Homotypic Targeting of [89Zr]Zr-Oxine Labeled PC3 and 4T1 Cells in Tumor-Bearing Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of [89Zr]Zr-Oxine
2.3. [89Zr]Zr-Oxine Labeling of PC3 and 4T1 Cells
2.4. Cellular Efflux of [89Zr]Zr-Oxine Labeled PC3 and 4T1 Cells
2.5. In Vivo Experiments
2.6. Statistics
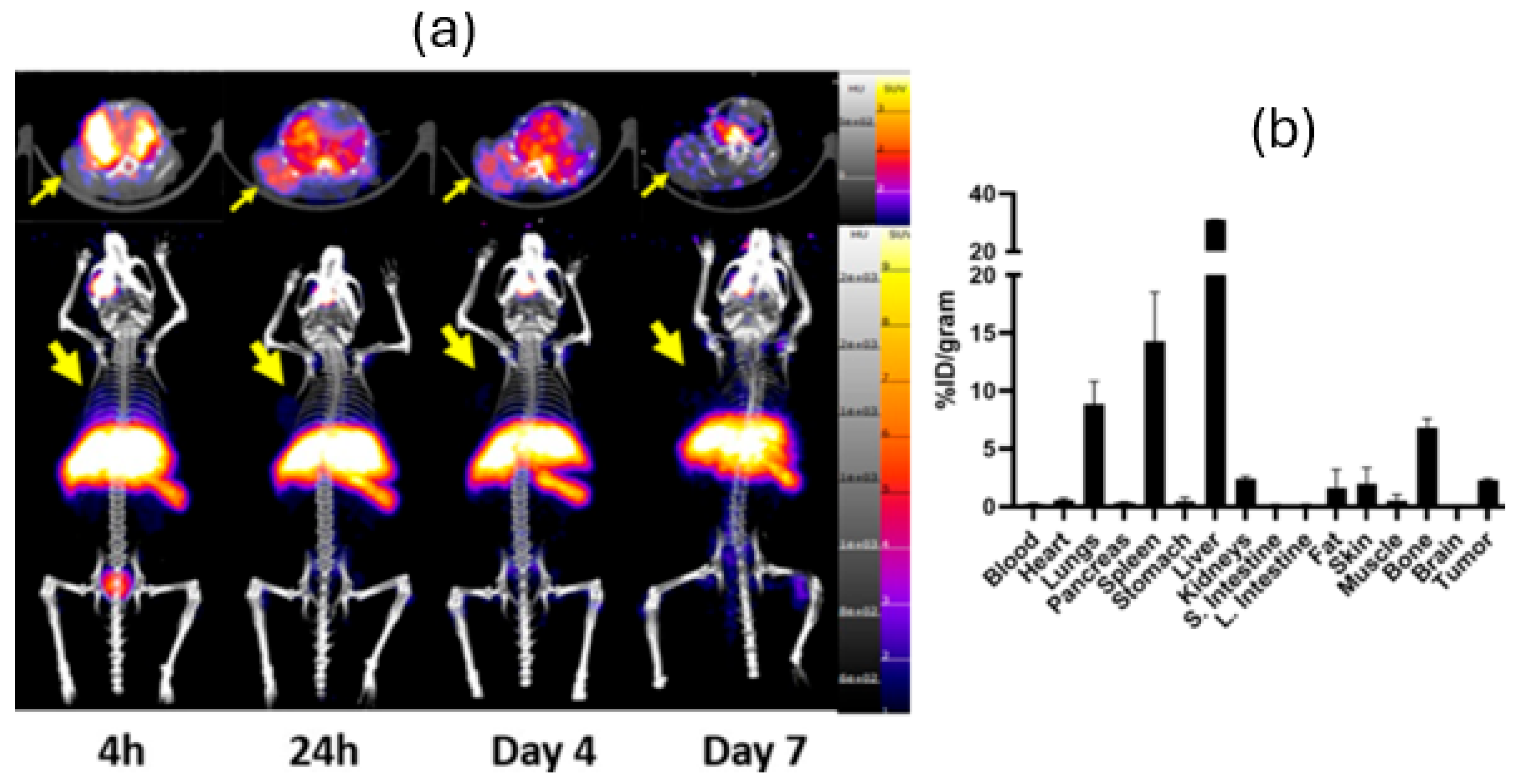
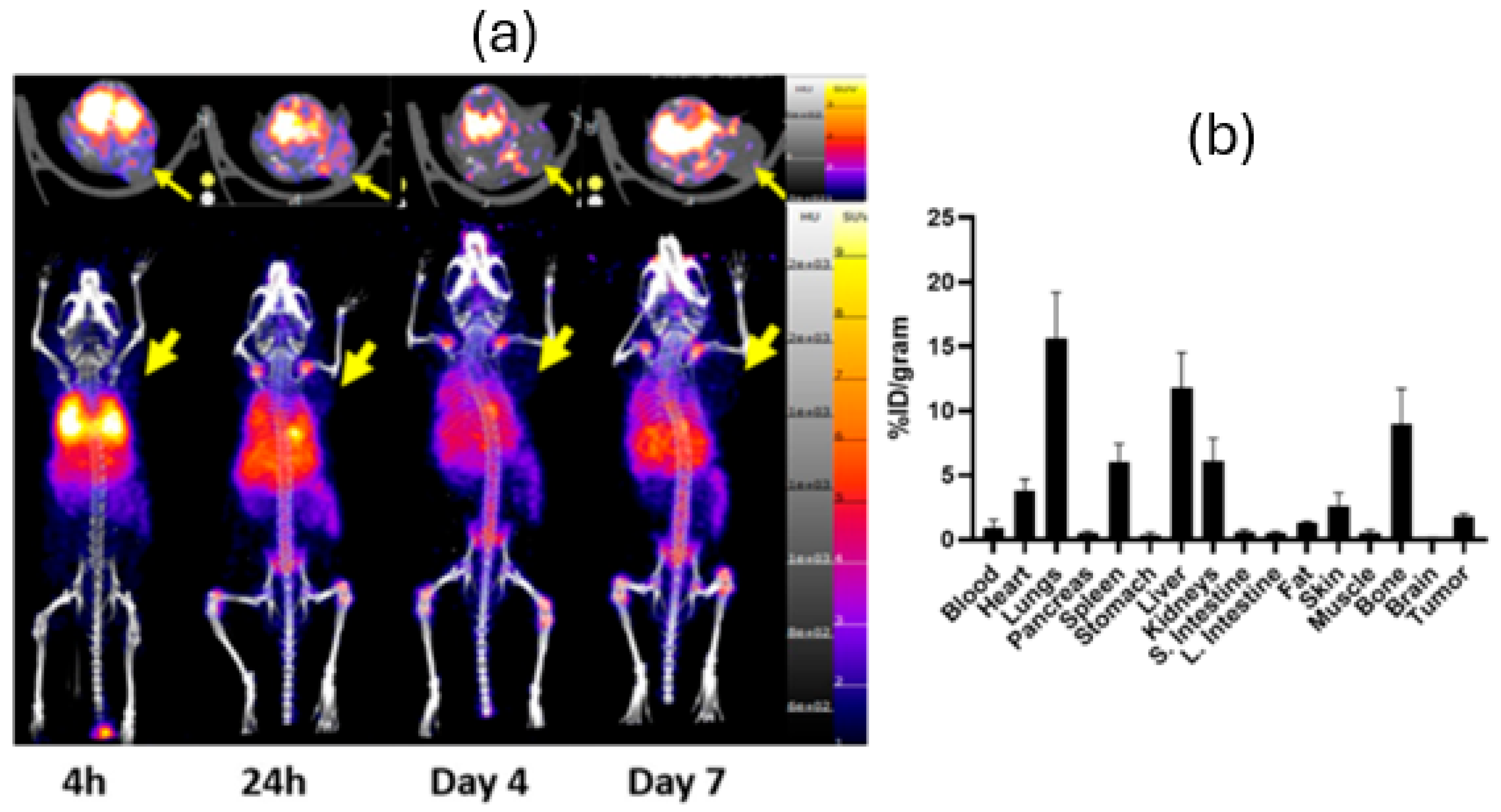
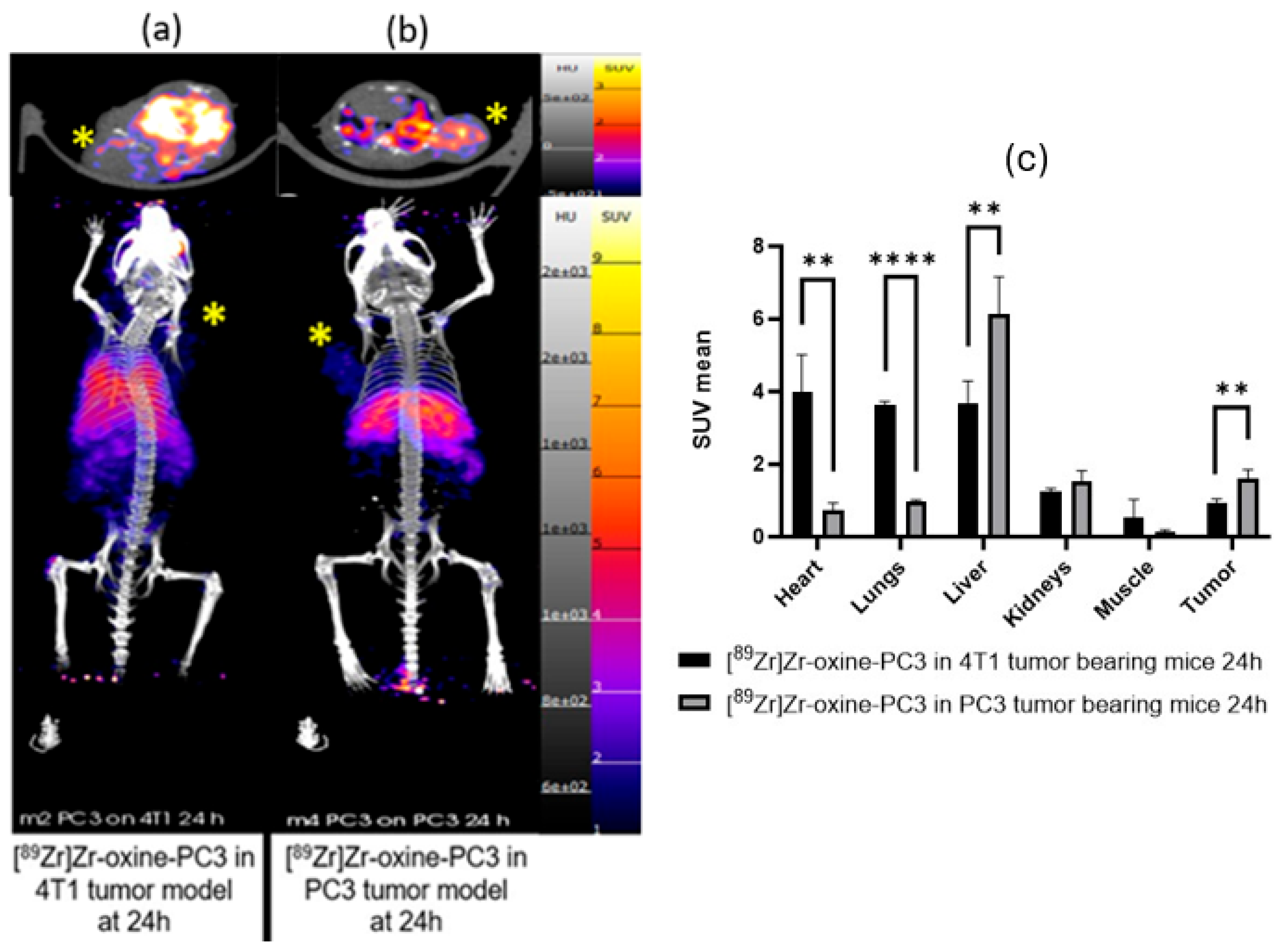
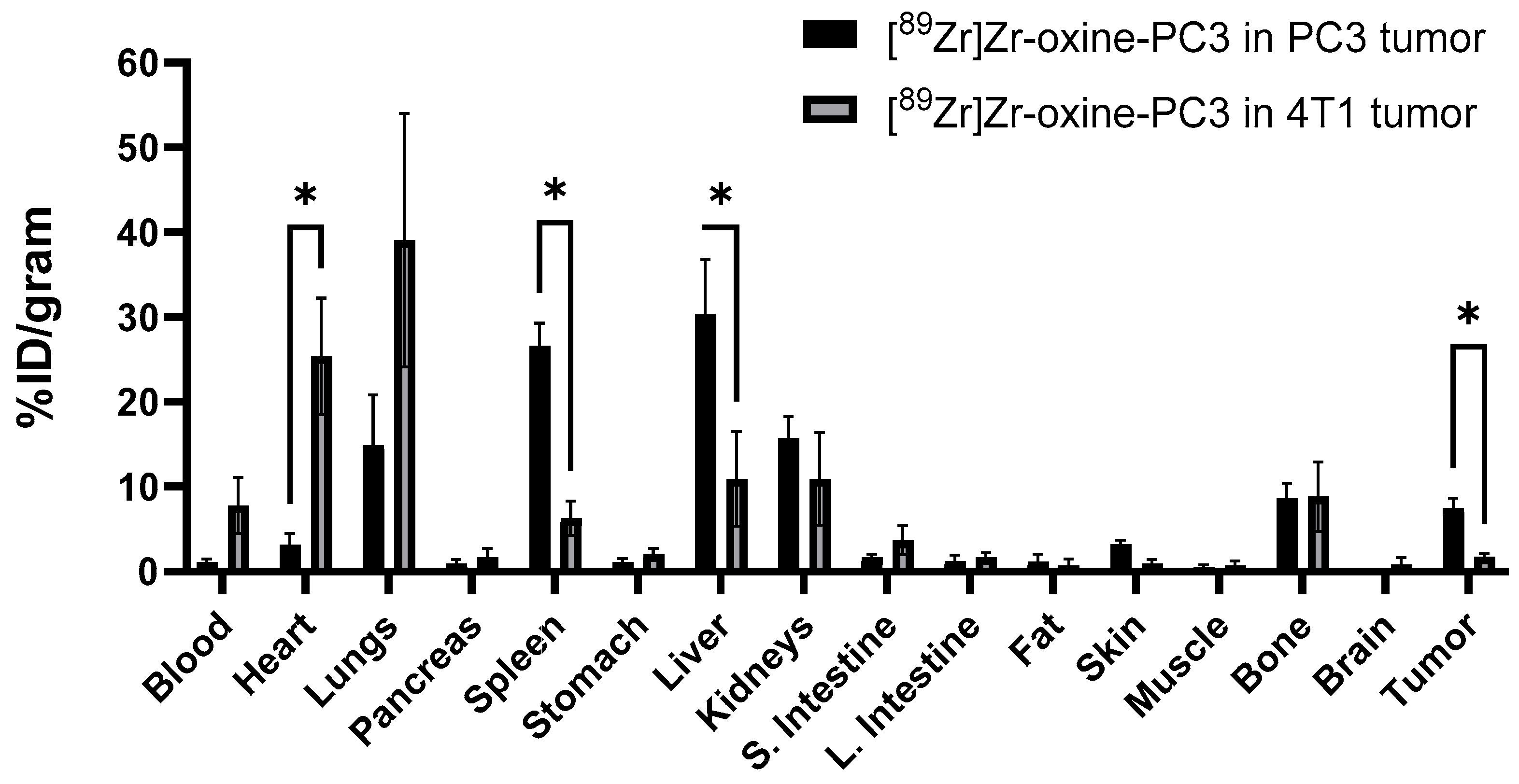
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PC3 | Human prostate adenocarcinoma cells |
| 4T1 | Murine mammary carcinoma cells |
| TLC | Thin-layer chromatography |
| HPLC | High-performance liquid chromatography |
| ATCC | American type culture collection |
| SUV | Standardized uptake value |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| %ID/gram | Injected dose per gram |
References
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Alimohammadvand, S.; Kaveh Zenjanab, M.; Mashinchian, M.; Shayegh, J.; Jahanban-Esfahlan, R. Recent advances in biomimetic cell membrane-camouflaged nanoparticles for cancer therapy. Biomed. Pharmacother. 2024, 177, 11695. [Google Scholar] [CrossRef]
