Nanoformulation-Based Transdermal Drug Delivery: A Paradigm Shift in Antiparasitic Therapy for Zoonotic Diseases
Abstract
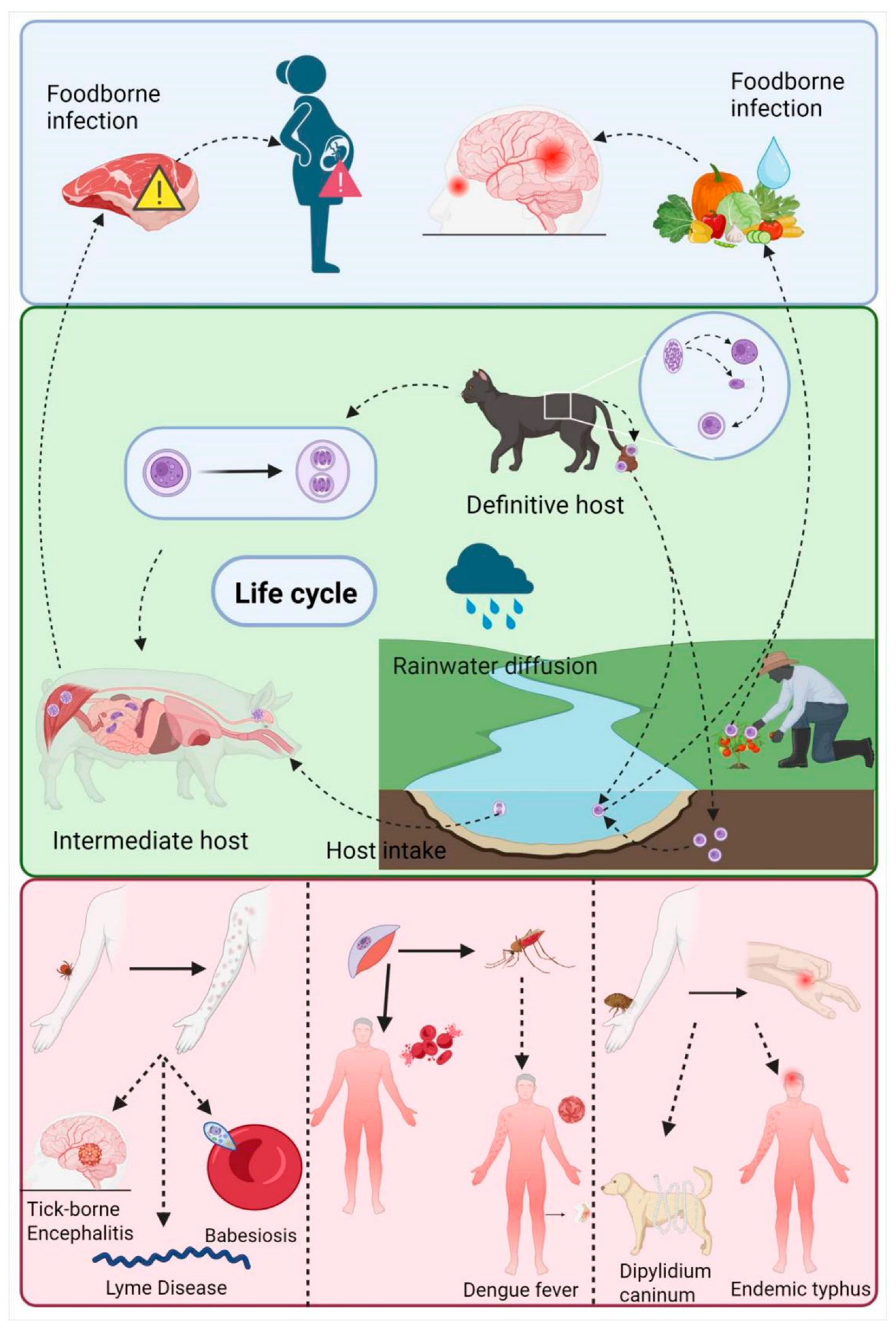
1. Introduction
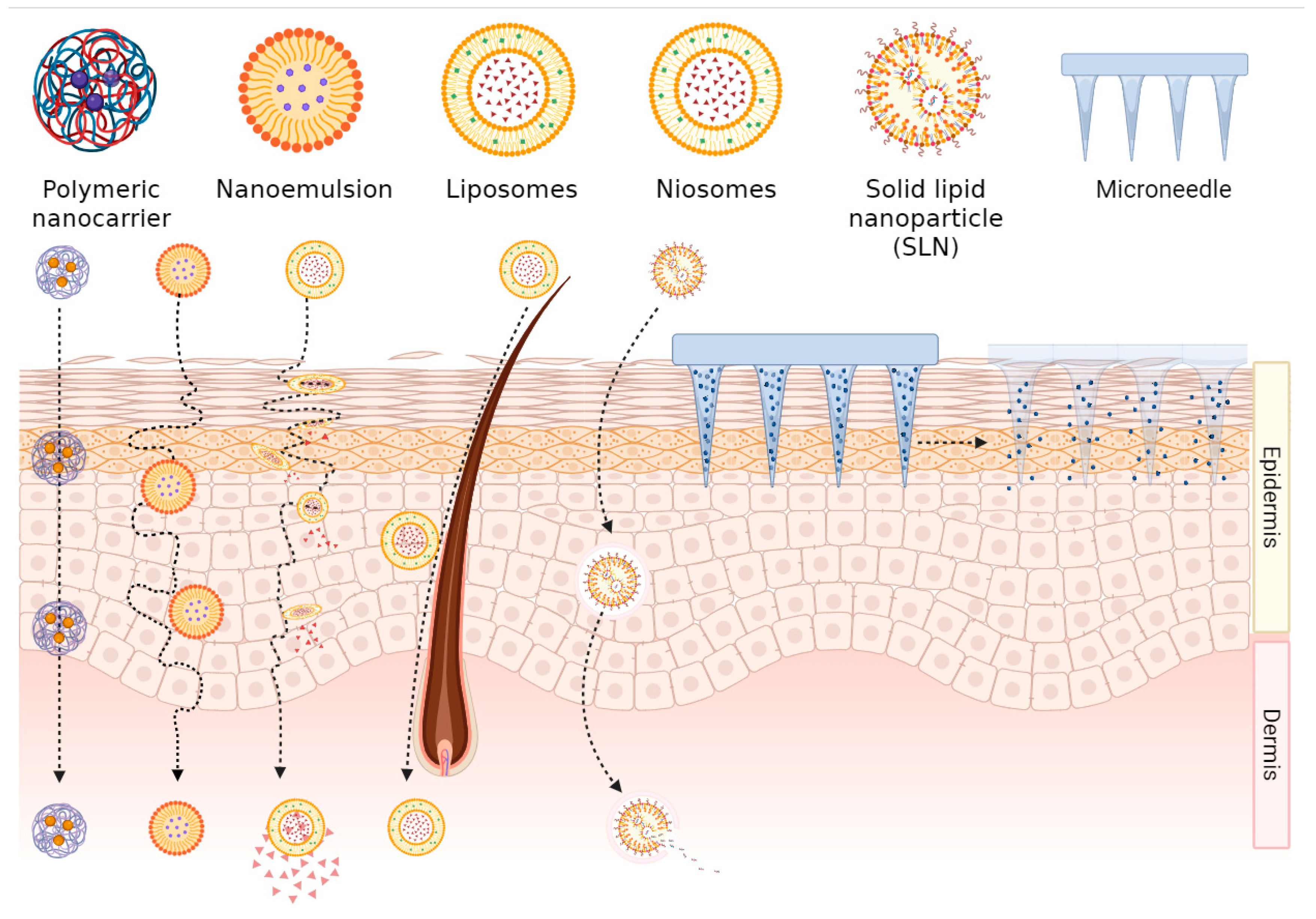
2. Advantages of Nano Transdermal Delivery System
2.1. Transdermal Mechanism of Nanoformulations
2.2. Controlled Release and Targeted Delivery of Nanoformulations
3. Application of Nanoformulations in the Treatment of Parasitic Diseases
3.1. Leishmaniasis
3.2. Malaria
4. Nanoformulations in Broad-Spectrum Antiparasitic Drugs
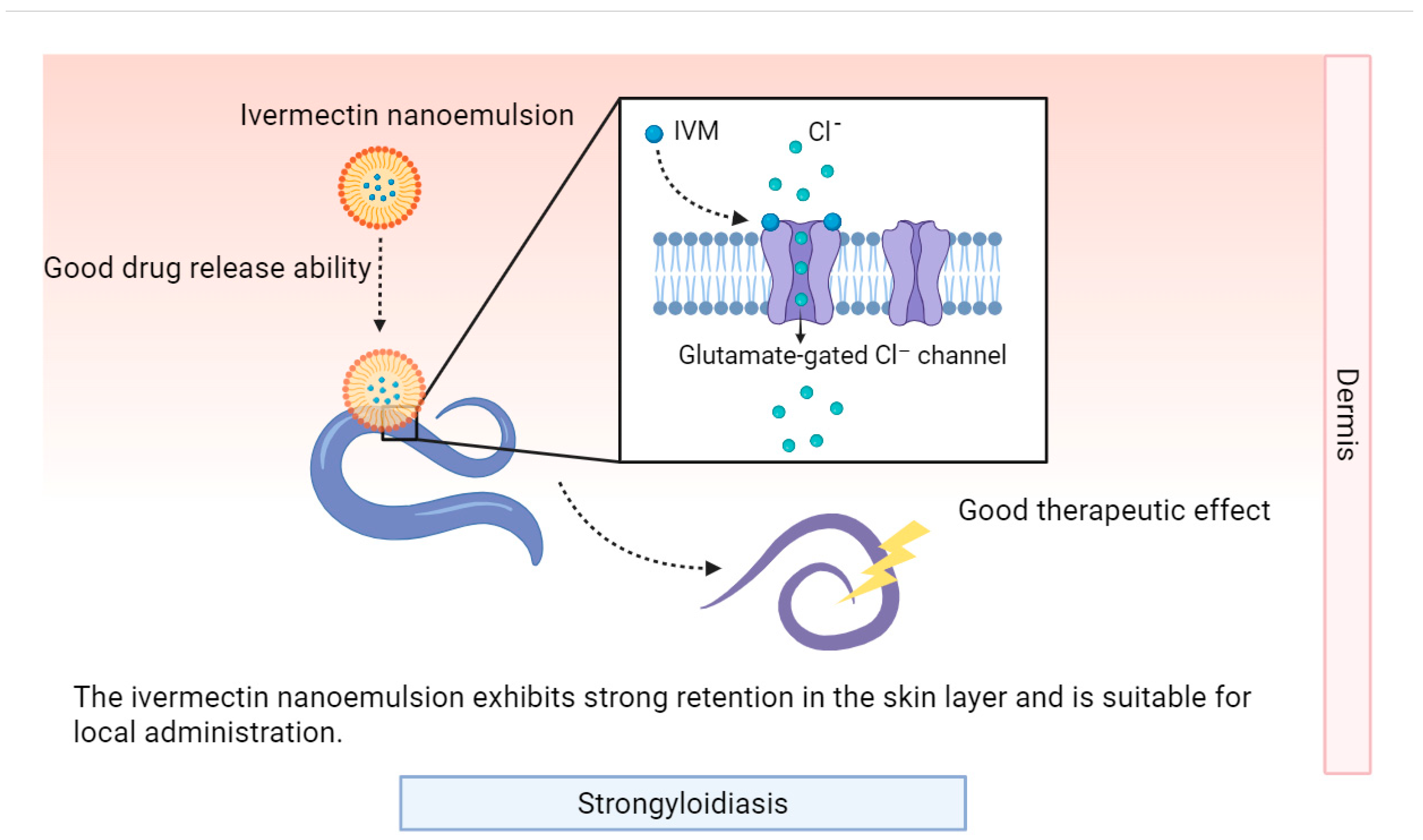
4.1. Avermectin-Class Drugs
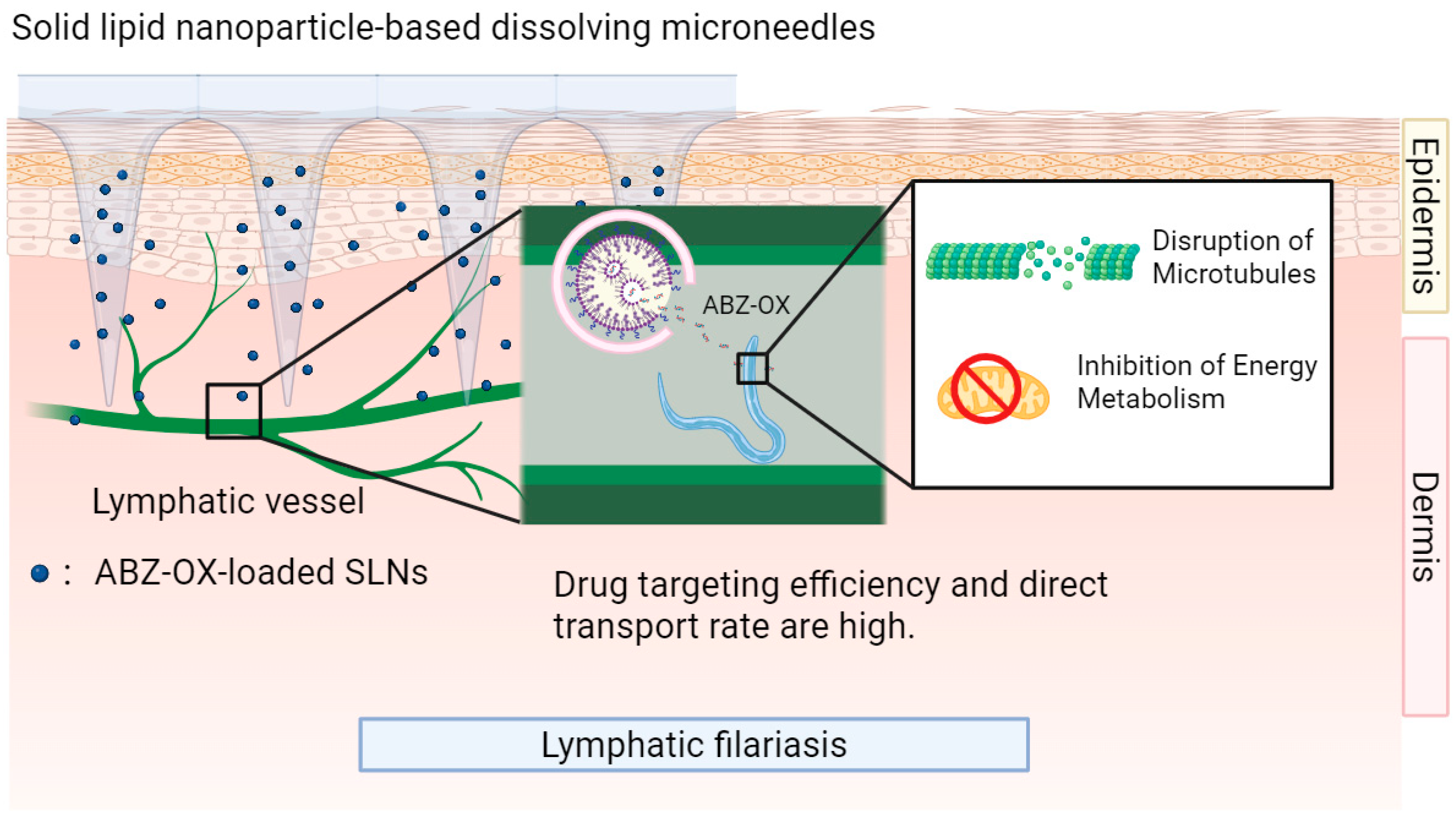
4.2. Benzimidazole-Class Drugs
5. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Report on Neglected Tropical Diseases 2024. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2024 (accessed on 1 May 2025).
- Otranto, D.; Strube, C.; Xiao, L. Zoonotic Parasites: The One Health Challenge. Parasitol. Res. 2021, 120, 4073–4074. [Google Scholar] [CrossRef] [PubMed]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Swartz, S.J.; Lopez, M.; Hsieh, M.H.; Lafferty, K.D.; Kuris, A.M.; Rickards, C.; De Leo, G.A. Global Assessment of Schistosomiasis Control Over the Past Century Shows Targeting the Snail Intermediate Host Works Best. PLoS Negl. Trop. Dis. 2016, 10, e0004794. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and Recent Advances in Peptide and Protein Drug Delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, R.R.; Desai, P.R.; Belay, K.; Singh, M.S. Trans Location of Cell Penetrating Peptide Engrafted Nanoparticles across Skin Layers. Biomaterials 2010, 31, 5598–5607. [Google Scholar] [CrossRef]
- Ashtikar, M.; Langelüddecke, L.; Fahr, A.; Deckert, V. Tip-Enhanced Raman Scattering for Tracking of Invasomes in the Stratum Corneum. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2017, 1861, 2630–2639. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin Permeabilization for Transdermal Drug Delivery: Recent Advances and Future Prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Yuan, L.; Pradhan, S.; Shrestha, K.; Pradhan, O.; Liu, H.; Li, W. Nano-Formulations for Transdermal Drug Delivery: A Review. Chin. Chem. Lett. 2018, 29, 1713–1724. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-Based Strategies for Treatment of Ocular Disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Kayser, O.; Kiderlen, A.F. Delivery Strategies for Antiparasitics. Expert Opin. Investig. Drugs 2003, 12, 197–207. [Google Scholar] [CrossRef]
- Laouini, A.; Charcosset, C.; Fessi, H.; Holdich, R.G.; Vladisavljević, G.T. Preparation of Liposomes: A Novel Application of Microengineered Membranes-From Laboratory Scale to Large Scale. Colloids Surf. B Biointerfaces 2013, 112, 272–278. [Google Scholar] [CrossRef]
- Kato, A.; Ishibashi, Y.; Miyake, Y. Effect of Egg Yolk Lecithin on Transdermal Delivery of Bunazosin Hydrochloride. J. Pharm. Pharmacol. 1987, 39, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Kirjavainen, M.; Urtti, A.; Jääskeläinen, I.; Marjukka Suhonen, T.; Paronen, P.; Valjakka-Koskela, R.; Kiesvaara, J.; Mönkkönen, J. Interaction of Liposomes with Human Skin In Vitro—The Influence of Lipid Composition and Structure. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1996, 1304, 179–189. [Google Scholar] [CrossRef]
