdiaki, A.; Nikitovic, D.; Katonis, P.; Afratis, N.; Hascall, V.C.; Karamanos, N.K.; Tzanakakis, G.N. Role of Receptor for Hyaluronic Acid-Mediated Motility (RHAMM) in Low Molecular Weight Hyaluronan (LMWHA)-Mediated Fibrosarcoma Cell Adhesion. J. Biol. Chem. 2011, 286, 38509–38520. [Google Scholar] [CrossRef]
- Schwertfeger, K.L.; Cowman, M.K.; Telmer, P.G.; Turley, E.A.; McCarthy, J.B. Hyaluronan, Inflammation, and Breast Cancer Progression. Front. Immunol. 2015, 6, 236. [Google Scholar] [CrossRef]
- Morla, S. Glycosaminoglycans and Glycosaminoglycan Mimetics in Cancer and Inflammation. Int. J. Mol. Sci. 2019, 20, 1963. [Google Scholar] [CrossRef]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavão, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key Players in Cancer Cell Biology and Treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chopin, E.; Xia, Y. The effects of mechanical loading and gadolinium concentration on the change of T1 and quantification of glycosaminoglycans in articular cartilage by microscopic MRI. Phys. Med. Biol. 2013, 58, 4535–4547. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Gray, M.L.; Hartke, J.; Burstein, D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn. Reson. Med. 1999, 41, 857–865. [Google Scholar] [CrossRef]
- Reddel, C.J.; Pennings, G.J.; Chen, V.M.; Gnanenthiran, S.; Kritharides, L. Colchicine as a Modulator of Platelet Function: A Systematic Review. Semin. Thromb. Hemost. 2022, 48, 552–567. [Google Scholar] [CrossRef]
- Thomas, C.V.; Sackrison, J.L.; Ryan, U.S.; Luikart, S.D. Effects of colchicine on sulfated glycosaminoglycan production and cell detachment in pre-capillary pulmonary endothelial cells. Tissue Cell 1987, 19, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.W.; Bornstein, P. Effects of antimicrotubular agents on glycosaminoglycan synthesis and secretion by embryonic chick cartilage and chondrocytes. Biochim. Biophys. Acta 1974, 362, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Agren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.M. Hyaluronan in Peritumoral Stroma and Malignant Cells Associates with Breast Cancer Spreading and Predicts Survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Bertrand, P.; Girard, N.; Delpech, B.; Duval, C.; d’Anjou, J.; Dauce, J.P. Hyaluronan (Hyaluronic Acid) and Hyaluronectin in the Extracellular Matrix of Human Breast Carcinomas: Comparison between Invasive and Non-Invasive Areas. Int. J. Cancer 1992, 52, 1–6. [Google Scholar] [CrossRef]
