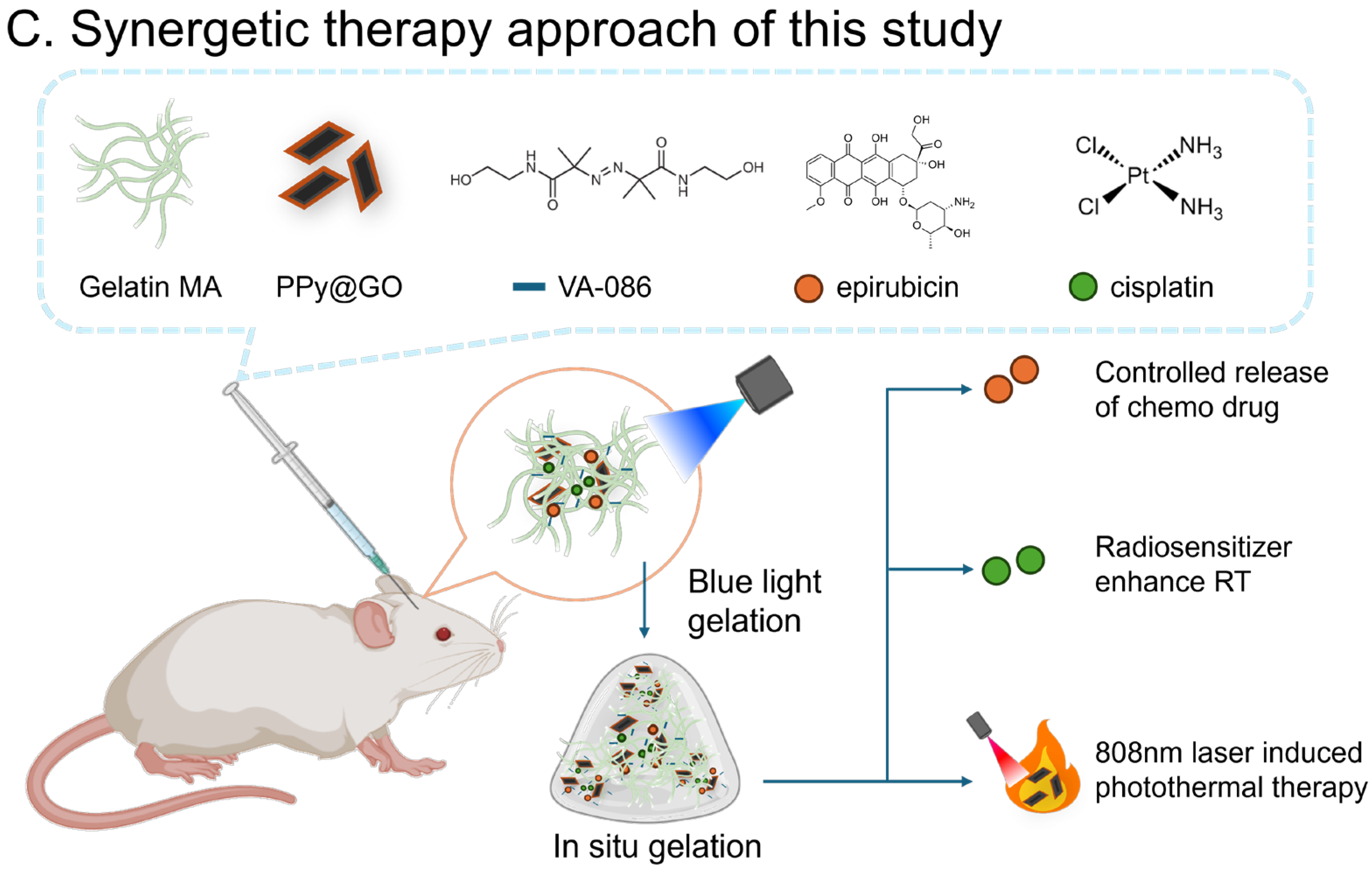
Gelatin-Based Rapid Blue Light-Irradiation In Situ Gelation Hydrogel Platform for Combination Therapy in Brain Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation
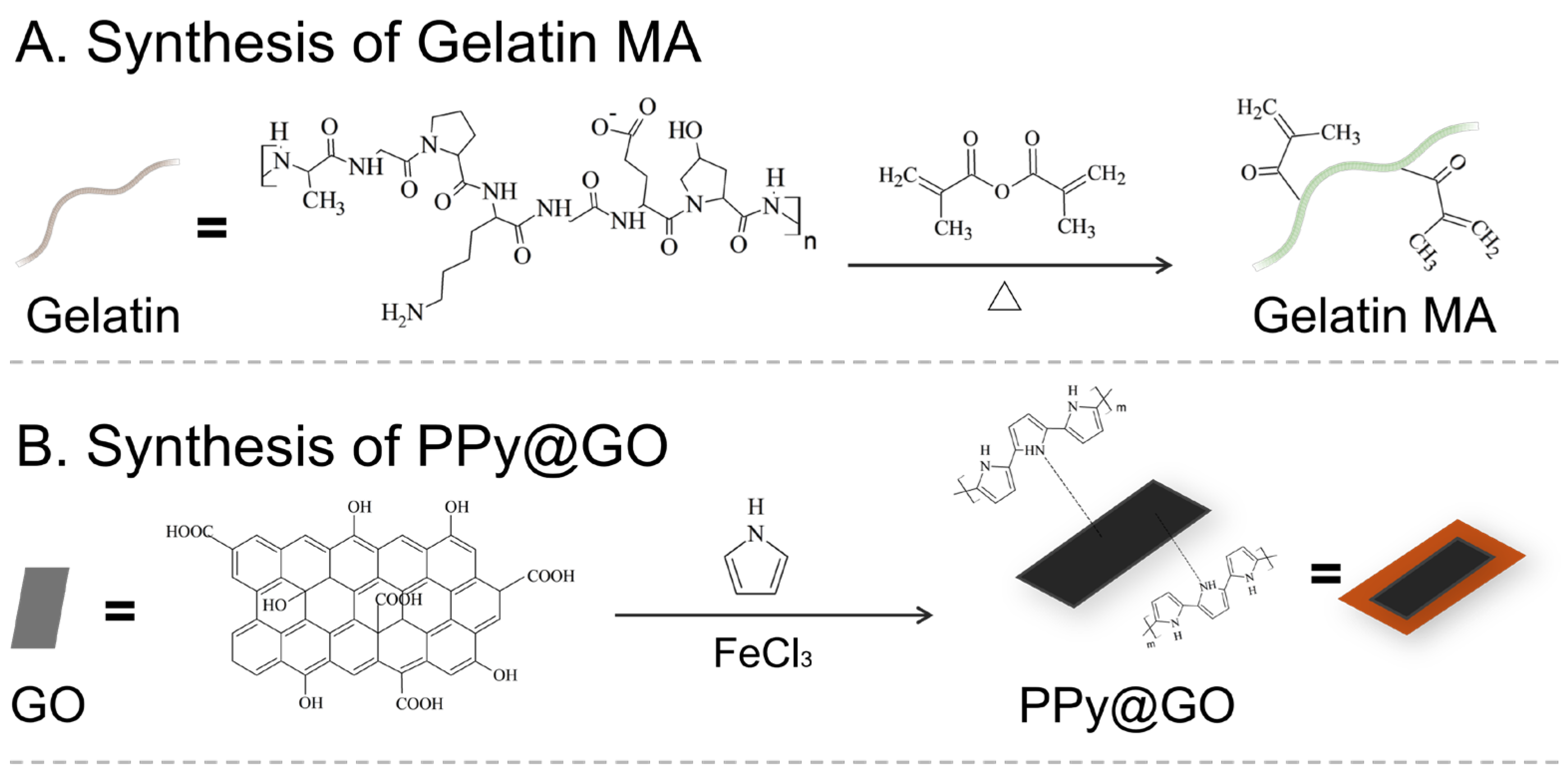
2.1.1. Gelatin Modification and Purification
2.1.2. Synthesis of Photothermal Material
Synthesis of Bovine Serum Albumin@Graphene (BSA@GO)
Synthesis of Polypyrrole@GO
2.1.3. Preparation of Drug and Material Solutions:
2.1.4. Light-Initiated Gelation
2.2. Characterization and Analysis of Hydrogel
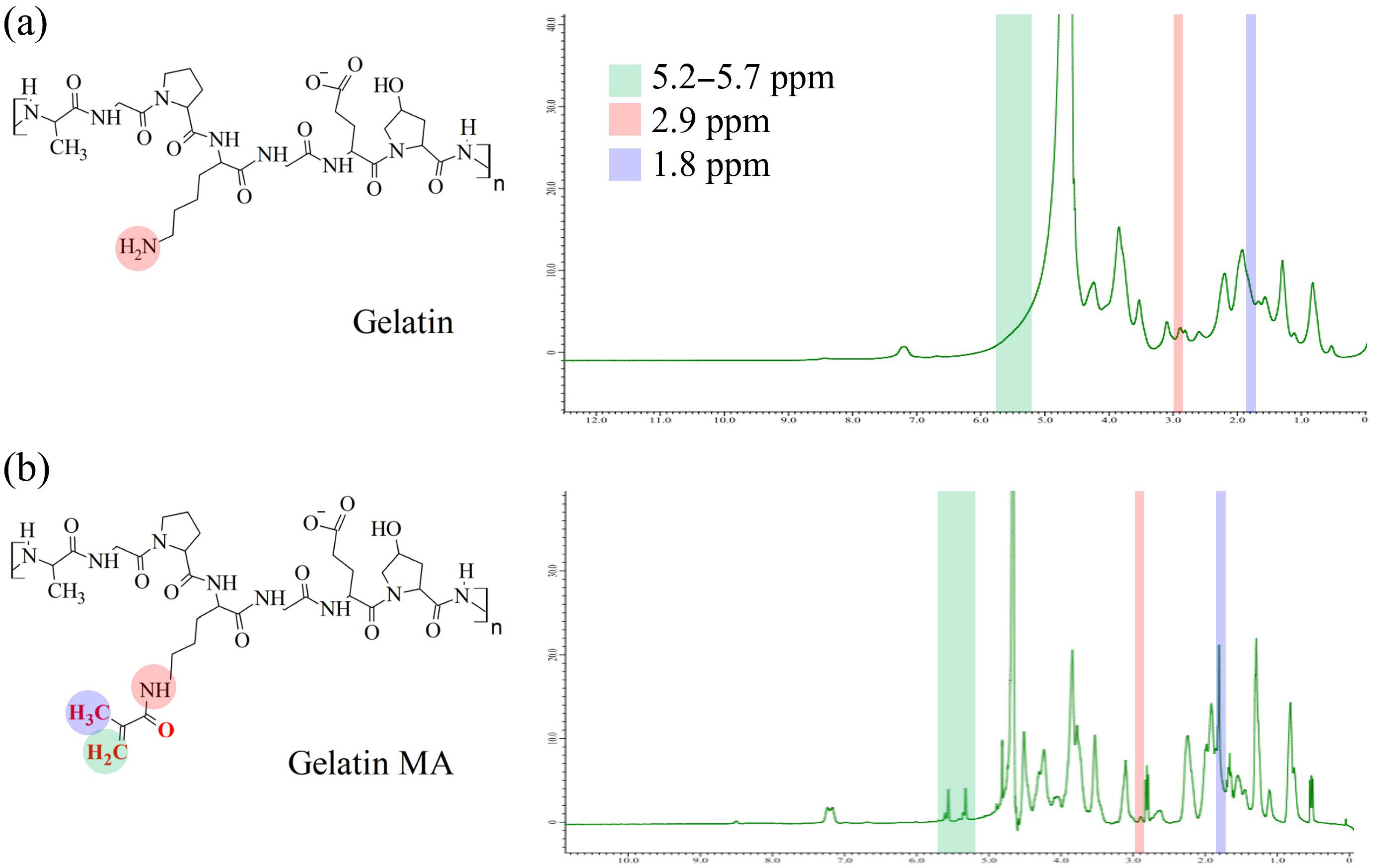
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Gelation Time Study
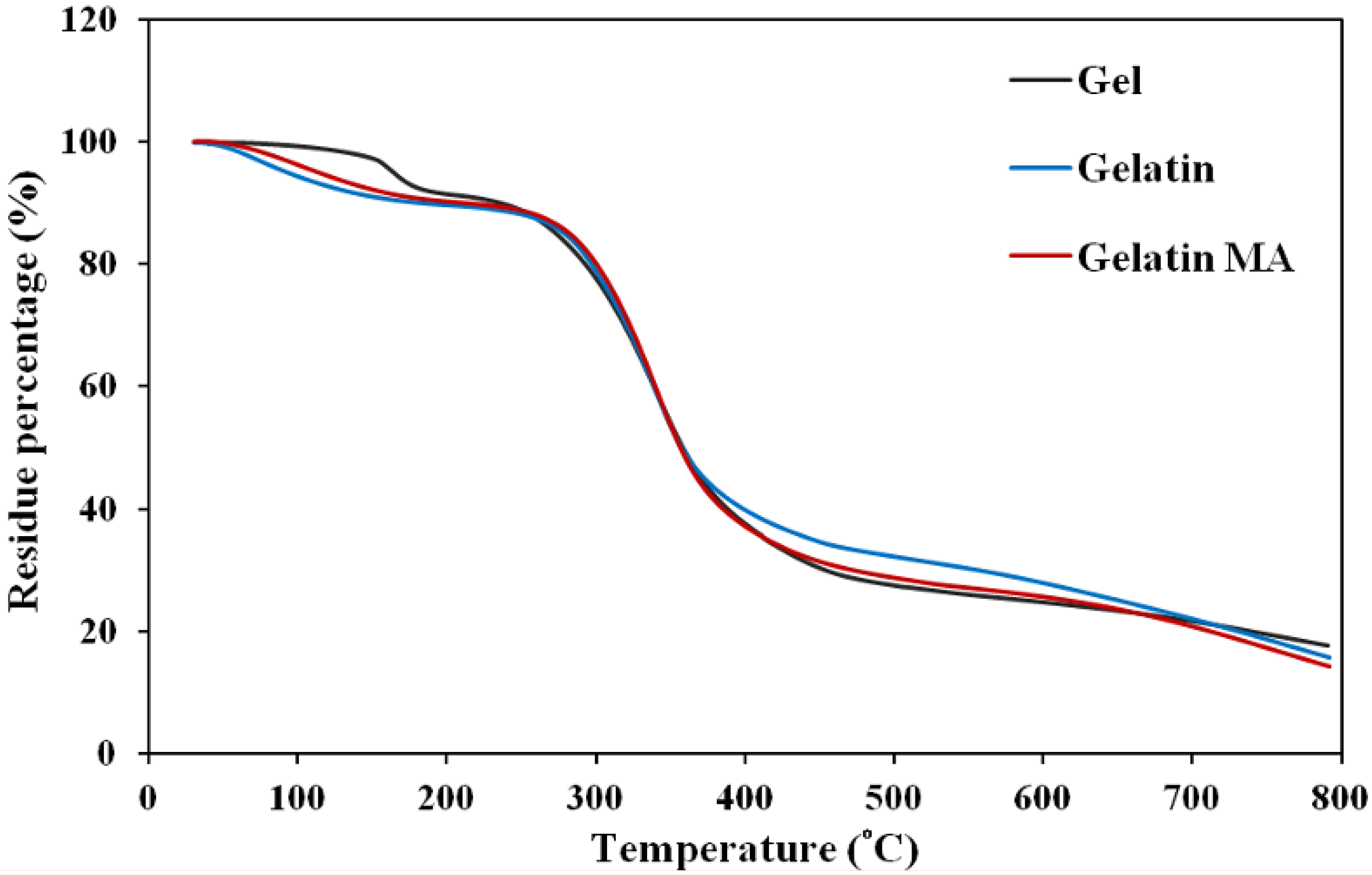
2.2.4. Thermogravimetric Analysis (TGA)
2.3. Photothermal Material Characterization and Analysis
2.3.1. Absorption Spectrum of PPy@GO
2.3.2. Transmission Electron Microscopy (TEM) Imaging
2.3.3. Evaluation of Photothermal Property
2.3.4. In Vitro Cytotoxicity of PPy@GO
2.4. Drug Release and Cytotoxicity of Drug-Loaded Hydrogels
2.4.1. EPI Release Profile
2.4.2. Cytotoxicity Assay
2.4.3. Fluorescence Imaging
2.5. Animal Experiments
2.5.1. In Vivo Gelation of Gelatin MA
2.5.2. In Vivo Photothermal Effect Study
2.5.3. Tumor Suppression Ability of Drug-Loaded Hydrogel In Vivo
3. Results and Discussion
3.1. Hydrogel Characterization and Analysis
3.1.1. Basic Properties
Chemical Structure Analysis