- Friberger, I.; Jussing, E.; Jinming, H.; Goos, J.A.C.M.; Siikanen, J.; Kaipe, H.; Labmert, M.; Harris, R.A.; Samen, E.; Carlsten, M.; et al. Optimisation of the synthesis and cell labelling conditions for [89Zr] Zr-oxine and [89Zr] Zr-DFO-NCS: A direct in vitro comparison in cell types with distinct therapeutic applications. Mol. Imaging Biol. 2021, 23, 952–962. [Google Scholar] [CrossRef]
- Massicano, A.V.F.; Bartel, J.L.; Jeffers, C.D.; Crenshaw, B.K.; Houson, H.; Mueller, C.; Younger, J.W.; Knapp, P.; McConathy, J.E.; Lapi, S.E. Production of [89Zr] Oxinate4 and cell radiolabeling for human use. J. Labelled Comp. Radiopharm. 2021, 64, 209–216. [Google Scholar] [CrossRef]
- Kumar, S.; Jiang, D.; Sun, B.; Seeley, K.V.; Engle, J.W.; Sia, E.; He, X.; Neelamegham, S.; Cai, W.; Lovell, J.J. Labeling of erythrocytes by porphyrin—Phospholipid. Adv. Nanobiomed. Res. 2021, 1, 16. [Google Scholar] [CrossRef]
- Jing-Yi, Z.; Zheng, D.W.; Xhang, M.K.; Yu, W.Y.; Qiu, W.X.; Hu, J.J.; Feng, J.; Zhang, X.Z. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016, 16, 5895–5901. [Google Scholar]
- Peter, J.G.; Man, F.; Blower, P.J.; Rosales, R.T.M. Direct cell radiolabeling for in vivo cell tracking with PET and SPECT imaging. Chem. Rev. 2022, 122, 10266–10318. [Google Scholar] [CrossRef] [PubMed]
- Valentina, O.; Graziella, V. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef]