- Kirjavainen, M.; Mönkkönen, J.; Saukkosaari, M.; Valjakka-Koskela, R.; Kiesvaara, J.; Urtti, A. Phospholipids Affect Stratum Corneum Lipid Bilayer Fluidity and Drug Partitioning into the Bilayers. J. Control. Release 1999, 58, 207–214. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B.W. Can Drug-Bearing Liposomes Penetrate Intact Skin? J. Pharm. Pharmacol. 2006, 58, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.W.; Jamshaid, H.; Rehman, F.U.; Zaeem, M.; Khan, J.Z.; Zeb, A. Transfersomes: A Revolutionary Nanosystem for Efficient Transdermal Drug Delivery. AAPS PharmSciTech 2021, 23, 7. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid Nanocarriers as Skin Drug Delivery Systems: Properties, Mechanisms of Skin Interactions and Medical Applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Raj, A.; Sarath, C.C.; Anoop, N.V.; Ivon, A.; Nazeera, F.N.M.; Neethu, N.P.P. Lipid-Based Vesicles: A Non-Invasive Tool for Transdermal Drug Delivery. J. Pharm. Innov. 2022, 17, 1039–1052. [Google Scholar] [CrossRef]
- Khan, A.U.; Jamshaid, H.; Ud Din, F.; Zeb, A.; Khan, G.M. Designing, Optimization and Characterization of Trifluralin Transfersomal Gel to Passively Target Cutaneous Leishmaniasis. J. Pharm. Sci. 2022, 111, 1798–1811. [Google Scholar] [CrossRef]
- Bashir, S.; Shabbir, K.; Din, F.U.; Khan, S.U.; Ali, Z.; Khan, B.A.; Kim, D.W.; Khan, G.M. Nitazoxanide and Quercetin Co-Loaded Nanotransfersomal Gel for Topical Treatment of Cutaneous Leishmaniasis with Macrophage Targeting and Enhanced Anti-Leishmanial Effect. Heliyon 2023, 9, e21939. [Google Scholar] [CrossRef]
- Shen, S.; Liu, S.-Z.; Zhang, Y.-S.; Du, M.-B.; Liang, A.-H.; Song, L.-H.; Ye, Z.-G. Compound Antimalarial Ethosomal Cataplasm: Preparation, Evaluation, and Mechanism of Penetration Enhancement. Int. J. Nanomed. 2015, 10, 4239–4253. [Google Scholar] [CrossRef] [PubMed]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A Critical Review of Synthesis Procedures, Applications and Future Potential of Nanoemulsions. Adv. Colloid. Interface Sci. 2021, 287, 102318. [Google Scholar] [CrossRef]
- Sghier, K.; Mur, M.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases. Gels 2024, 10, 45. [Google Scholar] [CrossRef]
- Coelho, D.; Veleirinho, B.; Mazzarino, L.; Alberti, T.; Buzanello, E.; Oliveira, R.E.; Yunes, R.A.; Moraes, M.; Steindel, M.; Maraschin, M. Polyvinyl Alcohol-Based Electrospun Matrix as a Delivery System for Nanoemulsion Containing Chalcone against Leishmania (Leishmania) Amazonensis. Colloids Surf. B Biointerfaces 2021, 198, 111390. [Google Scholar] [CrossRef]
- Garcia, D.J.; Fernandez-Culma, M.; Upegui, Y.A.; Rios-Vasquez, L.A.; Quinones, W.; Ocampo-Cardona, R.; Echeverri, F.; Velez, I.D.; Robledo, S.M. Nanoemulsions for Increased Penetrability and Sustained Release of Leishmanicidal Compounds. Arch. Pharm. 2023, 356, e202300108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Han, M.-R.; Park, J.-H. Polymer Microneedles for Transdermal Drug Delivery. J. Drug Target. 2013, 21, 211–223. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Jiang, X.; Li, L.; Wu, S.; Yuan, X.; Cheng, H.; Jiang, X.; Gou, M. 3D-Printed Microneedle Arrays for Drug Delivery. J. Control. Release 2022, 350, 933–948. [Google Scholar] [CrossRef]
- Gunawan, M.; Bestari, A.N.; Ramadon, D.; Efendi, A.; Boonkanokwong, V. Combination of Lipid-Based Nanoparticles with Microneedles as a Promising Strategy for Enhanced Transdermal Delivery Systems: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2025, 107, 106807. [Google Scholar] [CrossRef]
- Dumitriu Buzia, O.; Păduraru, A.M.; Stefan, C.S.; Dinu, M.; Cocoș, D.I.; Nwabudike, L.C.; Tatu, A.L. Strategies for Improving Transdermal Administration: New Approaches to Controlled Drug Release. Pharmaceutics 2023, 15, 1183. [Google Scholar] [CrossRef]
- Zare, M.R.; Khorram, M.; Barzegar, S.; Sarkari, B.; Asgari, Q.; Ahadian, S.; Zomorodian, K. Dissolvable Carboxymethyl Cellulose/Polyvinylpyrrolidone Microneedle Arrays for Transdermal Delivery of Amphotericin B to Treat Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2021, 182, 1310–1321. [Google Scholar] [CrossRef]
