Engineered Phage-Guided Nanotherapeutic Systems for Precision Antibacterial Therapy: Hacking Bacterial Resistance Mechanisms
Abstract
1. Introduction
2. Engineering Phage-Guided Nanocarriers to Enable Precise Pathogen Targeting
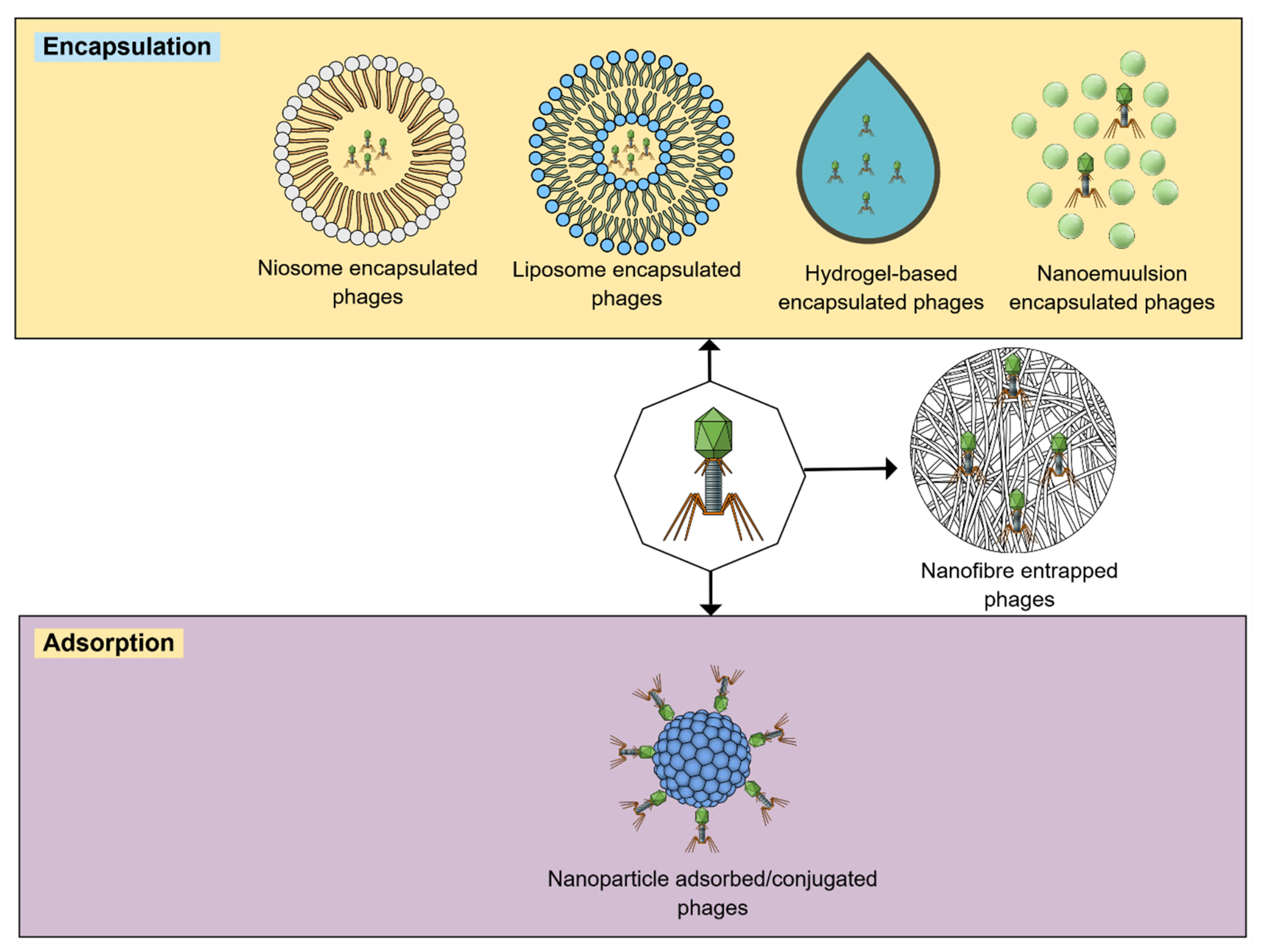
2.1. Encapsulation
2.2. Nanofibers
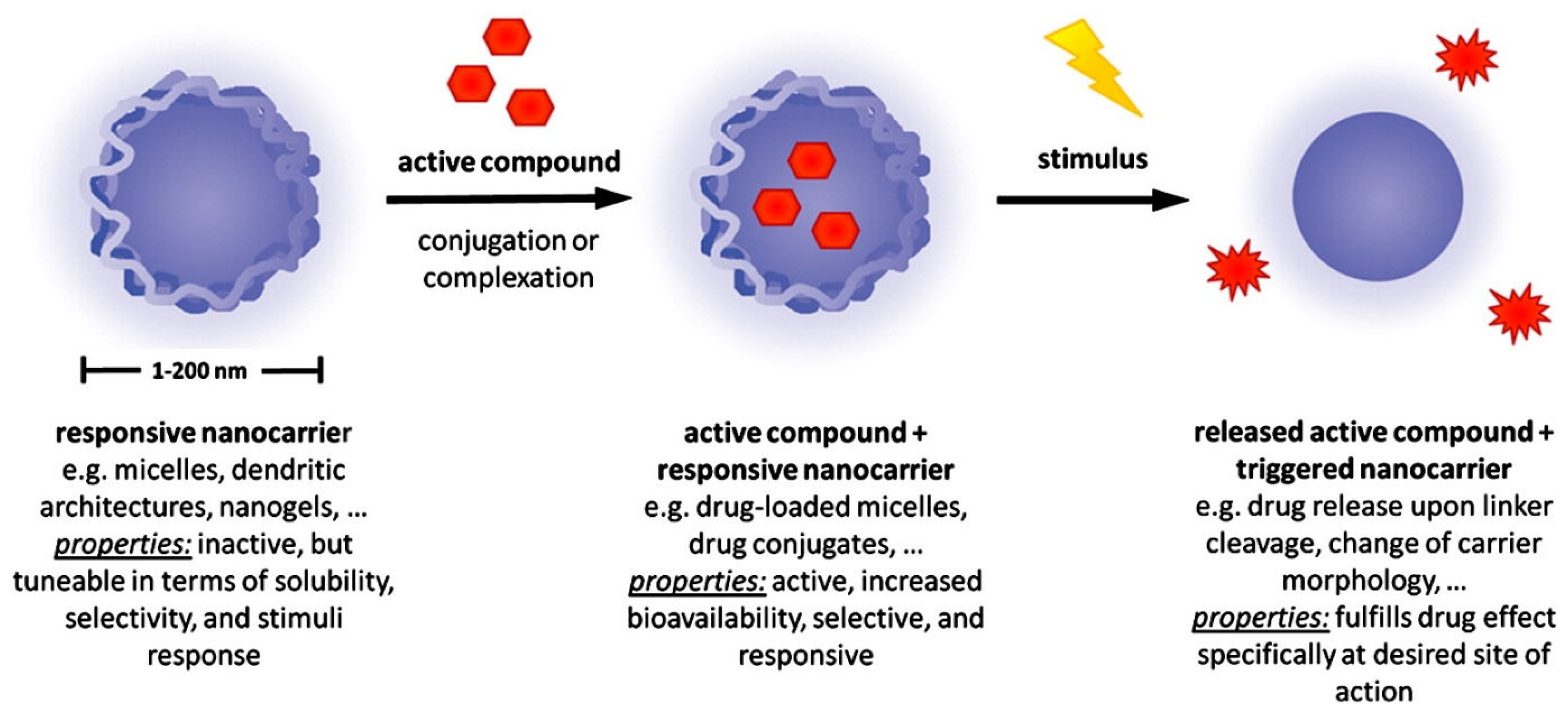
2.3. Stimuli-Responsive Nanocarriers
3. Mechanisms by Which Phage-Guided Nanocarriers Accurately Combat Bacterial Pathogens
4. Potential Applications of Engineered Phage-Guided Nanotherapeutic Systems for Precision Antibacterial Therapy
4.1. Management of Nosocomial Infections
4.2. Improved Wound Healing
4.3. Enhanced Antibacterial Activity
4.4. Disruption of Pathogenic Bacterial Biofilms
4.5. Enhanced Eradication of Bacteria by Using Phage/Nanocarrier Cocktails
5. Challenges and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—development, composition and regulation—therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef]
- Shinu, P.; Mouslem, A.K.A.; Nair, A.B.; Venugopala, K.N.; Attimarad, M.; Singh, V.A.; Nagaraja, S.; Alotaibi, G.; Deb, P.K. Progress report: Antimicrobial drug discovery in the resistance era. Pharmaceuticals 2022, 15, 413. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Pontes, J.T.C.d.; Toledo Borges, A.B.; Roque-Borda, C.A.; Pavan, F.R. Antimicrobial Peptides as an Alternative for the Eradication of Bacterial Biofilms of Multi-Drug Resistant Bacteria. Pharmaceutics 2022, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Abeleda, H.E.P.; Javier, A.P.; Murillo, A.Q.M.; Baculi, R.Q. Alpha-amylase conjugated biogenic silver nanoparticles as innovative strategy against biofilm-forming multidrug resistant bacteria. Biocatal. Agric. Biotechnol. 2020, 29, 101784. [Google Scholar] [CrossRef]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.d.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of Antibacterial Discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Szymczak, M.; Grygorcewicz, B.; Karczewska-Golec, J.; Decewicz, P.; Pankowski, J.A.; Országh-Szturo, H.; Bącal, P.; Dołęgowska, B.; Golec, P. Characterization of a Unique Bordetella bronchiseptica vB_BbrP_BB8 Bacteriophage and Its Application as an Antibacterial Agent. Int. J. Mol. Sci. 2020, 21, 1403. [Google Scholar] [CrossRef]
- Hopf, J.; Waters, M.; Kalwajtys, V.; Carothers, K.E.; Roeder, R.K.; Shrout, J.D.; Lee, S.W.; Nallathamby, P.D. Phage-mimicking antibacterial core–shell nanoparticles. Nanoscale Adv. 2019, 1, 4812–4826. [Google Scholar] [CrossRef]
- Aswani, V.H.; Shukla, S.K. An Early History of Phage Therapy in the United States: Is it Time to Reconsider? Clin. Med. Res. 2021, 19, 82–89. [Google Scholar] [CrossRef]
- Weaver, A.A.; Hasan, N.A.; Klaassen, M.; Karathia, H.; Colwell, R.R.; Shrout, J.D. Prosthetic joint infections present diverse and unique microbial communities using combined whole-genome shotgun sequencing and culturing methods. J. Med. Microbiol. 2019, 68, 1507–1516. [Google Scholar] [CrossRef]
- Bonine, N.G.; Berger, A.; Altincatal, A.; Wang, R.; Bhagnani, T.; Gillard, P.; Lodise, T. Impact of Delayed Appropriate Antibiotic Therapy on Patient Outcomes by Antibiotic Resistance Status From Serious Gram-negative Bacterial Infections. Am. J. Med. Sci. 2019, 357, 103–110. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Sachdeva, P.; Nath, G.; Jain, U. Phage based biosensors: Enhancing early detection of emerging pathogens in diagnostics. Talanta Open 2024, 10, 100345. [Google Scholar] [CrossRef]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Divya Ganeshan, S.; Hosseinidoust, Z. Phage Therapy with a Focus on the Human Microbiota. Antibiotics 2019, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Javaudin, F.; Bémer, P.; Batard, E.; Montassier, E. Impact of Phage Therapy on Multidrug-Resistant Escherichia coli Intestinal Carriage in a Murine Model. Microorganisms 2021, 9, 2580. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 457104. [Google Scholar] [CrossRef]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage—A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, 14, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Bullen, N.P.; Johnson, C.N.; Andersen, S.E.; Arya, G.; Marotta, S.R.; Lee, Y.-J.; Weigele, P.R.; Whitney, J.C.; Duerkop, B.A. An enterococcal phage protein inhibits type IV restriction enzymes involved in antiphage defense. Nat. Commun. 2024, 15, 6955. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, F.; Bai, J.; Che, Q.; Xiang, L.; Zhang, Z.; Wang, Y.; Sjöling, Å.; Martín–Rodríguez, A.J.; Zhu, B.; et al. Bacteriophage-resistant carbapenem-resistant Klebsiella pneumoniae shows reduced antibiotic resistance and virulence. Int. J. Antimicrob. Agents 2024, 64, 107221. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid. Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef]
