The crude extracts were previously submitted to a liquid–liquid partition to the obtention of the dichloromethane phase. After comprehensive fractioning with solvents of different polarities, a wide range of metabolites with good resolution was obtained from the dichloromethane phase, contrasting with the complex data from the previous study. Non-polar to polar compounds were eluted subsequently according to solvent gradient, resulting in a clearer spectrum of chemical profile. Still, an additional fractionation step through HPLC-MS/MS was performed to the dereplication.
The resulting dichloromethane phase was, therefore, submitted to a comprehensive fractioning of with solvents of different polarities, hexane, dichloromethane, ethyl acetate and methanol. For L. dendroidea, the hexane fraction resulted in 220 mg (12.94%), ethyl acetate fraction produced 420 mg (24.71%), dichloromethane fraction, 550 mg (32.35%) and the methanol fraction produced 550 mg (32.35%). For L. aldingensis, hexane fraction yielded 70 mg (4.11%), ethyl acetate, 290 mg (17.05%), dichloromethane fraction, 470 mg (27.64%), and methanolic fraction produced 240 mg (14.11%).
3.3. Metabolite Profiling
The metabolites analysis of
Laurencia aldingensis by HPLC-DAD-MS/MS (
Figure 1,
Table 6) reveals a complex chemical spectrum. Beginning with the compounds identified in peaks 4 to 7, a series of bromophenols was observed, including 5-bromo-3,4-dihydroxybenzaldehyde, and 3,5-dibromo-4-hydroxybenzoic acid—compounds known for their antioxidant properties [
18,
19]. The presence of these metabolites is characteristic of the
Laurencia genus and suggests a defensive or signaling role against pathogens and herbivores in the marine environment [
20,
21]. These compounds are also of interest in the search for novel bioactive molecules with pharmacological potential.
Continuing the analysis, several peaks—namely 8, 9, 10, 12, 13, 14, 18, 19, 23, 24, and 29—could not be identified based on their molecular formulas or observed fragment ions. These compounds share the presence of both bromine and chlorine atoms in their structures and the absence of previously isolated substances matching their molecular formulas, suggesting that they may represent novel compounds not yet reported in scientific literature. For a specific subset—peaks 18, 19, 23, and 24—data indicated a possible structural analogy with halogenated sesquiterpenes of the aldigenin type, previously isolated from
Laurencia aldingensis [
22,
23]. It is worth noting that the occurrence of halogenated structures is recurrent among red algal metabolites [
24,
25]. The possibility that these are new natural products highlights the relevance of future studies aimed at their isolation and full structural characterization, which could unveil new chemical entities and contribute to our understanding of marine biodiversity and its adaptive mechanisms.
The compound identified at peak 16, furocaespitanelactol—a member of the furocespitane class—is particularly intriguing, as it exhibits structural similarities to molecules previously reported in both algae and mollusks, suggesting the existence of a potentially conserved biosynthetic pathway [
26,
27,
28]. Such pathways may be associated with symbiotic interactions or adaptations to specific ecological niches in the marine environment.
Hydroxylated fatty acids, such as those observed at peaks 20 and 22, represent a class of compounds that perform a variety of cellular functions, including membrane formation and signaling. These fatty acids are important structural components in algae and have implications for both algal physiology and its interactions with the surrounding environment [
29,
30]. Finally, peak 35 suggests a lobophorolide derivative, indicating the presence of long chain polyketides in
Laurencia aldingensis. Polyketides are a class of secondary metabolites characterized by complex structures and a wide range of biological functions, ranging from chemical defense to the attraction of symbionts [
31,
32,
33]. In summary, the data highlights the chemical complexity of
L. aldingensis. The study of these compounds paves the way for a better understanding of the complex ecological interactions of this seaweed, as well as for the development of novel biotechnological and pharmaceutical resources. Mass spectrometry serves as a crucial tool for unraveling the chemical and biological complexity of these organisms, and each identified peak represents a potential starting point for future discoveries.
The data discussed above refers to the set of metabolites observed in the extracts of
L. aldingensis. In the following section, we provide a more detailed discussion of the distribution of these compounds across the extracts and partitions presented in
Figure 1,
Table 6.
