Effect of Crosslinking Using Heat on the Physicochemical Features of Bsa–Capsaicin Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of BSA NPs Loaded with Capsaicin
2.3. Quantification of BSA Transformed into NPs and Encapsulated Capsaicin
2.4. Measurement of Particle Size, PDI, and ζ-Potential
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. Raman Analysis
2.7. Transmission Electron Microscopy (TEM) and Morphometric Analysis of Nanoparticles
2.8. Sodium Dodecyl Sulphate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.9. Administration of BSA–Capsaicin NPs to a Murine Model
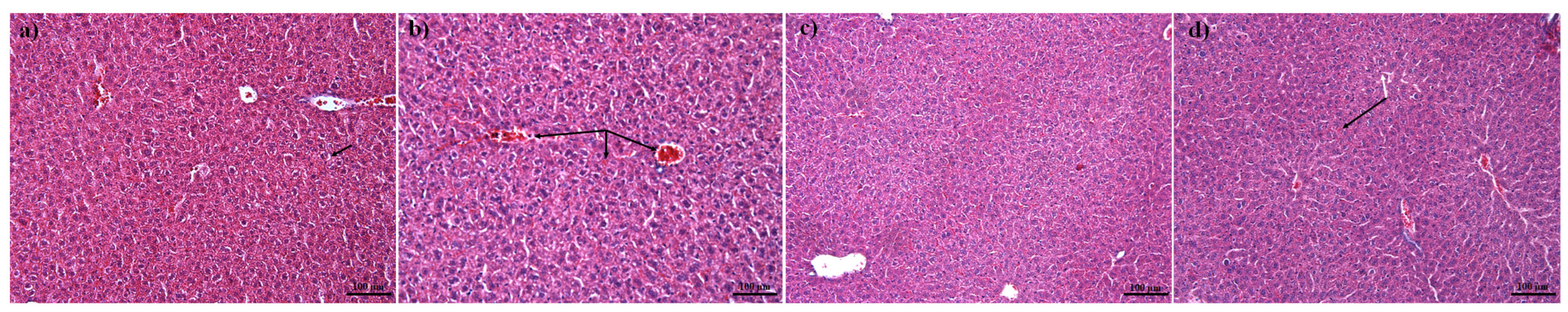
2.10. Histopathology of Mouse Liver
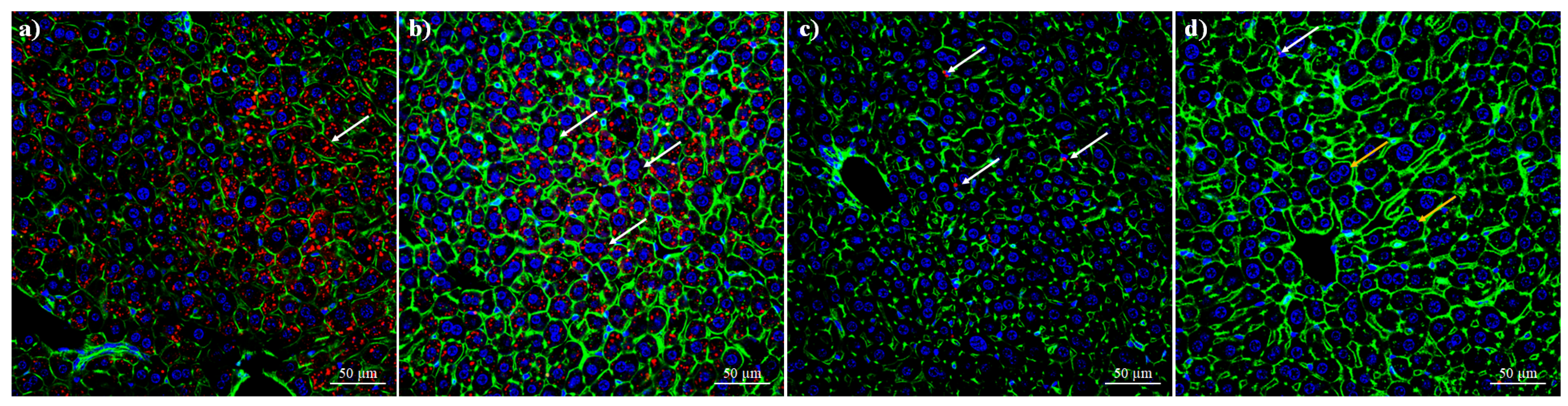
2.11. Multiphoton/Confocal Microscopy
3. Results and Discussion
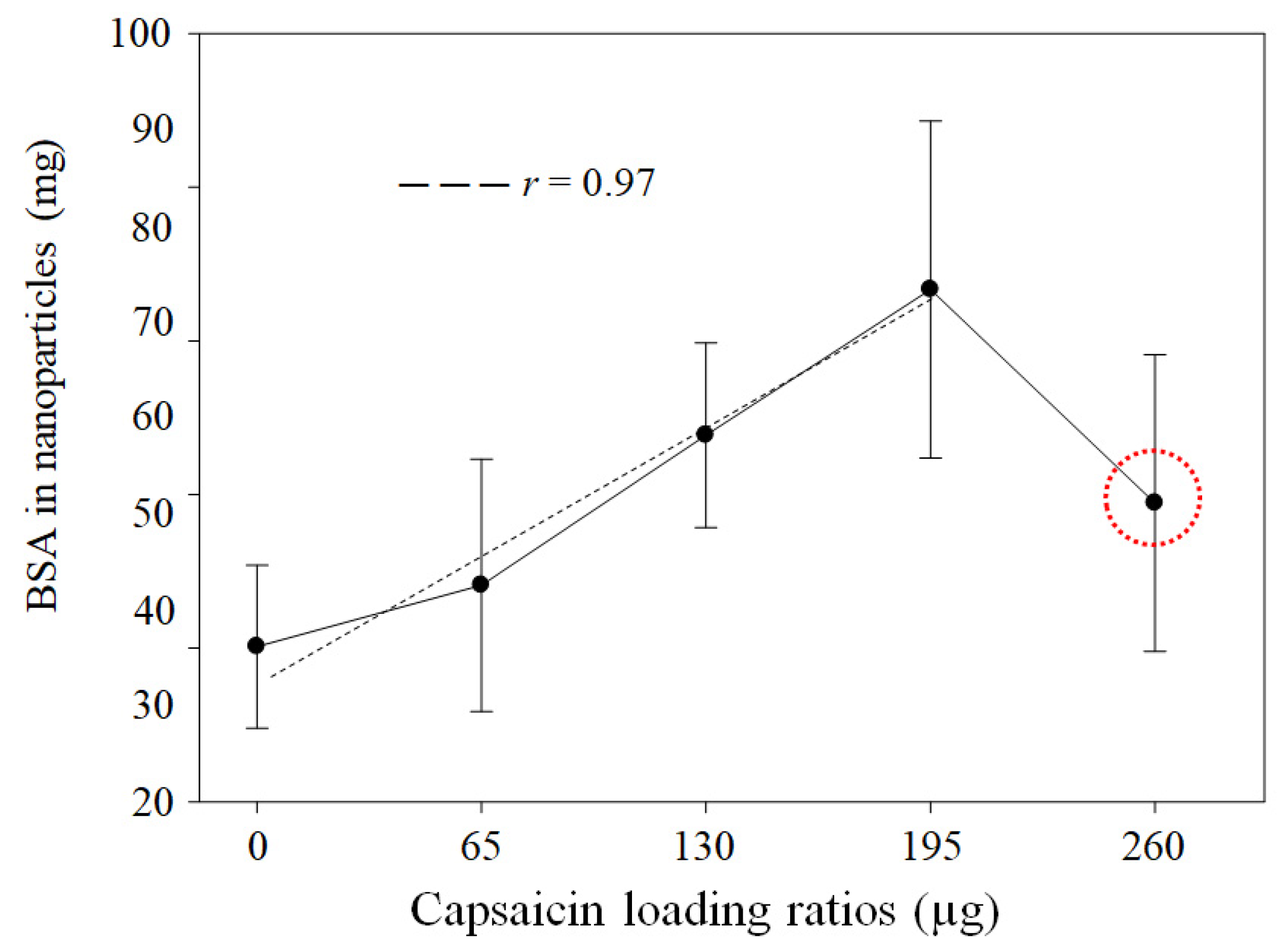
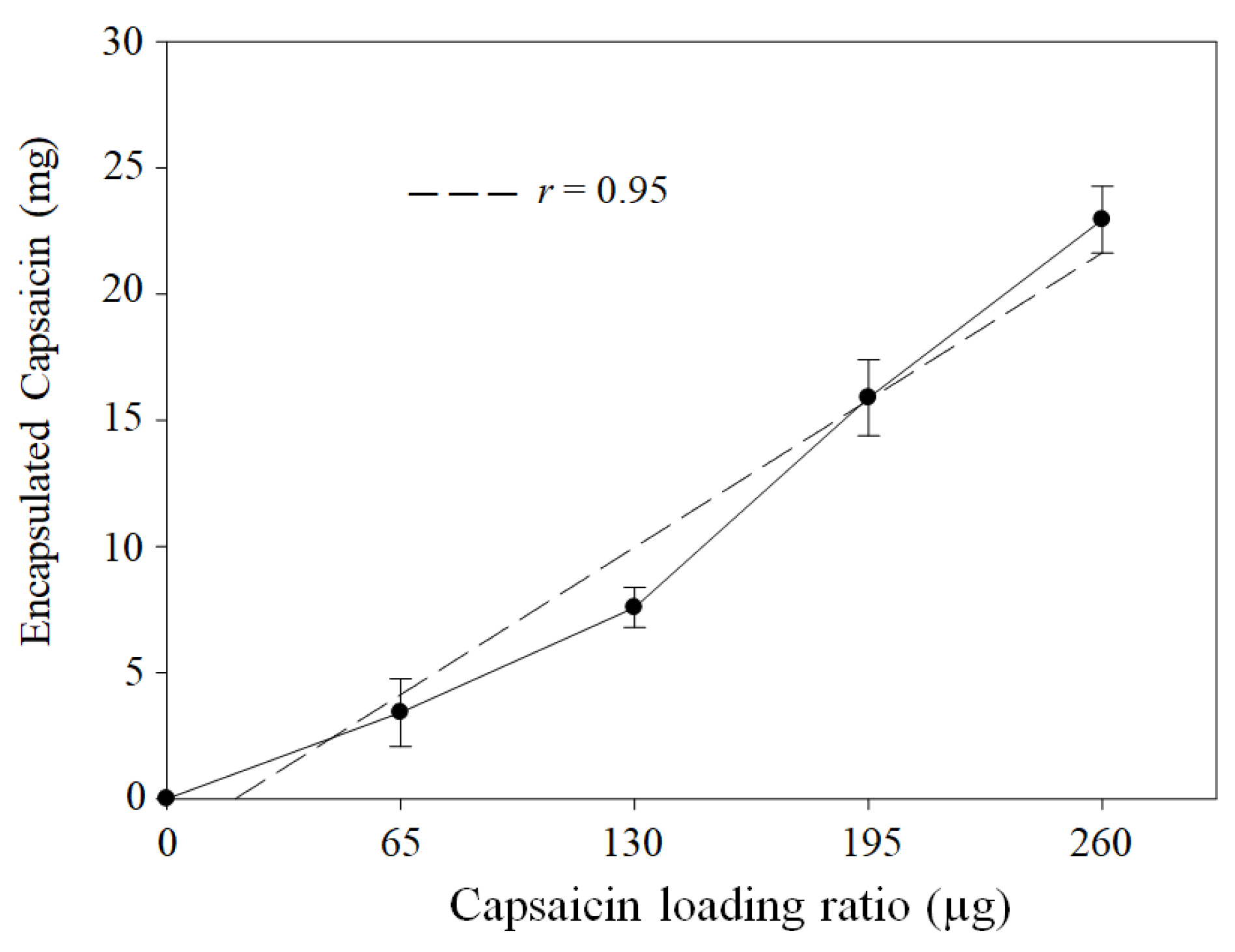
3.1. Yield of BSA Transformed to NPs and Quantification of Loaded Capsaicin
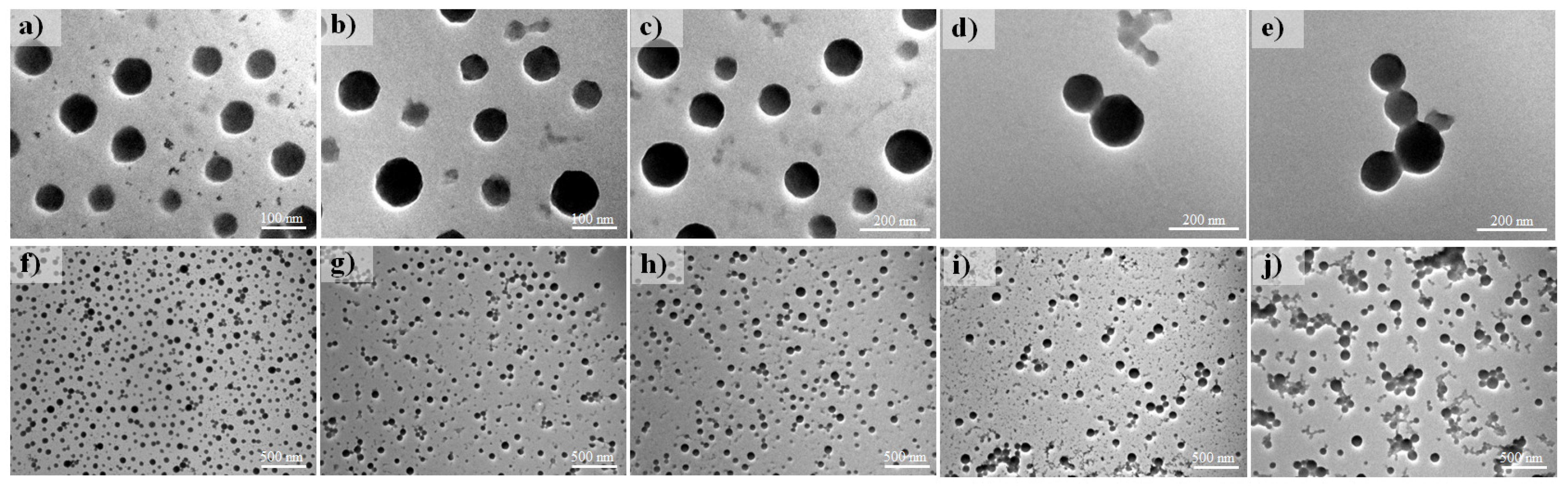
3.2. NP Morphology
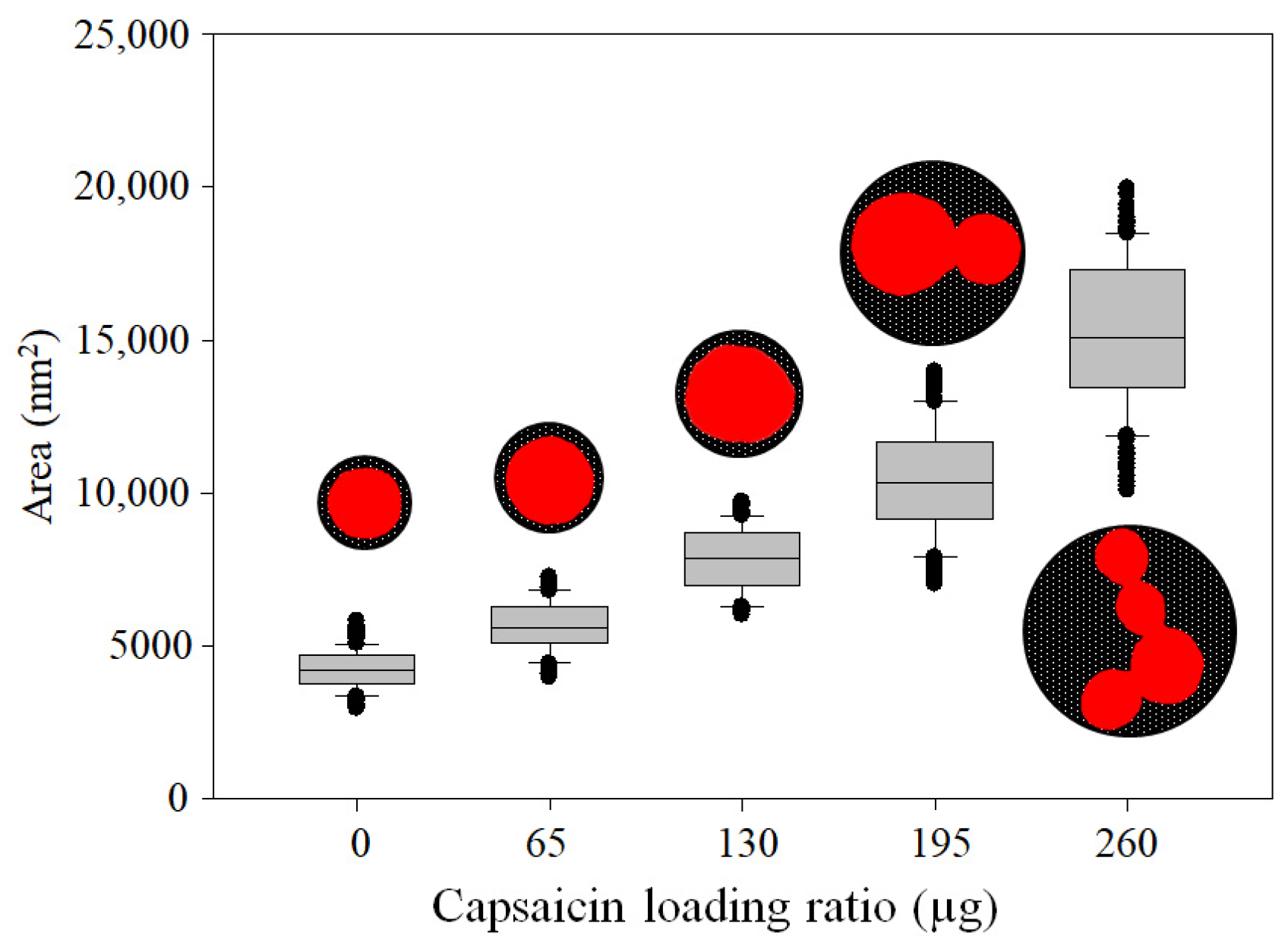
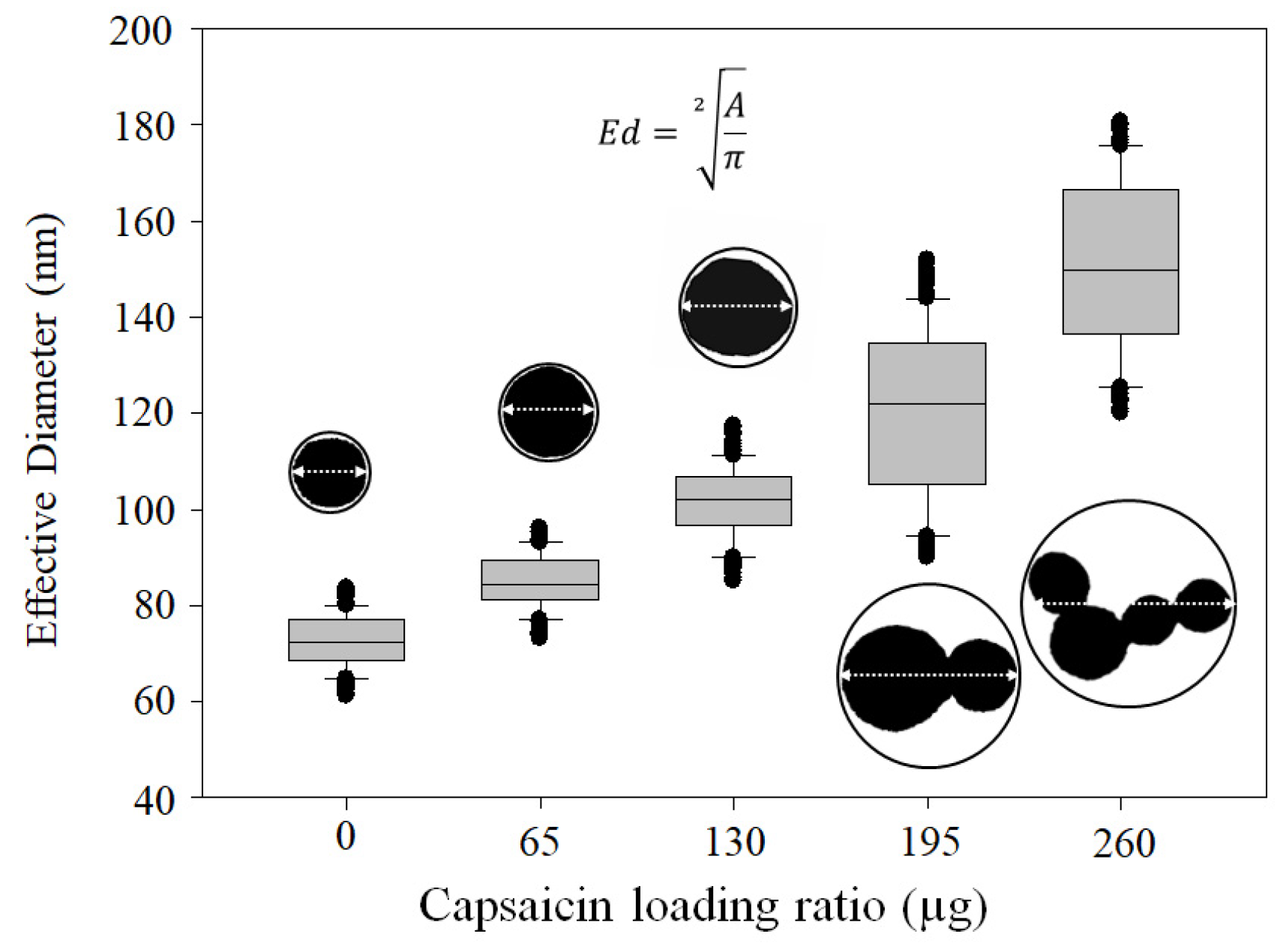
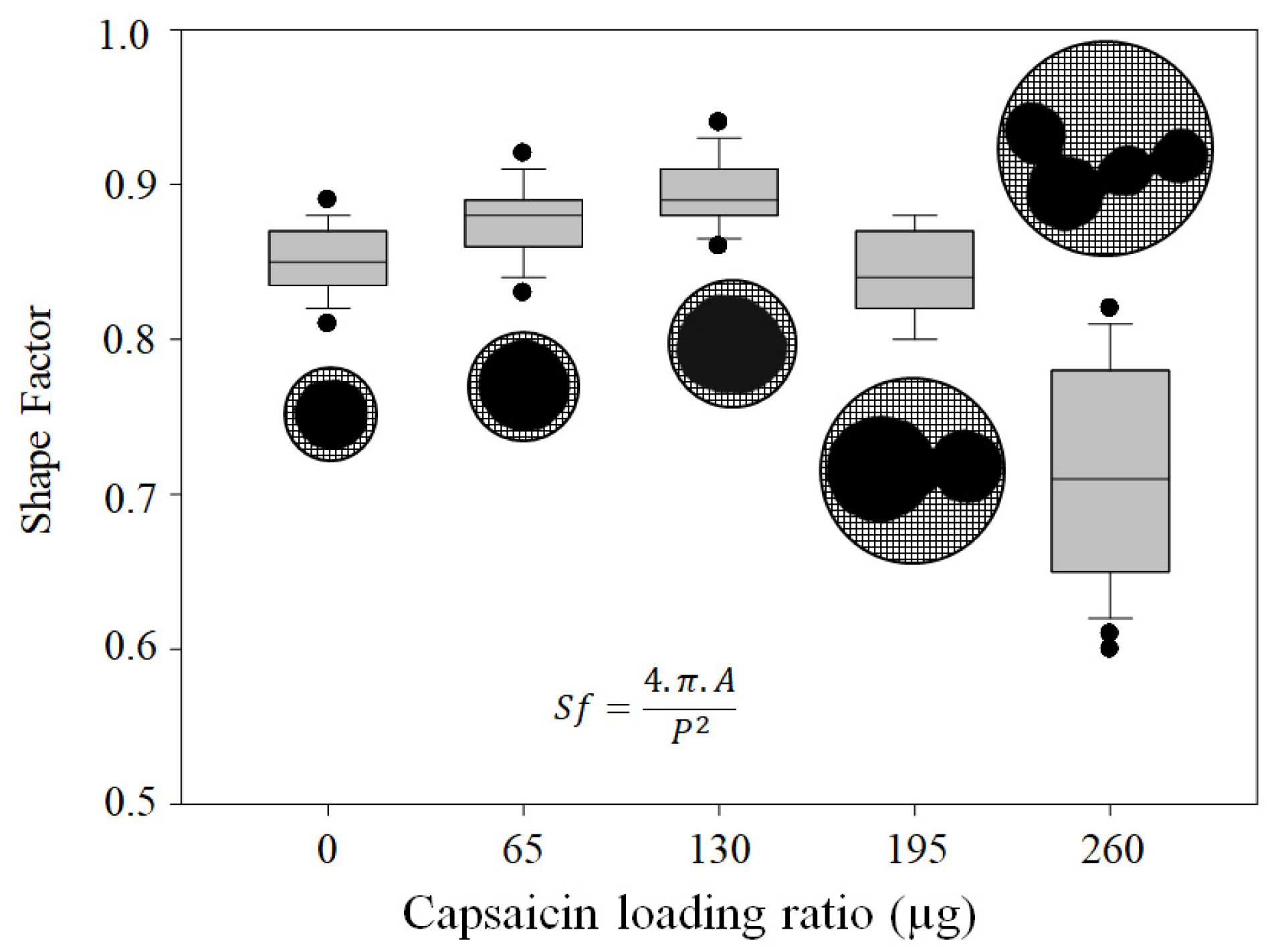
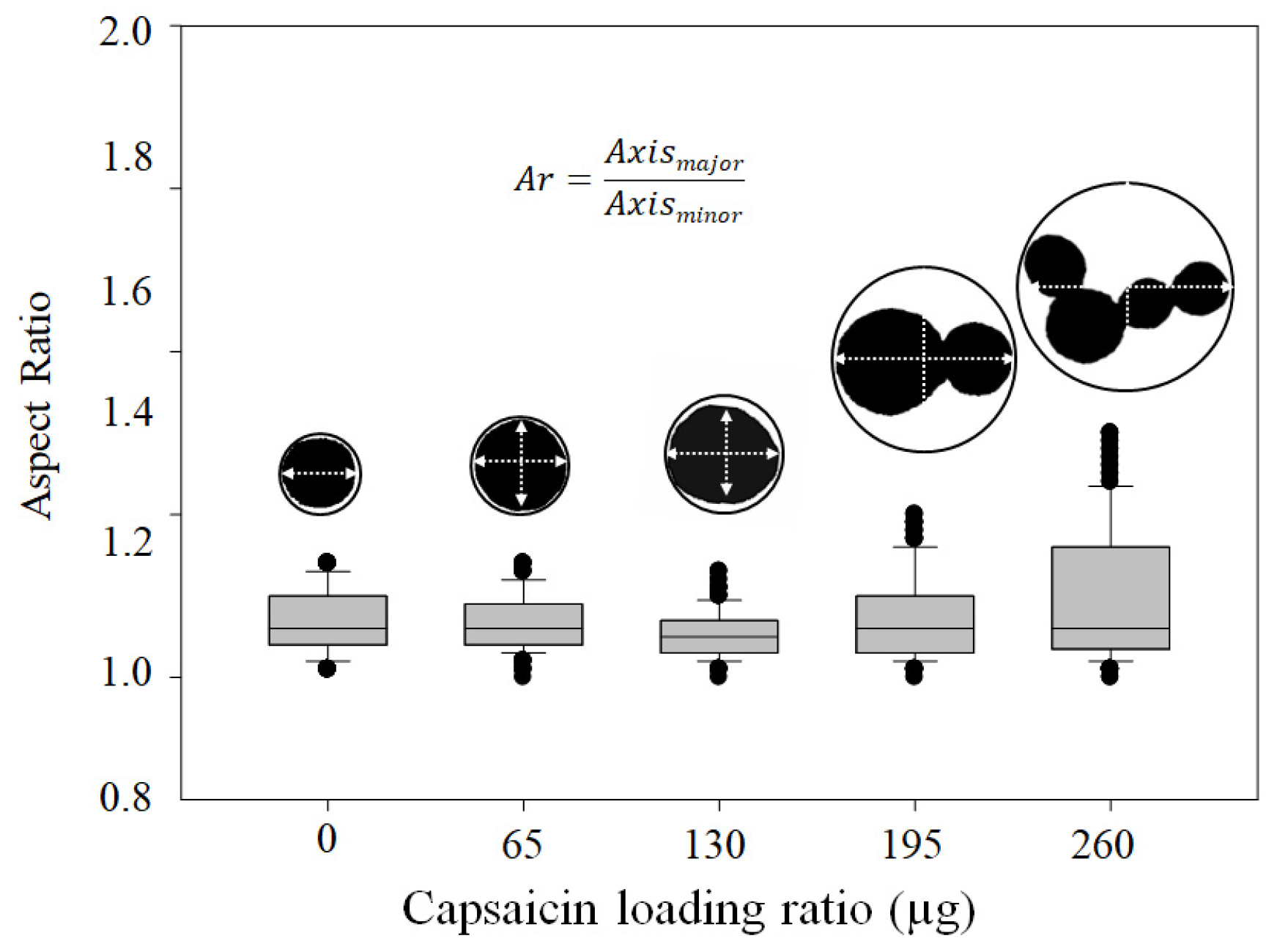
3.3. NP Morphometric Parameters
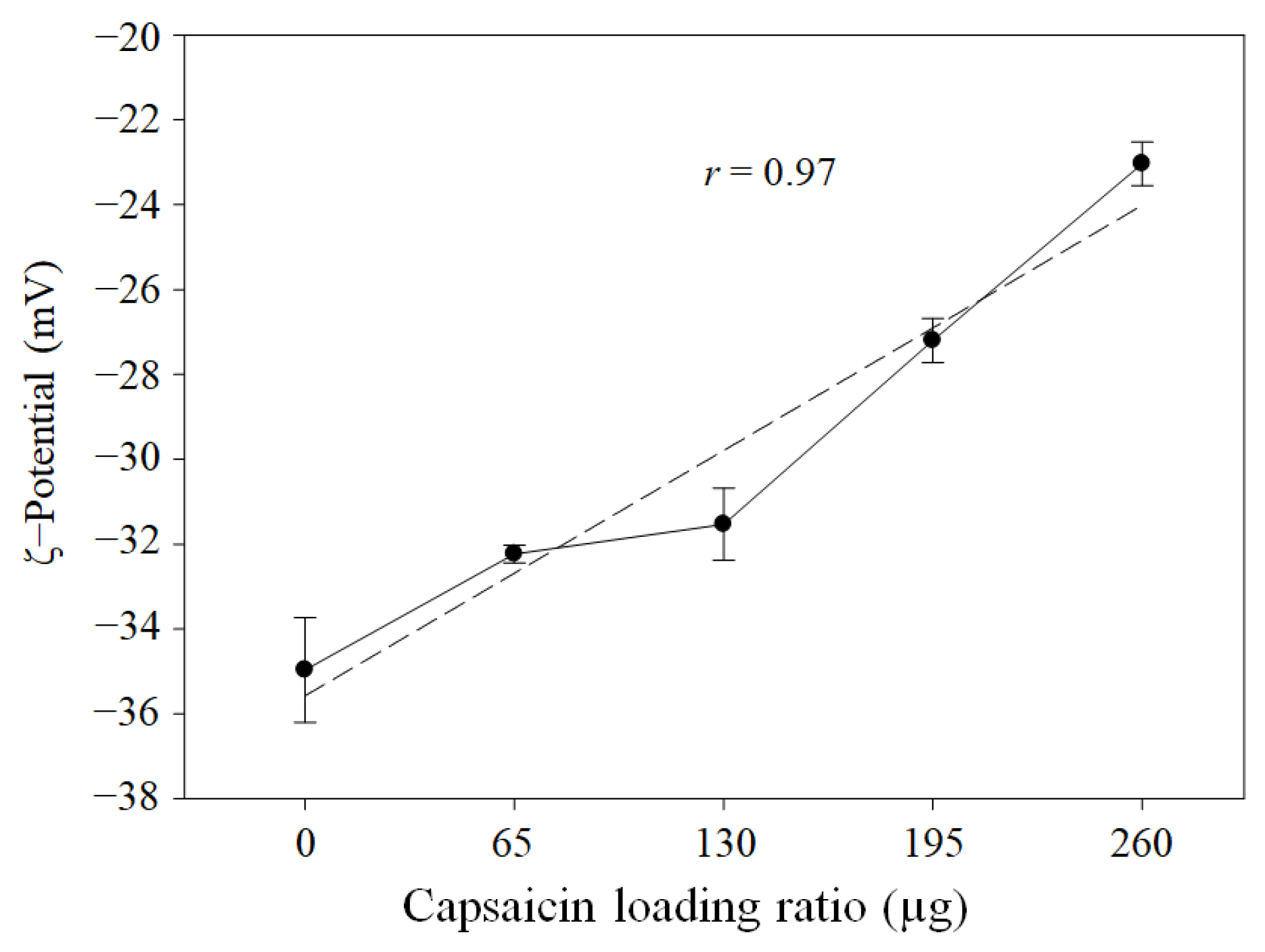
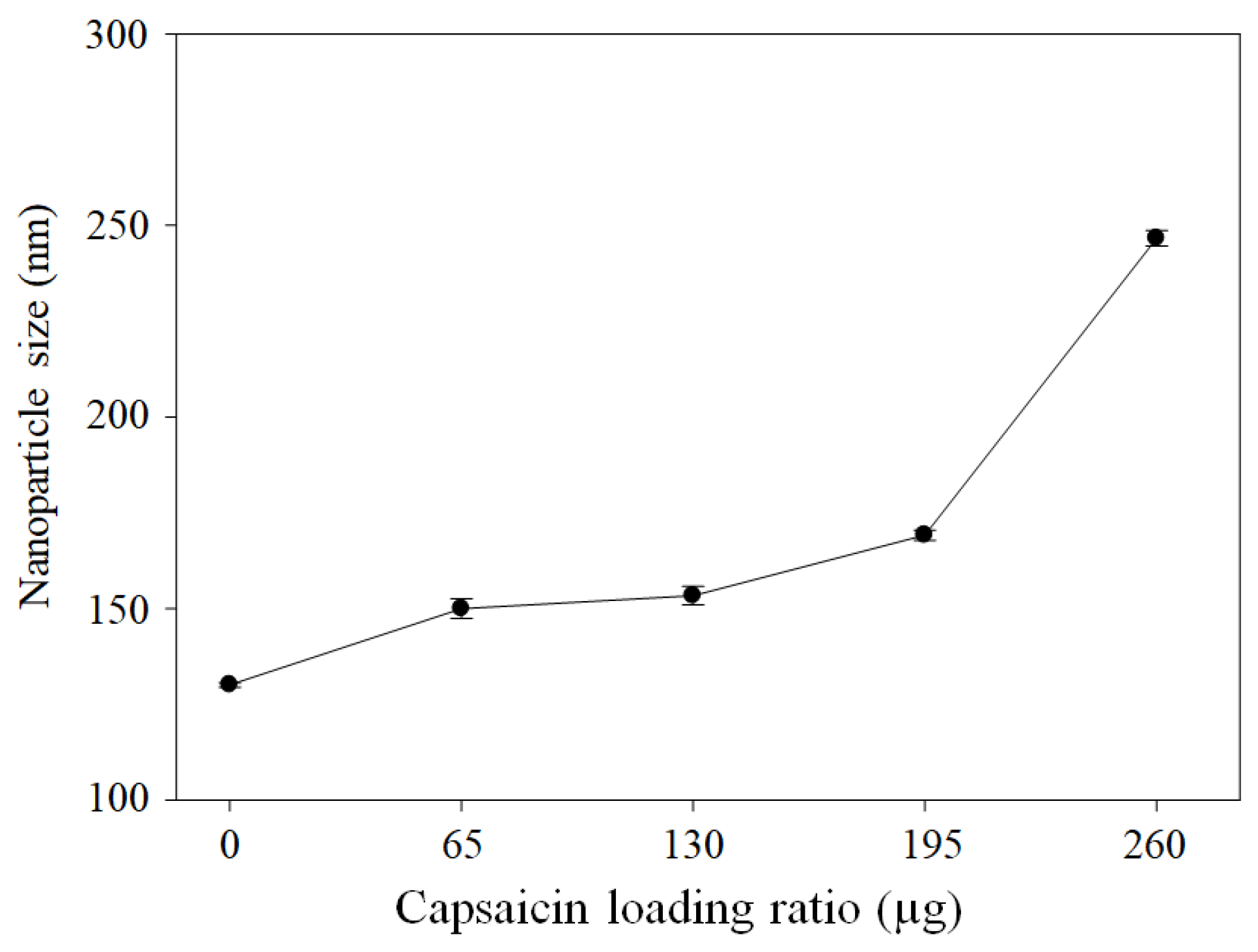
3.4. ζ-Potential, Hydrodynamic Diameter, and PDI
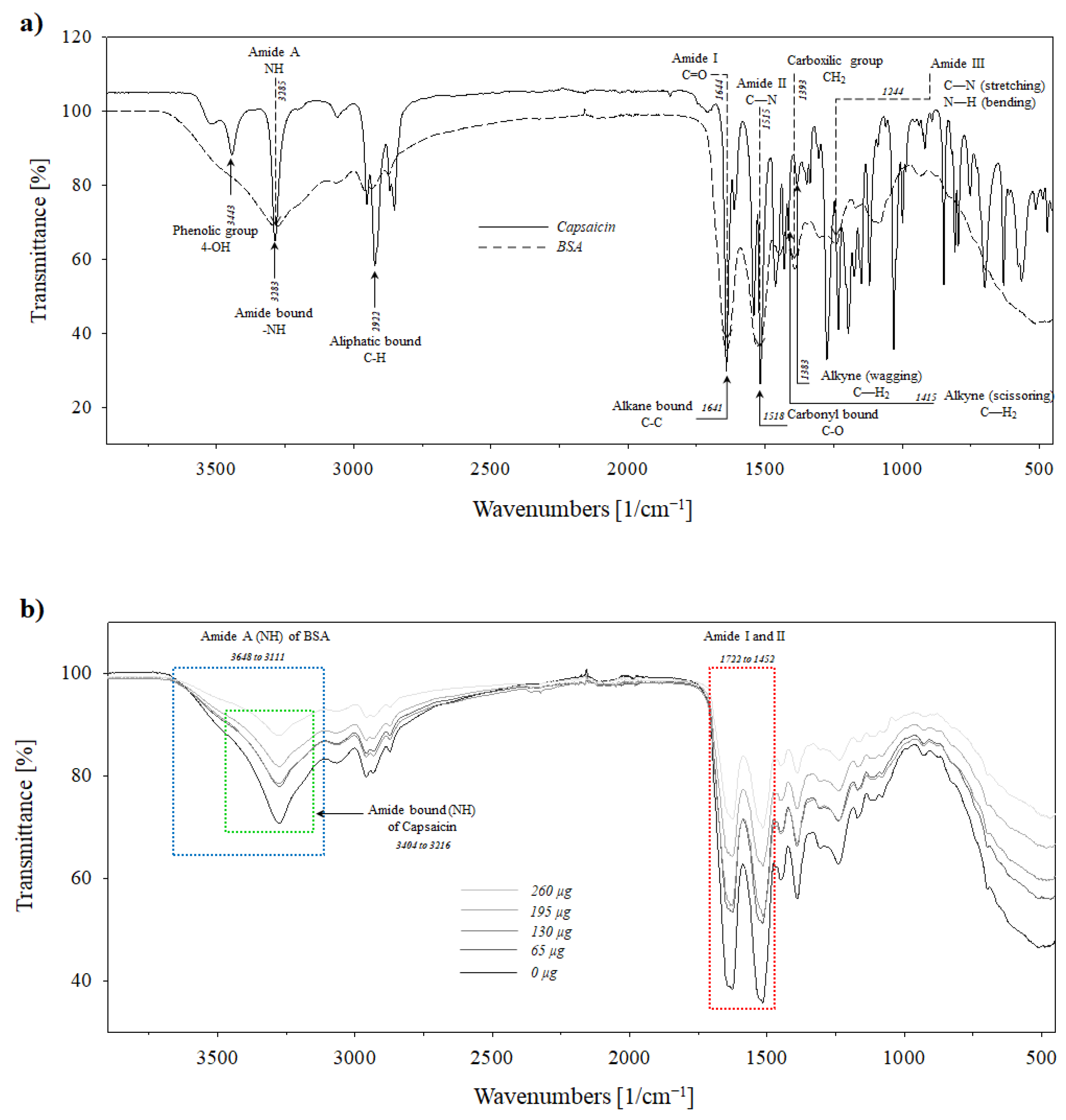
3.5. Effect of Heat Crosslinking on Molecular Interactions Between Nanoparticles
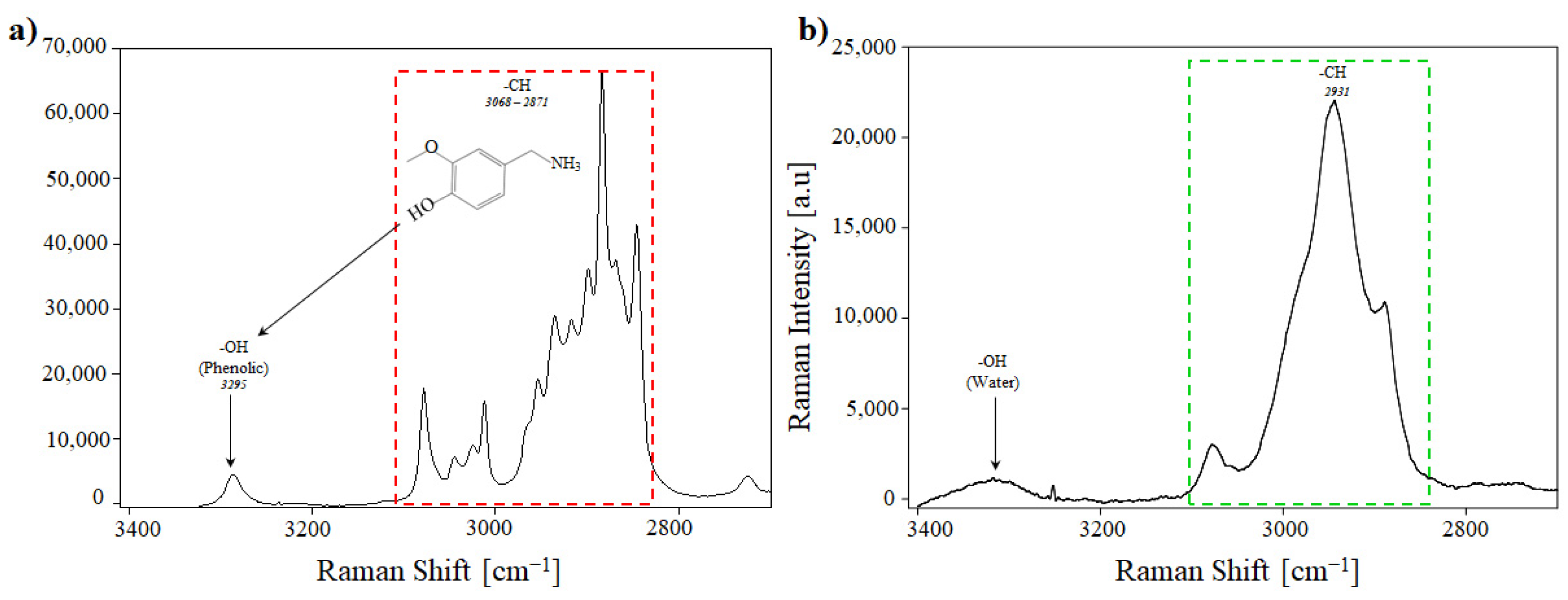
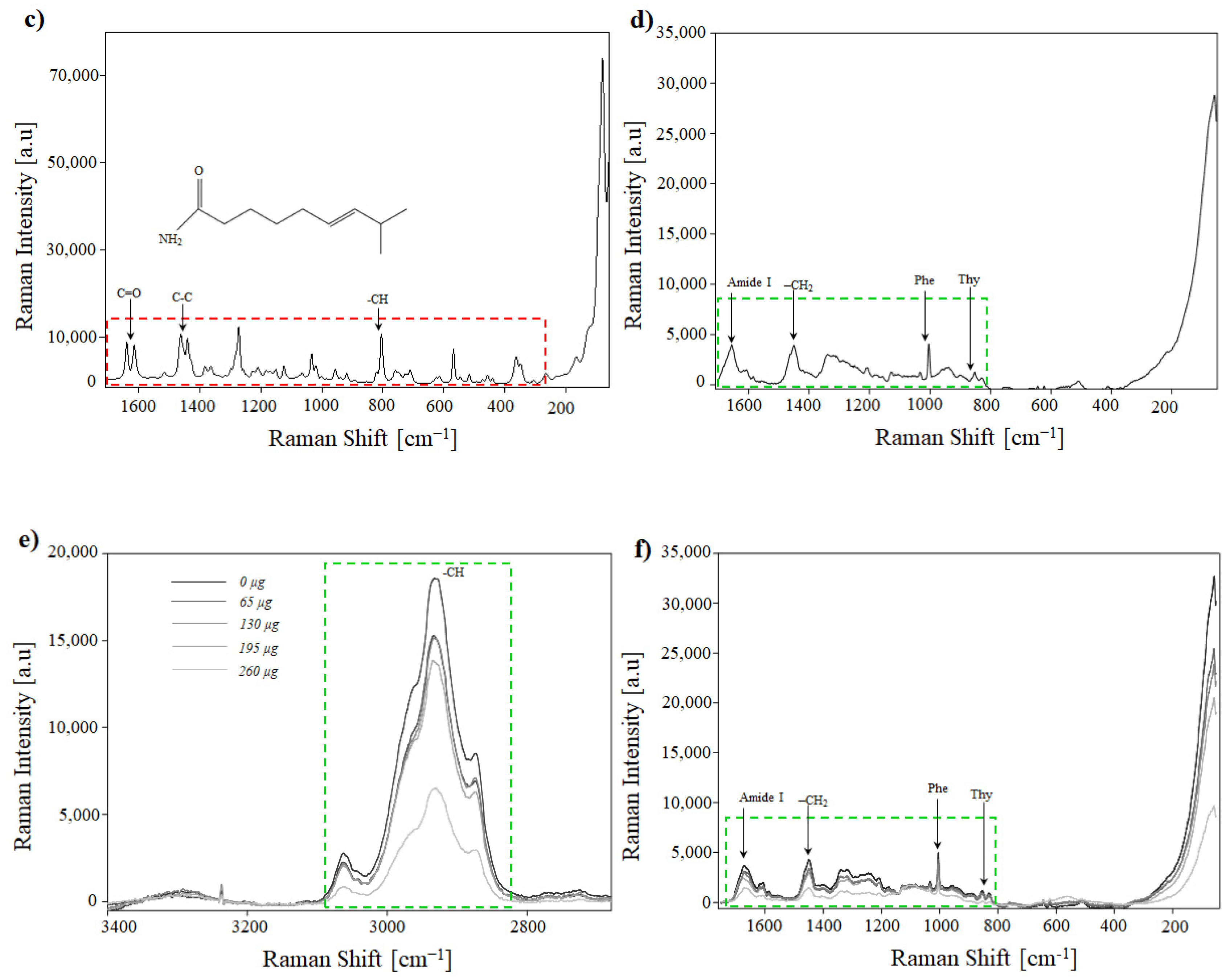
3.6. Raman Analysis
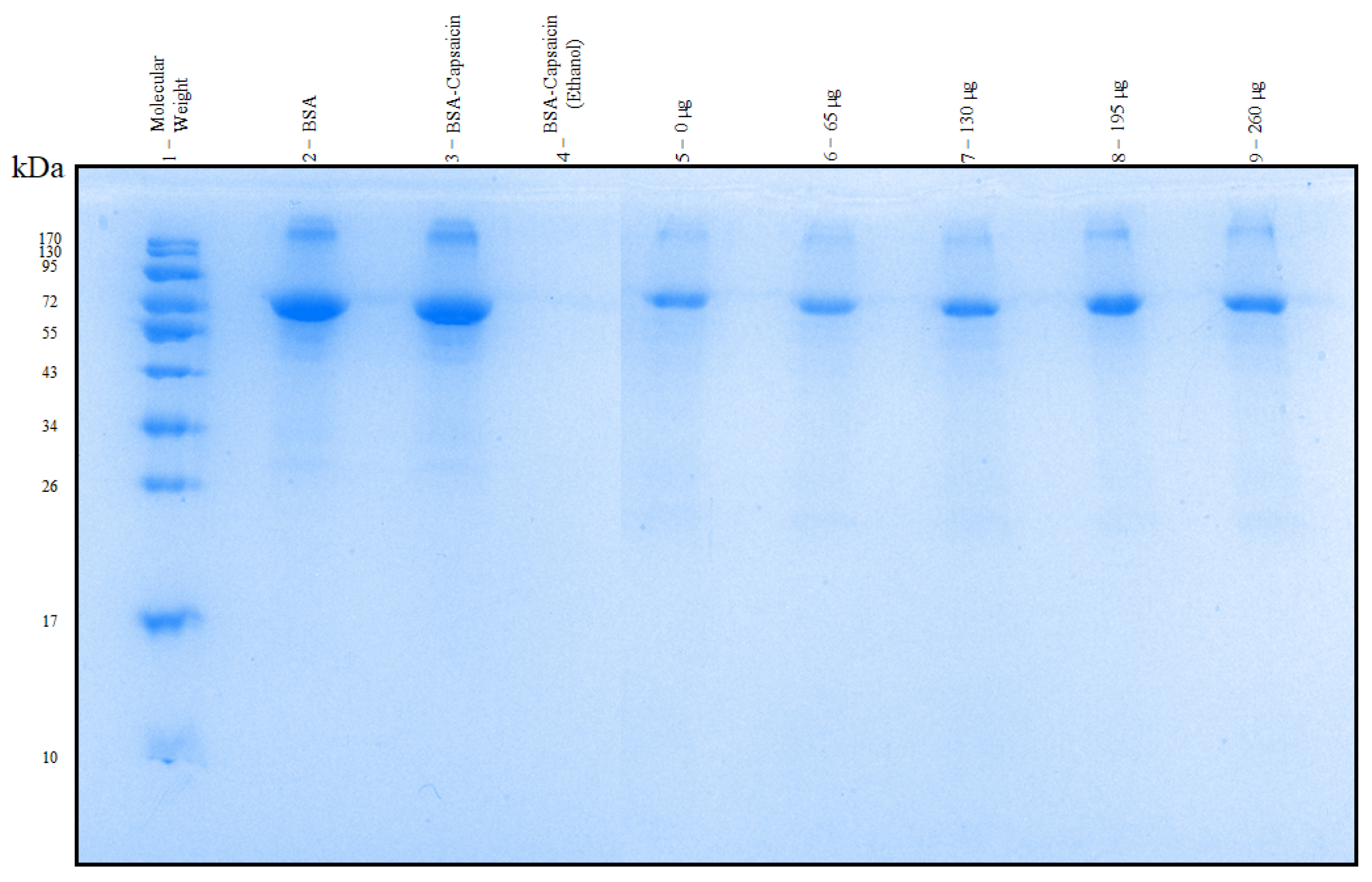
3.7. SDS-PAGE of the NPs
3.8. Biocompatibility of BSA–Capsaicin In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Area |
| Ar | Aspect ratio |
| BSA | Bovine serum albumin |
| dpi | Dots per inch |
| DLS | Dynamic light scattering |
| Ed | Effective diameter |
| EHT | Extra high tension |
| FTIR | Fourier transform infrared spectroscopy |
| g | g force |
| HPLC-UFLC | High-performance liquid chromatography–ultra-fast liquid chromatography |