- Tammi, R.H.; Kultti, A.; Kosma, V.-M.; Pirinen, R.; Auvinen, P.; Tammi, M.I. Hyaluronan in Human Tumors: Pathobiological and Prognostic Messages from Cell-Associated and Stromal Hyaluronan. Semin. Cancer Biol. 2008, 18, 288–295. [Google Scholar] [CrossRef]
- Kultti, A.; Li, X.; Jiang, P.; Thompson, C.B.; Frost, G.I.; Shepard, H.M. Therapeutic Targeting of Hyaluronan in the Tumor Stroma. Cancers 2012, 4, 873–903. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and Signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Toole, B.P.; Ghatak, S. Hyaluronan Constitutively Regulates Activation of Multiple Receptor Tyrosine Kinases in Epithelial and Carcinoma Cells. J. Biol. Chem. 2006, 281, 34936–34941. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, L. The Alterations and Roles of Glycosaminoglycans in Human Diseases. Polymers 2022, 14, 5014. [Google Scholar] [CrossRef]
- Subbarayan, K.; Leisz, S.; Wickenhauser, C.; Bethmann, D.; Massa, C.; Steven, A.; Seliger, B. Biglycan-mediated upregulation of MHC class I expression in HER-2/neu-transformed cells. Oncoimmunology 2018, 7, e1373233. [Google Scholar] [CrossRef]
- Ravikumar, M.; Smith, R.A.A.; Nurcombe, V.; Cool, S.M. Heparan Sulfate Proteoglycans: Key Mediators of Stem Cell Function. Front. Cell Dev. Biol. 2020, 8, 581213. [Google Scholar] [CrossRef]
- Kubaski, F.; Osago, H.; Mason, R.W.; Yamaguchi, S.; Kobayashi, H.; Tsuchiya, M.; Orii, T.; Tomatsu, S. Glycosaminoglycans detection methods: Applications of mass spectrometry. Mol. Genet. Metab. 2017, 120, 67–77. [Google Scholar] [CrossRef]
- Frazier, S.B.; Roodhouse, K.A.; Hourcade, D.E.; Zhang, L. The Quantification of Glycosaminoglycans: A Comparison of HPLC, Carbazole, and Alcian Blue Methods. Open Glycosci. 2008, 1, 31–39. [Google Scholar] [CrossRef]
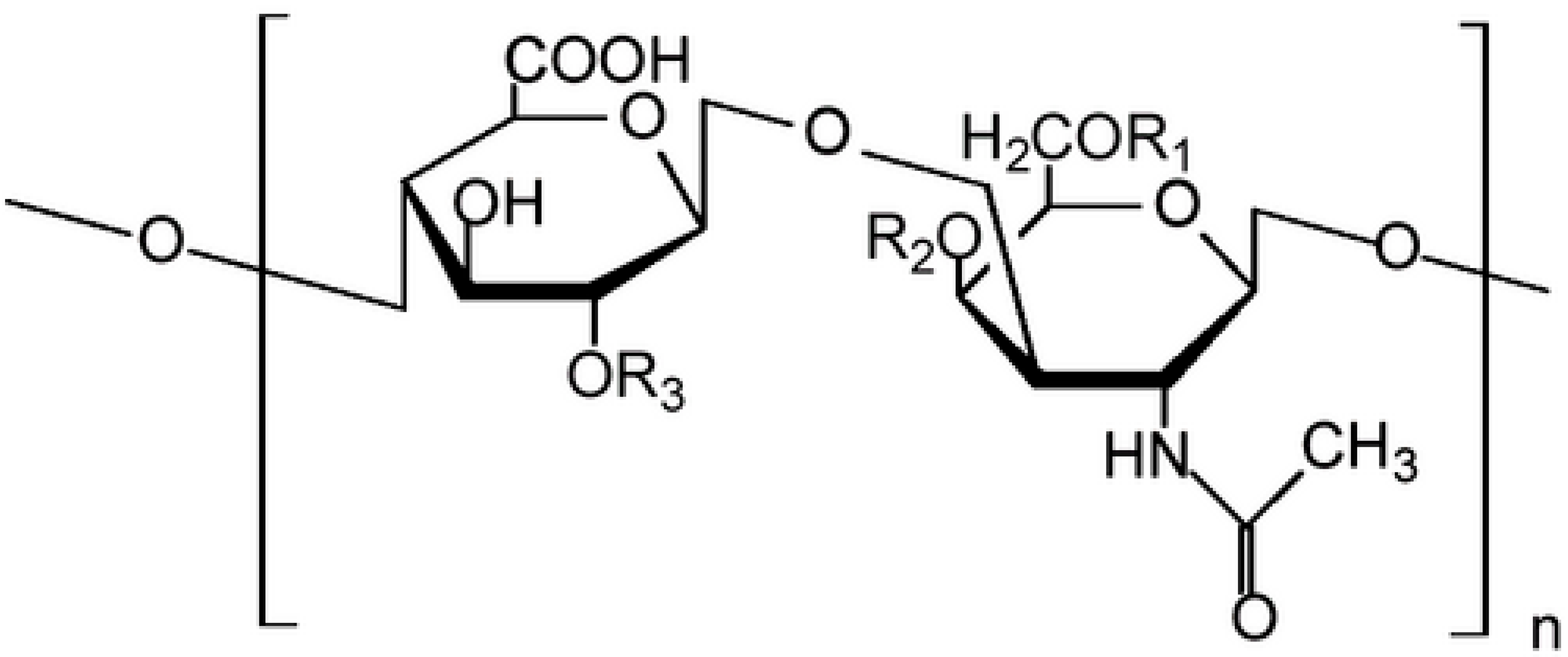
| Number of Weeks in Culture | Viability of Untreated HMEC | Viability of Untreated MCF-7 Her 2 | Viability of Untreated MCF-7 Neo 4 |
|---|---|---|---|
| 1 | 99 ± 2 | 98 ± 2 | 100 ± 2 |
| 3 | 95 ± 3 | 94 ± 3 | 95 ± 4 |
| 6 | 95 ± 5 | 94 ± 4 | 94 ± 4 |
| Number of Weeks in Culture | Viability of Treated HMEC | Viability of Treated MCF-7 Her 2 | Viability of Treated MCF-7 Neo 4 |
|---|---|---|---|
| 1 | 73 ± 2 | 78 ± 2 | 65 ± 1 |
