Barrier Products for Topical Delivery—Insight into Efficacy Testing and Barrier-Boosting Compounds
Abstract
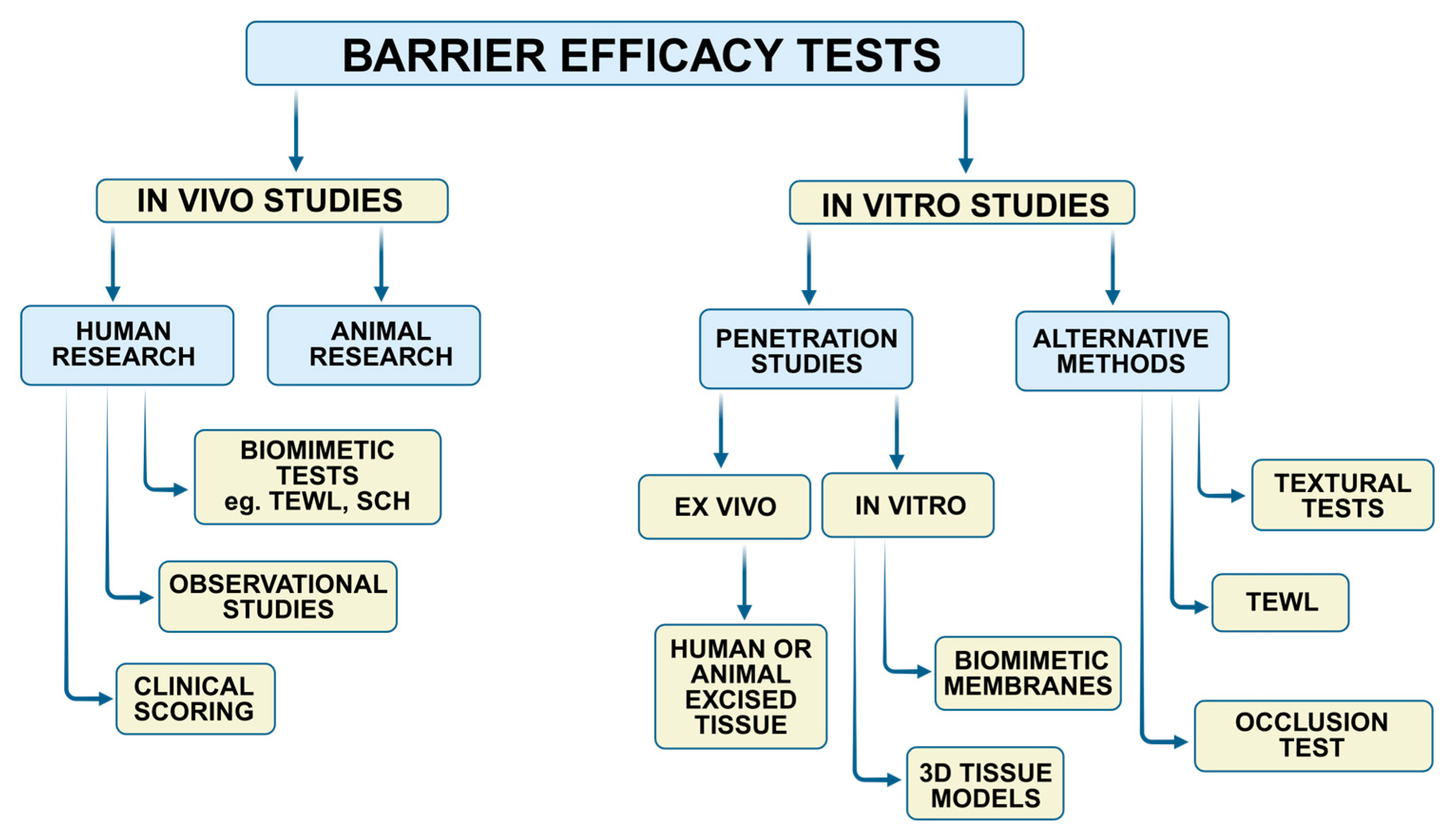
1. Introduction
2. Methodology
3. In Vivo Barrier Efficacy Assessment
3.1. Studies in Human Volunteers
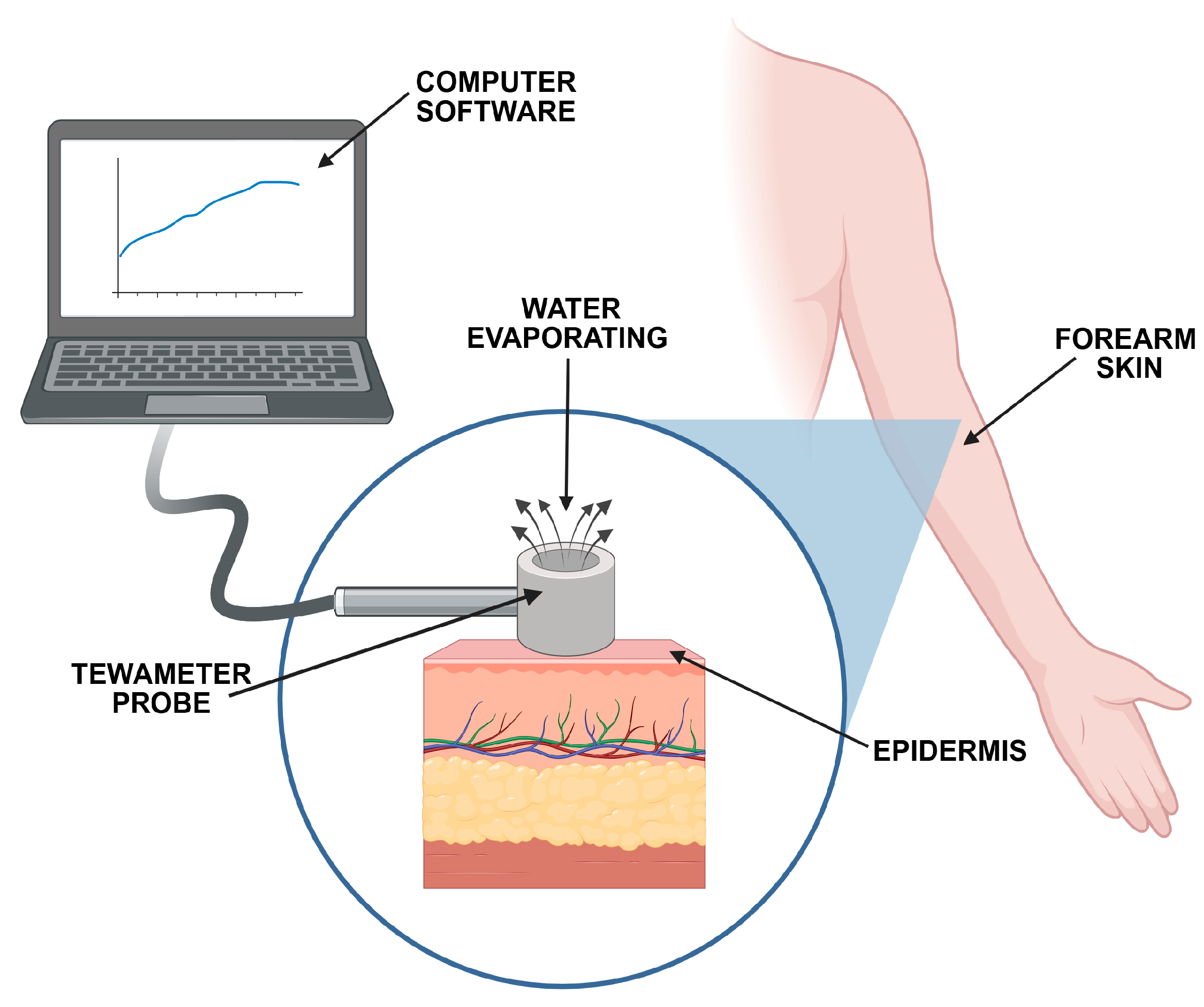
3.2. Biomimetic Studies
3.2.1. TEWL and SCH Measurements
3.2.2. Squamometry
3.3. Barrier Product Efficacy in Mucositis—Clinical Scoring
3.4. Barrier Product Efficacy in Dermatitis—Clinical Scoring
3.5. Animal Studies
4. In Vitro Barrier Efficacy Testing
4.1. Permeability Studies
4.1.1. Factors Affecting Penetration Measurements
The Type of Membrane
The Acceptor Solution
The Chemical Agent
4.1.2. General Considerations for Barrier Testing with an In Vitro Penetration Study
4.2. Studies with 3D Tissue Models
4.3. Alternative In Vitro Techniques for Barrier Efficacy Testing
5. Novel Barrier-Boosting Compounds
| Ingredient | Mode of Action | Outcome | Reference |
|---|---|---|---|
| Aloe vera extract | A hydrating, protective, and soothing ingredient with adhesive properties as well as film-forming ability | Creating a protective film, limiting the penetration of caffeine | Bassetto et al. [59] |
| Apigenin | Enhanced filaggrin expression and lamellar body production; upregulation of lipid synthetic enzymes | Improved skin barrier recovery | Hou et al. [4] |
| Cannabidiol | Enhanced expression of cytoprotective enzyme HMOX1 in keratinocytes; antioxidant, anti-inflammatory, and anti-apoptotic properties | Improved skin barrier recovery | Casares et al. [2] |
| Endocannabinoids (e.g., palmitoylethanolamide) | Anti-inflammatory effect; Stimulating the keratinocyte proliferation and differentiation; | Improved skin barrier recovery, increased epithelial hydration | Yuan et al. [93], Madnani et al. [94] McCormick et al. [95] |
| Enoxolone | Inhibition of endogenous hyaluronidase activity; increase in epidermal ceramide production | Enhanced skin hydration; improved integrity of intercellular cement | Zeichner et al. [98] |
| Pentetic acid | Chelating agent | Blocking the dermal accumulation of nickel | Magnano et al. [61,62] |
| Protopanaxatriol | Enhanced expression of transglutaminase, claudin, occludin, and filaggrin; stimulation of hyaluronic acid production; upregulation of Src/AKT/NF-κB signaling | Improved skin barrier recovery, increased epithelial hydration | Lee et al. [99] |
| Resveratrol | Antioxidant and anti-inflammatory activity; inhibition of free radical production and lipid peroxidation | Improved skin barrier integrity | Wagemaker et al. [97] |
| Microbiome therapeutic Lactobacillus strain LP51 | Modulating the skin microbiome composition; anti-inflammatory, antioxidant properties; increased the transcription of genes: HAS3, FLG, IVL, and LOR | Enhanced skin hydration; improved the skin barrier integrity; xerosis treatment | Kim et al. [5] |
| Tannic acid | Re-epithelialization enhancement; forming an impermeable layer with skin proteins | Skin barrier restoration: a physical barrier against irritants | Nakamura et al. [100] |
6. The Impact of Product Composition on Barrier Efficacy
7. Future Perspectives and Application Prospects in the Barrier Testing Approach
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEA | N-acetylethanolamine |
| AKT | protein kinase B |
| BCS | biopharmaceutic classification system |
| FLG | filaggrin |
| HAS3 | hyaluronan synthase 3 |
| HMOX1 | heme oxygenase-1 |
| IVL | involucrin |
| LOR | loricrin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PEA | N-palmitoylethanolamine |
| PEG | polyethylene glycol |
| POX | paraoxon |
| PTFE | polytetrafluoroethylene |
| SCH | stratum corneum hydration |
| SLS | sodium lauryl sulfate |
| Src | proto-oncogene tyrosine protein kinase |