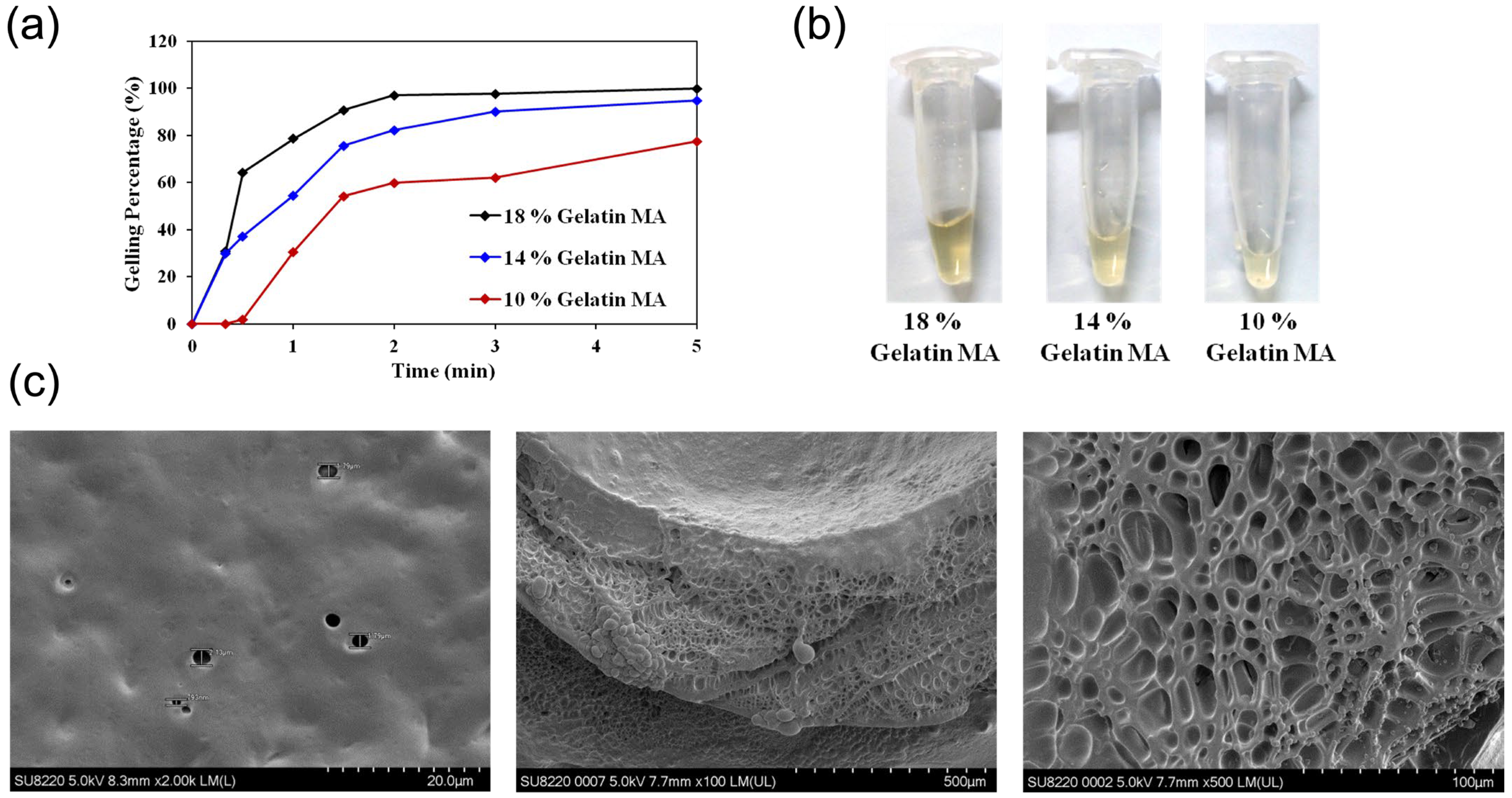
Gelation Test
3.1.2. Photothermal Material Analysis
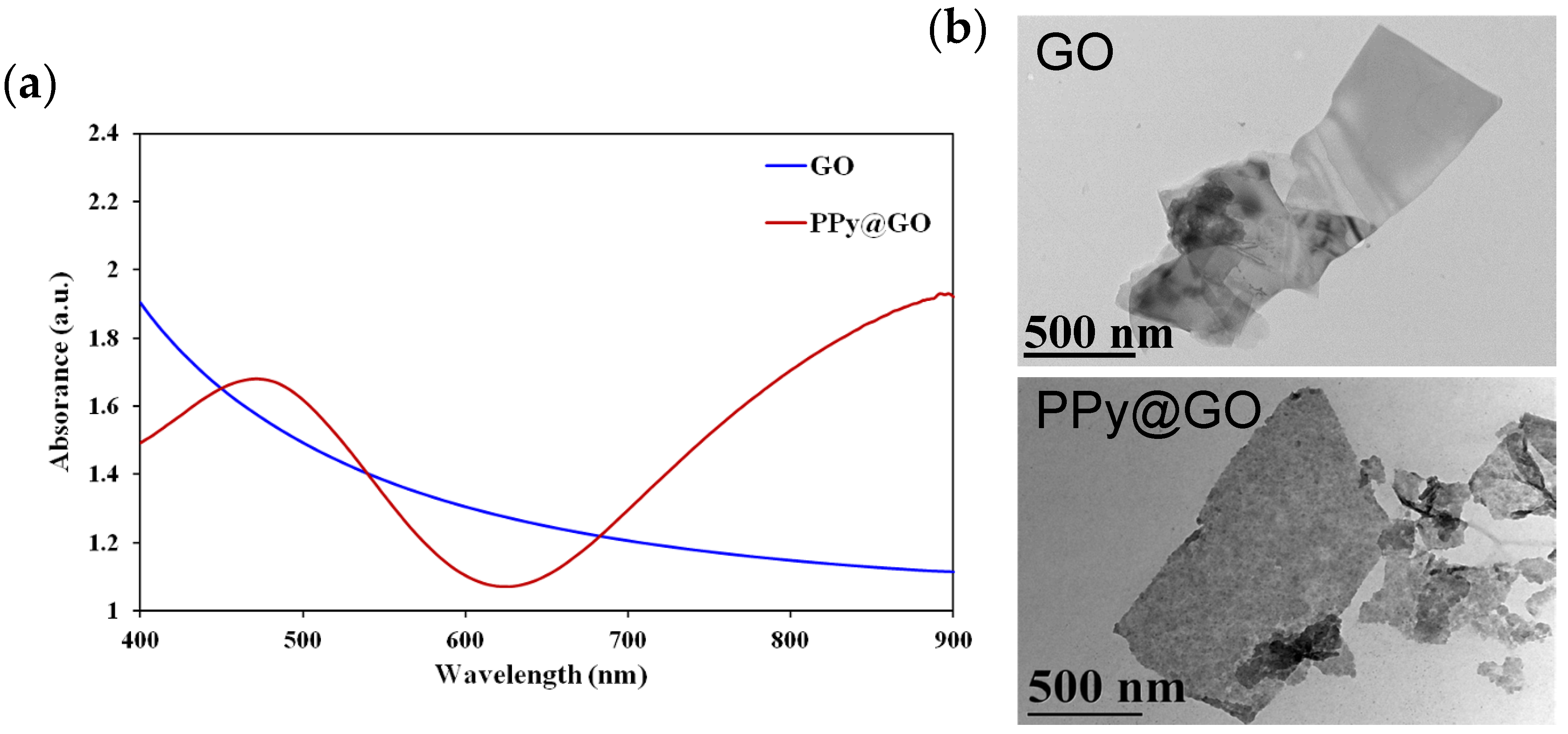
Physicochemical Characterization
In Vitro Photothermal Performance Test
Cell Cytotoxicity
3.2. Drug Release and Cytotoxicity of Drug-Loaded Hydrogels
3.2.1. Drug Release Profile
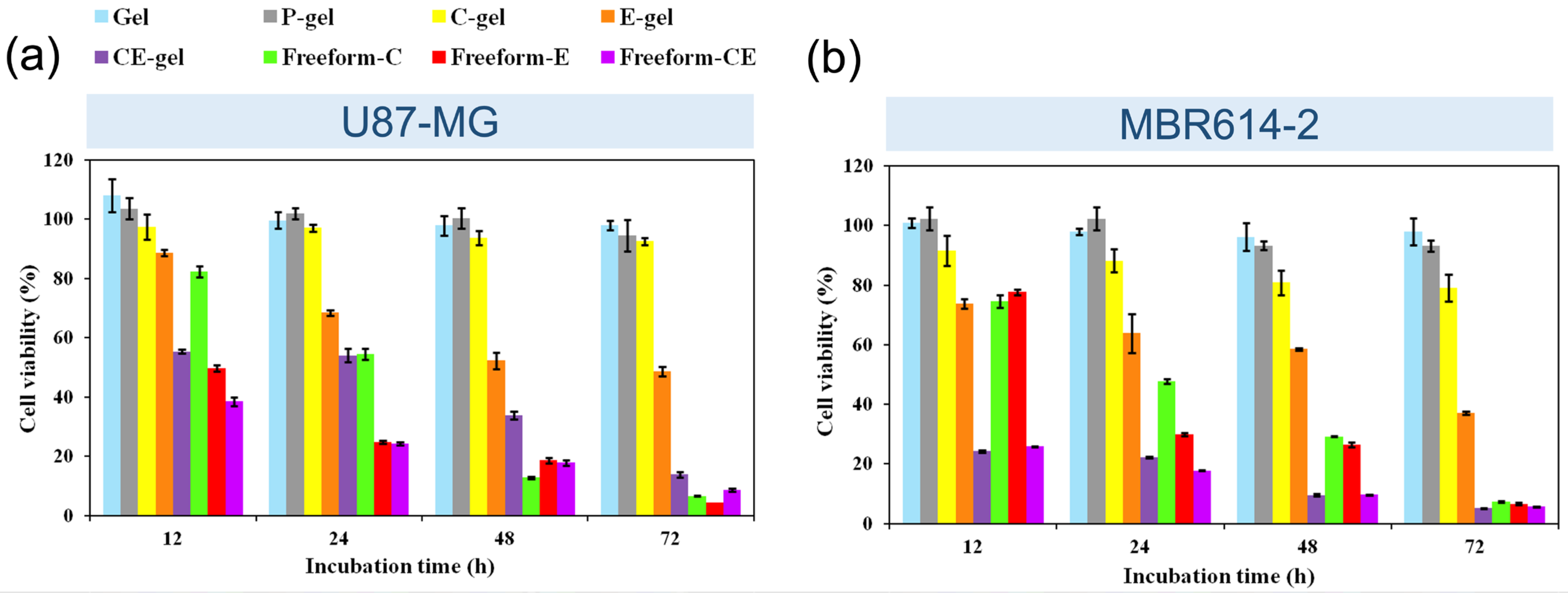
3.2.2. Cytotoxicity Assay
3.2.3. Cellular Fluorescence Imaging
3.3. Animal Experiments Results
3.3.1. In Vivo Gelation Test
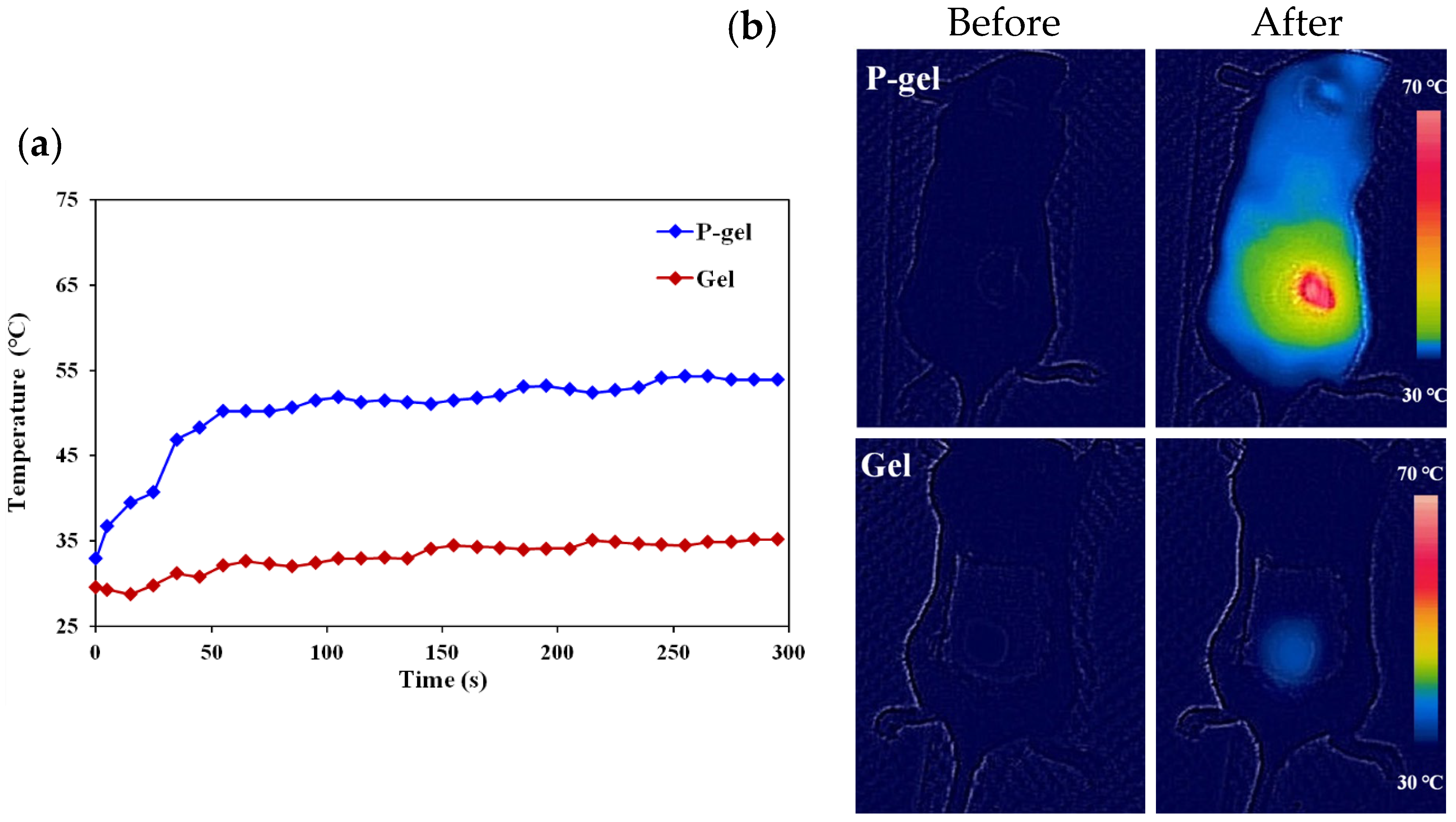
3.3.2. In Vivo Study of Photothermal Effect
3.3.3. Antitumor Efficacy of Hydrogels Loaded with Drugs and Photothermal Material
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovacs, A. Glioblastoma: Clinical Presentation, Multidisciplinary Management, and Long-Term Outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef]
- Seker-Polat, F.; Pinarbasi Degirmenci, N.; Solaroglu, I.; Bagci-Onder, T. Tumor Cell Infiltration into the Brain in Glioblastoma: From Mechanisms to Clinical Perspectives. Cancers 2022, 14, 443. [Google Scholar] [CrossRef]
- Vaz-Salgado, M.A.; Villamayor, M.; Albarran, V.; Alia, V.; Sotoca, P.; Chamorro, J.; Rosero, D.; Barrill, A.M.; Martin, M.; Fernandez, E.; et al. Recurrent Glioblastoma: A Review of the Treatment Options. Cancers 2023, 15, 4279. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, M.J.; Jung, H.H.; Chang, W.S.; Choi, H.S.; Rachmilevitch, I.; Zadicario, E.; Chang, J.W. One-Year Outcome of Multiple Blood–Brain Barrier Disruptions With Temozolomide for the Treatment of Glioblastoma. Front. Oncol. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Xiao, J.; Liu, Y. Nanomedicine-based combination therapies for overcoming temozolomide resistance in glioblastomas. Cancer Biol. Med. 2023, 20, 325–343. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Kobayashi, K.; Usami, N.; Porcel, E.; Lacombe, S.; Le Sech, C. Enhancement of radiation effect by heavy elements. Mutat. Res. Rev. Mutat. Res. 2010, 704, 123–131. [Google Scholar] [CrossRef]
- Setua, S.; Ouberai, M.; Piccirillo, G.S.; Watts, C.; Welland, M. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale 2014, 6, 10865–10873. [Google Scholar] [CrossRef]
- Marcu, L.; van Doorn, T.; Olver, I. Cisplatin and Radiotherapy in the Treatment of Locally Advanced Head and Neck Cancer. Acta Oncol. 2003, 42, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Cottin, Y.; Touzery, C.; Dalloz, F.; Coudert, B.; Toubeau, M.; Riedinger, A.; Louis, P.; Wolf, J.E.; Brunotte, F. Comparison of epirubicin and doxorubicin cardiotoxicity induced by low doses: Evolution of the diastolic and systolic parameters studied by radionuclide angiography. Clin. Cardiol. 1998, 21, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, L.; Belli, F.; Arena, M.G.; Carpano, S.; Paoletti, G.; Giannarelli, D.; Lopez, M. Epirubicin, cisplatin and docetaxel combination therapy for metastatic gastric cancer. Ann. Oncol. 2005, 16, 1498–1502. [Google Scholar] [CrossRef]