| kDa | Kilodalton |
| min | Minutes |
| mL | Millilitre |
| mg | Milligram |
| MW | Molecular weight |
| NP | Nanoparticles |
| PDI | Polydispersity index |
| RGB | Red, green, blue |
| rpm | Revolutions per minute |
| SDS-PAGE | Sodium dodecyl sulphate–polyacrylamide gel electrophoresis |
| Sf | Shape factor |
| TEM | Transmission electron microscopy |
| tif | Tagged image file |
| TM | Temperature melting |
| UV | Ultraviolet |
References
- Arce-Rodríguez, M.L.; Ochoa-Alejo, N. Biochemistry and molecular biology of capsaicinoid biosynthesis: Recent advances and perspectives. Plant Cell Rep. 2019, 38, 1017–1030. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, W.; Zhang, X.; Mao, J.; Zhang, Q.; Zhao, W.; Zhang, S.; Xie, J. Recent advances in analysis of capsaicin and its effects on metabolic pathways by mass spectrometry. Front. Nutr. 2023, 10, 1227517. [Google Scholar] [CrossRef]
- Maharjan, A.; Vasamsetti, B.; Park, J.H. A Comprehensive review of Capsaicin: Biosynthesis, Industrial productions, Processing to Applications, and Clinical uses. Heliyon 2024, 10, e39721. [Google Scholar] [CrossRef] [PubMed]
- Jincheng, W.; Xiaoyu, Z.; Sihao, C. Preparation and properties of nanocapsulated capsaicin by complex coacervation method. Chem. Eng. Commun. 2010, 197, 919–933. [Google Scholar] [CrossRef]
- Choi, A.J.; Kim, C.J.; Cho, Y.J.; Hwang, J.K.; Kim, C.T. Characterization of capsaicin-loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food Bioprocess Technol. 2011, 4, 1119–1126. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, S.; Xue, Y.; Lou, J. Structure and properties analysis of microcapsulated capsaicin prepared by diphase emulsion method. Adv. Mater. Res. 2011, 239–242, 3182–3185. [Google Scholar] [CrossRef]
- Sánchez-Segura, L.; García-Armenta, E.; Perea-Flores, M.J.; Téllez-Medina, I.D.; Carpio-Pedroza, C.J.; Hernández-Sánchez, H.; Alamilla-Beltrán, L.; Jiménez-Aparicio, R.A.; Gutiérrez-López, F.G. Food Nano- and Microconjugated Systems: The Case of Albumin–Capsaicin in Food Nanoscience and Nanotechnology; Springer Science+Business Media: New York, NY, USA, 2015. [Google Scholar]