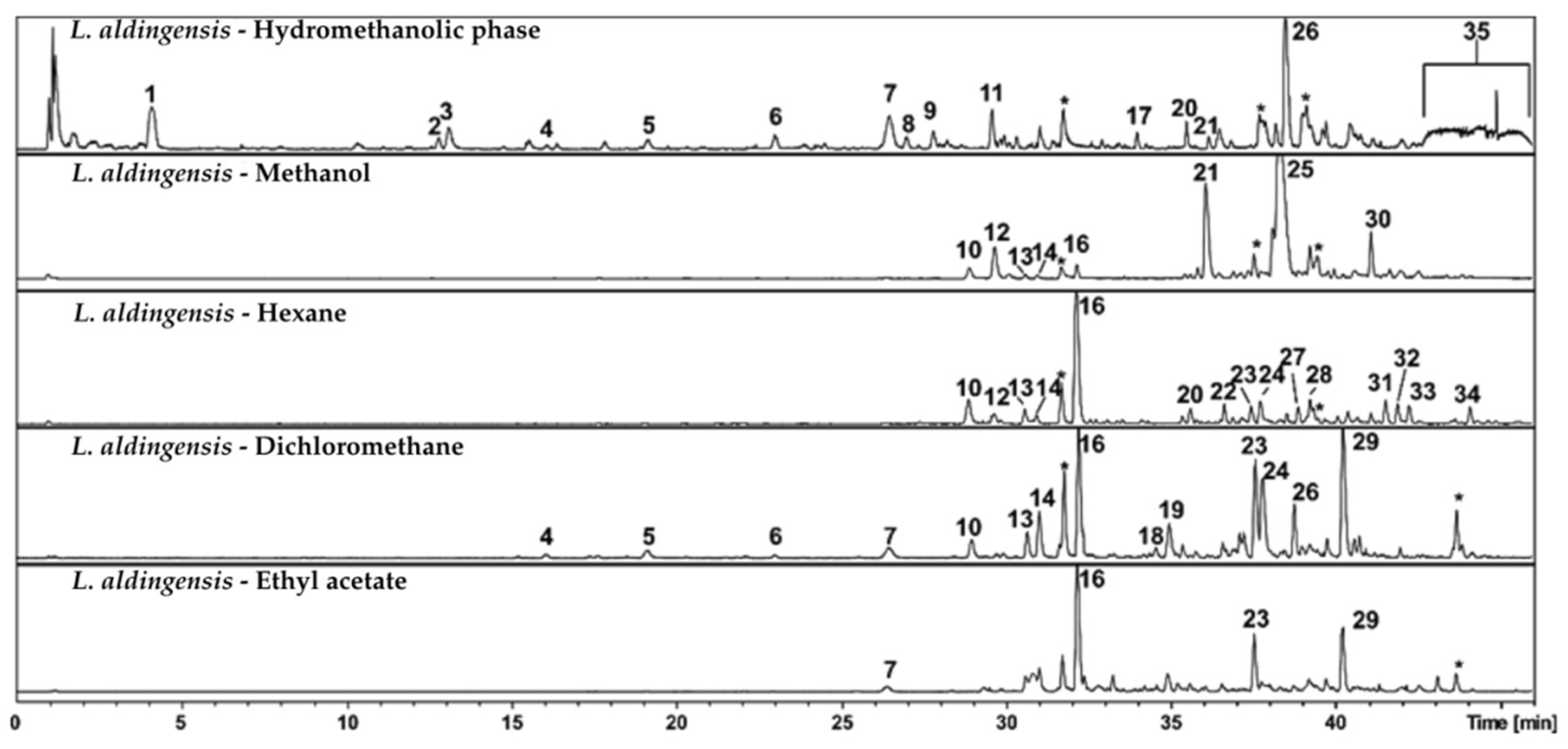
The hydromethanolic phase obtained through partitioning (
Figure 1—
L. aldingensis—Hydromethanolic phase), in which compound 1 and other highly polar compounds are prominent, as evidenced by their shorter retention times on the C18 column—indicating high affinity for water and the likely presence of polar functional groups. This fraction also contains polyketide 35, which was not detected in the other partitions, suggesting that the initial partitioning was effective in enriching this compound.
The hexane fraction was enriched in hydrophobic compounds, as evidenced by the prominent presence of peak 16 (furocaespitanelactol) and the more apolar aldingenin derivatives (peaks 23 and 29). In contrast, the dichloromethane (DCM) fraction exhibited a broader range of peaks, likely reflecting its ability to elute a wider spectrum of hydrophobic metabolites due to its relatively low polarity. Here, we observed a certain selectivity for furocaespitanelactol (peak 16) and aldingenin derivatives (peaks 18, 19, 23, 24, and 29), consistent with the expectation that DCM favors the extraction of compounds with higher hydrophobicity. This fraction also contained bromophenolic derivatives (peaks 4 to 7).