- Cui, L.; Kiga, K.; Kondabagil, K.; Węgrzyn, A. Current and future directions in bacteriophage research for developing therapeutic innovations. Sci. Rep. 2024, 14, 24404. [Google Scholar] [CrossRef]
- Fernandes, P.; Martens, E. Antibiotics in late clinical development. Biochem. Pharmacol. 2017, 133, 152–163. [Google Scholar] [CrossRef]
- Pelfrene, E.; Willebrand, E.; Cavaleiro Sanches, A.; Sebris, Z.; Cavaleri, M. Bacteriophage therapy: A regulatory perspective. J. Antimicrob. Chemother. 2016, 71, 2071–2074. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Osman, A.H.; Kotey, F.C.N.; Odoom, A.; Darkwah, S.; Yeboah, R.K.; Dayie, N.; Donkor, E.S. The Potential of Bacteriophage-Antibiotic Combination Therapy in Treating Infections with Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Khan Mirzaei, M.; Deng, L. Comparative evaluation of long-term preservation methods for morphologically distinct bacteriophages. Microbiol. Spectr. 2025, 13, e0144224. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kumari, A.; Kumari Negi, A.; Galav, V.; Thakur, S.; Agrawal, M.; Sharma, V. Nanotechnology Based Approaches in Phage Therapy: Overcoming the Pharmacological Barriers. Front. Pharmacol. 2021, 12, 699054. [Google Scholar] [CrossRef] [PubMed]
- González-Menéndez, E.; Fernández, L.; Gutiérrez, D.; Pando, D.; Martínez, B.; Rodríguez, A.; García, P. Strategies to Encapsulate the Staphylococcus aureus Bacteriophage phiIPLA-RODI. Viruses 2018, 10, 495. [Google Scholar] [CrossRef]
- Kim, M.K.; Chen, Q.; Echterhof, A.; Pennetzdorfer, N.; McBride, R.C.; Banaei, N.; Burgener, E.B.; Milla, C.E.; Bollyky, P.L. A blueprint for broadly effective bacteriophage-antibiotic cocktails against bacterial infections. Nat. Commun. 2024, 15, 9987. [Google Scholar] [CrossRef]
- Kebriaei, R.; Lehman, S.M.; Shah, R.M.; Stamper, K.C.; Coyne, A.J.K.; Holger, D.; Ghali, A.E.; Rybak, M.J. Optimization of Phage-Antibiotic Combinations against Staphylococcus aureus Biofilms. Microbiol. Spectr. 2023, 11, e04918–e04922. [Google Scholar] [CrossRef]
- Akturk, E.; Melo, L.D.R.; Oliveira, H.; Crabbé, A.; Coenye, T.; Azeredo, J. Combining phages and antibiotic to enhance antibiofilm efficacy against an in vitro dual species wound biofilm. Biofilm 2023, 6, 100147. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Gorji, M.; Alavigeh, M.Z.; Moghadam, M.T. Genetically engineered phages and engineered phage-derived enzymes to destroy biofilms of antibiotics resistance bacteria. Heliyon 2024, 10, e35666. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Aldhubiab, B.; Almuqbil, R.M.; Nair, A.B. Harnessing the Power of Nanocarriers to Exploit the Tumor Microenvironment for Enhanced Cancer Therapy. Pharmaceuticals 2025, 18, 746. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Nair, A.B.; Al-Dhubiab, B.E. 6—Dendrimers: An effective drug delivery and therapeutic approach. In Design and Applications of Theranostic Nanomedicines; Ray, S., Nayak, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 125–142. [Google Scholar]
- Jacob, S.; Nair, A.; Boddu, S.; Abuhijjleh, R.; Selvaraju, K.; Babu, T.; Gorain, B.; Shah, J.; Morsy, M. The emerging role of lipid nanosystems and nanomicelles in liver diseases. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8651–8680. [Google Scholar]
- Zhu, J.; Xie, R.; Gao, R.; Zhao, Y.; Yodsanit, N.; Zhu, M.; Burger, J.C.; Ye, M.; Tong, Y.; Gong, S. Multimodal nanoimmunotherapy engages neutrophils to eliminate Staphylococcus aureus infections. Nat. Nanotechnol. 2024, 19, 1032–1043. [Google Scholar] [CrossRef]
- Szymczak, M.; Pankowski, J.A.; Kwiatek, A.; Grygorcewicz, B.; Karczewska-Golec, J.; Sadowska, K.; Golec, P. An effective antibiofilm strategy based on bacteriophages armed with silver nanoparticles. Sci. Rep. 2024, 14, 9088. [Google Scholar] [CrossRef]
- Gandhi, S.; Shastri, D.H.; Shah, J.; Nair, A.B.; Jacob, S. Nasal Delivery to the Brain: Harnessing Nanoparticles for Effective Drug Transport. Pharmaceutics 2024, 16, 481. [Google Scholar] [CrossRef]