- Sánchez-Segura, L.; Ochoa-Alejo, N.; Carriles, R.; Zavala-García, L.E. Development of bovine serum albumin–capsaicin nanoparticles for biotechnological applications. Appl. Nanosci. 2018, 8, 1877–1886. [Google Scholar] [CrossRef]
- Sánchez-Arreguin, A.; Carriles, R.; Ochoa-Alejo, N.; López, M.G.; Sánchez-Segura, L. Generation of BSA-capsaicin nanoparticles and their hormesis effect on the Rhodotorula mucilaginosa yeast. Molecules 2019, 24, 2800. [Google Scholar] [CrossRef]
- Carriles, R.; Zavala-García, L.E.; Nava-Coronel, S.; Sánchez-Arreguín, A.; López, M.G.; Sánchez-Segura, L. Post-synthesis nanostructuration of BSA-capsaicin nanoparticles generated by sucrose excipient. Sci. Rep. 2021, 11, 7549. [Google Scholar] [CrossRef]
- Bartlett, B.A.; Klier, J.; Razavi, S. Preparation of bovine serum albumin nanospheres via desolvation: A study of synthesis, characterization, and aging. Nanoscale 2025, 17, 5715–5731. [Google Scholar] [CrossRef]
- Aly, G.A.; Sabra, S.A.; Haroun, M.; Helmy, M.W.; Moussa, N. Bovine serum albumin nanoparticles encapsulating Dasatinib and Celecoxib for oral cancer: Preparation, characterization, and in-vitro evaluation. Naunyn-Schmiedeb. Arch. Pharmacol. 2025, 398, 9291–9306. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, S.B.; Harper, C.; Demchuk, E.; Ingber, S.Z.; Wohlers, D.; Citra, M.J.; Kawa, M. Toxicological Profile for Glutaraldehyde; United States Department of Health and Human Services: Washington, DC, USA, 2017; pp. 1–302.
- Richards, F.M.; Knowles, J.R. Glutaraldehyde as a protein cross-linking reagent. J. Mol. Biol. 1968, 37, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, R.C. Solid colloidal drug delivery systems: Nanoparticles. Int. J. Pharm. 1981, 8, 217–234. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-Leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Galisteo-González, F.; Molina-Bolívar, J.A. Systematic study on the preparation of BSA nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 286–292. [Google Scholar] [CrossRef]