In the ethyl acetate fraction, compound 16 (furocaespitanelactol) and sesquiterpenoid structures related to the aldingenin skeleton, such as peaks 18 and 19, were prominent. These compounds suggest the presence of more complex and moderately polar structures that retain some solubility in this solvent. This indicates that these molecules possess structural features allowing moderate interactions with the silica stationary phase, favoring their elution with solvents of intermediate polarity.
Finally, the methanolic fraction was enriched in more polar metabolites. Specifically, compounds 21 (C
26H
40O
11) and 25 (C
28H
44O
11), although not fully characterized, share similar molecular formulas, suggesting they belong to the same class of compounds.
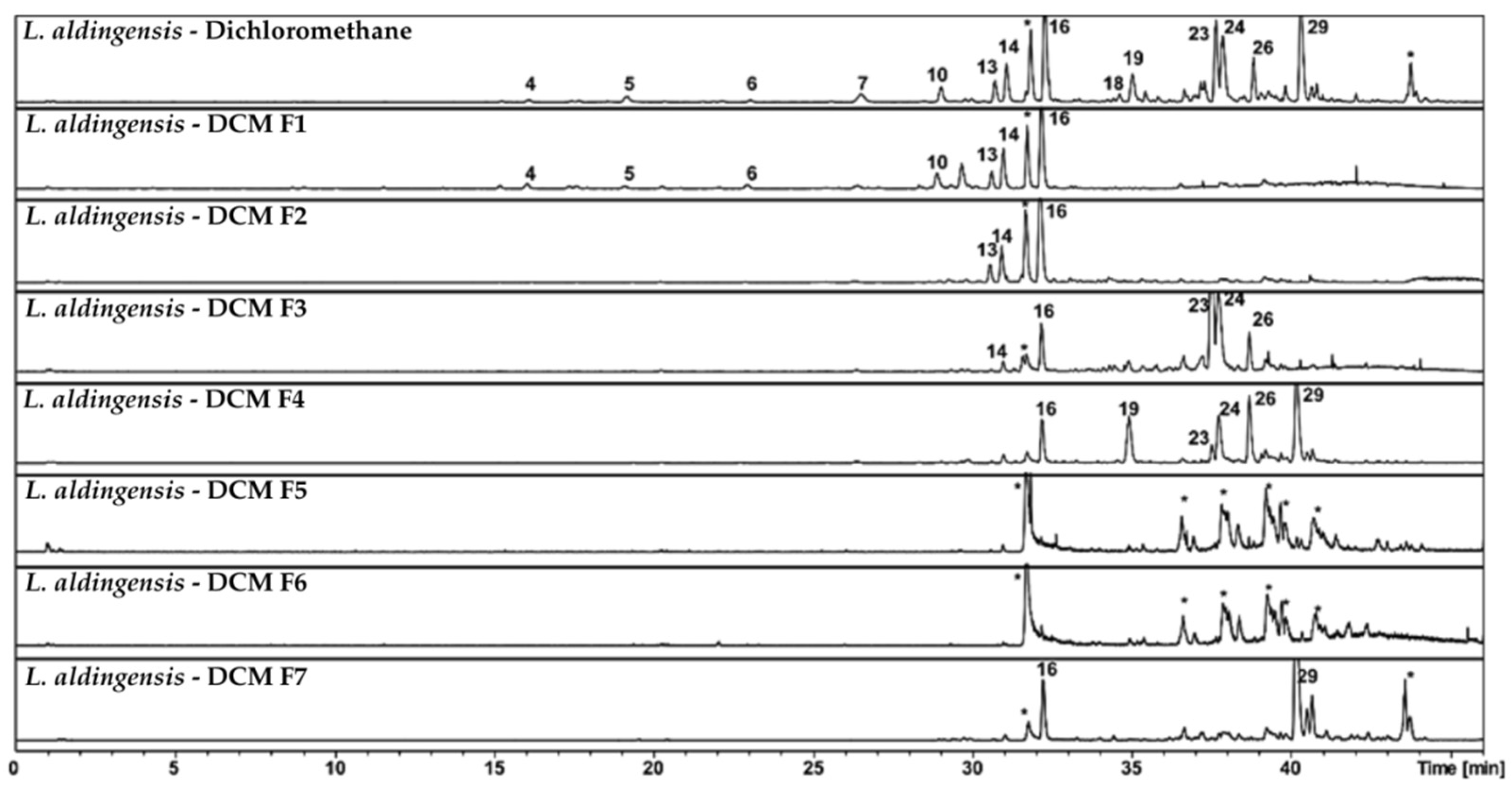
Figure 2 presents subfractions F1–F7, obtained through additional C8 column fractionation of the dichloromethane fraction (
L. aldingensis—DCM). It highlights the capacity of reversed-phase chromatography to discriminate molecules based on polarity and their affinity for the hydrophobic stationary phase.