- Shinu, P.; Nair, A.B.; Kumari, B.; Jacob, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Venugopala, K.N.; Attimarad, M.; Nagaraja, S. Recent Advances and Appropriate use of Niosomes for the Treatment of Skin Cancer. Indian J. Pharm. Educ. Res. 2022, 56, 1–14. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Międzybrodzki, R.; Drulis-Kawa, Z.; Cater, K.; Knežević, P.; Winogradow, C.; Amaro, K.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Rękas, J.; et al. Bacteriophages and antibiotic interactions in clinical practice: What we have learned so far. J. Biomed. Sci. 2022, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef] [PubMed]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Loh, B.; Gondil, V.S.; Manohar, P.; Khan, F.M.; Yang, H.; Leptihn, S. Encapsulation and Delivery of Therapeutic Phages. Appl. Environ. Microbiol. 2021, 87, e01979-20. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Abdelrahman, F.; Dawoud, A.; Connerton, I.F.; El-Shibiny, A. Encapsulation of E. coli phage ZCEC5 in chitosan–alginate beads as a delivery system in phage therapy. AMB Express 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e02071–e02121. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Singla, S.; Harjai, K.; Katare, O.P.; Chhibber, S. Bacteriophage-Loaded Nanostructured Lipid Carrier: Improved Pharmacokinetics Mediates Effective Resolution of Klebsiella pneumoniae–Induced Lobar Pneumonia. J. Infect. Dis. 2015, 212, 325–334. [Google Scholar] [CrossRef]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 2017, 43, 1532–1543. [Google Scholar] [CrossRef]
- Colom, J.; Cano-Sarabia, M.; Otero, J.; Cortés, P.; Maspoch, D.; Llagostera, M. Liposome-Encapsulated Bacteriophages for Enhanced Oral Phage Therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef]
- Singla, S.; Harjai, K.; Katare, O.P.; Chhibber, S. Encapsulation of Bacteriophage in Liposome Accentuates Its Entry in to Macrophage and Shields It from Neutralizing Antibodies. PLoS ONE 2016, 11, e0153777. [Google Scholar] [CrossRef]
- Chhibber, S.; Kaur, J.; Kaur, S. Liposome Entrapment of Bacteriophages Improves Wound Healing in a Diabetic Mouse MRSA Infection. Front. Microbiol. 2018, 9, 561. [Google Scholar] [CrossRef]
- Leung, S.S.Y.; Morales, S.; Britton, W.; Kutter, E.; Chan, H.-K. Microfluidic-assisted bacteriophage encapsulation into liposomes. Int. J. Pharm. 2018, 545, 176–182. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Sriwidodo; Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon 2022, 8, e08934. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, X.; Zhang, H.; Watts, A.B.; Ghosh, D. Manufacturing and ambient stability of shelf freeze dried bacteriophage powder formulations. Int. J. Pharm. 2018, 542, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, H.; Zou, G.; Song, Z.; Zhou, Y.; Li, H.; Tan, C.; Chen, H.; Fischetti, V.A.; Li, J. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Control. Release 2023, 353, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Vila, M.M.D.C.; Lima, R.; Del Fiol, F.S.; Tubino, M.; Teixeira, J.A.; Balcão, V.M. Structural and functional stabilization of bacteriophage particles within the aqueous core of a W/O/W multiple emulsion: A potential biotherapeutic system for the inhalational treatment of bacterial pneumonia. Process Biochem. 2018, 64, 177–192. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. New York Acad. Sci. USA 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Marchianò, V.; Duarte, A.C.; Agún, S.; Luque, S.; Marcet, I.; Fernández, L.; Matos, M.; Blanco, M.D.C.; García, P.; Gutiérrez, G. Phage Lytic Protein CHAPSH3b Encapsulated in Niosomes and Gelatine Films. Microorganisms 2024, 12, 119. [Google Scholar] [CrossRef]
- Bai, H.; Borjihan, Q.; Li, Z.; Qin, P.; Cheng, J.; Xiao, D.; Dong, A. Phage-Based antibacterial hydrogels for bacterial targeting and Ablation: Progress and perspective. Eur. J. Pharm. Biopharm. 2024, 198, 114258. [Google Scholar] [CrossRef]
- Abed, S.; Beig, M.; Barzi, S.M.; Shafiei, M.; Hashemi Shahraki, A.; Sadeghi, S.; Sohrabi, A. Development of phage-containing hydrogel for treating Enterococcus faecalis-infected wounds. PLoS ONE 2024, 19, e0312469. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Shah, J.; Nair, A.B. Innovations in Nanoemulsion Technology: Enhancing Drug Delivery for Oral, Parenteral, and Ophthalmic Applications. Pharmaceutics 2024, 16, 1333. [Google Scholar] [CrossRef]
- Franklyne, J.S.; Iyer, S.; Ebenazer, A.; Mukherjee, A.; Chandrasekaran, N. Essential oil nanoemulsions: Antibacterial activity in contaminated fruit juices. Int. J. Food Sci. Technol. 2019, 54, 2802–2810. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef] [PubMed]