- Luna-Valdez, J.G.; Balandrán-Quintana, R.R.; Azamar-Barrios, J.A.; Clamont-Montfort, G.R.; Mendoza-Wilson, A.M.; Mercado-Ruiz, J.N.; Madera-Santana, T.; Rascon-Chu, A.; Chaquilla-Quilca, G. Structural and physicochemical characterization of nanoparticles synthesized from an aqueous extract of wheat bran by a cold-set gelation/desolvation approach. Food Hydrocoll. 2017, 62, 165–173. [Google Scholar] [CrossRef]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin nanoparticle-based drug delivery systems. Int. J. Nanomed. 2024, 19, 6945–6980. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Kiselkov, D.; Zamorina, S.; Rayev, M. Synthesis and application of albumin nanoparticles loaded with prussian blue nanozymes. Colloids Interfaces 2022, 6, 29. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.; Bakeri, G. Investigation and modeling effective parameters influencing the size of BSA protein nanoparticles as colloidal carrier. Colloids Surf. A Physicochem. Eng. Asp. 2012, 412, 96–100. [Google Scholar] [CrossRef]
- Sganzerla, M.; Coutinho, P.J.; Tavares de Melo, M.A.; Godoy, T.H. Fast method for capsaicinoids analysis from Capsicum chinense fruits. Food Rest. Int. 2014, 64, 718–725. [Google Scholar] [CrossRef]
- Bhalekar, M.; Upadhaya, P.; Madgulkar, A. Formulation and characterization of solid lipid nanoparticles for an anti-retroviral drug darunavir. Appl. Nanosci. 2017, 7, 47–57. [Google Scholar] [CrossRef]
- Fan, Y.H.; Nazari, M.; Raval, G.; Khan, Z.; Patel, H.; Heerklotz, H. Utilizing zeta potential measurements to study the effective charge, membrane partitioning, and membrane permeation of the lipopeptide surfactin. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 2306–2312. [Google Scholar] [CrossRef]
- Syverud, K.; Chinga, G.; Per Olav, J.; Ingebjorg, L.; Knut, W. Analysis of lint particles from full-scale printing trials. Appita Technol. Innov. Manuf. Environ. 2007, 60, 286–290. [Google Scholar]