| TEWL | transepidermal water loss |
References
- Yu, G.; Lin, S.; Huang, X.; Gao, S.; Song, C.; Khalilov, F.; Chen, Q.; Issaro, N.; Xiao, J.; Xu, X.; et al. Expression of an epidermal growth factor-transdermal peptide fusion protein in Arabidopsis thaliana and its therapeutic effects on skin barrier repair. Front. Plant Sci. 2025, 16, 1573193. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Laneri, S.; Di Lorenzo, R.; Bernardi, A.; Sacchi, A.; Dini, I. Aloe barbadensis: A plant of nutricosmetic interest. Nat. Prod. Commun. 2020, 15, 1934578X20932744. [Google Scholar] [CrossRef]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical apigenin improves epidermal permeability barrier homeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef]
- Kim, S.; Rahim, M.A.; Tajdozian, H.; Barman, I.; Park, H.-A.; Yoon, Y.; Jo, S.; Lee, S.; Shuvo, M.S.H.; Bae, S.H.; et al. Clinical potential of novel microbial therapeutic LP51 based on xerosis-microbiome index. Cells 2024, 13, 2029. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Woo, K.; Hill, R.; LeBlanc, K.; Schultz, G.; Swanson, T.; Weir, D.; Mayer, D.O. Technological features of advanced skin protectants and an examination of the evidence base. J. Wound Care 2019, 28, 110–125. [Google Scholar] [CrossRef]
- Rawlings, A.V.; Lombard, K.J. A review on the extensive skin benefits of mineral oil. Int. J. Cosmet. Sci. 2012, 34, 511–518. [Google Scholar] [CrossRef]
- Labib, A.; Does, A.V.; Korbutov, J.; Yosipovitch, G. Silicone barrier cream in treatment of atopic dermatitis: A literature review. Dermatol. Ther. 2022, 35, e15884. [Google Scholar] [CrossRef]
- Casiraghi, A.; Ranzini, F.; Musazzi, U.M.; Franzè, S.; Meloni, M.; Minghetti, P. In vitro method to evaluate the barrier properties of medical devices for cutaneous use. Regul. Toxicol. Pharmacol. 2017, 90, 42–50. [Google Scholar] [CrossRef]
- Corazza, M.; Minghetti, S.; Bianchi, A.; Virgili, A.; Borghi, A. Barrier creams: Facts and controversies. Dermatitis 2014, 25, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kośnik, A.; Bagińska, Z.H.; Winnicka, K. Emulsions containing allantoin and D-panthenol as attractive moisturizing agents. Acta Pol. Pharm. 2023, 80, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Rego, L.; Martins, M.S.; Ferreira, M.S.; Cruz, M.T.; Sousa, E.; Almeida, I.F. How to Promote Skin Repair? In-Depth Look at Pharmaceutical and Cosmetic Strategies. Pharmaceuticals 2023, 16, 573. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Effect of barrier creams: Human skin in vivo. Contact Dermat. 1996, 35, 92–96. [Google Scholar] [CrossRef]
- Schoenfelder, H.; Liu, Y.; Lunter, D.J. Systematic investigation of factors, such as the impact of emulsifiers, which influence the measurement of skin barrier integrity by in-vitro trans-epidermal water loss (TEWL). Int. J. Pharm. 2023, 638, 122930. [Google Scholar] [CrossRef]
- Berkers, T.; Boiten, W.A.; Absalah, S.; van Smeden, J.; Lavrijsen, A.P.M.; Bouwstra, J.A. Compromising human skin in vivo and ex vivo to study skin barrier repair. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1103–1108. [Google Scholar] [CrossRef]
- Klotz, T.; Ibrahim, A.; Maddern, G.; Caplash, Y.; Wagstaff, M. Devices measuring transepidermal water loss: A systematic review of measurement properties. Ski. Res. Technol. 2022, 28, 497–539. [Google Scholar] [CrossRef]
- Frosch, P.J.; Kurte, A.; Pilz, B. Efficacy of skin barrier creams (III). The repetitive irritation test (RIT) in humans. Contact Dermat. 1993, 29, 113–118. [Google Scholar] [CrossRef]
- Berndt, U.; Wigger-Alberti, W.; Gabard, B.; Elsner, P. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermat. 2000, 42, 77–80. [Google Scholar] [CrossRef]