- Krekorian, M.; Sandker, G.G.W.; Cortenbach, K.R.G.; Tagit, O.; Riessen, N.K.; Raave, R.; Srinivas, M.; Fidgor, C.G.; Heskamp, S.; Aarntzen, E.H.J.G. characterization of intrinsically radiolabeled poly(lactic-co-glycolic acid) nanoparticles for ex vivo autologous cell labeling and in vivo tracking. Bioconjug. Chem. 2021, 32, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Freesmeyer, M.; Grober, S.; Greiser, J.; Seifert, P.; Guhne, F.; Drescher, R. PET/CT with [68Ga]Gallium-oxine-labeled heat denatured red blood cells for detection of dystopic splenic tissue. Eur. J. Nucl. Med. Mol. Imaging. 2020, 48, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Socan, A.; Petrik, M.; Peitl, P.K.; Krosejl, M.; Rangger, C.; Novy, Z.; Scajger, U.; Gmeiner, T.; Decristoforo, C. On-cartridge preparation and evaluation of 68Ga-, 89Zr-and 64Cu-precursors for cell radiolabelling. Nucl. Med. Biol. 2019, 71, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gawne, P.; Man, F.; Fonslet, J.; Radia, R.; Bordoloi, J.; Cleveland, M.; Jimenez-Royo, P.; Gabizon, A.; Blower, P.J.; Long, N.; et al. Manganese-52: Applications in cell radiolabelling and liposomal nanomedicine PET imaging using oxine (8-hydroxyquinoline) as an ionophore. Dalton Trans. 2018, 47, 9283–9293. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Stringaris, K.; Davidson-Moncada, J.K.; Reger, R.; Adler, S.S.; Dunbar, C.; Choyke, P.L.; Childs, R.W. In vivo tracking of adoptively transferred natural killer cells in rhesus macaques using 89Zirconium-oxine cell labeling and PET imaging. Clin. Cancer Res. 2020, 26, 2573–2581. [Google Scholar] [CrossRef]
- Asiedu, K.O.; Koyasu, S.; Szajek, L.P.; Choyke, P.L.; Sato, N. Bone marrow cell trafficking analyzed by 89Zr-oxine positron emission tomography in a murine transplantation model. Clin. Cancer Res. 2017, 23, 2759–2768. [Google Scholar] [CrossRef]
- Sato, N.; Wu, H.; Asiedu, K.O.; Szajek, L.P.; Griffiths, G.L.; Choyke, P.L. 89Zr-oxine complex PET cell imaging in monitoring cell-based therapies. Radiology 2015, 275, 490–500. [Google Scholar] [CrossRef]
- Charoenphun, P.; Meszaros, L.K.; Chuamsaamerkkee, K.; Sharif-Paghaleh, E.; Ballinger, J.R.; Ferris, T.J.; Went, M.J.; Mullen, G.E.D.; Blower, P.J. [89Zr]Oxinate4 for long-term in vivo cell tracking by positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 278–287. [Google Scholar] [CrossRef]
- Weist, M.; Starr, R.; Aguilar, B.; Chea, J.; Miles, J.K.; Poku, E.; Gerdts, E.; Yang, X.; Priceman, S.J.; Forman, S.J.; et al. PET of adoptively transferred chimeric antigen receptor T cells with 89Zr-oxine. J. Nucl. Med. 2018, 59, 1531–1537. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Choyke, P.L.; Sato, N. Imaging of cell-based therapy using 89Zr-oxine ex vivo cell labeling for positron emission tomography. Nanotheranostics 2021, 5, 27–35. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 8, 2495–2505. [Google Scholar] [CrossRef]
- Glukhova, M.; Deugnier, M.A.; Thiery, J.P. Tumor progression: The role of cadherins and integrins. Mol. Med. Today 1995, 1, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Herndon, M.E.; Gibson-Corley, K.; Wallrath, L.L.; Henry, M.D.; Stipp, C.S. The 4T1 breast carcinoma model possesses hybrid epithelial/mesenchymal traits linked to a highly metastatic phenotype. Dis. Model. Mech. 2024, 17, dmm050169. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.V.; Nima, Z.A.; Vang, K.B.; Kannarpady, G.; Nedosekin, A.D.; Zharov, V.P.; Griffin, R.J.; Biris, A.S.; Dings, R.P.M. Triple-negative breast cancer targeting and killing by EpCAM-directed, plasmonically active nanodrug systems. npj Precis. Oncol. 2017, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yamada, S. N-cadherin dependent collective cell invasion of prostate cancer cells is regulated by the N-terminus of α-catenin. PLoS ONE 2013, 8, e55069. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Muller, D.J.; Helenius, J. In PC3 prostate cancer cells ephrin receptors crosstalk to β1-integrins to strengthen adhesion to collagen type I. Sci Rep. 2015, 3, 5, 8206. [Google Scholar] [CrossRef]