- Parakhonskiy, B.; Zyuzin, V.M.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, J.W.; Skirtach, G.A. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnol. 2015, 13, 53. [Google Scholar] [CrossRef]
- Bouwman, M.A.; Bosma, C.J.; Vonk, P.; Wesselingh, A.J.; Frijlink, W.H. Which shape factor(s) best describe granules? Powder Technol. 2004, 146, 66–72. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- De Resende, L.F.T.; Basilio, F.C.; Alliprandini Filho, P.; Therézio, E.M.; Silva, R.A.; Oliveira, O.N., Jr.; Marletta, A.; Campana, P.T. Revisiting the conformational transition model for the pH dependence of BSA structure using photoluminescence, circular dichroism, and ellipsometric Raman spectroscopy. Int. J. Biol. Macromol. 2024, 259, 129142. [Google Scholar] [CrossRef]
- Kogure, K.; Goto, S.; Nishimura, M.; Yasumoto, M.; Abe, K.; Ohiwa, C.; Sassa, H.; Kusumi, T.; Terada, H. Mechanism of potent antiperoxidative effect of capsaicin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2002, 1573, 84–92. [Google Scholar] [CrossRef]
- Jun, J.Y.; Nguyen, H.H.; Paik, S.-Y.; Chun, H.S.; Kang, B.C.; Ko, S. Preparation of size-controlled bovine serum albumin (BSA) nanoparticles by a modified desolvation method. Food Chem. 2011, 127, 1892–1898. [Google Scholar] [CrossRef]
- Bansal, A.; Kapoor, D.; Kapil, R.; Chhabra, N.; Dhawan, S. Design and development of paclitaxel-loaded bovine serum albumin nanoparticles for brain targeting. Acta Pharm. 2011, 61, 141–156. [Google Scholar] [CrossRef]
- Sharma, L.G.; Pandey, L.M. Thermomechanical process induces unfolding and fibrillation of bovine serum albumin. Food Hydrocoll. 2021, 112, 106294. [Google Scholar] [CrossRef]
- Molodenskiy, D.; Shirshin, E.; Tikhonova, T.; Gruzinov, A.; Peters, G.; Spinozzi, F. Thermally induced conformational changes and protein–protein interactions of bovine serum albumin in aqueous solution under different pH and ionic strengths as revealed by SAXS measurements. Phys. Chem. Chem. Phys. 2017, 19, 17143–17155. [Google Scholar] [CrossRef]