- Vladisavljević, G.T.; Al Nuumani, R.; Nabavi, S.A. Microfluidic Production of Multiple Emulsions. Micromachines 2017, 8, 75. [Google Scholar] [CrossRef]
- Ziani, K.; Chang, Y.; McLandsborough, L.; McClements, D.J. Influence of Surfactant Charge on Antimicrobial Efficacy of Surfactant-Stabilized Thyme Oil Nanoemulsions. J. Agric. Food Chem. 2011, 59, 6247–6255. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A. Electrospun Nanofibers as Carriers of Microorganisms, Stem Cells, Proteins, and Nucleic Acids in Therapeutic and Other Applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun nanofibers: Exploring process parameters, polymer selection, and recent applications in pharmaceuticals and drug delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Nogueira, F.; Karumidze, N.; Kusradze, I.; Goderdzishvili, M.; Teixeira, P.; Gouveia, I.C. Immobilization of bacteriophage in wound-dressing nanostructure. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2475–2484. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, L.; Wang, J.; Jin, Q.; Ji, J. Stimuli-responsive nanoplatforms for antibacterial applications. WIREs Nanomed. Nanobiotechnology 2022, 14, e1775. [Google Scholar] [CrossRef]
- Jacob, S.; Rao, R.; Gorain, B.; Boddu, S.H.S.; Nair, A.B. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Anticancer Phytochemical Delivery: Advances, Challenges, and Future Prospects. Pharmaceutics 2025, 17, 1079. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, W.; Zhang, X.; Song, Z.; Tong, T. An Overview of Stimuli-Responsive Intelligent Antibacterial Nanomaterials. Pharmaceutics 2023, 15, 2113. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A technology evaluation. Expert. Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kumria, R.; Nair, A.B.; Al-Dhubiab, B.E. Loratidine buccal films for allergic rhinitis: Development and evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A Systematic Overview of Eudragit® Based Copolymer for Smart Healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Vinner, G.K.; Vladisavljević, G.T.; Clokie, M.R.J.; Malik, D.J. Microencapsulation of Clostridium difficile specific bacteriophages using microfluidic glass capillary devices for colon delivery using pH triggered release. PLoS ONE 2017, 12, e0186239. [Google Scholar] [CrossRef]
- Vinner, G.K.; Richards, K.; Leppanen, M.; Sagona, A.P.; Malik, D.J. Microencapsulation of Enteric Bacteriophages in a pH-Responsive Solid Oral Dosage Formulation Using a Scalable Membrane Emulsification Process. Pharmaceutics 2019, 11, 475. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Hathaway, H.; Alves, D.R.; Bean, J.; Esteban, P.P.; Ouadi, K.; Mark Sutton, J.; Jenkins, A.T.A. Poly(N-isopropylacrylamide-co-allylamine) (PNIPAM-co-ALA) nanospheres for the thermally triggered release of Bacteriophage K. Eur. J. Pharm. Biopharm. 2015, 96, 437–441. [Google Scholar] [CrossRef]
- Hathaway, H.; Ajuebor, J.; Stephens, L.; Coffey, A.; Potter, U.; Sutton, J.M.; Jenkins, A.T.A. Thermally triggered release of the bacteriophage endolysin CHAPK and the bacteriocin lysostaphin for the control of methicillin resistant Staphylococcus aureus (MRSA). J. Control. Release 2017, 245, 108–115. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Z.; Shi, J.; Zhang, X.; Zhang, F. Targeted polymeric drug delivery systems with stimuli-responsive release capabilities: Status and future perspectives. Nanomedicine 2025, 2025, 1–4. [Google Scholar] [CrossRef]
- Fatima, F.; Siddiqui, S.; Khan, W.A. Nanoparticles as Novel Emerging Therapeutic Antibacterial Agents in the Antibiotics Resistant Era. Biol. Trace Elem. Res. 2021, 199, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cao, F.; Gao, Y.; Zhang, C.; Qian, Z.; Zhang, J.; Mao, Z. Microenvironment-Activated Nanozyme-Armed Bacteriophages Efficiently Combat Bacterial Infection. Adv. Mater. 2023, 35, 2301349. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, I.A. Rapid Colorimetric Detection of Bacterial Species through the Capture of Gold Nanoparticles by Chimeric Phages. ACS Nano 2019, 13, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Xu, X.; Noman, M.; Wang, Q.; Li, B. Phage-guided nanocarriers: A precision strategy against bacterial pathogens. Trends Biotechnol. 2025, 43, 494–497. [Google Scholar] [CrossRef]
- Eugster, M.R.; Haug, M.C.; Huwiler, S.G.; Loessner, M.J. The cell wall binding domain of Listeria bacteriophage endolysin PlyP35 recognizes terminal GlcNAc residues in cell wall teichoic acid. Mol. Microbiol. 2011, 81, 1419–1432. [Google Scholar] [CrossRef]
