From Conventional to Next-Generation Strategies: Recent Advances in Polymeric Micelle Preparation for Drug Delivery
Abstract
1. Introduction
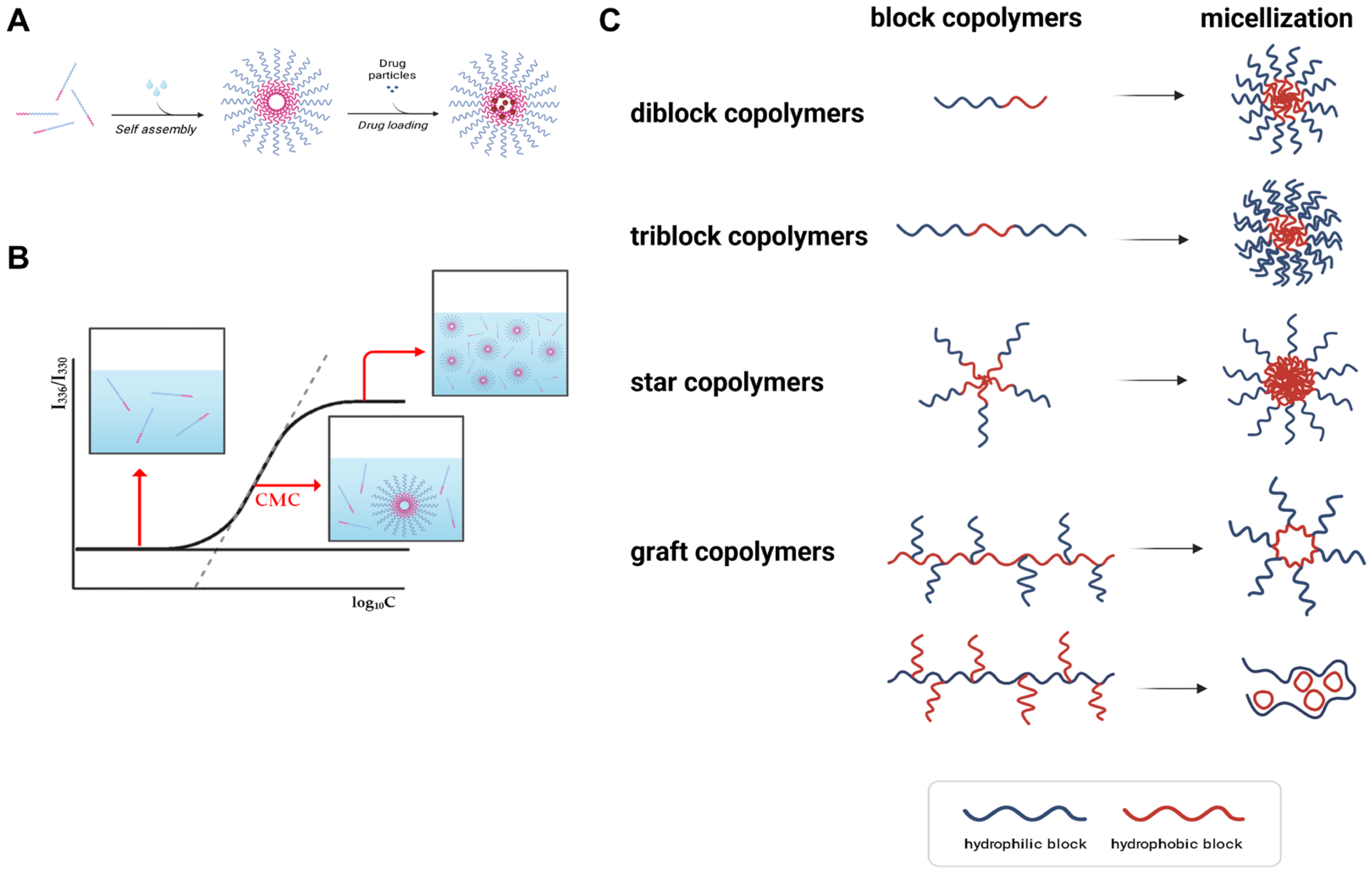
2. Fundamentals of Polymeric Micelles
3. Conventional Methods of Polymeric Micelle Preparation
3.1. Direct Dissolution

3.2. Dialysis

3.3. Emulsification and Solvent Evaporation
3.4. Thin-Film Hydration
3.5. Freeze-Drying (Lyophilization)

3.6. Comparative Evaluation of Conventional Methods
4. Emerging and Scalable Preparation Strategies
4.1. Microfluidic-Assisted Fabrication
4.2. Supercritical Fluid Processing
4.3. Stimuli-Triggered Micelle Formation
4.4. PEG-Assisted Method
4.5. Comparative Analysis and Scale-Up Considerations
5. Applications of Polymeric Micelles in Drug Delivery
5.1. Anticancer Application

5.2. Anti-Infective Applications

5.3. Anti-Inflammatory Applications

5.4. Route-Specific Micellar Formulations
6. Clinical Potential, Challenges, and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMC | Critical Micelle Concentration |
| DDS | Drug Delivery System |
| DPPC | Dipalmitoylphosphatidylcholine |
| DMSO | Dimethyl Sulfoxide |
| DENA | N,N-diethylniacinamide |
| EPR | Enhanced Permeability and Retention |
| FRET | Förster Resonance Energy Transfer |
| GMP | Good Manufacturing Practice |
| GEM | Germacrone |
| HPMA | N-(2-hydroxypropyl) methacrylamide |
| LCST | Lower Critical Solution Temperature |
| MAL | Maleimide |
| MMP | Matrix Metalloproteinase |
| mPEG | Methoxy Polyethylene Glycol |
| PGG | poly-(L-γ-glutamyl-glutamine) |
| P(2-VBOPNA) | poly(2-(4-(vinylbezyloxy)-N-picolylnicotinamide)) |
| Phe | Phenylalanine |
| P(VBODENA) | poly(2-(4-vinylbenzyloxy)-N,N-diethylnicotinamide) |
| PCL | Poly(ε-caprolactone) |
| PDCL | Poly(DL-caprolactone) |
| PDLLA | Poly(D,L-lactide) |
| PEG | Polyethylene Glycol |
| PLA | Poly(lactic acid) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PMPC | Poly(2-methacryloyloxyethyl phosphorylcholine) |
| QbD | Quality by Design |
| ROS | Reactive Oxygen Species |
| SAS | Supercritical Antisolvent |
| SCF | Supercritical Fluid |
| TPGS | D-α-Tocopheryl Polyethylene Glycol Succinate |
| uPAR | Urokinase Plasminogen Activator Receptor |
References
- Oh, J.H.; Kang, R.H.; Kim, J.; Bang, E.-K.; Kim, D. Thermally induced silane dehydrocoupling on porous silicon nanoparticles for ultra-long-acting drug release. Nanoscale 2021, 13, 15560–15568. [Google Scholar] [CrossRef]
- Kang, R.H.; Baek, S.W.; Oh, C.-K.; Kim, Y.H.; Kim, D. Recent Advances of Macrostructural Porous Silicon for Biomedical Applications. ACS Appl. Mater. Interfaces 2025, 17, 5609–5626. [Google Scholar] [CrossRef] [PubMed]
- Um, H.; Kang, R.H.; Kim, D. Iron-silicate-coated porous silicon nanoparticles for in situ ROS self-generation. Colloids Surf. B Biointerfaces 2023, 225, 113273. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Um, H.; Park, Y.K.; Kim, M.; Kim, D.; Bang, E.-K.; Kang, R.H.; Kim, D. Porous silicon surface modification via a microwave-induced in situ cyclic disulfide (S-S) cleavage and Si-S bond formation. Colloids Surf. B Biointerfaces 2023, 222, 113055. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.H.; Park, J.; Kim, J.; Chowdhury, T.; Oh, J.H.; Kim, J.; Shin, J.; Kim, M.; Park, C.-K.; Lee, S.; et al. A Deep Dive: SIWV Tetra-Peptide Enhancing the Penetration of Nanotherapeutics into the Glioblastoma. ACS Biomater. Sci. Eng. 2022, 8, 4163–4174. [Google Scholar] [CrossRef]
- Kang, R.-H.; Baek, S.W.; Ryu, T.-K.; Choi, S.-W. Fabrication of blue-fluorescent nanodiamonds modified with alkyl isocyanate for cellular bioimaging. Colloids Surf. B Biointerfaces 2018, 167, 191–196. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef]
- Geng, T.; Ding, L.; Liu, M.; Zou, X.; Gu, Z.; Lin, H.; Sun, L. Preservation of extracellular vesicles for drug delivery: A comparative evaluation of storage buffers. J. Drug Deliv. Sci. Technol. 2025, 107, 106850. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Kazunori, K.; Glenn, S.K.; Masayuki, Y.; Teruo, O.; Yasuhisa, S. Block copolymer micelles as vehicles for drug delivery. J. Control. Release 1993, 24, 119–132. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ding, Y.; Repp, L.; Kwon, G.S.; Hu, Q. Cell-Based Delivery Systems: Emerging Carriers for Immunotherapy. Adv. Funct. Mater. 2021, 31, 2100088. [Google Scholar] [CrossRef]
- Devine, D.V.; Wong, K.; Serrano, K.; Chonn, A.; Cullis, P.R. Liposome-complement interactions in rat serum: Implications for liposome survival studies. Biochim. Biophys. Acta. 1994, 1191, 43–51. [Google Scholar] [CrossRef]
- Kwon, G.S.; Okano, T. Polymeric micelles as new drug carriers. Adv. Drug Deliv. Rev. 1996, 21, 107–116. [Google Scholar] [CrossRef]
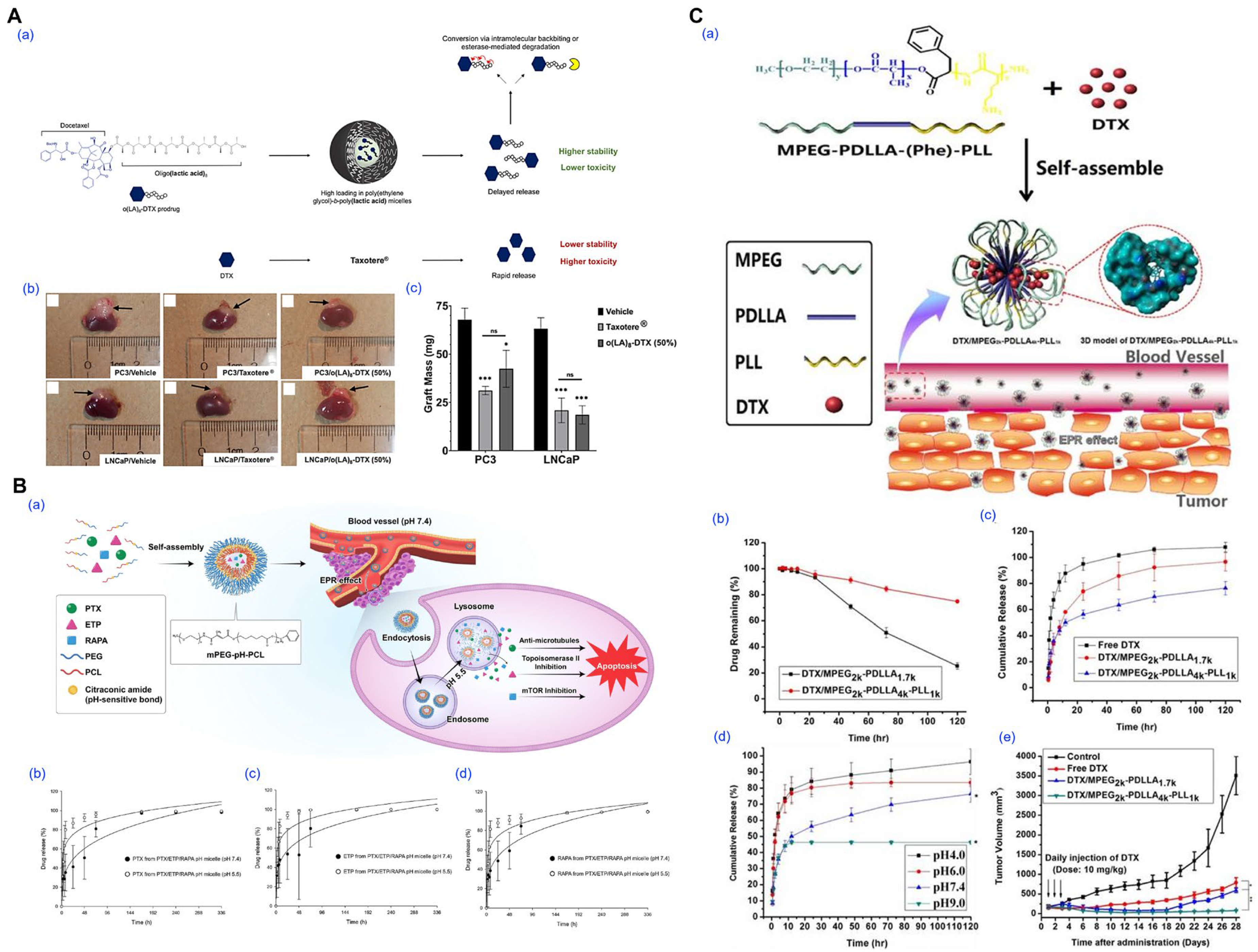