- Perrenoud, D.; Gallezot, D.; van Melle, G. The efficacy of a protective cream in a real-world apprentice hairdresser environment. Contact Dermat. 2001, 45, 134–138. [Google Scholar] [CrossRef]
- Sadhra, S.S.; Kurmi, O.P.; Mohammed, N.I.; Foulds, I.S. Protection afforded by controlled application of a barrier cream: A study in a workplace setting. Br. J. Dermatol. 2014, 171, 813–818. [Google Scholar] [CrossRef]
- Keurentjes, A.J.; Jakasa, I.; Kezic, S. Research Techniques Made Simple: Stratum Corneum Tape Stripping. J. Investig. Dermatol. 2021, 141, 1129–1133.e1. [Google Scholar] [CrossRef]
- Danby, S.G.; Chalmers, J.; Brown, K.; Williams, H.C.; Cork, M.J. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br. J. Dermatol. 2016, 175, 1011–1019. [Google Scholar] [CrossRef]
- Danby, S.G.; Andrew, P.V.; Brown, K.; Chittock, J.; Kay, L.J.; Cork, M.J. An Investigation of the Skin Barrier Restoring Effects of a Cream and Lotion Containing Ceramides in a Multi-vesicular Emulsion in People with Dry, Eczema-Prone, Skin: The RESTORE Study Phase 1. Dermatol. Ther. 2020, 10, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Roure, R.; Lanctin, M.; Nollent, V.; Bertin, C. Methods to assess the protective efficacy of emollients against climatic and chemical aggressors. Dermatol. Res. Pract. 2012, 2012, 864734. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, S.; Petri, M.; Elsner, P. Preventing irritant contact dermatitis with protective creams: Influence of the application dose. Contact Dermat. 2014, 70, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dykes, P.; Bradbury, S. Comparing the effectiveness and wash-off resistance of skin barrier creams: A healthy volunteer study. J. Wound Care 2017, 26, 552–557. [Google Scholar] [CrossRef]
- Kubota, T.; Watanabe, Y.; Takahashi, A.; Sekine, A. Usefulness of barrier function index based on water content and transepidermal water loss for evaluating efficacy of skin protective creams. Health Prim Car. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Shimizu, T.; Maibach, H.I. Squamometry: An evaluation method for a barrier protectant (tannic acid). Contact Dermat. 1999, 40, 189–191. [Google Scholar] [CrossRef]
- Yin, J.; Xie, J.; Lin, J.; Weng, C.; Lu, S.; Xu, P.; Zhang, S.; Luo, C.; Huang, Y.; Li, L.; et al. Evaluation of the efficacy of the anti-ulcer oral mucosal protective agent RADoralex® in the prevention and treatment of radiation-induced oral mucosal reactions induced during treatment of nasopharyngeal carcinoma. Cancer Biol. Ther. 2021, 23, 27–33. [Google Scholar] [CrossRef]
- Nasrollahi, H.; Khaki, S.; Ansari, M.; Ahmadloo, N.; Khanjani, N.; Hamedi, S.H.; Omidvari, S.; Mosalaei, A.; Mohammadianpanah, M.; Kadkhodaei, B. Evaluation of Mucosamin effect on treating radiation induced oral mucositis during and after radiotherapy amongst patients with oral cavity squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 3711–3715. [Google Scholar] [CrossRef]
- Cao, J.; Ye, L.; Li, X.; Song, Q.; Chai, Y. Early intervention with oral mucosal barrier protective agents in chronic oral graft-versus-host disease: A retrospective cohort study. BMC Oral Health 2024, 24, 958. [Google Scholar] [CrossRef] [PubMed]
- Ala, S.; Saeed, M.; Janbabai, G.; Ganji, R.; Azhdar, E.; Shiva, A. Efficacy of sucralfate mouth wash in prevention of 5-fluorouracil induced oral mucositis: A prospective, randomized, double-blind, controlled trial. Nutr. Cancer 2016, 68, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Soutome, S.; Yanamoto, S.; Kawashita, Y.; Yoshimatsu, M.; Murata, M.; Kojima, Y.; Funahara, M.; Umeda, M.; Saito, T. Effects of a bioadhesive barrier-forming oral liquid on pain due to radiation-induced oral mucositis in patients with head and neck cancer: A randomized crossover, preliminary study. J. Dent. Sci. 2021, 16, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Ambrad, A.A.; Arshoun, Y.; Carmel, R.J.; Ciuba, D.F.; Feldman, E.; Finkelstein, S.E.; Gandhavadi, R.; Heron, D.E.; Lane, S.C.; et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer 2014, 120, 1433–1440. [Google Scholar] [CrossRef]