In the original L. aldingensis—Dichloromethane fraction, we observed a wide array of compounds, including peaks 4, 5, 6, 7, 13, 14, 16, 18, 19, 23, 24, and 29. These represent a mixture of bromophenols, terpenoids, and other apolar compounds, as indicated by their respective retention times.
With refinement through reversed-phase fractionation, a clear segregation of components began to emerge. Subfraction DCM—F1 retained many of the more polar compounds from the original mixture, as evidenced by peaks 4, 5, and 6. This suggests that these metabolites exhibit moderate interaction with the C8 stationary phase and are eluted with less apolar solvents in the mobile phase.
Subfractions DCM—F2 and DCM—F3 began to show a shift toward more apolar compounds, such as peak 16 (furocaespitanelactol), which appears consistently across multiple fractions. The persistent presence of this compound suggests a significant affinity for the C18 stationary phase, requiring a stronger elution gradient to be displaced from the column, or alternatively, that it is present at high concentration, saturating the system and appearing in several subfractions.
Moving to subfractions DCM—F4 through DCM—F7, we observed a progressive decrease in compound diversity. In particular, DCM—F5 and DCM—F6 were predominantly composed of column related contaminants; however, it is also possible that these subfractions contain non-ionizable compounds that were not detected under the current MS conditions. Peak 16 remained dominant, and peak 29 occurred in DCM—F7, suggesting that this compound requires more specific elution conditions, possibly due to its higher hydrophobicity or larger molecular size.