- Mahfufah, U.; Fitri Sultan, N.A.; Nurul Fitri, A.M.; Elim, D.; Sya’ban Mahfud, M.A.; Wafiah, N.; Ardita Friandini, R.; Chabib, L.; Aliyah Permana, A.D. Application of Multipolymers System in the Development of Hydrogel-Forming Microneedle Integrated with Polyethylene Glycol Reservoir for Transdermal Delivery of Albendazole. Eur. Polym. J. 2023, 183, 111762. [Google Scholar] [CrossRef]
- Sharma, S.; Rawat, K.; Bohidar, H.B. Role of Nanomedicines in Controlling Malaria: A Review. Curr. Top. Med. Chem. 2023, 23, 1477–1488. [Google Scholar] [CrossRef]
- Nahanji, M.K.; Mahboobian, M.M.; Harchegani, A.L.; Mohebali, M.; Fallah, M.; Nourian, A.; Motavallihaghi, S.; Maghsood, A.H. Enhancing the Efficacy of Fluconazole against Leishmania Major: Formulation and Evaluation of FLZ-Nanoemulsions for Topical Delivery. Biomed. Pharmacother. 2024, 178, 117109. [Google Scholar] [CrossRef]
- Deshmukh, R. Exploring the Potential of Antimalarial Nanocarriers as a Novel Therapeutic Approach. J. Mol. Graph. Model. 2023, 122, 108497. [Google Scholar] [CrossRef]
- Nemati, S.; Mottaghi, M.; Karami, P.; Mirjalali, H. Development of Solid Lipid Nanoparticles-Loaded Drugs in Parasitic Diseases. Discov. Nano 2024, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.; Khan, S.A.; Osman, N.; Sadozai, S.K.; Khan, I. Ameliorated Delivery of Amphotericin B to Macrophages Using Chondroitin Sulfate Surface-Modified Liposome Nanoparticles. Drug Dev. Ind. Pharm. 2025, 51, 38–49. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Sadiku, E.; Jayaramudu, J.; Sinha Ray, S. Controlled Dual Release Study of Curcumin and a 4-aminoquinoline Analog from Gum Acacia Containing Hydrogels. J. Appl. Polym. Sci. 2015, 132, app.41613. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Mhlwatika, Z. Dual Release Kinetics of Antimalarials from Soy Protein Isolate-Carbopol-Polyacrylamide Based Hydrogels. J. Appl. Polym. Sci. 2016, 133, 43918. [Google Scholar] [CrossRef]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-Loaded Solid Lipid Nanoparticles: Preparation, Characterisation, Stability and Transdermal Behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef]
- Rahimi, M.; Seyyed Tabaei, S.J.; Ziai, S.A.; Sadri, M. Anti-Leishmanial Effects of Chitosan-Polyethylene Oxide Nanofibers Containing Berberine: An Applied Model for Leishmania Wound Dressing. Iran. J. Med. Sci. 2020, 45, 286–297. [Google Scholar] [CrossRef]
- Seyyed Tabaei, S.J.; Rahimi, M.; Akbaribazm, M.; Ziai, S.A.; Sadri, M.; Shahrokhi, S.R.; Rezaei, M.S. Chitosan-Based Nano-Scaffolds as Antileishmanial Wound Dressing in BALB/c Mice Treatment: Characterization and Design of Tissue Regeneration. Iran. J. Basic. Med. Sci. 2020, 23, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Golshirazi, A.; Mohammadzadeh, M.; Labbaf, S. The Synergistic Potential of Hydrogel Microneedles and Nanomaterials: Breaking Barriers in Transdermal Therapy. Macromol. Biosci. 2025, 25, 2400228. [Google Scholar] [CrossRef]
- Alex, M.; Alsawaftah, N.M.; Husseini, G.A. State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Appl. Sci. 2024, 14, 2926. [Google Scholar] [CrossRef]
- Dehghani, F.; Farhadian, N.; Goyonlo, V.M.; Ahmadi, O. A Novel Topical Formulation of the Leishmaniasis Drug Glucantime as a Nanostructured Lipid Carrier-Based Hydrogel. Am. J. Trop. Med. Hyg. 2023, 109, 301–314. [Google Scholar] [CrossRef]
- Rabia, S.; Khaleeq, N.; Batool, S.; Dar, M.J.; Kim, D.W.; Din, F.-U.; Khan, G.M. Rifampicin-Loaded Nanotransferosomal Gel for Treatment of Cutaneous Leishmaniasis: Passive Targeting Via Topical Route. Nanomedicine 2020, 15, 183–203. [Google Scholar] [CrossRef]
- Tyagi, R.K.; Garg, N.K.; Jadon, R.; Sahu, T.; Katare, O.P.; Dalai, S.K.; Awasthi, A.; Marepally, S.K. Elastic Liposome-Mediated Transdermal Immunization Enhanced the Immunogenicity of P. Falciparum Surface Antigen, MSP-119. Vaccine 2015, 33, 4630–4638. [Google Scholar] [CrossRef]