- Zelenkova, H.; Kerob, D.; Salah, S.; Demessant-Flavigny, A.-L. Impact of daily use of emollient ‘plus’ on corticosteroid consumption in patients with atopic dermatitis: An open, randomized controlled study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 27–34. [Google Scholar] [CrossRef]
- Green, M.; Feschuk, A.M.; Kashetsky, N.; Maibach, H.I. “Normal” TEWL-how can it be defined? A systematic review. Exp. Dermatol. 2022, 31, 1618–1631. [Google Scholar] [CrossRef]
- Mayrovitz, H.N. Transepidermal water loss and stratum corneum hydration in forearm versus hand palm. Ski. Res. Technol. 2023, 29, e13218. [Google Scholar] [CrossRef]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.-C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]
- Rogiers, V. EEMCO Group EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Ski. Pharmacol. Appl. Ski. Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Ski. Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Huygen, L.; Thys, P.M.; Wollenberg, A.; Gutermuth, J.; Krohn, I.K. 46 Skin Barrier Function Assessment: Electrical Impedance Spectroscopy is Less Influenced by Daily Routine Activities Than Transepidermal Water Loss. J. Investig. Dermatol. 2023, 143, S340. [Google Scholar] [CrossRef]
- di Nardo, A.; Sugino, K.; Wertz, P.; Ademola, J.; Maibach, H.I. Sodium lauryl sulfate (SLS) induced irritant contact dermatitis: A correlation study between ceramides and in vivo parameters of irritation. Contact Dermat. 1996, 35, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Black, D.; Boyer, J.; Lagarde, J.M. Image analysis of skin scaling using D-Squame® samplers: Comparison with clinical scoring and use for assessing moisturizer efficacy. Int. J. Cosmet. Sci. 2006, 28, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, D.; Nokhandani, A.M.; Otaghsaraei, M.T.; Moghadamnia, Y.; Kazemi, S.; Moghadamnia, A.A. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiother. Oncol. 2016, 120, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yatsuoka, W.; Ishiki, H.; Miyano, K.; Uezono, Y. Effects of an oral mucosa protective formulation on chemotherapy- and/or radiotherapy-induced oral mucositis: A prospective study. BMC Cancer 2022, 22, 90. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y.; Xiang, Y.; Hu, T.; Cheng, R.; Cai, H. The efficacy of mouthwashes on oral microorganisms and gingivitis in patients undergoing orthodontic treatment: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 204. [Google Scholar] [CrossRef]
- Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. [Google Scholar] [CrossRef]
- Fowler, J.F. Efficacy of a skin-protective foam in the treatment of chronic hand dermatitis. Am. J. Contact Dermat. 2000, 11, 165–169. [Google Scholar] [CrossRef]
- Fowler, J.F. A Skin Moisturizing Cream Containing Quaternium-18-BentoniteEffectively Improves Chronic Hand Dermatitis. JCMS 2001, 5, 201–205. [Google Scholar] [CrossRef]
- Rowe, J.; McCall, E.; Kent, B. Clinical effectiveness of barrier preparations in the prevention and treatment of nappy dermatitis in infants and preschool children of nappy age. JBI Evid. Implement. 2008, 6, 3. [Google Scholar] [CrossRef]
- Mostosi, C.; Simonart, T. Effectiveness of barrier creams against irritant contact dermatitis. Dermatology 2016, 232, 353–362. [Google Scholar] [CrossRef]
- ISO 10993-2:2022; Biological Evaluation of Medical Devices, Part 2: Animal Welfare Requirements. International Organization for Standardization (ISO): Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/78866.html (accessed on 4 July 2025).