- Yap, E.S.P.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P. Influence of hot air drying on capsaicinoids, phenolics, flavonoids and antioxidant activities of ‘Super Hot’ chilies. PeerJ 2022, 10, e13423. [Google Scholar] [CrossRef]
- Bustamante, K.; Guajardo, J.I.A.; Cahill, T. Thermal degradation of capsaicin and dihydrocapsaicin during cooking. J. Ariz. Acad. Sci. 2022, 49, 99–108. [Google Scholar] [CrossRef]
- Ou, C.; Bi, X.; Liu, Y.; Fu, T.; Ouyang, Q.; Li, S.; Chen, C. Thermal degradation of capsaicin and dihydrocapsaicin: Mechanism and hazards of volatile products. J. Anal. Appl. Pyrolysis 2024, 182, 106714. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Pedersen, J.N.; Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 2020, 11, 2337. [Google Scholar] [CrossRef]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Hydrodynamic diameter and zeta potential of nanostructured lipid carriers: Emphasizing some parameters for correct measurements. Colloids Surf. A 2021, 620, 126610. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Solouk, A.; Saber-Samandari, S.; Akbari, S.; Ghanbari, H.; Brycki, B.E. Capsaicin-loaded alginate nanoparticles embedded polycaprolactone-chitosan nanofibers as a controlled drug delivery nanoplatform for anticancer activity. J. Colloid Interface Sci. 2023, 638, 616–628. [Google Scholar] [CrossRef]
- Razzak, M.A.; Cho, S.J. Molecular characterization of capsaicin binding interactions with ovalbumin and casein. Food Hydrocoll. 2022, 133, 107991. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution–a perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press Limited: London, UK, 1991; pp. 1–503. [Google Scholar]
- Çınar, M.; Alım, B.; Alım, Z.; Şakar, E. Determination of the molecular structure and spectroscopic properties of capsaicin. Radiat. Phys. Chem. 2023, 208, 110879. [Google Scholar] [CrossRef]