- Nielsen, D.; Dombernowsky, P.; Larsen, S.K.; Hansen, O.P.; Skovsgaard, T. Epirubicin or epirubicin and cisplatin as first-line therapy in advanced breast cancer. A phase III study. Cancer Chemother. Pharmacol. 2000, 46, 459–466. [Google Scholar] [CrossRef]
- Fuith, L.C.; Bazzanella, A.; Hetzel, H. Combination chemotherapy with epirubicin and cisplatin in ovarian carcinoma. Arch. Gynecol. Obstet. 1988, 244, 15–21. [Google Scholar] [CrossRef]
- Kanat, O.; Ertas, H.; Caner, B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J. Clin. Oncol. 2017, 8, 329–335. [Google Scholar] [CrossRef]
- Ryberg, M.; Nielsen, D.; Skovsgaard, T.; Hansen, J.; Jensen, B.V.; Dombernowsky, P. Epirubicin cardiotoxicity: An analysis of 469 patients with metastatic breast cancer. J. Clin. Oncol. 1998, 16, 3502–3508. [Google Scholar] [CrossRef]
- Sultana, N.; Pathak, R.; Samanta, S.; Sen Sarma, N. A comprehensive analysis of photothermal therapy (PTT) and photodynamic therapy (PDT) for the treatment of cancer. Process Biochem. 2025, 148, 17–31. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef]