- Peng, H.; Borg, R.E.; Dow, L.P.; Pruitt, B.L.; Chen, I.A. Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages. Proc. Natl. Acad. Sci. USA 2020, 117, 1951–1961. [Google Scholar] [CrossRef]
- Yan, J.; Lyu, X.; Jiang, Y.; Ng, K.R.; Yang, R.; Zhang, F.; Zhao, W. Precise Photothermal Treatment of Methicillin-Resistant S. aureus Infection via Phage Lysin-Cell Binding Domain-Modified Gold Nanosheets. ACS Appl. Mater. Interfaces 2023, 15, 6514–6525. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Pletzer, D.; Mansour, S.C.; Wuerth, K.; Rahanjam, N.; Hancock, R.E. New Mouse Model for Chronic Infections by Gram-Negative Bacteria Enabling the Study of Anti-Infective Efficacy and Host-Microbe Interactions. mBio 2017, 8, e00140-17. [Google Scholar] [CrossRef]
- Ciepluch, K.; Maciejewska, B.; Gałczyńska, K.; Kuc-Ciepluch, D.; Bryszewska, M.; Appelhans, D.; Drulis-Kawa, Z.; Arabski, M. The influence of cationic dendrimers on antibacterial activity of phage endolysin against P. aeruginosa cells. Bioorg. Chem. 2019, 91, 103121. [Google Scholar] [CrossRef] [PubMed]
- Shemyakin, I.G.; Firstova, V.V.; Fursova, N.K.; Abaev, I.V.; Filippovich, S.Y.; Ignatov, S.G.; Dyatlov, I.A. Next-Generation Antibiotics, Bacteriophage Endolysins, and Nanomaterials for Combating Pathogens. Biochemistry 2020, 85, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Klekotka, U.; Zambrzycka, M.; Zambrowski, G.; Święcicka, I.; Kalska-Szostko, B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. 2023, 400, 133960. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Lee, S.-Y.; Siddiqi, M.Z.; Balusamy, S.R.; Ashrafudoulla, M.; Rupa, E.J.; Huq, M.A. Ecofriendly Synthesis of Silver Nanoparticles by Terrabacter humi sp. nov. and Their Antibacterial Application against Antibiotic-Resistant Pathogens. Int. J. Mol. Sci. 2020, 21, 9746. [Google Scholar] [CrossRef]
- Martínez Espinosa, J.C.; Carrera Cerritos, R.; Ramírez Morales, M.A.; Sánchez Guerrero, K.P.; Silva Contreras, R.A.; Macías, J.H. Characterization of Silver Nanoparticles Obtained by a Green Route and Their Evaluation in the Bacterium of Pseudomonas aeruginosa. Crystals 2020, 10, 395. [Google Scholar] [CrossRef]
- Ramírez Saenz, D.; Martínez Espinosa, J.C.; Mancillas, A.G.V.; Lechuga Arana, A.A.; Silva Contreras, R.A.; Gutiérrez Chávez, A.J. Silver Nanoparticles Conjugated with BK510Lys Endolysin for Gram-Negative Bacteria Inhibition. Appl. Sci. 2024, 14, 6493. [Google Scholar] [CrossRef]
- Piewngam, P.; Otto, M. Staphylococcus aureus colonisation and strategies for decolonisation. Lancet Microbe 2024, 5, e606–e618. [Google Scholar] [CrossRef]
- Linz, M.S.; Mattappallil, A.; Finkel, D.; Parker, D. Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics 2023, 12, 557. [Google Scholar] [CrossRef]
- Almuqbil, R.M.; Aldhubiab, B. Bioadhesive Nanoparticles in Topical Drug Delivery: Advances, Applications, and Potential for Skin Disorder Treatments. Pharmaceutics 2025, 17, 229. [Google Scholar] [CrossRef]
- Lacey, K.A.; Geoghegan, J.A.; McLoughlin, R.M. The Role of Staphylococcus aureus Virulence Factors in Skin Infection and Their Potential as Vaccine Antigens. Pathogens 2016, 5, 22. [Google Scholar] [CrossRef]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E. Apitherapeutics and phage-loaded Nanofibers As Wound Dressings With Enhanced Wound Healing and Antibacterial Activity. Nanomedicine 2017, 12, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Najafpour, Z.; Cheraghian, B.; Keliddar, I.; Mombeyni, R. The Extra Length of Stay, Costs, and Mortality Associated With Healthcare-Associated Infections: A Case-Control Study. Health Sci. Rep. 2024, 7, e70168. [Google Scholar] [CrossRef]
- Faruk, O.; Jewel, Z.A.; Bairagi, S.; Rasheduzzaman, M.; Bagchi, H.; Tuha, A.S.M.; Hossain, I.; Bala, A.; Ali, S. Phage treatment of multidrug-resistant bacterial infections in humans, animals, and plants: The current status and future prospects. Infect. Med. 2025, 4, 100168. [Google Scholar] [CrossRef]
- Esteban, P.P.; Alves, D.R.; Enright, M.C.; Bean, J.E.; Gaudion, A.; Jenkins, A.T.A.; Young, A.E.R.; Arnot, T.C. Enhancement of the antimicrobial properties of bacteriophage-K via stabilization using oil-in-water nano-emulsions. Biotechnol. Prog. 2014, 30, 932–944. [Google Scholar] [CrossRef]