- Repp, L.; Rasoulianboroujeni, M.; Lee, H.J.; Kwon, G.S. Acyl and oligo(lactic acid) prodrugs for PEG-b-PLA and PEG-b-PCL nano-assemblies for injection. J. Control. Release 2021, 330, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, J.; Zhang, J.; Wang, J.; Xiang, D.; Luo, S.; Li, J.; Liu, X. Puerarin-loaded PEG-PE micelles with enhanced anti-apoptotic effect and better pharmacokinetic profile. Drug Deliv. 2018, 25, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Serras, A.; Faustino, C.; Pinheiro, L. Functionalized Polymeric Micelles for Targeted Cancer Therapy: Steps from Conceptualization to Clinical Trials. Pharmaceutics 2024, 16, 1047. [Google Scholar] [CrossRef] [PubMed]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef]
- Cheng, Z.; Huang, H.; Yin, M.; Liu, H. Applications of liposomes and lipid nanoparticles in cancer therapy: Current advances and prospects. Exp. Hematol. Oncol. 2025, 14, 11. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Patel, M.; Bajwa, N.; Prasad, R.; Vora, L.K. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape. Small 2025, 21, e2502315. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Tomoda, K.; Tam, Y.T.; Cho, H.; Buehler, D.; Kozak, K.R.; Kwon, G.S. Triolimus: A Multi-Drug Loaded Polymeric Micelle Containing Paclitaxel, 17-AAG, and Rapamycin as a Novel Radiosensitizer. Macromol. Biosci. 2017, 17, 1600194. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Poly(ethylene oxide)-block-poly(l-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 169–190. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.C.; Allen, C. Overcoming the Road Blocks: Advancement of Block Copolymer Micelles for Cancer Therapy in the Clinic. Mol. Pharm. 2017, 14, 2503–2517. [Google Scholar] [CrossRef]
- Gou, M.; Zheng, X.; Men, K.; Zhang, J.; Wang, B.; Lv, L.; Wang, X.; Zhao, Y.; Luo, F.; Chen, L.; et al. Self-Assembled Hydrophobic Honokiol Loaded MPEG-PCL Diblock Copolymer Micelles. Pharm. Res. 2009, 26, 2164–2173. [Google Scholar] [CrossRef]
- Mazzotta, E.; Chieffallo, M.; Muzzalupo, R.; Spingola, M.; Caputo, P.; Romeo, M.; Ioele, G. Formulation of Polymeric Micelles to Increase the Solubility and Photostability of Caffeic Acid. Molecules 2024, 29, 3329. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, F.; Cai, C.; Zhang, L. Toward understanding polymeric micelle stability regulated by diverse physico-chemical strategies and their stabilized mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131880. [Google Scholar] [CrossRef]
- Emami, J.; Kazemi, M.; Mirian, M. Synthesis and in vitro evaluation of self-assembling biocompatible heparin-based targeting polymeric micelles for delivery of doxorubicin to leukemic cells. Res. Pharm. Sci. 2025, 20, 142–164. [Google Scholar] [CrossRef]
- Wang, Y.; Thies-Weesie, D.M.E.; Bosman, E.D.C.; van Steenbergen, M.J.; van den Dikkenberg, J.; Shi, Y.; Lammers, T.; van Nostrum, C.F.; Hennink, W.E. Tuning the size of all-HPMA polymeric micelles fabricated by solvent extraction. J. Control. Release 2022, 343, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.J.; Shin, H.J.; Yoon, M.S.; Kim, S.Y.; Jin, C.E.; Park, C.W.; Kim, J.S.; Shin, D.H. Evaluation of pH-Sensitive Polymeric Micelles Using Citraconic Amide Bonds for the Co-Delivery of Paclitaxel, Etoposide, and Rapamycin. Pharmaceutics 2023, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Faisal, K.S.; Clulow, A.J.; Albrecht, H.; Krasowska, M.; Blencowe, A. Influence of Lyophilization and Cryoprotection on the Stability and Morphology of Drug-Loaded Poly(ethylene glycol-b-ε-caprolactone) Micelles. Polymers 2023, 15, 1974. [Google Scholar] [CrossRef] [PubMed]
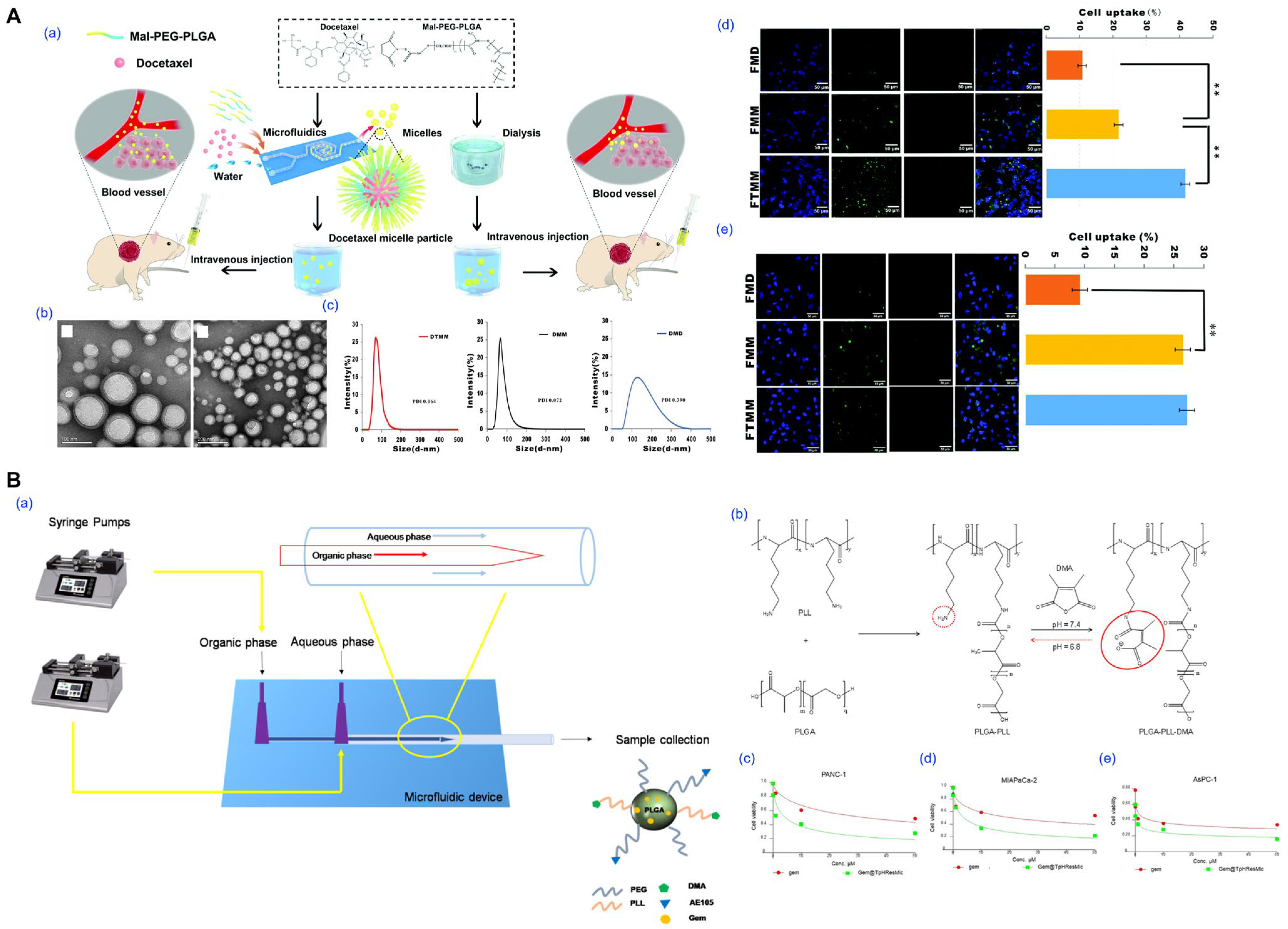
- Bao, Y.; Deng, Q.; Li, Y.; Zhou, S. Engineering docetaxel-loaded micelles for non-small cell lung cancer: A comparative study of microfluidic and bulk nanoparticle preparation. RSC Adv. 2018, 8, 31950–31966. [Google Scholar] [CrossRef]
- Tyrrell, Z.; Winoto, W.; Shen, Y.; Radosz, M. Block Copolymer Micelles Formed in Supercritical Fluid Can Become Water-Dispensable Nanoparticles: Poly(ethylene glycol)−block-Poly(ϵ-caprolactone) in Trifluoromethane. Ind. Eng. Chem. Res. 2009, 48, 1928–1932. [Google Scholar] [CrossRef]
- Farhoudi, L.; Hosseinikhah, S.M.; Kazemi-Beydokhti, A.; Arabi, L.; Alavizadeh, S.H.; Moosavian, S.A.; Jaafari, M.R. pH-sensitive polymeric micelles enhance the co-delivery of doxorubicin and docetaxel: An emerging modality for treating breast cancer. Cancer Nanotechnol. 2024, 15, 37. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Repp, L.; Lee, H.J.; Kwon, G.S. Production of paclitaxel-loaded PEG-b-PLA micelles using PEG for drug loading and freeze-drying. J. Control. Release 2022, 350, 350–359. [Google Scholar] [CrossRef]
- Iacobazzi, R.M.; Arduino, I.; Di Fonte, R.; Lopedota, A.A.; Serratì, S.; Racaniello, G.; Bruno, V.; Laquintana, V.; Lee, B.C.; Silvestris, N.; et al. Microfluidic-Assisted Preparation of Targeted pH-Responsive Polymeric Micelles Improves Gemcitabine Effectiveness in PDAC: In Vitro Insights. Cancers 2021, 14, 5. [Google Scholar] [CrossRef]