- Dachir, S.; Barness, I.; Fishbine, E.; Meshulam, J.; Sahar, R.; Eisenkraft, A.; Amir, A.; Kadar, T. Dermostyx (IB1)—High efficacy and safe topical skin protectant against percutaneous toxic agents. Chem. Biol. Interact. 2017, 267, 25–32. [Google Scholar] [CrossRef]
- Chilcott, R.P.; Dalton, C.H.; Hill, I.; Davison, C.M.; Blohm, K.L.; Clarkson, E.D.; Hamilton, M.G. Evaluation of a barrier cream against the chemical warfare agent VX using the domestic white pig. Basic. Clin. Pharmacol. Toxicol. 2005, 97, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Millerioux, J.; Cruz, C.; Bazire, A.; Lallement, G.; Lefeuvre, L.; Josse, D. In vitro selection and efficacy of topical skin protectants against the nerve agent VX. Toxicol. Vitr. 2009, 23, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Alonso, C.; Calpena, A.C.; Pérez-García, M.L.; Clares-Naveros, B.; Ramos, A.; Martí, M. Permeation protection by waterproofing mucosal membranes. Pharmaceutics 2023, 15, 2698. [Google Scholar] [CrossRef] [PubMed]
- Bignon, C.; Amigoni, S.; Devers, T.; Guittard, F. Barrier cream based on CeO2 nanoparticles grafted polymer as an active compound against the penetration of organophosphates. Chem. Biol. Interact. 2017, 267, 17–24. [Google Scholar] [CrossRef]
- Bassetto, R.; Perin, S.; Amadio, E.; Zanatta, S.; Nenzioni, D.; Bertin, W. Safety and efficacy of substance-based medical devices: Design of an in vitro barrier effect test. Front. Drug Saf. Regul. 2023, 3, 1124873–1124882. [Google Scholar] [CrossRef]
- Millerioux, J.; Cruz, C.; Bazire, A.; Polly, V.; Lallement, G.; Lefeuvre, L.; Josse, D. Evaluation of in vitro tests to assess the efficacy of formulations as topical skin protectants against organophosphorus compounds. Toxicol. Vitr. 2009, 23, 127–133. [Google Scholar] [CrossRef]
- Magnano, G.C.; Carton, F.; Boccafoschi, F.; Marussi, G.; Cocetta, E.; Crosera, M.; Adami, G.; Voinovich, D.; Larese Filon, F. Evaluating the role of protective creams on the cutaneous penetration of Ni nanoparticles. Environ. Pollut. 2023, 328, 121654. [Google Scholar] [CrossRef]
- Magnano, G.C.; Marussi, G.; Crosera, M.; Hasa, D.; Adami, G.; Lionetti, N.; Larese Filon, F. Probing the effectiveness of barrier creams against human skin penetration of nickel powder. Int. J. Cosmet. Sci. 2024, 46, 39–50. [Google Scholar] [CrossRef]
- Nimmansophon, P.; Wanasathop, A.; Li, S.K. Lateral transport during membrane permeation in diffusion cell: In Silico study on edge effect and membrane blocking. J. Pharm. Sci. 2023, 112, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Lane, M.E. Topical and transdermal delivery of caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Buist, H.; Craig, P.; Dewhurst, I.; Bennekou, H.S.; Kneuer, C.; Machera, K.; Pieper, C.; Court Marques, D.; Guillot, G.; et al. Guidance on dermal absorption. EFSA J. 2017, 15, e04873. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD). Guidance Notes on Dermal Absorption Studies. 2022. Available online: https://one.oecd.org/document/ENV/JM/MONO(2011)36/REV1/en/pdf (accessed on 4 September 2025).
- The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Basic Criteria for the in Vitro Assessment of Dermal Absorption of Cosmetic Ingredients. 2003. Available online: https://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out231_en.pdf (accessed on 4 September 2025).
- Wanasathop, A.; Choi, H.A.; Nimmansophon, P.; Murawsky, M.; Krishnan, D.G.; Li, S.K. Permeability of Fresh and Frozen Porcine and Human Gingiva and the Effect of Storage Duration. Pharmaceutics 2023, 15, 1492. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Ski. Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef]
- Herbig, M.E.; Houdek, P.; Gorissen, S.; Zorn-Kruppa, M.; Wladykowski, E.; Volksdorf, T.; Grzybowski, S.; Kolios, G.; Willers, C.; Mallwitz, H.; et al. A custom tailored model to investigate skin penetration in porcine skin and its comparison with human skin. Eur. J. Pharm. Biopharm. 2015, 95, 99–109. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Effect of Frozen Human Epidermis Storage Duration and Cryoprotectant on Barrier Function using Two Model Compounds. Ski. Pharmacol. Physiol. 2016, 29, 31–40. [Google Scholar] [CrossRef]
- Harrison, S.M.; Barry, B.W.; Dugard, P.H. Effects of freezing on human skin permeability. J. Pharm. Pharmacol. 1984, 36, 261–262. [Google Scholar] [CrossRef]
- Lane, M.E. In vitro permeation testing for the evaluation of drug delivery to the skin. Eur. J. Pharm. Sci. 2024, 201, 106873. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- di Cagno, M.; Bibi, H.; Bauer-Brandl, A. New Biomimetic Barrier Permeapad(TM) for Efficient Investigation of Passive Permeability of Drugs. Eur. J. Pharm. Sci. 2024, 73, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.; Silva, C.; Chaves, L. Tissue-Based In Vitro and Ex Vivo Models for Intestinal Permeability Studies. In Concepts and Models for Drug Permeability Studies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 203–236. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2023; Volume 1.