- Zheng, K.; Sui, B.; Ilyas, K.; Boccaccini, A.R. Porous bioactive glass micro- and nanospheres with controlled morphology: Developments, properties and emerging biomedical applications. Mater. Horiz. 2021, 8, 300–335. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Meng, X.; Xu, Z.; Wang, C.; Patitz, J.; Boccaccini, A.R.; Burkovski, A.; Zheng, K. Surface engineering of mesoporous bioactive glass nanoparticles with bacteriophages for enhanced antibacterial activity. Colloids Surf. B Biointerfaces 2024, 234, 113714. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Yakoup, A.Y.; Khaled, Y.; Safwat, A.; El-Shibiny, A. The synergistic effect of using bacteriophages and chitosan nanoparticles against pathogenic bacteria as a novel therapeutic approach. Int. J. Biol. Macromol. 2023, 228, 374–384. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Leung, C.Y.; Henry, M.; Morello, E.; Singh, D.; Di Santo, J.P.; Weitz, J.S.; Debarbieux, L. Synergy between the Host Immune System and Bacteriophage Is Essential for Successful Phage Therapy against an Acute Respiratory Pathogen. Cell Host Microbe 2017, 22, 38–47.e34. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Bleriot, I.; González de Aledo, M.; Fernández-García, L.; Pacios, O.; Oliveira, H.; López, M.; Ortiz-Cartagena, C.; Fernández-Cuenca, F.; Pascual, Á.; et al. Development of an Anti-Acinetobacter baumannii Biofilm Phage Cocktail: Genomic Adaptation to the Host. Antimicrob. Agents Chemother. 2022, 66, e0192321. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Ivanova, K.; Hoyo, J.; Perelshtein, I.; Owen, G.; Haegert, A.; Lin, Y.-Y.; LeBihan, S.; Gedanken, A.; Häfeli, U.O.; et al. Novel Lignin-Capped Silver Nanoparticles against Multidrug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 22098–22109. [Google Scholar] [CrossRef]
- Pareek, V.; Devineau, S.; Sivasankaran, S.K.; Bhargava, A.; Panwar, J.; Srikumar, S.; Fanning, S. Silver Nanoparticles Induce a Triclosan-Like Antibacterial Action Mechanism in Multi-Drug Resistant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 638640. [Google Scholar] [CrossRef]
- Gouyau, J.; Duval, R.E.; Boudier, A.; Lamouroux, E. Investigation of Nanoparticle Metallic Core Antibacterial Activity: Gold and Silver Nanoparticles against Escherichia coli and Staphylococcus aureus. Int. J. Mol. Sci. 2021, 22, 1905. [Google Scholar] [CrossRef]
- Najjuka, C.F.; Kateete, D.P.; Kajumbula, H.M.; Joloba, M.L.; Essack, S.Y. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes 2016, 9, 235. [Google Scholar] [CrossRef]
- Szymczak, M.; Golec, P. Long-Term Effectiveness of Engineered T7 Phages Armed with Silver Nanoparticles Against Escherichia coli Biofilm. Int. J. Nanomed. 2024, 19, 10097–10105. [Google Scholar] [CrossRef]
- Wang, L.; Tkhilaishvili, T.; Jiang, Z.; Pirlar, R.F.; Ning, Y.; Millán Laleona, A.; Wang, J.; Tang, J.; Wang, Q.; Trampuz, A.; et al. Phage-liposome nanoconjugates for orthopedic biofilm eradication. J. Control. Release 2024, 376, 949–960. [Google Scholar] [CrossRef]
- Wdowiak, M.; Raza, S.; Grotek, M.; Zbonikowski, R.; Nowakowska, J.; Doligalska, M.; Cai, N.; Luo, Z.; Paczesny, J. Phage/nanoparticle cocktails for a biocompatible and environmentally friendly antibacterial therapy. Appl. Microbiol. Biotechnol. 2025, 109, 129. [Google Scholar] [CrossRef]
- Ling, H.; Lou, X.; Luo, Q.; He, Z.; Sun, M.; Sun, J. Recent advances in bacteriophage-based therapeutics: Insight into the post-antibiotic era. Acta Pharm. Sin. B 2022, 12, 4348–4364. [Google Scholar] [CrossRef]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Piątek, B. Phage Therapy-Challenges, Opportunities and Future Prospects. Pharmaceuticals 2023, 16, 1638. [Google Scholar] [CrossRef]
- Terwilliger, A.L.; Gu Liu, C.; Green, S.I.; Clark, J.R.; Salazar, K.C.; Hernandez Santos, H.; Heckmann, E.R.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research: Personalized Infectious Disease Medicine for the Most Vulnerable At-Risk Patients. Phage 2020, 1, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Kruger, D.H. Approaches to optimize therapeutic bacteriophage and bacteriophage-derived products to combat bacterial infections. Virus Genes 2020, 56, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Varghese, D.; Pabary, R.; Langley, R.J. The potential of bacteriophage therapy in the treatment of paediatric respiratory infections. Paediatr. Respir. Rev. 2022, 44, 70–77. [Google Scholar] [CrossRef]
- Melo, L.D.R.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef]
- Segall, A.M.; Roach, D.R.; Strathdee, S.A. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Curr. Opin. Microbiol. 2019, 51, 46–50. [Google Scholar] [CrossRef]