- Hou, X.; Cao, B.; He, Y.; Guo, T.; Li, Z.; Liu, Y.; Zhang, Y.; Feng, N. Improved self-assembled micelles based on supercritical fluid technology as a novel oral delivery system for enhancing germacrone oral bioavailability. Int. J. Pharm. 2019, 569, 118586. [Google Scholar] [CrossRef]
- Li, M.; Ling, L.; Xia, Q.; Li, X. A reduction-responsive drug delivery with improved stability: Disulfide crosslinked micelles of small amiphiphilic molecules. RSC Adv. 2021, 11, 12757–12770. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Kang, R.H.; Klukas, M.; Kwon, G.S. Crystallization of supersaturated PEG-b-PLA for the production of drug-loaded polymeric micelles. J. Control. Release 2025, 380, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, S.; Tyler, J.Y.; Park, K.; Cheng, J.X. Blood-stable, tumor-adaptable disulfide bonded mPEG-(Cys)4-PDLLA micelles for chemotherapy. Biomaterials 2013, 34, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.e.; Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. Int. J. Pharm. 2020, 578, 119105. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef]
- Negi, P.; Sharma, G.; Verma, C.; Garg, P.; Rathore, C.; Kulshrestha, S.; Lal, U.R.; Gupta, B.; Pathania, D. Novel thymoquinone loaded chitosan-lecithin micelles for effective wound healing: Development, characterization, and preclinical evaluation. Carbohydr. Polym. 2020, 230, 115659. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, L.; Jiang, Q.; Sun, Y.; Zhao, D.; Sun, M.; He, Z.; Sun, J.; Wang, Y. Intestinal OCTN2- and MCT1-targeted drug delivery to improve oral bioavailability. Asian J. Pharm. Sci. 2020, 15, 158–173. [Google Scholar] [CrossRef]
- Almeida, A.; Linares, V.; Mora-Castaño, G.; Casas, M.; Caraballo, I.; Sarmento, B. 3D printed systems for colon-specific delivery of camptothecin-loaded chitosan micelles. Eur. J. Pharm. Biopharm. 2021, 167, 48–56. [Google Scholar] [CrossRef]
- Bauer, T.A.; Horvat, N.K.; Marques, O.; Chocarro, S.; Mertens, C.; Colucci, S.; Schmitt, S.; Carrella, L.M.; Morsbach, S.; Koynov, K.; et al. Core Cross-Linked Polymeric Micelles for Specific Iron Delivery: Inducing Sterile Inflammation in Macrophages. Adv. Healthc. Mater. 2021, 10, e2100385. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Teng, J.; Zhao, C.-X. FRET Ratiometric Nanoprobes for Nanoparticle Monitoring. Biosensors 2021, 11, 505. [Google Scholar] [CrossRef]
- Arafa, W.M.; Elkomy, M.H.; Aboud, H.M.; Ali, M.I.; Abdel Gawad, S.S.; Aboelhadid, S.M.; Mahdi, E.A.; Alsalahat, I.; Abdel-Tawab, H. Tunable Polymeric Mixed Micellar Nanoassemblies of Lutrol F127/Gelucire 44/14 for Oral Delivery of Praziquantel: A Promising Nanovector against Hymenolepis nana in Experimentally-Infected Rats. Pharmaceutics 2022, 14, 2023. [Google Scholar] [CrossRef]
- Galdopórpora, J.M.; Martinena, C.; Bernabeu, E.; Riedel, J.; Palmas, L.; Castangia, I.; Manca, M.L.; Garcés, M.; Lázaro-Martinez, J.; Salgueiro, M.J.; et al. Inhalable Mannosylated Rifampicin-Curcumin Co-Loaded Nanomicelles with Enhanced In Vitro Antimicrobial Efficacy for an Optimized Pulmonary Tuberculosis Therapy. Pharmaceutics 2022, 14, 959. [Google Scholar] [CrossRef]
- Alshamrani, S.; Kumar, A.; Aldughaim, M.S.; Alghamdi, K.M.; Hussain, M.D.; Alanazi, F.K.; Kazi, M. Development of Polymeric Micelles for Combined Delivery of Luteolin and Doxorubicin for Cancer Therapy. J. Cancer 2024, 15, 4717–4730. [Google Scholar] [CrossRef]
- Lecot, N.; Fernández-Lomónaco, M.; Cerecetto, H.; Gambini, J.P.; Cabral, P.; Glisoni, R. Indocyanine green within glycosylated polymeric micelles as potential image agents to map sentinel lymph nodes and breast cancer. RSC Pharm. 2024, 1, 57–67. [Google Scholar] [CrossRef]
- Jiang, C.; Bai, R.; Somavarapu, S. Inhalable TPGS/DPPC Micelles Coloaded with Curcumin and Icariin for Targeted Lung Cancer Therapy. ACS Omega 2025, 10, 15400–15411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tian, K.; Zhu, R.; Ding, M.; Xu, Z. Self-Assembly of Amphiphilic Comb-like Copolymers into Micelles and Vesicles in Solution. Polymers 2025, 17, 1870. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Bandyopadhyay, R.; Debnath, B.; Dutta, G.; Sugumaran, A. Review on various activator-assisted polymer grafting techniques for smart drug delivery applications. RSC Adv. 2025, 15, 23025–23044. [Google Scholar] [CrossRef]