- Chedik, L.; Baybekov, S.; Cosnier, F.; Marcou, G.; Varnek, A.; Champmartin, C. An update of skin permeability data based on a systematic review of recent research. Sci. Data 2024, 11, 224. [Google Scholar] [CrossRef]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the Influence of Alcohol: The Effect of Ethanol and Methanol on Lipid Bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef]
- Benfeldt, E.; Serup, J.; Menné, T. Effect of barrier perturbation on cutaneous salicylic acid penetration in human skin: In vivo pharmacokinetics using microdialysis and non-invasive quantification of barrier function. Br. J. Dermatol. 1999, 140, 739–748. [Google Scholar] [CrossRef]
- Kasting, G.B.; Miller, M.A.; Xu, L.; Yu, F.; Jaworska, J. In Vitro Human Skin Absorption of Solvent-deposited Solids: Niacinamide and Methyl Nicotinate. J. Pharm. Sci. 2022, 111, 727–733. [Google Scholar] [CrossRef]
- Wigger-Alberti, W.; Elsner, P. Petrolatum prevents irritation in a human cumulative exposure model in vivo. Dermatology 1997, 194, 247–250. [Google Scholar] [CrossRef]
- Chilcott, R.P.; Jenner, J.; Hotchkiss, S.A.M.; Rice, P. Evaluation of Barrier Creams against Sulphur Mustard. Ski. Pharmacol. Physiol. 2002, 15, 225–235. [Google Scholar] [CrossRef]
- Treffel, P.; Gabard, B.; Juch, R. Evaluation of barrier creams: An in vitro technique on human skin. Acta Derm. Venereol. 1994, 74, 7–11. [Google Scholar] [CrossRef]
- Lau, W.M.; Ng, K.W. Finite and Infinite Dosing. In Percutaneous Penetration Enhancers Drug Penetration in-to/Through the Skin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–44. [Google Scholar] [CrossRef]
- Alépée, N.; Grandidier, M.-H.; Cotovio, J. Usefulness of the EpiSkinTM reconstructed human epidermis model within Integrated Approaches on Testing and Assessment (IATA) for skin corrosion and irritation. Toxicol. Vitr. 2019, 54, 147–167. [Google Scholar] [CrossRef]
- Netzlaff, F.; Lehr, C.-M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin, SkinEthic and EpiDerm: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A.; Ptaszek, A.; Kruk, J. Effective tool for assessment of the quality of barrier creams—Relationships between rheological, textural and sensory properties. Regul. Toxicol. Pharmacol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Goffin, V.; Piérard-Franchimont, C.; Piérard, G.E. Shielded corneosurfametry and corneoxenometry: Novel bioassays for the assessment of skin barrier products. Dermatology 1998, 196, 434–437. [Google Scholar] [CrossRef]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In vitro evaluation of barrier function against oral bacteria of dense and expanded polytetrafluoroethylene (PTFE) membranes for guided bone regeneration. Clin. Implant. Dent. Relat. Res. 2018, 20, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Antonijević, M.; Novac, O.; O’Hagan, B. Can emollients of similar composition be assumed to be therapeutically. Clin. Cosmet. Investig. Dermatol. 2018, 11, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, X.-M.; Guichard, A.; Tan, Y.-M.; Qian, C.-Y.; Yang, L.-J.; Humbert, P. N-palmitoylethanolamine and N-acetylethanolamine are effective in asteatotic eczema: Results of a randomized, double-blind, controlled study in 60 patients. Clin. Interv. Aging 2014, 9, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Madnani, N.; Deo, J.; Dalal, K.; Benjamin, B.; Murthy, V.V.; Hegde, R.; Shetty, T. Revitalizing the skin: Exploring the role of barrier repair moisturizers. J. Cosmet. Dermatol. 2024, 23, 1533–1540. [Google Scholar] [CrossRef]
- McCormick, E.; Han, H.; Azim, S.A.; Whiting, C.; Bhamidipati, N.; Kiss, A.; Efimova, T.; Berman, B.; Friedman, A. Topical nanoencapsulated cannabidiol cream as an innovative strategy combating UV-A–induced nuclear and mitochondrial DNA injury: A pilot randomized clinical study. J. Am. Acad. Dermatol. 2024, 91, 855–862. [Google Scholar] [CrossRef]
- Zonari, A.; Brace, L.E.; Buhrer, L.B.; Harder, N.H.O.; Harker, C.; Aronson, A.B.; Tse, C.N.; Oliveira, C.R.; Boroni, M.; Carvalho, J.L. OS-01 Peptide Topical Formulation Improves Skin Barrier Function and Reduces Systemic Inflammation Markers: A Pilot 12-Week Clinical Trial. J. Cosmet. Dermatol. 2025, 24, e70169. [Google Scholar] [CrossRef]
- Wagemaker, T.A.L.; Maia Campos, P.M.B.G.; Shimizu, K.; Kyotani, D.; Yoshida, D. Antioxidant-based topical formulations influence on the inflammatory response of Japanese skin: A clinical study using non-invasive techniques. Eur. J. Pharm. Biopharm. 2017, 117, 195–202. [Google Scholar] [CrossRef]
- Zeichner, J.; Bussmann, T.; Weise, J.M.; Maass, E.; Krüger, A.; Schade, A.-K.; Lain, E.; Mariwalla, K.; Kirchner, F.; Draelos, Z.D. Evaluation of Antioxidants’ Ability to Enhance Hyaluronic-acid Based Topical Moisturizers. J. Clin. Aesthet. Dermatol. 2024, 17, 48–51. [Google Scholar] [CrossRef]
- Lee, J.-O.; Hwang, S.-H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yoshida, N.; Yasoshima, M.; Kojima, Y. Effect of tannic acid on skin barrier function. Exp. Dermatol. 2018, 27, 824–826. [Google Scholar] [CrossRef] [PubMed]
- Kurpiewska, J.; Liwkowicz, J. The composition of waterproof barrier creams’ ingredients and their barrier properties. Chemik. 2012, 66, 991–996. [Google Scholar]