These results illustrate the principle that reversed-phase C8 chromatography can be fine-tuned to separate compounds based on subtle differences in polarity and solubility. With appropriate optimization of the elution gradient, compounds such as furocaespitanelactol and aldingenin derivatives—which may possess biological or pharmaceutical relevance—can be effectively isolated for further analysis.
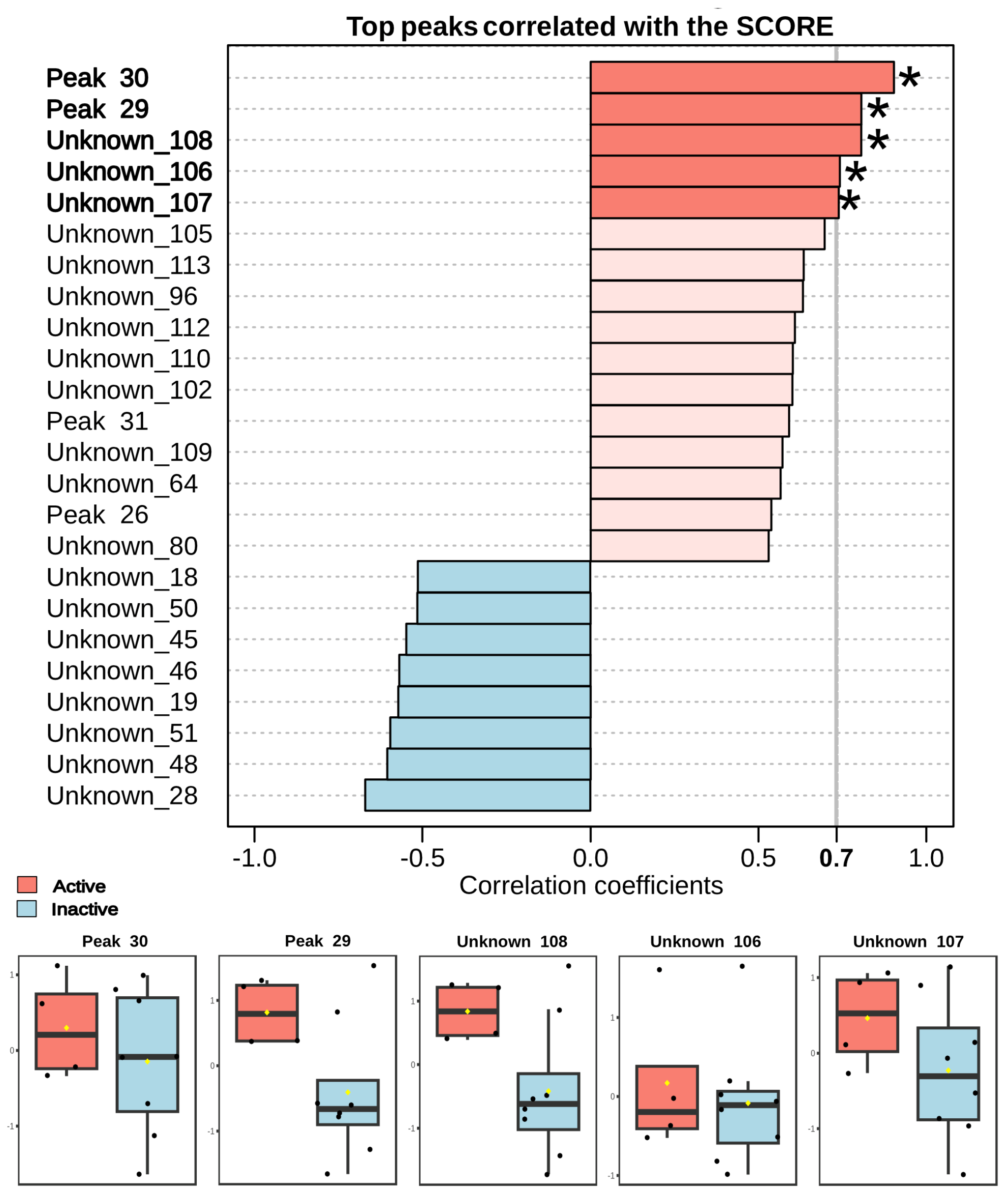
The Pearson correlation analysis (
Figure 3) clearly highlights specific chemical compounds highly associated with the observed anthelminthic activity in fractions of
Laurencia aldingensis. Notably, compounds annotated as Peak 30, Peak 29, Unknown_108, Unknown_106, and Unknown_107 demonstrated strong positive correlations (correlation coefficient > 0.7). Boxplots provided below the correlation graph further emphasize that these metabolites exhibit substantially higher intensities in active fractions compared to inactive ones, reinforcing their potential key role in the observed biological activity.
Furthermore, the unknown metabolites (Unknown_106, Unknown_107, and Unknown_108) also display high correlation, likely representative of novel bioactive compounds not previously reported. Future research focusing on the isolation, characterization, and biological evaluation of these metabolites is critical and could significantly advance therapeutic strategies against schistosomiasis. Conversely, metabolites showing negative correlations (e.g., Unknown_28, Unknown_48, Unknown_51) may serve as negative chemical markers to streamline and optimize bioactivity guided purification protocols.