- Porello, I.; Stucchi, F.; Sbaruffati, G.; Cellesi, F. Tailoring copolymer architectures and macromolecular interactions for enhanced nanotherapeutic delivery: A design-by-architecture approach. Eur. Polym. J. 2024, 220, 113455. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Polymeric Micellar Systems-A Special Emphasis on “Smart” Drug Delivery. Pharmaceutics 2023, 15, 976. [Google Scholar] [CrossRef]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer–drug conjugates: Recent advances and future perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Li, G.; Xu, S.; Liu, H. Construction of Core-Cross-Linked Polymer Micelles with High Biocompatibility and Stability for pH/Reduction Controllable Drug Delivery. Langmuir 2023, 39, 12671–12679. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Yamato, M.; Aoyagi, T.; Sakurai, Y.; Okano, T. Thermo-responsive drug delivery from polymeric micelles constructed using block copolymers of poly(N-isopropylacrylamide) and poly(butylmethacrylate). J. Control. Release 1999, 62, 115–127. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, L.; Shen, C.; He, Y.; Yang, F.; Zhang, G.; Jia, M.; Zeng, R.; Li, C.; Qiao, R. Reactive oxygen species-responsive amino acid-based polymeric nanovehicles for tumor-selective anticancer drug delivery. Mater. Sci. Eng. C 2020, 106, 110159. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [CrossRef]
- Iurciuc, C.E.; Popa, M.; Atanase, L.I.; Popa, O.; Ochiuz, L.; Postolache, P.; Ghizdovat, V.; Irimiciuc, S.A.; Agop, M.; Volovat, C.; et al. Multi-fractal modeling of curcumin release mechanism from polymeric nanomicelles. Drug Deliv. 2022, 29, 2883–2896. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Kim, S.; Yunzhou, S.; Young, K.J.; Kinam, P.; Cheng, J.-X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.; Liu, F.; Duan, Y.; Li, S. Novel biodegradable polylactide/poly(ethylene glycol) micelles prepared by direct dissolution method for controlled delivery of anticancer drugs. Pharm. Res. 2009, 26, 2332–2342. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Sotoudegan, F.; Talebkhan Garoosi, Y.; Afshar, S.H.; Barkhordari, F.; Davami, F. Anti-Aβ-scFv-loaded polymeric nano-micelles with enhanced plasma stability. J. Pharm. Pharmacol. 2021, 73, 460–472. [Google Scholar] [CrossRef]
- Chen, F.; Rice, K.C.; Liu, X.-M.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Triclosan-Loaded Tooth-Binding Micelles for Prevention and Treatment of Dental Biofilm. Pharm. Res. 2010, 27, 2356–2364. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Qi-Long, W.; Michael, A.-F.; Jian, L.; Kang-Yi, Z.; Xi-Ming, X.; Yu, J.-N. Preparation and evaluation of isoliquiritigenin-loaded F127/P123 polymeric micelles. Drug Dev. Ind. Pharm. 2019, 45, 1224–1232. [Google Scholar] [CrossRef]
- Ganguly, R.; Kunwar, A.; Dutta, B.; Kumar, S.; Barick, K.C.; Ballal, A.; Aswal, V.K.; Hassan, P.A. Heat-induced solubilization of curcumin in kinetically stable pluronic P123 micelles and vesicles: An exploit of slow dynamics of the micellar restructuring processes in the aqueous pluronic system. Colloids Surf. B Biointerfaces 2017, 152, 176–182. [Google Scholar] [CrossRef]
- Pepić, I.; Hafner, A.; Lovrić, J.; Pirkić, B.; Filipović-Grcčić, J. A Nonionic Surfactant/Chitosan Micelle System in an Innovative Eye Drop Formulation. J. Pharm. Sci. 2010, 99, 4317–4325. [Google Scholar] [CrossRef]
- Kang Moo, H.; Hyun Su, M.; Sang Cheon, L.; Hong Jae, L.; Sungwon, K.; Kinam, P. A new hydrotropic block copolymer micelle system for aqueous solubilization of paclitaxel. J. Control. Release 2008, 126, 122–129. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Q.; Wang, Y.; Liu, X.; Lu, M.; Li, Z.; Jiang, X.; Ji, J. Preparation of Glutathione-Responsive Paclitaxel Prodrug Based on Endogenous Molecule of L-Glutathione Oxidized for Cancer Therapy. Pharmaceutics 2024, 16, 1178. [Google Scholar] [CrossRef]
- Yang, X.; Wu, S.; Wang, Y.; Li, Y.; Chang, D.; Luo, Y.; Ye, S.; Hou, Z. Evaluation of self-assembled HCPT-loaded PEG-b-PLA nanoparticles by comparing with HCPT-loaded PLA nanoparticles. Nanoscale Res. Lett. 2014, 9, 2408. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Ozturk, M.-R.; Popa, M.; Rata, D.M.; Cadinoiu, A.N.; Parfait, F.; Delaite, C.; Atanase, L.I.; Solcan, C.; Daraba, O.M. Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. Int. J. Mol. Sci. 2022, 23, 9382. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, Y.; Hao, L.; Yang, Y.; Gao, Y.; Zhang, N.; Zhang, X.; Song, Y. CD44/Folate Dual Targeting Receptor Reductive Response PLGA-Based Micelles for Cancer Therapy. Front. Pharmacol. 2022, 13, 829590. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Kim, D.H.; Chung, C.W.; Yoo, J.J.; Choi, K.H.; Kim, C.H.; Ha, S.H.; Kang, D.H. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-co-glycolide) copolymer. Int. J. Nanomed. 2011, 6, 1415–1427. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; YT, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636. [Google Scholar] [CrossRef]
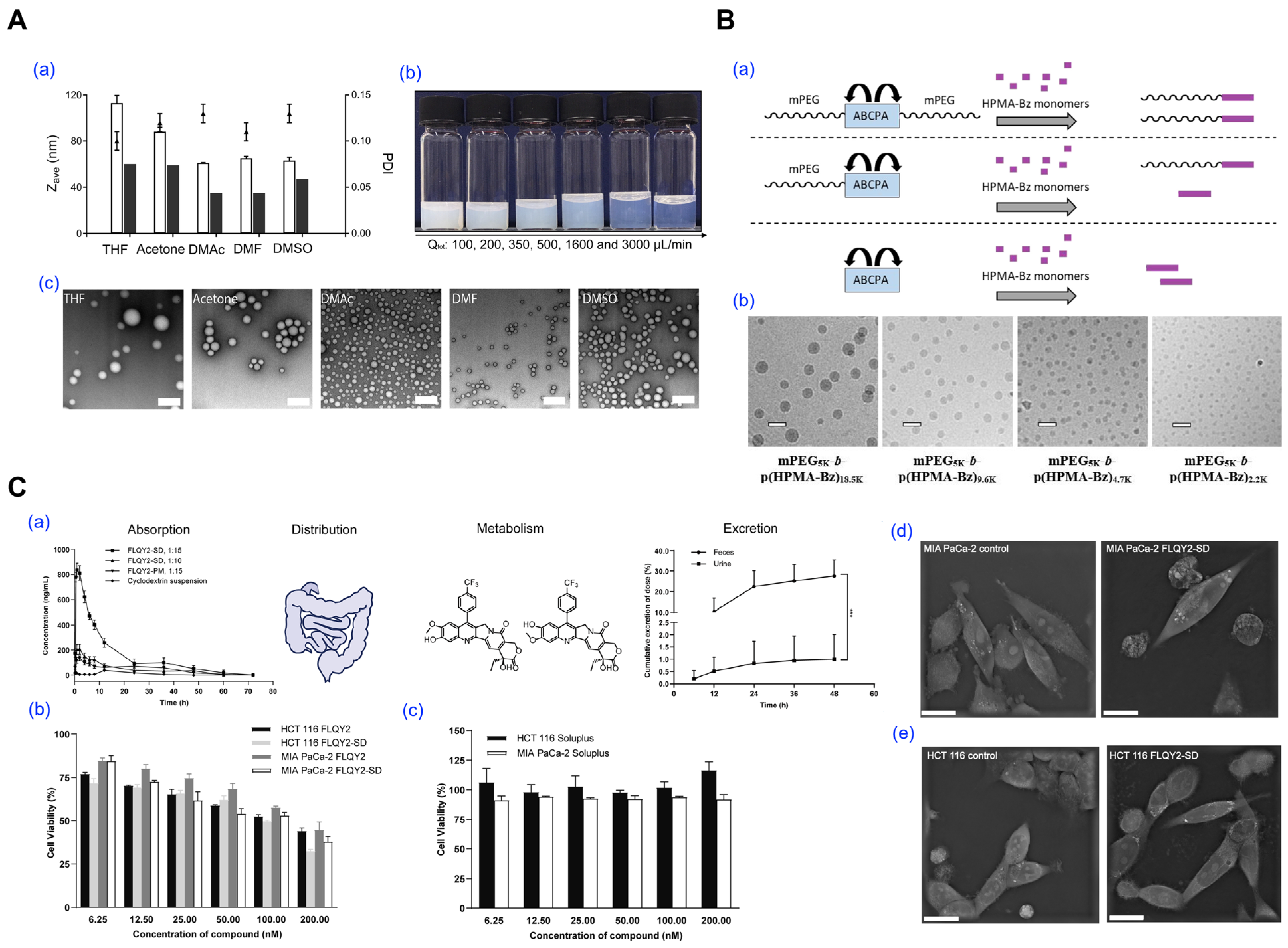