- Sano, K.; Uzawa, Y.; Kaneshima, I.; Nakasato, S.; Hashimoto, M.; Tanaka, Y.; Nakatani, S.; Kobata, K. Vanillin reduction in the biosynthetic pathway of capsiate, a non-pungent component of Capsicum fruits, is catalyzed by cinnamyl alcohol dehydrogenase. Sci. Rep. 2022, 12, 12384. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Chikashi, O.; Kazufumi, T. Behavior of Bovine Serum Albumin Molecules in Molecular Crowding Environments Investigated by Raman Spectroscopy. Langmuir 2016, 32, 7372–7382. [Google Scholar] [CrossRef]
- Hernández, B.; Pflüger, F.; Kruglik, S.G.; Ghomi, M. Characteristic Raman lines of phenylalanine analyzed by a multiconformational approach. J. Raman Spectrosc. 2013, 44, 827–833. [Google Scholar] [CrossRef]
- Ojeda-Galván, H.J.; Hernández-Arteaga, A.C.; Rodríguez-Aranda, M.C.; Toro-Vazquez, J.F.; Cruz-González, N.; Ortíz-Chávez, S.; Comas-García, M.; Rodríguez, A.G.; Navarro-Contreras, H.R. Application of Raman spectroscopy for the determination of proteins denaturation and amino acids decomposition temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121941. [Google Scholar] [CrossRef]
- Hernández, B.; Coïc, Y.M.; Kruglik, S.G.; Sanchez-Cortes, S.; Ghomi, M. The relationship between the tyrosine residue 850–830 cm−1 Raman doublet intensity ratio and the aromatic side chain χ1 torsion angle. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 308, 123681. [Google Scholar] [CrossRef]
- Anand, B.G.; Dubey, K.; Shekhawat, D.S.; Kar, K. Capsaicin-coated silver nanoparticles inhibit amyloid fibril formation of serum albumin. Biochemistry 2016, 55, 3345–3348. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Amighi, F.; Emam-Djomeh, Z.; Labbafi-Mazraeh-Shahi, M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J. Iran. Chem. Soc. 2020, 17, 1223–1235. [Google Scholar] [CrossRef]
- Da Silva, N.I.O.; Salvador, E.A.; Franco, I.R.; de Souza, G.A.P.; de Souza Morais, S.M.; Rocha, R.P.; Novaes, R.D.; Corsetti, P.P.; Malaquias, L.C.C.; Coelho, L.F.L. Bovine serum albumin nanoparticles induce histopathological changes and inflammatory cell recruitment in the skin of treated mice. Biomed. Pharmacother. 2018, 107, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, A.M.; Yun, J.W. Capsaicin induces ATP-dependent thermogenesis via the activation of TRPV1/β3-AR/α1-AR in 3T3-L1 adipocytes and mouse model. Arch. Biochem. Biophys. 2024, 755, 109975. [Google Scholar] [CrossRef]