- Kingwell, K. Bacteriophage therapies re-enter clinical trials. Nat. Rev. Drug Discov. 2015, 14, 515–516. [Google Scholar] [CrossRef]
- Kakkar, A.; Kandwal, G.; Nayak, T.; Jaiswal, L.K.; Srivastava, A.; Gupta, A. Engineered bacteriophages: A panacea against pathogenic and drug resistant bacteria. Heliyon 2024, 10, e34333. [Google Scholar] [CrossRef]
- Botka, T.; Pantůček, R.; Mašlaňová, I.; Benešík, M.; Petráš, P.; Růžičková, V.; Havlíčková, P.; Varga, M.; Žemličková, H.; Koláčková, I.; et al. Lytic and genomic properties of spontaneous host-range Kayvirus mutants prove their suitability for upgrading phage therapeutics against staphylococci. Sci. Rep. 2019, 9, 5475. [Google Scholar] [CrossRef]
- García, R.; Latz, S.; Romero, J.; Higuera, G.; García, K.; Bastías, R. Bacteriophage Production Models: An Overview. Front. Microbiol. 2019, 10, 1187. [Google Scholar] [CrossRef]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef]
- Anyaegbunam, N.J.; Anekpo, C.C.; Anyaegbunam, Z.K.G.; Doowuese, Y.; Chinaka, C.B.; Odo, O.J.; Sharndama, H.C.; Okeke, O.P.; Mba, I.E. The resurgence of phage-based therapy in the era of increasing antibiotic resistance: From research progress to challenges and prospects. Microbiol. Res. 2022, 264, 127155. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e288. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mirshekari, H.; Moosavi Basri, S.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Caflisch, K.M.; Suh, G.A.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert. Rev. Anti Infect. Ther. 2019, 17, 1011–1041. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid. Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Kumar, R.N.; Srilatha, P.; Muhammad, T.; Nagaraja, K.V.; Karthik, K.; Kumar, R.; Gowda, R.J.P. Numerical Study on Nanoparticles Aggregation with Brownian Motion in Fluid Flow Induced by Squeezing Porous Slider. BioNanoScience 2024, 14, 2446–2456. [Google Scholar] [CrossRef]
- Lim, S.H.; Wong, T.W.; Tay, W.X. Overcoming colloidal nanoparticle aggregation in biological milieu for cancer therapeutic delivery: Perspectives of materials and particle design. Adv. Colloid. Interface Sci. 2024, 325, 103094. [Google Scholar] [CrossRef]


| Features | Antibiotic Therapy | Phage Therapy | References |
|---|---|---|---|
| Antimicrobial spectrum | Broad-spectrum, thus affects more than the targeted organism | Selectively infect and eliminate bacteria with a high degree of specificity. | [17] |
| Disruption of beneficial microbiota | Disrupts the balance of the host’s beneficial microbiome, resulting in dysbiosis and potential secondary infections | Minimal impact on the beneficial microbiome | [18,19] |
| Dosing | Continuous dosing is required to maintain therapeutic levels in the body | Self-amplification in target bacteria after initial administration and enhanced concentration at the target site, therefore, reduces the frequency of administration | [20,21] |
| Anti-biofilm activity | Ineffective/less effective | Effectively penetrates and destroys biofilms | [22] |
| Resistance Development | More recurrently used to treat infections; therefore, there is an increased occurrence of a wide range of bacteria developing resistance | Phages co-evolve with bacteria, therefore limited chances of developing resistance | [23,24] |
| Side effects | Numerous side effects are observed | Side effects related to phage therapy have rarely been reported | [25] |
| Impact on the immune system | Directly affects the immune system via various immunomodulatory antibiotics | Repeated administration of phages may result in the development of anti-phage antibodies that can neutralize therapeutic phages | [26,27] |
| Regulatory pathway | Standardized | Complex | [28,29] |
| Discovery process | Slow | Rapid | [30,31] |
| Storage stability | Typically show higher and more consistent long-term storage stability | Exhibit variable stability depending on the storage method and phage type | [32] |
| Phage-Guided Nanotherapeutic Systems | Target Pathogens | Study Outcomes | References |
|---|---|---|---|
| BK510 commercial phage endolysins conjugated with silver nanocapsules (NCs) | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species | Use of the conjugates showed a higher inhibitory effect as compared to silver NCs in over 65% of the Gram-negative bacteria. | [107] |