- Bagheri, M.; Bresseleers, J.; Varela-Moreira, A.; Sandre, O.; Meeuwissen, S.A.; Schiffelers, R.M.; Metselaar, J.M.; van Nostrum, C.F.; van Hest, J.C.M.; Hennink, W.E. Effect of Formulation and Processing Parameters on the Size of mPEG-b-p(HPMA-Bz) Polymeric Micelles. Langmuir 2018, 34, 15495–15506. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yu, E.; Zhuang, W.; Sun, X.; Wang, H.; Li, Q. Preparation of a camptothecin analog FLQY2 self-micelle solid dispersion with improved solubility and bioavailability. J. Nanobiotechnol. 2022, 20, 402. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Elhasi, S.; Mahmud, A.; Gulamhusein, R.; Mahdipoor, P.; Lavasanifar, A. Encapsulation of hydrophobic drugs in polymeric micelles through co-solvent evaporation: The effect of solvent composition on micellar properties and drug loading. Int. J. Pharm. 2007, 329, 158–165. [Google Scholar] [CrossRef]
- Czysch, C.; Medina-Montano, C.; Dal, N.-J.K.; Dinh, T.; Fröder, Y.; Winterwerber, P.; Maxeiner, K.; Räder, H.-J.; Schuppan, D.; Schild, H.; et al. End Group Dye-Labeled Polycarbonate Block Copolymers for Micellar (Immuno-)Drug Delivery. Macromol. Rapid Commun. 2022, 43, 2200095. [Google Scholar] [CrossRef]
- Khodaverdi, E.; Tayarani-Najaran, Z.; Minbashi, E.; Alibolandi, M.; Hosseini, J.; Sepahi, S.; Kamali, H.; Hadizadeh, F. Docetaxel-Loaded Mixed Micelles and Polymersomes Composed of Poly (caprolactone)-Poly (ethylene glycol) (PEG-PCL) and Poly (lactic acid)-Poly (ethylene glycol) (PEG-PLA): Preparation and In-vitro Characterization. Iran J. Pharm. Res. 2019, 18, 142–155. [Google Scholar]
- Repp, L.; Unterberger, C.J.; Ye, Z.; Feltenberger, J.B.; Swanson, S.M.; Marker, P.C.; Kwon, G.S. Oligo(Lactic Acid)8-Docetaxel Prodrug-Loaded PEG-b-PLA Micelles for Prostate Cancer. Nanomaterials 2021, 11, 2745. [Google Scholar] [CrossRef]
- Tan, L.; Peng, J.; Zhao, Q.; Zhang, L.; Tang, X.; Chen, L.; Lei, M.; Qian, Z. A Novel MPEG-PDLLA-PLL Copolymer for Docetaxel Delivery in Breast Cancer Therapy. Theranostics 2017, 7, 2652–2672. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, J.; Yuan, S.; Li, Y.; Juan, W.; Sha, X.; Fang, X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: Formulation, optimization and in vitro characterization. Int. J. Pharm. 2009, 376, 176–185. [Google Scholar] [CrossRef]
- Lamch, Ł.; Gancarz, R.; Tsirigotis-Maniecka, M.; Moszyńska, I.M.; Ciejka, J.; Wilk, K.A. Studying the “Rigid–Flexible” Properties of Polymeric Micelle Core-Forming Segments with a Hydrophobic Phthalocyanine Probe Using NMR and UV Spectroscopy. Langmuir 2021, 37, 4316–4330. [Google Scholar] [CrossRef]
- Yassin, A.E.B.; Massadeh, S.; Alshwaimi, A.A.; Kittaneh, R.H.; Omer, M.E.; Ahmad, D.; Aodah, A.H.; Shakeel, F.; Halwani, M.; Alanazi, S.A.; et al. Tween 80-Based Self-Assembled Mixed Micelles Boost Valsartan Transdermal Delivery. Pharmaceuticals 2024, 17, 19. [Google Scholar] [CrossRef]
- He, Z.; Wan, X.; Schulz, A.; Bludau, H.; Dobrovolskaia, M.A.; Stern, S.T.; Montgomery, S.A.; Yuan, H.; Li, Z.; Alakhova, D.; et al. A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anti-cancer activity. Biomaterials 2016, 101, 296–309. [Google Scholar] [CrossRef]
- Deng, C.; Xu, C.; Zhang, X.; Yao, J.U.; Zhang, Y.; Yu, B.O.; Lee, R.J.; Jiang, C. A Novel Paclitaxel-Loaded Polymeric Micelle System with Favorable Biocompatibility and Superior Antitumor Activity. Anticancer Res. 2018, 38, 219. [Google Scholar]
- Wang, Q.; Liu, Y.; Pu, C.; Zhang, H.; Tan, X.; Gou, J.; He, H.; Yin, T.; Zhang, Y.; Wang, Y.; et al. Drug-Polymer Interaction, Pharmacokinetics and Antitumor Effect of PEG-PLA/Taxane Derivative TM-2 Micelles for Intravenous Drug Delivery. Pharm. Res. 2018, 35, 208. [Google Scholar] [CrossRef]
- Jin, M.-j.; Piao, S.-j.; Jin, T.-x.; Jin, Z.-h.; Yin, X.-z.; Gao, Z.-g. Improved anti-tumor efficiency against prostate cancer by docetaxel-loaded PEG-PCL micelles. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 66–75. [Google Scholar] [CrossRef]
- Yang, Z.L.; Li, X.R.; Yang, K.W.; Liu, Y. Amphotericin B-loaded poly(ethylene glycol)–poly(lactide) micelles: Preparation, freeze-drying, and in vitro release. J. Biomed. Mater. Res. A 2008, 85A, 539–546. [Google Scholar] [CrossRef]
- Lee, S.C.; Huh, K.M.; Lee, J.; Cho, Y.W.; Galinsky, R.E.; Park, K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: In vitro and in vivo characterization. Biomacromolecules 2007, 8, 202–208. [Google Scholar] [CrossRef]
- Gao, L.; Gao, L.; Fan, M.; Li, Q.; Jin, J.; Wang, J.; Lu, W.; Yu, L.; Yan, Z.; Wang, Y. Hydrotropic polymer-based paclitaxel-loaded self-assembled nanoparticles: Preparation and biological evaluation. RSC Adv. 2017, 7, 33248–33256. [Google Scholar] [CrossRef]
- Mihyar, R.; Shalmani, A.A.; Wildt, V.; Sheybanifard, M.; Wang, A.; May, J.-N.; Shahzad, S.; Buhl, E.M.; Rütten, S.; Behrens, D.; et al. Microfluidic formulation, cryoprotection and long-term stability of paclitaxel-loaded π electron-stabilized polymeric micelles. J. Control. Release 2024, 375, 614–626. [Google Scholar] [CrossRef]