- Tyagi, R.K.; Garg, N.K.; Dalai, S.K.; Awasthi, A. Transdermal Immunization of P-Falciparum Surface Antigen (MSP-119) via Elastic Liposomes Confers Robust Immunogenicity. Hum. Vaccines Immunother. 2016, 12, 990–992. [Google Scholar] [CrossRef]
- Puttappa, N.; Kumar, R.S.; Yamjala, K. Artesunate-Quercetin/Luteolin Dual Drug Nanofacilitated Synergistic Treatment for Malaria: A Plausible Approach to Overcome Artemisinin Combination Therapy Resistance. Med. Hypotheses 2017, 109, 176–180. [Google Scholar] [CrossRef]
- Courtenay, O.; Peters, N.C.; Rogers, M.E.; Bern, C. Combining Epidemiology with Basic Biology of Sand Flies, Parasites, and Hosts to Inform Leishmaniasis Transmission Dynamics and Control. PLoS Pathog. 2017, 13, e1006571. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, T.; Deschacht, M.; da Luz, R.A.I.; Maes, L.; Cos, P. Leishmania–Macrophage Interactions: Insights into the Redox Biology. Free Radic. Biol. Med. 2011, 51, 337–351. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Al-Rawahi, A.; Green, I.R.; Gibbons, S. Fruitful Decade for Antileishmanial Compounds from 2002 to Late 2011. Chem. Rev. 2014, 114, 10369–10428. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous Leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Olliaro, P.L.; Guerin, P.J.; Gerstl, S.; Haaskjold, A.A.; Rottingen, J.-A.; Sundar, S. Treatment Options for Visceral Leishmaniasis: A Systematic Review of Clinical Studies Done in India, 1980–2004. Lancet Infect. Dis. 2005, 5, 763–774. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An Update on Pharmacotherapy for Leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Varma, D.M.; Redding, E.A.; Bachelder, E.M.; Ainslie, K.M. Nano- and Microformulations to Advance Therapies for Visceral Leishmaniasis. ACS Biomater. Sci. Eng. 2021, 7, 1725–1741. [Google Scholar] [CrossRef]
- Riaz, A.; Khan, M.F.A.; Jalil, A.; ur Rehman, A.; Ahmed, N.; Mubarak, Z.; Parhizkar, M.; Muqaddas, H. Lipid-Based Nanocarriers for Topical Therapy of Cutaneous Leishmaniasis: An Insight into the Mechanism of Action. ACS Omega 2025, 10, 23873–23888. [Google Scholar] [CrossRef]
- Romero, A.H.; Gonzalez, K.N.; Sabino, M.A. Application of Nano and Microformulations to Improve the Leishmanicidal Response of Quinoline Compounds: A Brief Review. Front. Chem. 2025, 13, 1622566. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.; Marins, D.S.S.; Mathias, S.L.; Monteiro, L.M.; Yukuyama, M.N.; Scarim, C.B.; Löbenberg, R.; Bou-Chacra, N.A. Promising Nanotherapy in Treating Leishmaniasis. Int. J. Pharm. 2018, 547, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.S.; de Araújo, R.V.; Giarolla, J.; Seoud, O.E.; Ferreira, E.I. Searching for Drugs for Chagas Disease, Leishmaniasis and Schistosomiasis: A Review. Int. J. Antimicrob. Agents 2020, 55, 105906. [Google Scholar] [CrossRef]
- Alishahi, M.; Khorram, M.; Asgari, Q.; Davani, F.; Goudarzi, F.; Emami, A.; Arastehfar, A.; Zomorodian, K. Glucantime-Loaded Electrospun Core-Shell Nanofibers Composed of Poly(Ethylene Oxide)/Gelatin-Poly(Vinyl Alcohol)/Chitosan as Dressing for Cutaneous Leishmaniasis. Int. J. Biol. Macromol. 2020, 163, 288–297. [Google Scholar] [CrossRef]
- Mahmoud Abd-Alaziz, D.; Mansour, M.; Nasr, M.; Sammour, O. Tailored Green Synthesized Silymarin-Selenium Nanoparticles: Topical Nanocarrier of Promising Antileishmanial Activity. Int. J. Pharm. 2024, 660, 124275. [Google Scholar] [CrossRef] [PubMed]
- Lanza, J.S.; Vucen, S.; Flynn, O.; Donadei, A.; Cojean, S.; Loiseau, P.M.; Fernandes, A.P.S.M.; Frézard, F.; Moore, A.C. A TLR9-Adjuvanted Vaccine Formulated into Dissolvable Microneedle Patches or Cationic Liposomes Protects against Leishmaniasis after Skin or Subcutaneous Immunization. Int. J. Pharm. 2020, 586, 119390. [Google Scholar] [CrossRef] [PubMed]