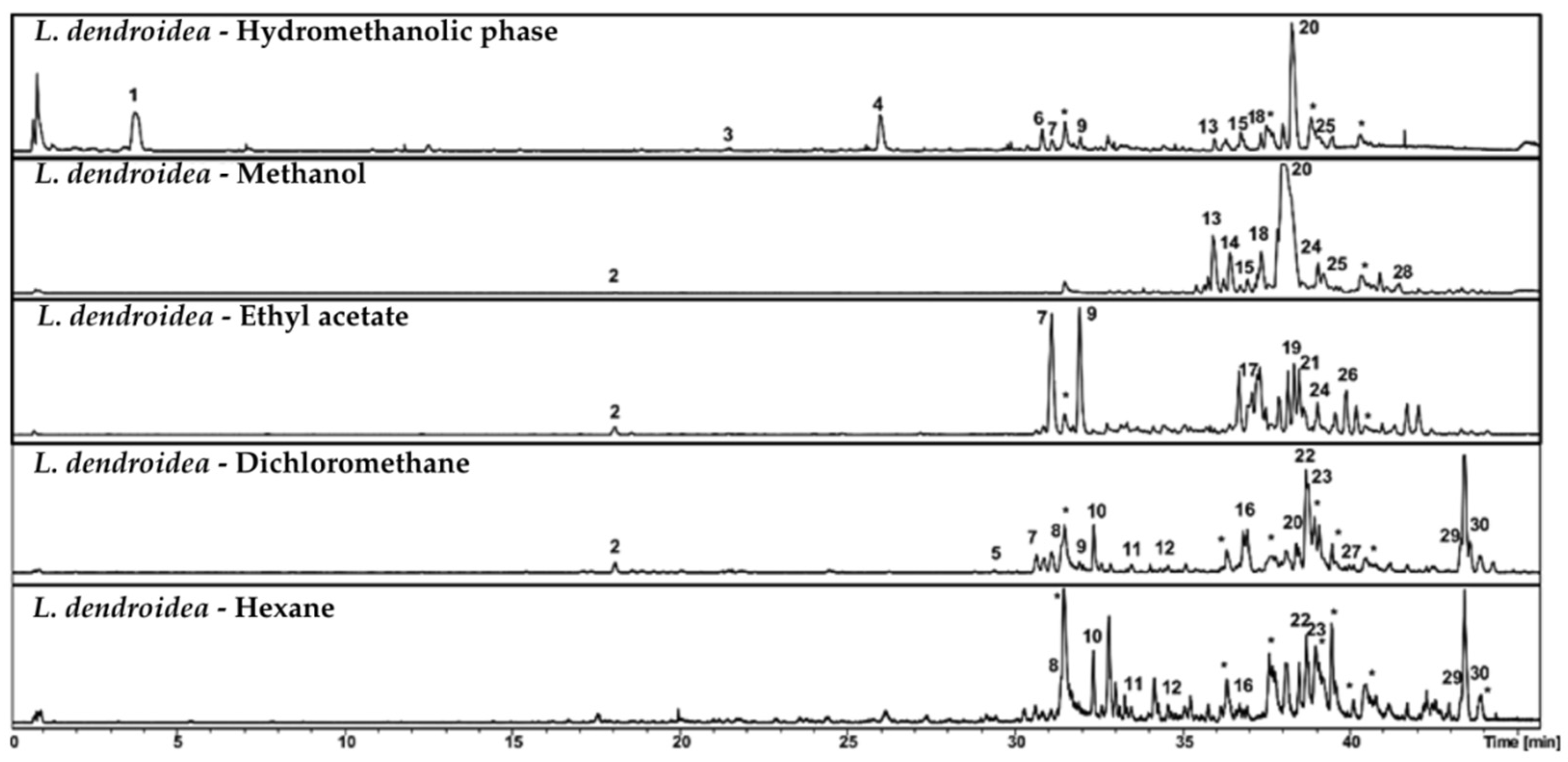
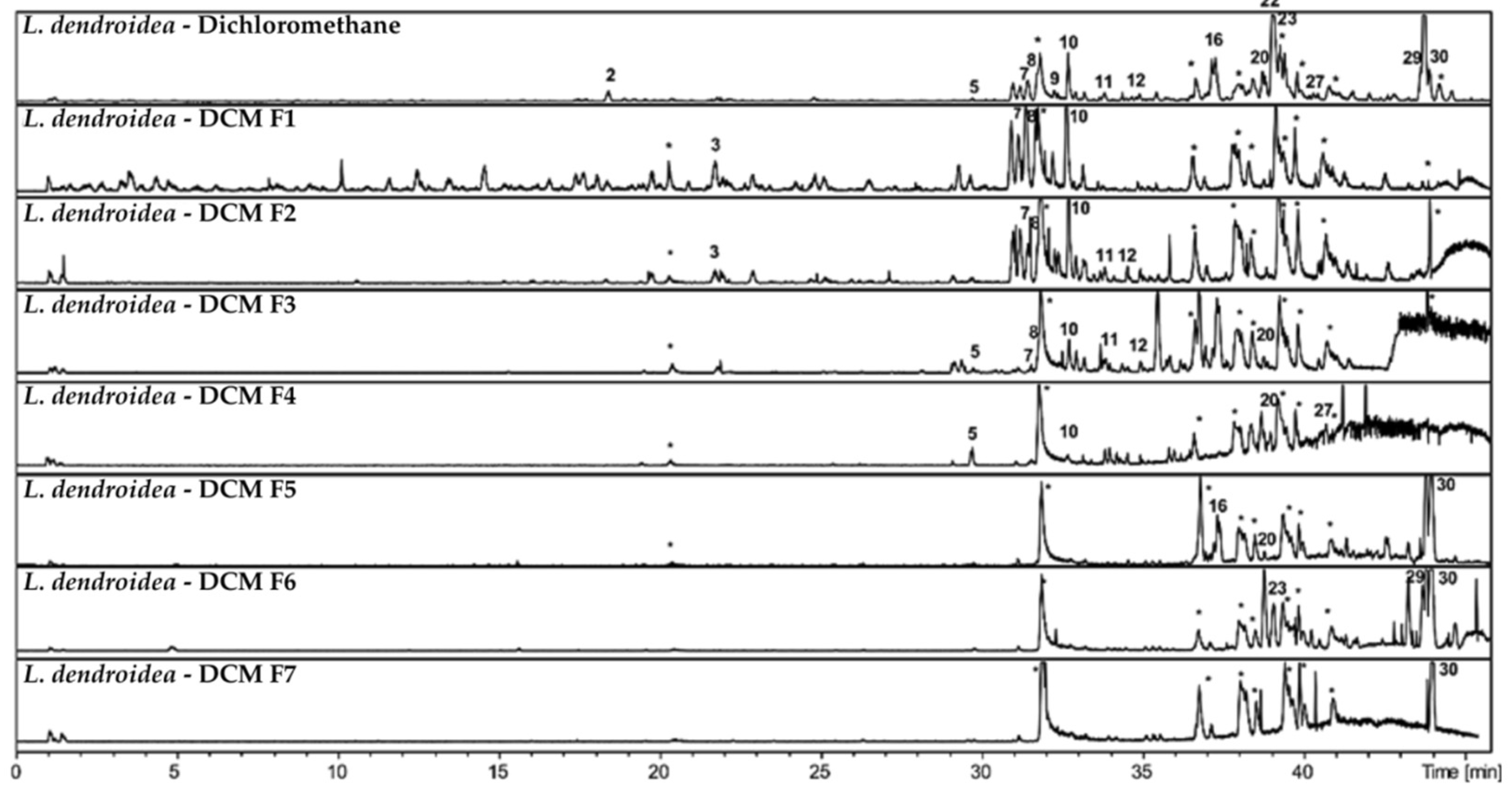
Chromatographic analysis by HPLC-DAD-MS/MS revealed a high chemical diversity in fractions from
Laurencia dendroidea, comparable to that observed in
L. aldingensis (
Figure 4 and
Figure 5). The dichloromethane fraction was selected for subfractionation due to its remarkable activity and its shared compounds with other fractions.
Major compounds in this fraction included oxygenated sesquiterpenes, fatty acid derivatives, and halogenated metabolites (
Table 7). Peaks 8 and 10 were annotated as laurecomin D and hydroxy laurecomin D, while Peaks 2, 7, 8, and 11 presented fragmentation patterns and molecular formulas consistent with yet undescribed sesquiterpenoid derivatives.
Among fatty acid derivatives, Peak 29 was identified as hydroxy eicosanoic acid [
34]. Peaks 22 and 23 were annotated as penicitide A isomers, a polyketide previously isolated from
Penicillium chrysogenum, an endophytic fungus associated with
Laurencia species [
35]. Peak 30 was identified as the marine-derived steroid muristeroid G [
36].
Chromatographic profiles of subfractions F1–F7 (
Figure 4), obtained from the dichloromethane fraction via reverse-phase C8 column, showed effective separation by polarity. More polar compounds were concentrated in the initial subfractions, while less polar compounds were found in later ones. Several peaks absent or at low intensity in the original fraction became enriched in the subfractions, highlighting the efficiency of the chromatographic process.