- Gupta, A.; Costa, A.P.; Xu, X.; Burgess, D.J. Continuous processing of paclitaxel polymeric micelles. Int. J. Pharm. 2021, 607, 120946. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhang, Y. Preparation and Characterization of Paclitaxel Loaded SF/PLLA-PEG-PLLA Nanoparticles via Solution-Enhanced Dispersion by Supercritical CO2. J. Nanomater. 2015, 2015, 913254. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, H.; Gu, L.; Fu, J.; Guo, J.; Du, L.; Zhou, X.; Yu, X.; Huang, Y.; Wang, H. Aminoglucose-functionalized, redox-responsive polymer nanomicelles for overcoming chemoresistance in lung cancer cells. J. Nanobiotechnol. 2017, 15, 87. [Google Scholar] [CrossRef]
- Hami, Z.; Amini, M.; Ghazi-Khansari, M.; Rezayat, S.M.; Gilani, K. Synthesis and in vitro evaluation of a pH-sensitive PLA-PEG-folate based polymeric micelle for controlled delivery of docetaxel. Colloids Surf. B Biointerfaces 2014, 116, 309–317. [Google Scholar] [CrossRef]
- Cui, F.; Li, Y.; Zhou, S.; Jia, M.; Yang, X.; Yu, F.; Ye, S.; Hou, Z.; Xie, L. A comparative in vitro evaluation of self-assembled PTX-PLA and PTX-MPEG-PLA nanoparticles. Nanoscale Res. Lett. 2013, 8, 301. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Saad, W.S.; Prud’homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Park, I.H.; Sohn, J.H.; Kim, S.B.; Lee, K.S.; Chung, J.S.; Lee, S.H.; Kim, T.Y.; Jung, K.H.; Cho, E.K.; Kim, Y.S.; et al. An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer. Cancer Res. Treat. 2017, 49, 569–577. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, Y.-M.; Kim, Y.T.; Kang, S.B. An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: A Korean Gynecologic Oncology Group study (KGOG-3016). J. Gynecol Oncol. 2017, 28, e26. [Google Scholar] [CrossRef]
- Choi, J.; Ko, E.; Chung, H.K.; Lee, J.H.; Ju, E.J.; Lim, H.K.; Park, I.; Kim, K.S.; Lee, J.H.; Son, W.C.; et al. Nanoparticulated docetaxel exerts enhanced anticancer efficacy and overcomes existing limitations of traditional drugs. Int. J. Nanomed. 2015, 10, 6121–6132. [Google Scholar] [CrossRef]
- Fraile, M.; Buratto, R.; Gómez, B.; Martín, Á.; Cocero, M.J. Enhanced Delivery of Quercetin by Encapsulation in Poloxamers by Supercritical Antisolvent Process. Ind. Eng. Chem. Res. 2014, 53, 4318–4327. [Google Scholar] [CrossRef]
- Maravajjala, K.S.; Swetha, K.L.; Sharma, S.; Padhye, T.; Roy, A. Development of a size-tunable paclitaxel micelle using a microfluidic-based system and evaluation of its in-vitro efficacy and intracellular delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 102041. [Google Scholar] [CrossRef]
- Yoshida, T.; Lai, T.C.; Kwon, G.S.; Sako, K. pH- and ion-sensitive polymers for drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1497–1513. [Google Scholar] [CrossRef]
- Tam, Y.T.; Huang, C.; Poellmann, M.; Kwon, G.S. Stereocomplex Prodrugs of Oligo(lactic acid)n-Gemcitabine in Poly(ethylene glycol)-block-poly(d,l-lactic acid) Micelles for Improved Physical Stability and Enhanced Antitumor Efficacy. ACS Nano 2018, 12, 7406–7414. [Google Scholar] [CrossRef]
- Gong, F.; Wang, R.; Zhu, Z.; Duan, J.; Teng, X.; Cui, Z.K. Drug-interactive mPEG-b-PLA-Phe(Boc) micelles enhance the tolerance and anti-tumor efficacy of docetaxel. Drug Deliv. 2020, 27, 238–247. [Google Scholar] [CrossRef]
- Ahmadi, M.; Siavashy, S.; Ayyoubzadeh, S.M.; Kecili, R.; Ghorbani-Bidkorbeh, F. Controllable Synthesis of Polymeric Micelles by Microfluidic Platforms for Biomedical Applications: A Systematic Review. Iran J. Pharm. Res. 2021, 20, 229–240. [Google Scholar] [CrossRef]
- De Marco, I. Supercritical Fluids and Nanoparticles in Cancer Therapy. Micromachines 2022, 13, 1449. [Google Scholar] [CrossRef]
- Shalmani, A.A.; Wang, A.; Ahmed, Z.; Sheybanifard, M.; Mihyar, R.; Buhl, E.M.; Pohl, M.; Hennink, W.E.; Kiessling, F.; Metselaar, J.M.; et al. Tunable polymeric micelles for taxane and corticosteroid co-delivery. Drug Deliv. Transl. Res. 2024, 14, 2642–2654. [Google Scholar] [CrossRef]
- Xue, R.; Pan, Y.; Xia, L.; Li, J. Non-viral vectors combined delivery of siRNA and anti-cancer drugs to reverse tumor multidrug resistance. Biomed. Pharmacother. 2024, 178, 117119. [Google Scholar] [CrossRef]
- Zhou, L.; Lei, D.; Wang, Q.; Ouyang, Y.; Luo, X. Rational Design of Polyphosphorylcholine-Based Micelles for Superior Anti-Biofilm Activity. Macromol. Mater. Eng. 2022, 307, 2100806. [Google Scholar] [CrossRef]
- Sun, X.; Lin, Y.; Zhong, X.; Fan, C.; Liu, Z.; Chen, X.; Luo, Z.; Wu, J.; Tima, S.; Zhang, Z.; et al. Alendronate-functionalized polymeric micelles target icaritin to bone for mitigating osteoporosis in a rat model. J. Control. Release 2024, 376, 37–51. [Google Scholar] [CrossRef]
- Que, Y.; Yang, Y.; Zafar, H.; Wang, D. Tetracycline-grafted mPEG-PLGA micelles for bone-targeting and osteoporotic improvement. Front. Pharmacol. 2022, 13, 993095. [Google Scholar] [CrossRef]