- Maria Jose Alves De, O.; Regina, M.; Lucia Almeida, B.; Ademar Benevolo, L.; Valdir Sabbaga, A.; Duclerc Fernandes, P. Topical Treatment of Cutaneous Leishmaniasis: Wound Reduction in Mice Using N-Methyl Glucamine from PVP and Nano Clay Membranes. J. Dermatol. Res. Ther. 2016, 2, 10–23937. [Google Scholar] [CrossRef]
- Oliveira, M.J.A.D.; Villegas, G.M.E.; Motta, F.D.; Fabela-Sánchez, O.; Espinosa-Roa, A.; Fotoran, W.L.; Peixoto, J.C.; Tano, F.T.; Lugão, A.B.; Vásquez, P.A.S. Influence of Gamma Radiation on Amphotericin B Incorporated in PVP Hydrogel as an Alternative Treatment for Cutaneous Leishmaniosis. Acta Trop. 2021, 215, 105805. [Google Scholar] [CrossRef]
- Puttappa, N.; Kumar, R.S.; Kuppusamy, G.; Radhakrishnan, A. Nano-Facilitated Drug Delivery Strategies in the Treatment of Plasmodium Infection. Acta Trop. 2019, 195, 103–114. [Google Scholar] [CrossRef]
- Mishra, A.; Qamar, F.; Ashrafi, K.; Fatima, S.; Samim, M.; Mohmmed, A.; Abdin, M.Z. Emerging Nanotechnology-Driven Drug Delivery Solutions for Malaria: Addressing Drug Resistance and Improving Therapeutic Success. Int. J. Pharm. 2025, 670, 125163. [Google Scholar] [CrossRef]
- Baruah, U.K.; Gowthamarajan, K.; Ravisankar, V.; Karri, V.V.S.R.; Simhadri, P.K.; Singh, V. Optimisation of Chloroquine Phosphate Loaded Nanostructured Lipid Carriers Using Box-Behnken Design and Its Antimalarial Efficacy. J. Drug Target. 2018, 26, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Kuntworbe, N.; Martini, N.; Shaw, J.; Al-Kassas, R. Malaria Intervention Policies and Pharmaceutical Nanotechnology as a Potential Tool for Malaria Management. Drug Dev. Res. 2012, 73, 167–184. [Google Scholar] [CrossRef]
- Santos-Magalhães, N.S.; Mosqueira, V.C.F. Nanotechnology Applied to the Treatment of Malaria. Adv. Drug Deliv. Rev. 2010, 62, 560–575. [Google Scholar] [CrossRef]
- Eastman, R.T.; Fidock, D.A. Artemisinin-Based Combination Therapies: A Vital Tool in Efforts to Eliminate Malaria. Nat. Rev. Microbiol. 2009, 7, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Kenechukwu, F.C.; Ugwu, K.C.; Chukwu, G.C.; Anikwe, C.C.; Okaudu, M.C.; Momoh, M.A.; Ricci-Junior, E.; Attama, A.A. Transdermal Films and Nanogels as Toolboxes for Novel Generation of Non-Invasive Antimalarial Pharmaceuticals in Dermatology: A Comprehensive Review. Biomed. Mater. Devices 2025, 3, 1–38. [Google Scholar] [CrossRef]
- Zech, J.; Dzikowski, R.; Simantov, K.; Golenser, J.; Maeder, K. Transdermal Delivery of Artemisinins for Treatment of Pre-Clinical Cerebral Malaria. Int. J. Parasitol.-Drugs Drug Resist. 2021, 16, 148–154. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; Fox, L.T.; Brümmer, A.; Gerber, M.; Du Preez, J.L.; Haynes, R.K.; Du Plessis, J. In Vitro Skin Permeation of Artemisone and Its Nano-Vesicular Formulations. Int. J. Pharm. 2016, 503, 1–7. [Google Scholar] [CrossRef]
- Dandekar, P.P.; Jain, R.; Patil, S.; Dhumal, R.; Tiwari, D.; Sharma, S.; Vanage, G.; Patravale, V. Curcumin-Loaded Hydrogel Nanoparticles: Application in Anti-Malarial Therapy and Toxicological Evaluation. J. Pharm. Sci. 2010, 99, 4992–5010. [Google Scholar] [CrossRef]
- Alfrd Mavondo, G.A.; Tagumirwa, M.C. Asiatic Acid-Pectin Hydrogel Matrix Patch Transdermal Delivery System Influences Parasitaemia Suppression and Inflammation Reduction in P. Berghei Murine Malaria Infected Sprague—Dawley Rats. Asian Pac. J. Trop. Med. 2016, 9, 1172–1180. [Google Scholar] [CrossRef]
- Nnamani, P.O.; Hansen, S.; Windbergs, M.; Lehr, C.-M. Development of Artemether-Loaded Nanostructured Lipid Carrier (NLC) Formulation for Topical Application. Int. J. Pharm. 2014, 477, 208–217. [Google Scholar] [CrossRef]
- Ananda, P.W.R.; Elim, D.; Zaman, H.S.; Muslimin, W.; Tunggeng, M.G.R.; Permana, A.D. Combination of Transdermal Patches and Solid Microneedles for Improved Transdermal Delivery of Primaquine. Int. J. Pharm. 2021, 609, 121204. [Google Scholar] [CrossRef]