- Martini, J.; Huertas, C.; Turlier, V.; Saint-Martory, C.; Delarue, A. Efficacy of an emollient cream in the treatment of xerosis in diabetic foot: A double-blind, randomized, vehicle-controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 743–747. [Google Scholar] [CrossRef]
- Spada, F.; Harrison, I.P.; Barnes, T.M.; Greive, K.A.; Daniels, D.; Townley, J.P.; Mostafa, N.; Fong, A.T.; Tong, P.L.; Shumack, S. A daily regimen of a ceramide-dominant moisturizing cream and cleanser restores the skin permeability barrier in adults with moderate eczema: A randomized trial. Dermatol. Ther. 2021, 34, e14970. [Google Scholar] [CrossRef]
- Brunina, L. Bridging the gap: Evaluating emollients and emulsifiers in dermatology for long-term skin health and barrier recovery. Medicni Perspekt. 2025, 30, 149–155. [Google Scholar] [CrossRef]
- Reuter, M.; Schoenfelder, H.; Gaiser, A.; Volc, S.; Lunter, D. Emulsifier-Induced changes to the human skin barrier: Connection to ceramide profiles and assessment as a skin lesion model. Ski. Pharmacol. Physiol. 2025, 38, 79–91. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, J.; Jeong, E.T.; Hwang, S.J.; Kang, N.; Lee, J. Penetration rates into the stratum corneum layer: A novel quantitative indicator for assessing skin barrier function. Ski. Res. Technol. 2024, 30, e13655. [Google Scholar] [CrossRef]
- Tsai, J.C.; Sheu, H.M.; Hung, P.L.; Cheng, C.L. Effect of barrier disruption by acetone treatment on the permeability of compounds with various lipophilicities: Implications for the permeability of compromised skin. J. Pharm. Sci. 2001, 9, 1242–1254. [Google Scholar] [CrossRef]
- Alsamad, F.; Stamatas, G.N. Directional assessment of the skin barrier function in vivo. Ski. Res. Technol. 2023, 29, e13346. [Google Scholar] [CrossRef]
- Chilcott, R.P.; Dalton, C.H.; Emmanuel, A.J.; Allen, C.E.; Bradley, S.T. Transepidermal water loss does not correlate with skin barrier function in vitro. J. Invest. Dermatol. 2002, 118, 871–875. [Google Scholar] [CrossRef]
- Koseki, K.; Kawakami, E.; Kawasaki, H.; Atsugi, T.; Nakanishi, M.; Mizuno, M.; Naru, E.; Ebihara, T.; Amagai, M. 360 Assessment of skin barrier function from skin images with topological data analysis. J. Invest. Dermatol. 2019, 139, S62. [Google Scholar] [CrossRef]



| Method of Barrier Efficacy | Participant /Disease | Control Group | Type of Barrier Product | Reference |
|---|---|---|---|---|
| TEWL and SCH test | Volunteers with quiescent atopic dermatitis | No treatment, no skin protection | Skin care emollient cream (petrolatum, paraffin, silicone polymer) Commercial emollient gel (isopropyl myristate, paraffin, carbomer) | Danby et al. [23] |
| SCH test | Volunteers with eczema-prone skin | Reference emollient creams (skin care products) | Skin care cream and lotion containing ceramides (1, 3, and 6-II), cholesterol, and triglycerides | Danby et al. [24] |
| TEWL test | Healthy volunteers | Petrolatum ointment | Skin care creams differed in water content (20–70%, w/w) | Casiraghi et al. [10] |
| TEWL test | Healthy volunteers | No treatment, no skin protection | Skin care emollient with glycerin and sodium glycine | Roure et al. [25] |
| TEWL and SCH tests | Healthy participants with induced contact dermatitis | No treatment, no skin protection | Cream A (aluminium chlorohydrate, paraffin, urea, petrolatum, beeswax, cholesterol, zinc stearate, lanolin) Cream B (petrolatum, paraffin, glycerin, cera alba, zinc stearate) Cream C (kaolin, paraffin, petrolatum, oxozinc) | Schliemann et al. [26] |
| Resistance to wash-off, SCH tests | Healthy volunteers | No treatment, no skin protection | Commercial skin care silicone-based products | Dykes et al. [27] |
| TEWL and skin hydration tests | Healthy volunteers | No treatment, no skin protection | Formulated skin care creams (glycerin glyceryl isostearate, isopropyl myristate, squalene, retinol palmitate, ascorbyl tetraisopalmitate, stearic acid, simethicone) with or without film-forming agents (poly-perfluoromethyl isopropyl ether, silsesquioxane, myristoyl pullulan) | Kubota et al. [28] |
| Squamometric analysis | Healthy volunteers | No treatment, no skin protection | Tannic acid solution | Shimizu et al. [29] |
| Clinical scoring | Oncologic patients with radiation-induced mucositis | Sodium bicarbonate solution | Mouthwash (no information about composition) | Yin et al. [30] |
| Clinical scoring | Oncologic patients with radiotherapy-induced mucositis | Combination of nystatin, diphenhydramine, magnesium, and aluminum hydroxide | Commercial oral spray (medical device, sodium hyaluronate, glycine, L-leucine, L-lysine) | Nasrollahi et al. [31] |
| Clinical scoring, salivary flow | Patients with chronic oral graft-versus-host disease | No treatment | Commercial oromucosal preparation (medical device containing polyvinylpyrrolidone, trisodium glycyrrhizinate) | Cao et al. [32] |
| Clinical scoring | Oncologic patients with chemotherapy-induced mucositis | No treatment | Mouthwash with sucralfate (drug product) | Ala et al. [33] |
| Clinical scoring | Oncologic patients with radiation-induced mucositis | Dexamethasone cream | Commercial oral liquid (medical device comprising soy phosphatidylcholine and glycerol dioleate) | Soutome et al. [34] |