| Phage-mimicking antibacterial NCs composed of silver-coated gold nanospheres | Corynebacterium striatum, Enterococcus faecalis, Pseudomonas aeruginosa, and Staphylococcus aureus | The bacterial growth of these 4 bacteria was delayed (by up to 5 h) and suppressed (21% to 90%). | [10] |
| Phage K incorporated poly(N-isopropylacrylamide) nanospheres copolymerized with allylamine | Methicillin-resistant Staphylococcus aureus | The developed phage incorporated Poly(N-isopropylacrylamide) nanospheres copolymerized with allylamine successfully lysed Staphylococcus aureus at 37 °C, while the growth of bacteria remained unchanged at 25 °C, therefore providing a thermally triggered phage release. | [90] |
| Polyvinyl alcohol, honey, and chitosan nanofibers were electrospun and loaded with bee venom, propolis and/or phage | Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Escherichia coli, multidrug-resistant Pseudomonas aeruginosa | Among various nanofiber formulations, the combination of polyvinyl alcohol, honey, and chitosan-bee venom/phage exhibited the highest antibacterial efficacy against both Gram-positive and Gram-negative bacteria, achieving near-complete elimination of Pseudomonas aeruginosa. | [113] |
| Phage K-loaded nano-emulsion | Staphylococcus aureus strains H560, H325, and Btn766 | Phage-loaded nano-emulsion formulations demonstrated superior antibacterial activity compared to freely suspended phages. Additionally, these nano-emulsions achieved rapid and complete eradication of three distinct Staphylococcus aureus strains. | [117] |
| Engineered Mesoporous bioactive glass nanoparticles (MBGNs) by utilizing phage PFPV25.1 | Salmonella Typhimurium strain LT2 | MBGNs were modified with amine groups to improve the affinity between phages and the surfaces of MBGNs. As compared to non-functionalized MBGNs, an increased level of phages was found to be bound onto amine-functionalized MBGNs. In addition, because of this increased phage binding, amine-functionalized MBGNs showed higher antibacterial properties as compared to phage-bound MBGNs. | [120] |
| Combination of 3 phages (ZCPA5, ZCSS1, and ZCSE6) with 3 green synthesized NCs including silver-chitosan-NCs, pH-sensitive chitosan-NCs, and propolis-chitosan-NCs | Pseudomonas aeruginosa, Staphylococcus sciuri, and Salmonella Typhimurium | Among the NCs, silver-chitosan-NCs showed higher bactericidal properties in combination with phages. In addition, a substantial killing capacity was exhibited by silver-chitosan-NCs (16.5–30.1 μg/mL) in combination with phages. | [121] |
| Engineered T7 phages armed with silver NCs | Escherichia coli | In comparison with the silver NCs or phages alone, the developed biomaterial markedly improved biofilm eradication, especially following 48 h of treatment. | [129] |
| Novel bioactive nanoconjugate of antibiotic-loaded liposomes and phage Sb-1 | Methicillin-resistant Staphylococcus aureus | Upon exposure to biofilms, the Sb-1 phage disrupted the extracellular polymeric substance structure, enhancing bacterial susceptibility to antibiotics and facilitating deeper antibiotic penetration into the biofilm. Additionally, the liposome-phage nanoconjugates effectively reduced bacterial load in the infected area and significantly promoted recovery from osteomyelitis in a rat model of prosthetic joint infection. | [130] |
| T7 phages armed with silver NCs | Escherichia coli | The developed silver NC-binding phages efficiently eradicated bacterial biofilms, even at lower concentrations. In addition, these silver NC-binding phages were not toxic to eukaryotic cells. | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhubiab, B.; Almuqbil, R.M. Engineered Phage-Guided Nanotherapeutic Systems for Precision Antibacterial Therapy: Hacking Bacterial Resistance Mechanisms. Pharmaceutics 2025, 17, 1288. https://doi.org/10.3390/pharmaceutics17101288
Aldhubiab B, Almuqbil RM. Engineered Phage-Guided Nanotherapeutic Systems for Precision Antibacterial Therapy: Hacking Bacterial Resistance Mechanisms. Pharmaceutics. 2025; 17(10):1288. https://doi.org/10.3390/pharmaceutics17101288
Chicago/Turabian StyleAldhubiab, Bandar, and Rashed M. Almuqbil. 2025. "Engineered Phage-Guided Nanotherapeutic Systems for Precision Antibacterial Therapy: Hacking Bacterial Resistance Mechanisms" Pharmaceutics 17, no. 10: 1288. https://doi.org/10.3390/pharmaceutics17101288
APA StyleAldhubiab, B., & Almuqbil, R. M. (2025). Engineered Phage-Guided Nanotherapeutic Systems for Precision Antibacterial Therapy: Hacking Bacterial Resistance Mechanisms. Pharmaceutics, 17(10), 1288. https://doi.org/10.3390/pharmaceutics17101288