- Gomes, D.C.; Medeiros, T.S.; Alves Pereira, E.L.; Da Silva, J.F.O.; De Freitas Oliveira, J.W.; Fernandes-Pedrosa, M.D.F.; De Sousa Da Silva, M.; Da Silva-Júnior, A.A. From Benznidazole to New Drugs: Nanotechnology Contribution in Chagas Disease. Int. J. Mol. Sci. 2023, 24, 13778. [Google Scholar] [CrossRef]
- Permana, A.D.; Paredes, A.J.; Zanutto, F.V.; Amir, M.u.h.N.; Ismail, I.; Bahar, M.u.h.A.; Sumarheni Palma, S.D.; Donnelly, R.F. Albendazole Nanocrystal-Based Dissolving Microneedles with Improved Pharmacokinetic Performance for Enhanced Treatment of Cystic Echinococcosis. ACS Appl. Mater. Interfaces 2021, 13, 38745–38760. [Google Scholar] [CrossRef]
- Devineni, J.; Pravallika, C.h.D.; Sudha Rani, B.; Nalluri, B.N. Effective Single Drug Treatment of Lymphatic Filariasis through Enhanced Transdermal Delivery of Ivermectin Liposomes Using Solid and Dissolving Microneedles. Indian J. Pharm. Educ. Res. 2020, 54, s492–s504. [Google Scholar] [CrossRef]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.C.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid Lipid Nanoparticle-Based Dissolving Microneedles: A Promising Intradermal Lymph Targeting Drug Delivery System with Potential for Enhanced Treatment of Lymphatic Filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef]
- Manna, O.; Nditanchou, R.; Siewe Fodjo, J.; Colebunders, R. Utilization of Health Facilities and Schools in Onchocerciasis Elimination Efforts. IJID Reg. 2025, 16, 100677. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Torii, A.; Suzuki, D.; Ito, K.; Iwade, K.; Takahashi, K. A Case Report of Disseminated Strongyloidiasis Following Chemoradiotherapy for Lung Cancer in a Patient Living in a Non-Endemic Area in Japan. Intern. Med. 2025, 1–6. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; Macbeath, C.; Liu, Y.; Shlieout, G. Development of Microemulsion Based Topical Ivermectin Formulations: Pre-Formulation and Formulation Studies. Colloids Surf. B Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Rawas-Qalaji, M.; El Hosary, R.; Jagal, J.; Ahmed, I.S. Formulation and Optimization of Ivermectin Nanocrystals for Enhanced Topical Delivery. Int. J. Pharm. X 2023, 6, 100210. [Google Scholar] [CrossRef] [PubMed]
- Steenekamp, E.M.; Liebenberg, W.; Lemmer, H.J.R.; Gerber, M. Formulation and Ex Vivo Evaluation of Ivermectin Within Different Nano-Drug Delivery Vehicles for Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1466. [Google Scholar] [CrossRef]
- Velho, M.C.; Funk, N.L.; Deon, M.; Benvenutti, E.V.; Buchner, S.; Hinrichs, R.; Pilger, D.A.; Beck, R.C.R. Ivermectin-Loaded Mesoporous Silica and Polymeric Nanocapsules: Impact on Drug Loading, In Vitro Solubility Enhancement, and Release Performance. Pharmaceutics 2024, 16, 325. [Google Scholar] [CrossRef]
- Mao, Y.; Hao, T.; Zhang, H.; Gu, X.; Wang, J.; Shi, F.; Chen, X.; Guo, L.; Gao, J.; Shen, Y.; et al. Penetration Enhancer-Free Mixed Micelles for Improving Eprinomectin Transdermal c Efficiency in Animal Parasitic Infections Therapy. Int. J. Nanomed. 2024, 19, 11071–11085. [Google Scholar] [CrossRef]
- Berger, S.N.; Rustum, A.M. A Comprehensive Study to Identify Major Degradation Products of Avermectin Active Ingredient Using High Resolution LCMS and NMR. J. Liq. Chromatogr. Relat. Technol. 2025, 1–11. [Google Scholar] [CrossRef]
- de Souza, D.K.; Bockarie, M.J. Current Perspectives in the Epidemiology and Control of Lymphatic Filariasis. Clin. Microbiol. Rev. 2025, 38, e00126-23. [Google Scholar] [CrossRef] [PubMed]
- Karakoyun, Ö.; Ayhan, E.; Yıldız, İ. Effect of Ivermectin on Scabies: A Retrospective Evaluation. BMC Infect. Dis. 2025, 25, 937. [Google Scholar] [CrossRef] [PubMed]