- Lu, W.; Melancon, M.P.; Xiong, C.; Huang, Q.; Elliott, A.; Song, S.; Zhang, R.; Flores, L.G.; Gelovani, J.G.; Wang, L.V.; et al. Effects of Photoacoustic Imaging and Photothermal Ablation Therapy Mediated by Targeted Hollow Gold Nanospheres in an Orthotopic Mouse Xenograft Model of Glioma. Cancer Res. 2011, 71, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Diez, J.; Foti, A.; Casanova-Carvajal, O.; Marrodan, L.; Granado, N.; Satriano, C.; Martinez-Murillo, R.; Serrano-Olmedo, J.J.; Ramos-Gomez, M. Effect of Photothermal Therapy Using Gold Nanoparticles Conjugated with Hyaluronic Acid in an Intracranial Murine Glioblastoma Model. Int. J. Nanomed. 2025, 20, 9327–9346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Yan, F.; Zhao, Y.; Wang, S.; Guo, B.; He, J.; Zou, D. Vapor deposition synthesis of polypyrrole nanoparticles with a tunable photothermal conversion capacity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126073. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, S.-T.; Wang, H.; Chang, Y.; Cao, A.; Liu, Y. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 2012, 7, 1801–1812. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Huang, H.; Zhang, H.; Hou, L.; Zhang, Z. Functionalized graphene oxide-based thermosensitive hydrogel for near-infrared chemo-photothermal therapy on tumor. J. Biomater. Appl. 2016, 30, 1230–1241. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Tian, B.; Zhang, Y.; Liu, Z. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power. Biomaterials 2012, 33, 2206–2214. [Google Scholar] [CrossRef]