- Cong, Y.; Quan, C.; Liu, M.; Liu, J.; Huang, G.; Tong, G.; Yin, Y.; Zhang, C.; Jiang, Q. Alendronate-decorated biodegradable polymeric micelles for potential bone-targeted delivery of vancomycin. J. Biomater. Sci. Polym. Ed. 2015, 26, 629–643. [Google Scholar] [CrossRef]
- Lu, X.; Wan, X.; Lian, J.; Peng, J.; Jing, P.; Guo, Q.; Liao, Y.; Jiang, Y.; Yang, C.; Jin, L.; et al. Antibiotic-based micelles with bone-targeting and pH-responsive properties for infectious osteomyelitis treatment. J. Colloid Interface Sci. 2025, 685, 648–660. [Google Scholar] [CrossRef]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Hiebner, D.W.; Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. J. Nanobiotechnol. 2021, 19, 104. [Google Scholar] [CrossRef]
- Uthaman, S.; Parvinroo, S.; Mathew, A.P.; Jia, X.; Hernandez, B.; Proctor, A.; Sajeevan, K.A.; Nenninger, A.; Long, M.-J.; Park, I.-K.; et al. Inhibiting the cGAS-STING Pathway in Ulcerative Colitis with Programmable Micelles. ACS Nano 2024, 18, 12117–12133. [Google Scholar] [CrossRef]
- Guo, R.-B.; Zhang, L.; Liu, Y.; Kong, L.; Yu, Y.; Yang, B.; Wang, Z.-J.; Zhang, J.-Y.; Li, X.-T. Treatment of rheumatoid arthritis using dual-targeted and dual-response intelligent micelles: A “three birds with one stone” strategy. J. Nanobiotechnol. 2025, 23, 71. [Google Scholar] [CrossRef]
- Lima, A.C.; Reis, R.L.; Ferreira, H.; Neves, N.M. Glutathione Reductase-Sensitive Polymeric Micelles for Controlled Drug Delivery on Arthritic Diseases. ACS Biomater. Sci. Eng. 2021, 7, 3229–3241. [Google Scholar] [CrossRef]
- Han, J.; Na, R.; Zhao, N.; Yuan, X.; Fu, L.; Jing, J.; Qian, A.; Ye, W. Macrophage-Targeted Dextran Sulfate-Dexamethasone Conjugate Micelles for Effective Treatment of Rheumatoid Arthritis. Molecules 2023, 28, 591. [Google Scholar] [CrossRef]
- Cherian, I.V.; Vijukumar, A.; Islam, M.M.; Janvi; Vikal, A. Assessing the therapeutic potential of quercetin, a widely spread flavonoid, in the prevention and management of chronic and degenerative diseases through a modern Chinese medicine perspective. Pharmacol. Res.-Mod. Chin. Med. 2025, 15, 100630. [Google Scholar] [CrossRef]
- Abdollahi, A.R.; Firouzian, F.; Haddadi, R.; Nourian, A. Indomethacin loaded dextran stearate polymeric micelles improve adjuvant-induced arthritis in rats: Design and in vivo evaluation. Inflammopharmacology 2021, 29, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, Z.; Huang, Y.; Wang, Y.; Jin, Q.; Shentu, X.; Ye, J.; Ji, J.; Yao, K.; Han, H. Anti-Oxidative and Anti-Inflammatory Micelles: Break the Dry Eye Vicious Cycle. Adv. Sci. 2022, 9, 2200435. [Google Scholar] [CrossRef] [PubMed]
- Szalai, B.; Jójárt-Laczkovich, O.; Kovács, A.; Berkó, S.; Sipos, B.; Katona, G.; Budai-Szűcs, M. Comparative Study of Dexamethasone-Loaded Thermoresponsive In Situ Gels and Polymeric Micelles for Ocular Drug Delivery. Int. J. Mol. Sci. 2025, 26, 8414. [Google Scholar] [CrossRef] [PubMed]


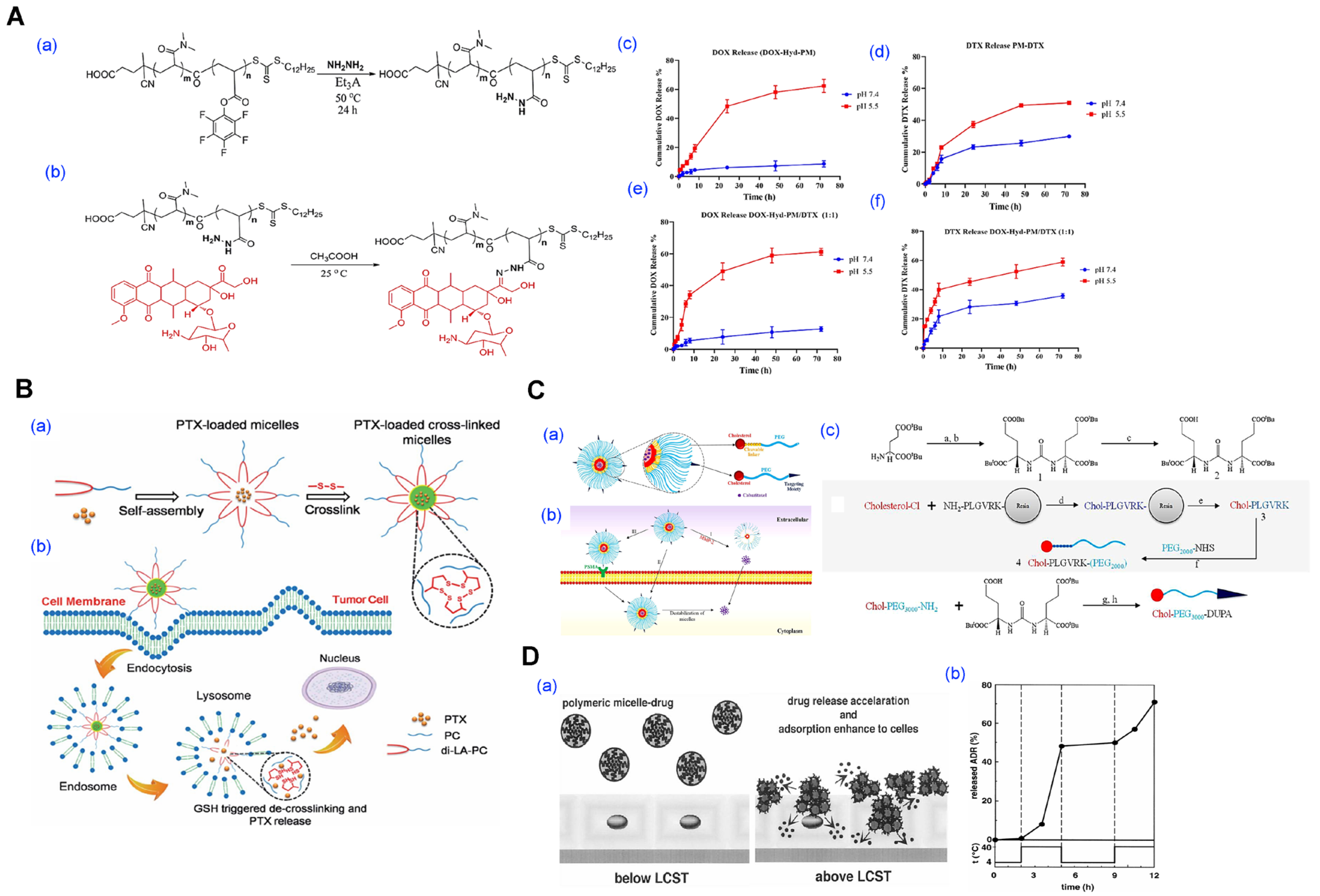
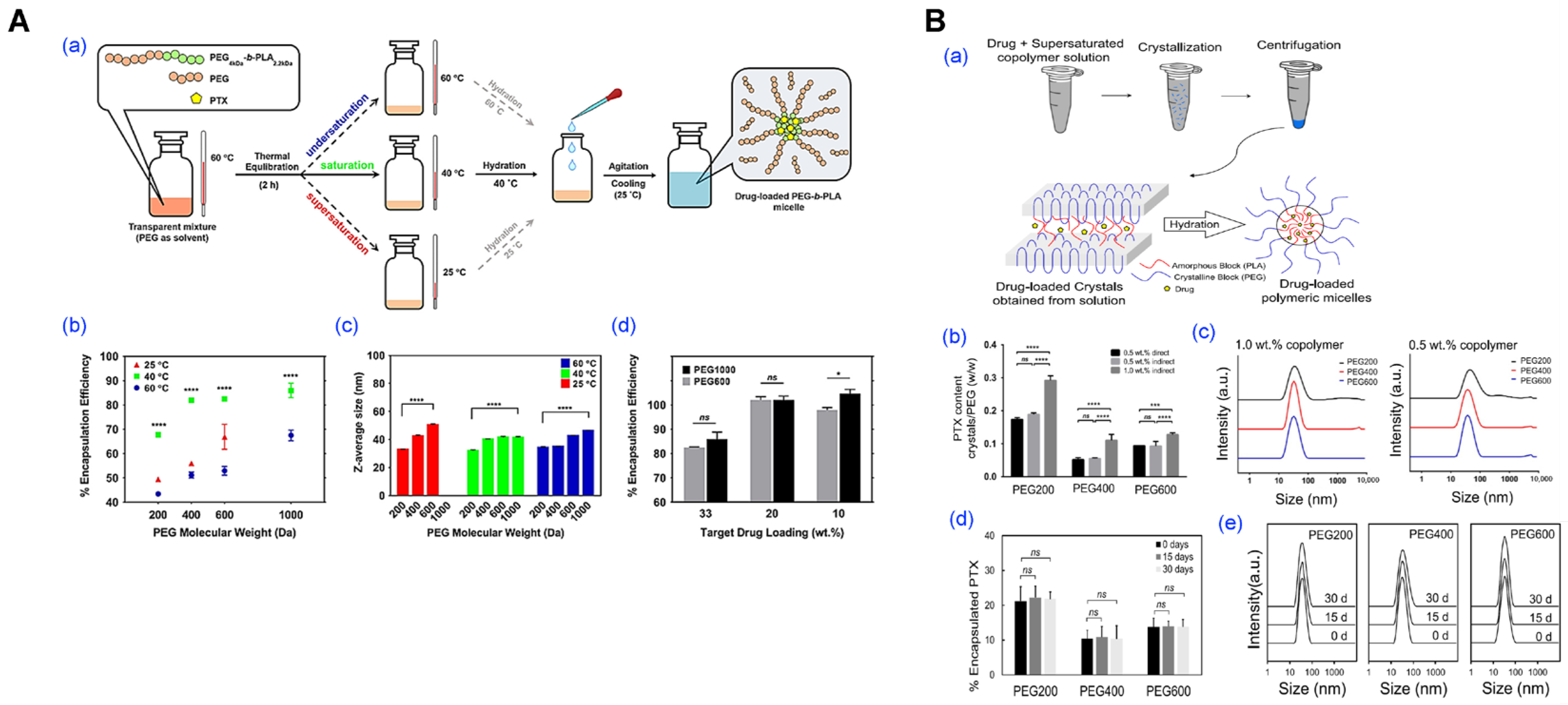
| Methods | Key Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|
| Direct Dissolution | Polymer and drug directly mixed in aqueous medium. | Simple, solvent-free, fast. | Low drug loading, poor stability for highly hydrophobic drugs. | [27,28,68,69,70,71,72,73,74,78] |
| Dialysis | Polymer-drug solution in organic solvent dialyzed into water. | Uniform particle size, stable micelles. | Labor-intensive, slow, not scalable. | [29,30,65,76,77,78,79,80,81,97] |
| Emulsification & Evaporation | Polymer and drug in organic solvent emulsified and solvent evaporated. | High drug loading, tunable size. | Toxic solvents used, batch variability, difficult scale-up. | [31,82,83,84,85,86,98] |
| Thin-Film Hydration | Polymer and drug dissolved in volatile solvent, film formed and hydrated. | Versatile, compatible with various drugs. | Sensitive to hydration/film parameters, needs rehydration optimization. | [32,52,87,88,89,90,91,92,93,94] |
| Freeze-Drying | Micelles lyophilized with or without cryoprotectants, rehydrated later. | Enhances shelf-life, useful post-processing method. | Risk of drug crystallization, collapse without cryoprotectants. | [33,95,96] |
| Methods | Polymer | API | Size (nm) | PDI | DL% | References |
|---|---|---|---|---|---|---|
| Direct Dissolution | PEG-PLA | PTX | 178–276 | 0.15~0.19 | 30.6~52.2 | [68] |
| PEG-b–P(2-VBOPNA) | PTX | 25–90 | NR | NR | [74] | |
| Dialysis | PEG-P(VBODENA) | PTX | 105–120 | NR | 18.4–37.4 | [97] |
| PEG-PLA | PTX | NR | NR | 20–28 | [68] | |
| Emulsification & Evaporation | PGG-DENA | PTX | ~70 | 0.219 | 11.7 | [98] |
| PEG-PLA | DTX | ~36.19 | 0.249 | ≤20% | [86] | |
| Thin-Film Hydration | Genexol-PM (mPEG–PDLLA/PTX) | PTX | 25–30 | <0.1 | 16 | [92] |
| mPEG-PDLLA-Phe(Fmoc) | PTX | ~45 | 0.112 | NR | [93] | |
| PEG–PLA | PTX | 25–104.5 | <0.21 | 15–20 | [94] | |
| Freeze-Drying | PEG-PLA | DTX | ~30.6 | 0.18 | 9.7 | [87] |