| Clinical scoring | Oncologic patients with chemotherapy-induced mucositis | Saline solution | Commercial oral carbomer-based hydrogel (medical device) | Allison et al. [35] |
| Clinical scoring | Patients with atopic dermatitis | Conventional emollient cream (no information about composition) | Commercial skin care product with Aqua posae filiformis, canola oil, niacinamide | Zeleknova et al. [36] |
| Volunteer-Linked Variables | Recommendations |
|---|---|
| Sex, age, race |
|
| Anatomical side |
|
| Skin surface temperature and sweating |
|
| Skin damage/disease |
|
| Daily routine |
|
| Circadian rhythm |
|
| Product removal procedure |
|
| Skin cleansing |
|
| Environmental variables | |
| Temperature and humidity |
|
| Light source |
|
| Air circulation |
|
| Single experiment |
|
| Repeated or long-term experiments |
|
| Instrumental variables | |
| Measuring probe |
|
| Analysis time | As short as possible to avoid occlusion Zero value should be displayed before measurement |
| Membrane Model | Type of Irritant Agent | Control | Type of Barrier Product | Reference |
|---|---|---|---|---|
| Human abdominal skin, porcine ear skin, silicone membrane | Warfare agent XV | Unprotected membranes | Non-proprietary skin care cream with perfluorinated compounds; Commercial emulsion o/w with silicone, perfluorinated polymers, polyvinylpyrrolidone, glyceryl and PEG-100 stearate; Skin care emulsion w/o with polyvinylpyrrolidone, polyquaternium 51, and cetyl dimeticone copolyol | Millerioux et al. [56] |
| Porcine sublingual epithelium, polycarbonate membrane | Caffeine, ibuprofen, dexamethasone, ivermectin | Unprotected membranes | Hydrophobic skin care formulations (based on petrolatum, lecithin, isopropyl myristate, or medium chain triglycerides); Hydrophilic skin care formulations (with chitosan, CMC, poloxamer, alginate sodium, or hyaluronate sodium); Liposomal formulations (with ceramides-3, -6, cholesterol, or phosphatidylcholine) | Coderch et al. [57] |
| Human skin, Strat-M, silicone membrane | Paraoxon | Unprotected membranes | Non-proprietary cream formulations with cerium oxide nanoparticles grafted to methacrylic acid, 2,2,2-trifluoroethyl methacrylate | Bignon et al. [58] |
| Cellulose membrane impregnated with phospholipids, cholesterol, and n-octanol | Caffeine, acyclovir | Unprotected membrane (negative control), membrane covered with petrolatum (positive control) | Oral hydrogel with xyloglucan, aloe vera extract, glycerol, and polyvinylpyrrolidone (medical device) | Bassetto et al. [59] |
| Porcine ear skin, silicone membrane | Paraoxon | Unprotected membranes | Non-proprietary cream with perfluorinated compounds (medical device); Emulsion o/w with silicone, perfluorinated polymers, polyvinylpyrrolidone, glyceryl and PEG-100 stearate; Emulsion w/o with polyvinylpyrrolidone, polyquaternium 51, and cetyl dimeticone copolyol | Millerioux et al. [60] |
| Porcine ear skin | Warfare agent VX | Unprotected membrane | Cream with polyperfluoromethylisopropyl ether (FomblinTM HC/R) and polytetrafluoroethylene | Chilcott et al. [55] |
| Human abdominal skin | Nickel nanoparticles | Unprotected membranes; membrane covered with vehicle (without chelating agent) | Commercial skin care product with, e.g., pentetic acid, cetostearyl alcohol, chitosan, paraffin; Commercial cream formulation with, e.g., ceramide 3, palmitamide MEA, cholesterol, squalane, and xanthan gum | Magnano et al. [61] |
| Human abdominal skin | Nickel powder | Unprotected membranes | Commercial skin care product with, e.g., pentetic acid, cetostearyl alcohol, chitosan, paraffin; Commercial cream formulation with, e.g., ceramide 3, palmitamide MEA, cholesterol, squalane, and xanthan gum | Magnano et al. [62] |
| 3D skin model Episkin™ | Caffeine | Membrane covered with petrolatum | Commercial cream products differed in composition and water content (20–70%, w/w) | Casiraghi et al. [10] |
| Variables | Recommendations |
|---|---|
| Membrane-linked variables | |
| Tissue sample |
|
| Synthetic membrane |
|
| Process parameters | |
| Acceptor solution |
|
| Model chemical agent |
|
| Data presentation |
|
| Control studies |
|
| Instrumental variables | |
| Measuring system |
|
| Analysis time and sampling schedule |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagińska, Z.H.; Szymańska, E. Barrier Products for Topical Delivery—Insight into Efficacy Testing and Barrier-Boosting Compounds. Pharmaceutics 2025, 17, 1361. https://doi.org/10.3390/pharmaceutics17111361
Bagińska ZH, Szymańska E. Barrier Products for Topical Delivery—Insight into Efficacy Testing and Barrier-Boosting Compounds. Pharmaceutics. 2025; 17(11):1361. https://doi.org/10.3390/pharmaceutics17111361
Chicago/Turabian StyleBagińska, Zofia Helena, and Emilia Szymańska. 2025. "Barrier Products for Topical Delivery—Insight into Efficacy Testing and Barrier-Boosting Compounds" Pharmaceutics 17, no. 11: 1361. https://doi.org/10.3390/pharmaceutics17111361
APA StyleBagińska, Z. H., & Szymańska, E. (2025). Barrier Products for Topical Delivery—Insight into Efficacy Testing and Barrier-Boosting Compounds. Pharmaceutics, 17(11), 1361. https://doi.org/10.3390/pharmaceutics17111361