- Kang, J.H.; Tsuyoshi, G.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar]
- Sung, J.; Yang, J.; Kim, Y.; Kim, M.; Jeong, H.S.; Lee, J. Effect of defatted pepper (Capsicum annuum L.) seed extracts on high-fat diet-induced obesity in C57BL/6J mice. Food Sci. Biotechnol. 2016, 25, 1457–1461. [Google Scholar] [CrossRef]
- Kim, H.J.; You, M.K.; Wang, Z.; Lee, Y.H.; Kim, H.A. Red pepper seed water extract suppresses high-fat diet-induced obesity in C57BL/6 mice. Food Sci. Biotechnol. 2020, 29, 275–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Segura, L.; Zaina, S.; Kú-González, A.F.; Guzmán-López, J.A.; Zavala-García, L.E.; López, M.G. Effect of Crosslinking Using Heat on the Physicochemical Features of Bsa–Capsaicin Nanoparticles. Pharmaceutics 2025, 17, 1306. https://doi.org/10.3390/pharmaceutics17101306
Sánchez-Segura L, Zaina S, Kú-González AF, Guzmán-López JA, Zavala-García LE, López MG. Effect of Crosslinking Using Heat on the Physicochemical Features of Bsa–Capsaicin Nanoparticles. Pharmaceutics. 2025; 17(10):1306. https://doi.org/10.3390/pharmaceutics17101306
Chicago/Turabian StyleSánchez-Segura, Lino, Silvio Zaina, Angela F. Kú-González, José Alfredo Guzmán-López, Laura E. Zavala-García, and Mercedes G. López. 2025. "Effect of Crosslinking Using Heat on the Physicochemical Features of Bsa–Capsaicin Nanoparticles" Pharmaceutics 17, no. 10: 1306. https://doi.org/10.3390/pharmaceutics17101306
APA StyleSánchez-Segura, L., Zaina, S., Kú-González, A. F., Guzmán-López, J. A., Zavala-García, L. E., & López, M. G. (2025). Effect of Crosslinking Using Heat on the Physicochemical Features of Bsa–Capsaicin Nanoparticles. Pharmaceutics, 17(10), 1306. https://doi.org/10.3390/pharmaceutics17101306