- Sung, S.Y.; Wu, I.H.; Chuang, P.H.; Petros, J.A.; Wu, H.C.; Zeng, H.J.; Huang, W.C.; Chung, L.W.; Hsieh, C.L. Targeting L1 cell adhesion molecule expression using liposome-encapsulated siRNA suppresses prostate cancer bone metastasis and growth. Oncotarget 2014, 5, 20, 9911–9929. [Google Scholar] [CrossRef]
- Queern, S.L.; Aweda, T.A.; Massicano, A.V.F.M.; Clanton, N.A.; Sayed, R.E.; Sader, J.A.; Zyuzin, A.; Lapi, S.E. Production of Zr-89 using sputtered yttrium coin targets. Nucl. Med. Biol. 2017, 50, 11–16. [Google Scholar] [CrossRef]
- Friberger, I.; Nilsson, J.N.; Lu, L.; Siikanen, J.; Ardenfors, O.; Milton, S.; Samen, E.; Goos, J.A.C.M.; Carlsten, M.; Holmin, S.; et al. Comparative in vivo biodistribution of cells labelled with [89Zr]Zr-(oxinate)4 or [89Zr] Zr-DFO-NCS using PET. EJNMMI Res. 2023, 13, 73. [Google Scholar] [CrossRef]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef]
- Seo, B.B.; Jahed, Z.; Coggan, J.A.; Chau, Y.Y.; Rogowski, J.L.; Gu, F.X.; Wen, W.; Mofrad, M.R.K.; Tsui, T.Y. Mechanical contact characteristics of pc3 human prostate cancer cells on complex-shaped silicon micropillars. Materials 2017, 10, 892. [Google Scholar] [CrossRef]