- Karpstein, T.; Kalamatianou, A.; Keller, S.; Spane, P.; Haberli, C.; Odermatt, A.; Blacque, O.; Cariou, K.; Gasser, G.; Keiser, J. Synthesis and Multidisciplinary Preclinical Investigations of Ferrocenyl, Ruthenocenyl, and Benzyl Derivatives of Thiabendazole as New Drug Candidates against Soil-Transmitted Helminth Infections. ACS Infect. Dis. 2025, 11, 2037–2047. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29, 1218. [Google Scholar] [CrossRef] [PubMed]

| Indication | Nanoformulation | Compounds | Advantages | Study Type and Model | Formulation Characteristics | Year | References |
|---|---|---|---|---|---|---|---|
| Leishmaniasis | Transfersomes | Trifluralin | High transdermal efficiency, high encapsulation efficiency, sustained release, reduced IC50 for Leishmania pathogens, high inhibition rate of amastigotes | In vivo (Albino Wistar rats) | PS: 140.3 ± 2.3 nm; PDI: 0.006 ± 0.002; %EE: 86 ± 0.5% and 43.5 ± 1.0% | 2022 | [20] |
| Transfersomes | Nitazoxanide-quercetin | Enhanced skin permeation, increased macrophage uptake, higher CC50, smaller lesion size, low systemic toxicity | In vitro (PMs) In vivo (Male Albino Wistar rats, BALB/c mice) | PS: 210 nm; PDI: 0.16; ZP: −15.1 mV; EE of NTZ and QUR was 88% and 85%. | 2023 | [21] | |
| Nanoemulsions | 3′-(Trifluoromethyl)-chalcone | High dermal permeation amount, high skin retention | In vitro (TH-1) in vivo (Pigs) | PS: 179.0 ± 1.0 nm; PDI: <0.3; %EE: ~100%; |ZP|: > 30 mV | 2021 | [25] | |
| Nanoemulsions | C6I/TC1/TC2 | Enhanced skin permeation, Sustained release, high parasiticidal activity, low macrophage cytotoxicity | In vitro (U-937-CRL-1593.2) | / | 2023 | [26] | |
| Electrospun core–shell nanofibers | Glucantime | Stable drug release, maintenance of effective drug concentration for a long time, sustained therapeutic effect | In vitro (NIH3T3, Franz diffusion cell) | / | 2020 | [62] | |
| Nanoparticles | Silymarin-selenium | High drug loading capacity, high skin deposition rate, significant reduction in local treatment toxicity | In vitro (NHDFa) In vivo (Male Wistar rats) | %LE: 58.22 ± 0.56%; HD: 245.77 ± 11.12 nm; PDI: 0.19 ± 0.01; ZP: −30.63 ± 0.40 mV | 2024 | [63] | |
| Transfersomes | Rifampicin | High skin permeability, targeted reduction of IC50 value | In vitro (Macrophages) and in vivo (Albino Wistar rats, female BALB/c mice) | PS: 190 nm, %EE: 83%; 3-fold permeation vs. free RIF. | 2020 | [46] | |
| Nanostructured lipid carrier | Glucantime | Controlled drug release, enhanced skin retention, reduced systemic toxicity | In vitro (L. major) and in vivo (female BALB/c mice) | PS: 93.87 ± 0.1 nm; PDI: 0.295 ± 0.015; ZP: −30.31 ± 0.25 mV; %LE: 74 ± 0.37% | 2023 | [45] | |
| Nanofibers | Berberine | Gradient release, excellent biocompatibility, stable release rate | In vitro (J774A.1, L. major) and in vivo (female BALB/c mice) | PS: 10.51 ± 0.24 nm; PDI: 0.19 ± 0.03; ZP: −0.41 ± 0.17 mV | 2023, 2024 | [32,33] | |
| Microneedle | Aphotericin B | Improved skin permeability, minimal cellular damage | In vitro (HT-29,) In vivo (Sprague-Dawley male rats) | Drug Loading: 182 ± 4 μg per (22 × 22) MN array | 2021 | [31] | |
| Malaria | Ethosomes | Artesunate and Febrifugine | High cumulative permeation, high efficiency | In vivo (Specific-pathogen-free male Kunming mice) | PS: 26.48 ± 0.12 nm; ZP: −28.0 ± 1.6 mV; PDI: 0.195 ± 0.005; %EE: 71.81 ± 2.57% | 2015 | [22] |
| Solid lipid nanoparticles | Artemisone | High skin delivery concentration | In vitro (Caucasian female skin obtained by abdominoplasty) | PS: 295 ± 18 nm; ZP: −12 ± 3 mV; %EE: 79 ± 5.00% | 2016 | [75] | |
| Elastic liposomes | PfMSP-119 | Efficient targeting, long-lasting immune response | / | / | 2016 | [49] | |
| Nanoparticles | Curcumin | High antimalarial activity, prolonged drug circulation time, high bioavailability, enhanced therapeutic efficacy | Albino nude rat, Balb/c mice | / | 2015, 2016, 2010 | [47,48,76] | |