| PEG–PLA | Amphotericin B | 50–91 | <0.141 | NR | [96] | |
| Microfluidic-Assisted | Mal-PEG-PLA | DTX | 72 ± 1 | 0.072 | 11.12 ± 1.17 | [34] |
| mPEG-b-p(HPMAm-Bz) | PTX | ~80 | <0.1 | ~80% (EE) | [99] | |
| PEG-PLA | PTX | 15–70 | 0.18 ± 0.02 | ~20% | [100] | |
| Supercritical Fluid (SCF) | Soluplus®, DSPE-PEG2000 and Lipoid S-75 | GEM | 86.3 ± 3.7 | 0.106 ± 0.008 | 5.93 ± 0.2 | [39] |
| PLLA-PEG-PLLA | PTX | ~651 | NR | 18.1 | [101] | |
| Stimuli-Responsive | AG-PEG-SS-PLA | PTX | 85 ± 2.5 | NR | ~8 | [102] |
| PLA–PEG–folate | DTX | ~181 | 0.29 | 47.15 | [103] | |
| PEG-Assisted | PEG-PLA | PTX | ~50 | NR | ~33 | [37] |
| PEG-PLA | PTX | ~40 | NR | ~30 | [41] |
| Methods | Scalability | Reproducibility | Solvent Concerns | Drug Loading Efficiency (%) | References |
|---|---|---|---|---|---|
| Direct Dissolution | Good–excellent | Moderate | Excellent (no/benign solvent) | Variable (Low–mid) | [68,74,97] |
| Dialysis | Poor | Moderate | Good–excellent (depends on volume/time/membrane) | Low–moderate | [76,104,105] |
| Emulsification & Evaporation | Good | Moderate | Poor | Good | [98,106] |
| Thin-Film Hydration | Moderate | Moderate | Moderate–poor | Moderate | [88,107,108] |
| Microfluidic-Assisted | Excellent | Excellent | Good–excellent | Variable | [99,100] |
| Supercritical Fluid (SCF) | Good | Excellent | excellent | Good | [35,39,109,110] |
| Stimuli-Responsive | Moderate | Excellent | Good–excellent | Variable | [1,36,62,63] |
| PEG-Assisted | Good | Good | Good | Good | [37,41] |
| Methods | Key Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|
| Microfluidic-Assisted | Rapid mixing of an organic phase containing the polymer and drug with an aqueous phase | Narrow size distribution, improved batch-to-batch reproducibility, enhanced cytotoxicity | Difficult scale-up, complex optimization, low throughput, solvent compatibility issues | [34,38,99,100,111] |
| Supercritical Fluid (SCF) | Processing of polymers and drugs using supercritical fluids | Avoidance of toxic solvents, solvent-free or solvent-minimized conditions, formation of dry micellar powders | Requirement for high-pressure equipment, narrow processing window, need for specialized formulations | [35,39,109,110] |
| Stimuli-Responsive | Self-assembly is triggered or modulated by environmental stimuli (e.g., pH, redox, enzymes) | Site-specific release, reduced systemic toxicity, enhanced therapeutic index | Synthetic complexity, batch variability, undefined pathways, trigger inconsistency | [1,36,40,62,63,64,65] |
| PEG-Assisted | Co-dissolution of amphiphilic block copolymers and drugs in low-molecular-weight PEGs | Simple protocol, high encapsulation efficiency, solvent-free process, scalability, regulatory alignment | Requires mild heating, PEG–drug solubility mismatch, hydration condition sensitivity | [37,41] |
| Administration Route | Key Application | Micelle Strategy | Therapeutic Effect | References |
|---|---|---|---|---|
| Intravenous (IV) | Systemic anticancer therapy | Core-crosslinked micelles, PEG-assisted micelles | Prolonged circulation, enhanced tumor accumulation, and reduced off-target toxicity | [37,42,49,87] |
| Oral (PO) | Gastrointestinal delivery of poorly water-soluble drugs | pH-sensitive micelles, transporter-targeted systems | Improved stability and drug solubility, enhanced permeability and bioavailability | [43,46,47] |
| Transdermal | Non-invasive delivery of lipophilic agents | Hyaluronan-based micelles, lecithin-integrated micelles | Sustained skin deposition, wound healing efficacy, potential in dermatological and regenerative medicine | [45,50] |
| Pulmonary | Local or systemic lung drug delivery | TPGS/DPPC mixed micelles | Effective aerosolization, increased lung deposition, cytotoxic and antimicrobial activity | [51,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Rasoulianboroujeni, M.; Kang, R.H.; Kwon, G.S. From Conventional to Next-Generation Strategies: Recent Advances in Polymeric Micelle Preparation for Drug Delivery. Pharmaceutics 2025, 17, 1360. https://doi.org/10.3390/pharmaceutics17101360
Cho S, Rasoulianboroujeni M, Kang RH, Kwon GS. From Conventional to Next-Generation Strategies: Recent Advances in Polymeric Micelle Preparation for Drug Delivery. Pharmaceutics. 2025; 17(10):1360. https://doi.org/10.3390/pharmaceutics17101360
Chicago/Turabian StyleCho, Suhyeon, Morteza Rasoulianboroujeni, Rae Hyung Kang, and Glen S. Kwon. 2025. "From Conventional to Next-Generation Strategies: Recent Advances in Polymeric Micelle Preparation for Drug Delivery" Pharmaceutics 17, no. 10: 1360. https://doi.org/10.3390/pharmaceutics17101360
APA StyleCho, S., Rasoulianboroujeni, M., Kang, R. H., & Kwon, G. S. (2025). From Conventional to Next-Generation Strategies: Recent Advances in Polymeric Micelle Preparation for Drug Delivery. Pharmaceutics, 17(10), 1360. https://doi.org/10.3390/pharmaceutics17101360