- Zhang, X.J.; Cai, W.B.; Hao, L.Y.; Hu, X.H.; Wei, X.J.; Wang, X.Y.; Lin, Q. Preparation of thermo/pH-sensitive reduced graphene oxide interpenetrating hydrogel nanocomposites for co-delivery of paclitaxel and epirubicin. Mater. Technol. 2018, 33, 245–252. [Google Scholar] [CrossRef]
- Yin, T.; Liu, J.; Zhao, Z.; Zhao, Y.; Dong, L.; Yang, M. Redox Sensitive Hyaluronic Acid-Decorated Graphene Oxide for Photothermally Controlled Tumor-Cytoplasm-Selective Rapid Drug Delivery. Adv. Funct. Mater. 2017, 27, 1604620. [Google Scholar]
- Rajput, P.; Khanchandani, S. A review of chitosan-functionalized graphene oxide nanocomposites: A revolutionary drug delivery vehicle for cancer therapy. Int. J. Biol. Macromol. 2025, 311, 143999. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Soysal, F.; Çıplak, Z.; Getiren, B.; Gökalp, C.; Yıldız, N. Fabrication of polypyrrole enveloped reduced graphene oxide/iron oxide and determination of its photothermal properties. Mater. Res. Bull. 2022, 150, 111792. [Google Scholar] [CrossRef]
- Marimuthu, A.; Basu, H.; Singh, S.; Saha, S.; Basu, R.; Chandwadkar, P.; Acharya, C.; Patra, C.N. Intercalation of V(2)O(5) and Polypyrrole into a Graphene Oxide Layer: A Hybrid Multifunctional Photothermal Structure for Efficient Solar Evaporation, Water Purification, Disinfection, and Power Production. ACS Appl. Mater. Interfaces 2024, 16, 45063–45077. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Paola, O.; Roberta, V.; Laura, R.; Laura, C.; Matteo, M.; Marco, R. VA-086 methacrylate gelatine photopolymerizable hydrogels: A parametric study for highly biocompatible 3D cell embedding. J. Biomed. Mater. Res. Part A 2015, 103, 2109–2117. [Google Scholar] [CrossRef]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef]
- Hoch, E.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Chemical tailoring of gelatin to adjust its chemical and physical properties for functional bioprinting. J. Mater. Chem. B 2013, 1, 5675–5685. [Google Scholar] [CrossRef]
- Klein, M.P.; Hackenhaar, C.R.; Lorenzoni, A.S.G.; Rodrigues, R.C.; Costa, T.M.H.; Ninow, J.L.; Hertz, P.F. Chitosan crosslinked with genipin as support matrix for application in food process: Support characterization and β-d-galactosidase immobilization. Carbohydr. Polym. 2016, 137, 184–190. [Google Scholar] [CrossRef]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Nicodemus, G.D.; Bryant, S.J. Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]






| Gelatin MA 250 mg/mL | EPI 80 mg/mL | Cisplatin 5 mg/mL | PPy@GO 0.5 mg/mL | D.I. H2O | VA-086 200 mg/mL | |
|---|---|---|---|---|---|---|
| Gel only | 144 | 26 | 30 | |||
| C-gel | 144 | 14 | 12 | 30 | ||
| E-gel | 144 | 6.6 | 19.4 | 30 | ||
| CE-gel | 144 | 6.6 | 14 | 5.4 | 30 | |