| Nanostructured lipid carrier | Artemether | High cumulative permeation rate, high drug stability, high release controllability | In vitro (Caucasian female skin obtained by abdominoplasty) | / | 2014 | [78] | |
| Microneedles | Primaquine | Optimal performance, safe for long-term medication | In vitro and in vivo (Wistar rats) | In vitro and ex vivo permeation/release: 31.31 ± 5.25% and 22.55 ± 4.35%. | 2021 | [79] | |
| Cystic Echinococcosis | Microneedle | Albendazole | High transdermal permeation amount, safe | In vitro and in vivo (rats) | / | 2023 | [32] |
| Microneedles-Nanocrystals system | Albendazole | High transdermal depth, high peak drug concentration, long half-life | Wistar rats | / | 2021 | [81] | |
| Lymphatic filariasis | Solid lipid nanoparticle—microneedle system | Albendazole | High drug targeting efficiency, high direct transport rate, Sustained release, reduced metabolite generation, low drug distribution in liver and kidney | In vitro and in vivo (female Sprague–Dawley rats) | PS: 95.25 ± 9.26 nm; PDI: 0.273 ± 0.02 | 2019 | [83] |
| Scabies, Rosacea, Head lice, Trichuriasis, Onchocerciasis, Lymphatic filariasis, Strongyloidiasis | Microemulsion | Ivermectin | Improved membrane permeability, high solubility | / | PS: 18–54 nm; PDI: 0.3–0.5 | 2020 | [86] |
| Nanocrystals | Ivermectin | High equilibrium solubility, fast dissolution rate, high dermal deposition, low side effects | In vitro (Adult pig ear skin) | PS: 186 nm; PDI: 0.4 | 2023 | [87] | |
| Nanoemulsion (NE) | Ivermectin | Highest drug concentration in stratum corneum and epidermis/dermis junction | In vitro (HaCaT, BJ-5ta, female skin obtained by abdominoplasty) | PS: 57.157 ± 0.455nm; PDI: 0.165 ± 0.014; ZP: −30.600 ± 1.300 mV | 2024 | [88] | |
| Nanoemulsion gel (NEG) | Ivermectin | Fastest delivery rate | PS: 106.900 ± 0.490nm; PDI: 0.287 ± 0.037; ZP: −40.400 ± 1.283 mV | ||||
| Colloidal system (CS) | Ivermectin | Highest drug diffusion percentage, efficient delivery | ZP: −36.200 ± 0.666 mV | ||||
| Solid lipid nanoparticles | Ivermectin | Sustained-release efficacy, targeting, low systemic toxicity, few side effects | / | %EE: 99.9±0.0%; %LC: 5.0 ± 0.0%, | 2018 | [40] | |
| Nanoparticles | Ivermectin | suitable for acute infection management | / | DL: 10.7%; >90% drug recovery | 2024 | [89] | |
| Nanocapsules | Ivermectin | suitable for chronic prevention | / | Z-average: 202 ± 2 nm; ZP: −17 ± 0.5 mV; PDI: 0.12 ± 0.01 | |||
| Hybrid micelles | Eprinomectin | High permeability, low toxicity | In vitro & In vivo (Male Sprague–Dawley rats, Male ICR mice) | PS: 13.97 ± 0.16 nm; PDI: 0.132; %LE: 0.49%; %EE: 95.81% | 2024 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xiu, R.; Wang, C.; Wang, J.; Guo, D.; Luo, W.; Jiang, S.; Ge, Z.; Gao, X. Nanoformulation-Based Transdermal Drug Delivery: A Paradigm Shift in Antiparasitic Therapy for Zoonotic Diseases. Pharmaceutics 2025, 17, 1216. https://doi.org/10.3390/pharmaceutics17091216
Zhao Y, Xiu R, Wang C, Wang J, Guo D, Luo W, Jiang S, Ge Z, Gao X. Nanoformulation-Based Transdermal Drug Delivery: A Paradigm Shift in Antiparasitic Therapy for Zoonotic Diseases. Pharmaceutics. 2025; 17(9):1216. https://doi.org/10.3390/pharmaceutics17091216
Chicago/Turabian StyleZhao, Yuan, Ruoxuan Xiu, Chengxiang Wang, Junqi Wang, Dawei Guo, Wanhe Luo, Shanxiang Jiang, Zhiyi Ge, and Xiuge Gao. 2025. "Nanoformulation-Based Transdermal Drug Delivery: A Paradigm Shift in Antiparasitic Therapy for Zoonotic Diseases" Pharmaceutics 17, no. 9: 1216. https://doi.org/10.3390/pharmaceutics17091216
APA StyleZhao, Y., Xiu, R., Wang, C., Wang, J., Guo, D., Luo, W., Jiang, S., Ge, Z., & Gao, X. (2025). Nanoformulation-Based Transdermal Drug Delivery: A Paradigm Shift in Antiparasitic Therapy for Zoonotic Diseases. Pharmaceutics, 17(9), 1216. https://doi.org/10.3390/pharmaceutics17091216