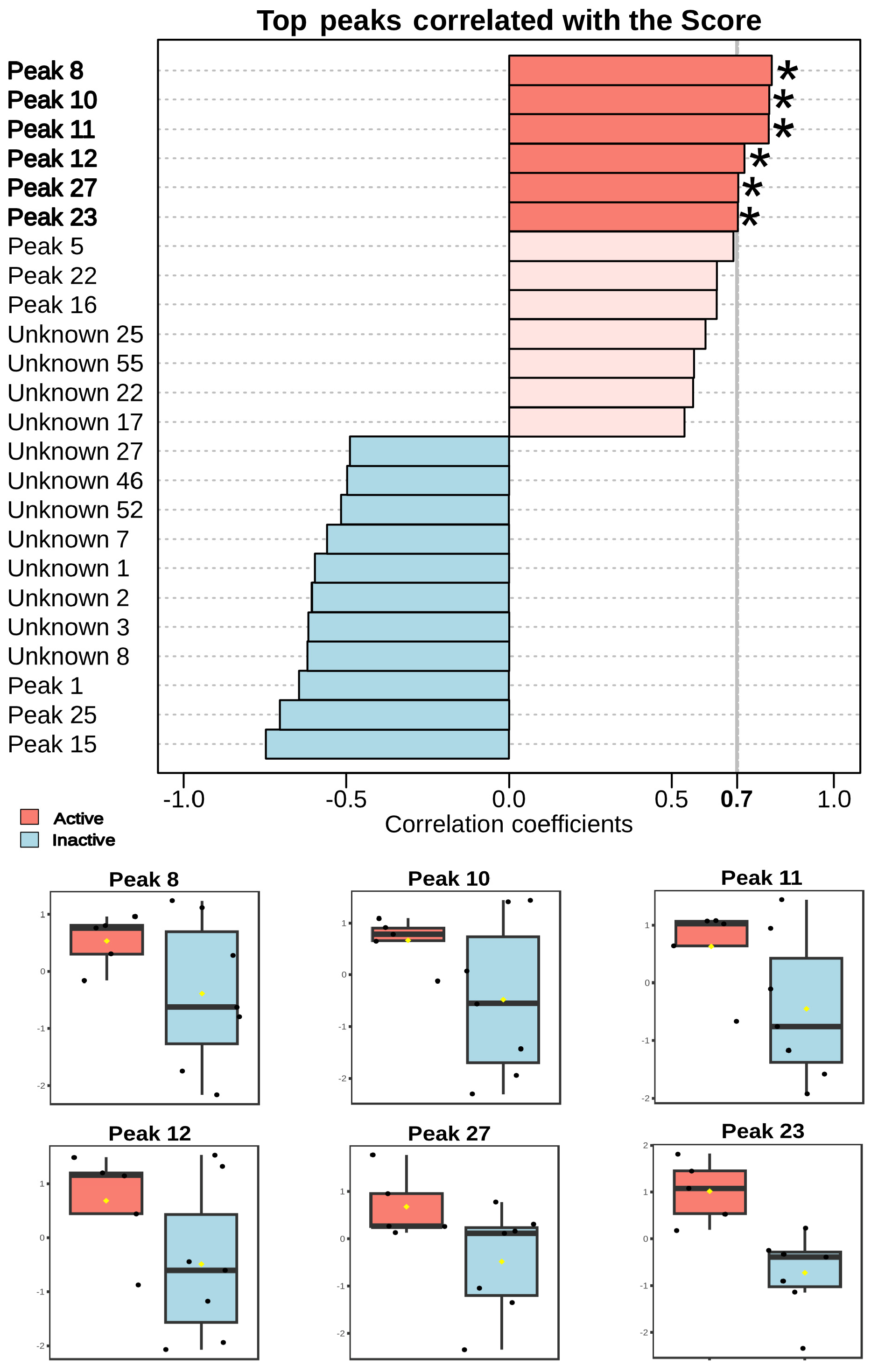
Statistical analysis revealed that several LC-MS peaks showed a significant positive correlation (
p < 0.05) with biological activity against
Schistosoma mansoni (
Figure 6).
One of most notable was penicitide A (23, r = 0.744;
p = 0.0056), which was exclusive to active fractions. Other significantly correlated peaks included 11 (r = 0.799;
p = 0.0018), 12 (r = 0.723;
p = 0.0078), and 27 (r = 0.704;
p = 0.011), all being halogenated compounds. Additionally, laurecoin D (8, r = 0.808;
p = 0.0015) and hydroxy laurecomin D (10, r = 0.800;
p = 0.0018) also showed strong correlations with biological activity. Both are halogenated sesquiterpenes previously reported in
Laurencia composita [
37].
The strong correlation between schistosomicidal activity and penicitide A (23) suggests a direct involvement of these compounds in the biological effect of
Laurencia dendroidea. Penicitide A belongs to a class of polyketides previously reported for antimicrobial properties, although its activity against
Schistosoma mansoni has not yet been described [
35]. The exclusive presence of 23 in active fractions supports its potential as a bioactive lead compound.
Peaks 11, 12, and 27, annotated as halogenated but uncharacterized molecules, also showed positive correlations with biological activity. The presence of bromine and chlorine atoms in their structures is consistent with typical metabolites from the
Laurencia genus, which are known for their biochemical diversity and broad pharmacological potential. Halogenated marine natural products have been associated with enzyme inhibition and disruption of membrane integrity in helminths [
38].
Sesquiterpenes such as laurecomin D and its hydroxylated derivative (10 and 8) also showed high correlation coefficients. These compounds have been previously described in
Laurencia composita with antimicrobial and cytotoxic activities [
37]. While their role in schistosomicidal activity remains to be confirmed, their presence in active fractions suggests potential involvement (
Figure 6).
The observed diversity of compounds and their distribution across fractions and subfractions suggests that the schistosomicidal effect may result from synergistic interactions among different classes of metabolites, particularly halogenated sesquiterpenes and fatty acid derivatives. This pattern is consistent with findings in L. aldingensis, where bioactivity has also been linked to chemical synergy.
Overall, these results underscore the pharmacological potential of Laurencia dendroidea as a source of novel schistosomicidal agents. Further work is needed to isolate and structurally characterize the peaks most strongly correlated with activity—especially the penicitide A—and to evaluate their bioactivity through in vitro and in vivo assays. The elucidation of yet uncharacterized halogenated metabolites (e.g., 11, 12, and 27) may also lead to the discovery of new marine-derived bioactive scaffolds.