| P-gel | 144 | 5 | 21 | 30 | ||
| CEP-gel | 144 | 6.6 | 14 | 5 | 0.4 | 30 |
| Gelatin | Gelatin MA | Gel |
|---|---|---|
| 71.6 | 102.7 | 163.4 |
| 338.4 | 340.4 | 341.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Yang, H.-W.; Wang, H.-C.; Hsu, C.-Y.; Wei, K.-C.; Chen, P.-Y.; Pang, H.-H. Gelatin-Based Rapid Blue Light-Irradiation In Situ Gelation Hydrogel Platform for Combination Therapy in Brain Tumors. Pharmaceutics 2025, 17, 1353. https://doi.org/10.3390/pharmaceutics17101353
Huang C-Y, Yang H-W, Wang H-C, Hsu C-Y, Wei K-C, Chen P-Y, Pang H-H. Gelatin-Based Rapid Blue Light-Irradiation In Situ Gelation Hydrogel Platform for Combination Therapy in Brain Tumors. Pharmaceutics. 2025; 17(10):1353. https://doi.org/10.3390/pharmaceutics17101353
Chicago/Turabian StyleHuang, Chiung-Yin, Hung-Wei Yang, Hung-Chun Wang, Chia-Yu Hsu, Kuo-Chen Wei, Pin-Yuan Chen, and Hao-Han Pang. 2025. "Gelatin-Based Rapid Blue Light-Irradiation In Situ Gelation Hydrogel Platform for Combination Therapy in Brain Tumors" Pharmaceutics 17, no. 10: 1353. https://doi.org/10.3390/pharmaceutics17101353
APA StyleHuang, C.-Y., Yang, H.-W., Wang, H.-C., Hsu, C.-Y., Wei, K.-C., Chen, P.-Y., & Pang, H.-H. (2025). Gelatin-Based Rapid Blue Light-Irradiation In Situ Gelation Hydrogel Platform for Combination Therapy in Brain Tumors. Pharmaceutics, 17(10), 1353. https://doi.org/10.3390/pharmaceutics17101353