| 3 | 75 ± 6 | 64 ± 7 | 55 ± 4 |
| 6 | 56 ± 10 | 49 ± 10 | 47 ± 6 |
| Number of Weeks in Culture | T1 (ms) Untreated HMEC | T1 (ms) Treated HMEC | T1 (ms) Untreated MCF-7 Her 2 | T1 (ms) Treated MCF-7 Her 2 | T1 (ms) Untreated MCF-7 Neo 4 | T1 (ms) Treated MCF-7 Neo 4 |
|---|---|---|---|---|---|---|
| 1 | 2876 ± 111 | 2278 ± 34 | 2613 ± 209 | 2114 ± 76 | 2573 ± 112 | 2023 ± 36 |
| 3 | 2230 ± 67 | 1876 ± 12 | 2430 ± 216 | 1987 ± 31 | 2320 ± 162 | 1972 ± 11 |
| 6 | 1542 ± 56 | 1454 ± 10 | 1712 ± 111 | 1654 ± 16 | 1654 ± 110 | 1354 ± 10 |
| Number of Weeks in Culture | T2 (ms) Untreated HMEC | T2 (ms) Treated HMEC | T2 (ms) Untreated MCF-7 Her 2 | T2 (ms) Treated MCF-7 Her 2 | T2 (ms) Untreated MCF-7 Neo 4 | T2 (ms) Treated MCF-7 Neo 4 |
|---|---|---|---|---|---|---|
| 1 | 156 ± 11 | 123 ± 10 | 148 ± 19 | 121 ± 11 | 121 ± 10 | 119 ± 09 |
| 3 | 134 ± 10 | 122 ± 10 | 114 ± 11 | 109 ± 10 | 109 ± 12 | 98 ± 23 |
| 6 | 109 ± 4 | 91 ± 11 | 113 ± 20 | 91 ± 11 | 98 ± 16 | 78 ± 17 |
| Number of Weeks in Culture | Untreated HMEC | Treated HMEC | Untreated MCF-7 Her 2 | Treated MCF-7 Her 2 | Untreated MCF-7 Neo 4 | Treated MCF-7 Neo 4 |
|---|---|---|---|---|---|---|
| 6 | 7.3 ± 3 | 9.1 ± 4 | 3.3 ± 3 | 2.5 ± 2 | 12.6 ± 4 | 8.6 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnecka-Czapczyńska, M.; Przygórzewska, A.; Dynarowicz, K.; Bartusik-Aebisher, D.; Aebisher, D.; Kawczyk-Krupka, A. Glycosaminoglycans Targeted by Colchicine in MCF-7 Cells. Pharmaceutics 2025, 17, 1368. https://doi.org/10.3390/pharmaceutics17111368
Czarnecka-Czapczyńska M, Przygórzewska A, Dynarowicz K, Bartusik-Aebisher D, Aebisher D, Kawczyk-Krupka A. Glycosaminoglycans Targeted by Colchicine in MCF-7 Cells. Pharmaceutics. 2025; 17(11):1368. https://doi.org/10.3390/pharmaceutics17111368
Chicago/Turabian StyleCzarnecka-Czapczyńska, Magdalena, Agnieszka Przygórzewska, Klaudia Dynarowicz, Dorota Bartusik-Aebisher, David Aebisher, and Aleksandra Kawczyk-Krupka. 2025. "Glycosaminoglycans Targeted by Colchicine in MCF-7 Cells" Pharmaceutics 17, no. 11: 1368. https://doi.org/10.3390/pharmaceutics17111368
APA StyleCzarnecka-Czapczyńska, M., Przygórzewska, A., Dynarowicz, K., Bartusik-Aebisher, D., Aebisher, D., & Kawczyk-Krupka, A. (2025). Glycosaminoglycans Targeted by Colchicine in MCF-7 Cells. Pharmaceutics, 17(11), 1368. https://doi.org/10.3390/pharmaceutics17111368