- Hoffmann, E.; Gerwing, M.; Niland, S.; Niehoff, R.; Masthoff, M.; Geyer, C.; Wachsmuth, L.; Wilken, E.; Höltke, C.; Heindel, W.L.; et al. Profiling specific cell populations within the inflammatory tumor microenvironment by oscillating-gradient diffusion-weighted MRI. J. Immunother. Cancer 2023, 11, e006092. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin, V.; Archer, N.E.; Fernandez, S.R.; Houson, H.A.; Bartels, J.L.; Lapi, S.E. Homotypic Targeting of [89Zr]Zr-Oxine Labeled PC3 and 4T1 Cells in Tumor-Bearing Mice. Pharmaceutics 2025, 17, 1259. https://doi.org/10.3390/pharmaceutics17101259
Tekin V, Archer NE, Fernandez SR, Houson HA, Bartels JL, Lapi SE. Homotypic Targeting of [89Zr]Zr-Oxine Labeled PC3 and 4T1 Cells in Tumor-Bearing Mice. Pharmaceutics. 2025; 17(10):1259. https://doi.org/10.3390/pharmaceutics17101259
Chicago/Turabian StyleTekin, Volkan, Noel E. Archer, Solana R. Fernandez, Hailey A. Houson, Jennifer L. Bartels, and Suzanne E. Lapi. 2025. "Homotypic Targeting of [89Zr]Zr-Oxine Labeled PC3 and 4T1 Cells in Tumor-Bearing Mice" Pharmaceutics 17, no. 10: 1259. https://doi.org/10.3390/pharmaceutics17101259
APA StyleTekin, V., Archer, N. E., Fernandez, S. R., Houson, H. A., Bartels, J. L., & Lapi, S. E. (2025). Homotypic Targeting of [89Zr]Zr-Oxine Labeled PC3 and 4T1 Cells in Tumor-Bearing Mice. Pharmaceutics, 17(10), 1259. https://doi.org/10.3390/pharmaceutics17101259







