Emerging Insulin Analogues: A Glimpse into How Insulin Analogues May Look in the near Future
Abstract
1. Introduction
2. Methods of Literature Search
3. General Physiology of Insulin
3.1. Insulin Signalling
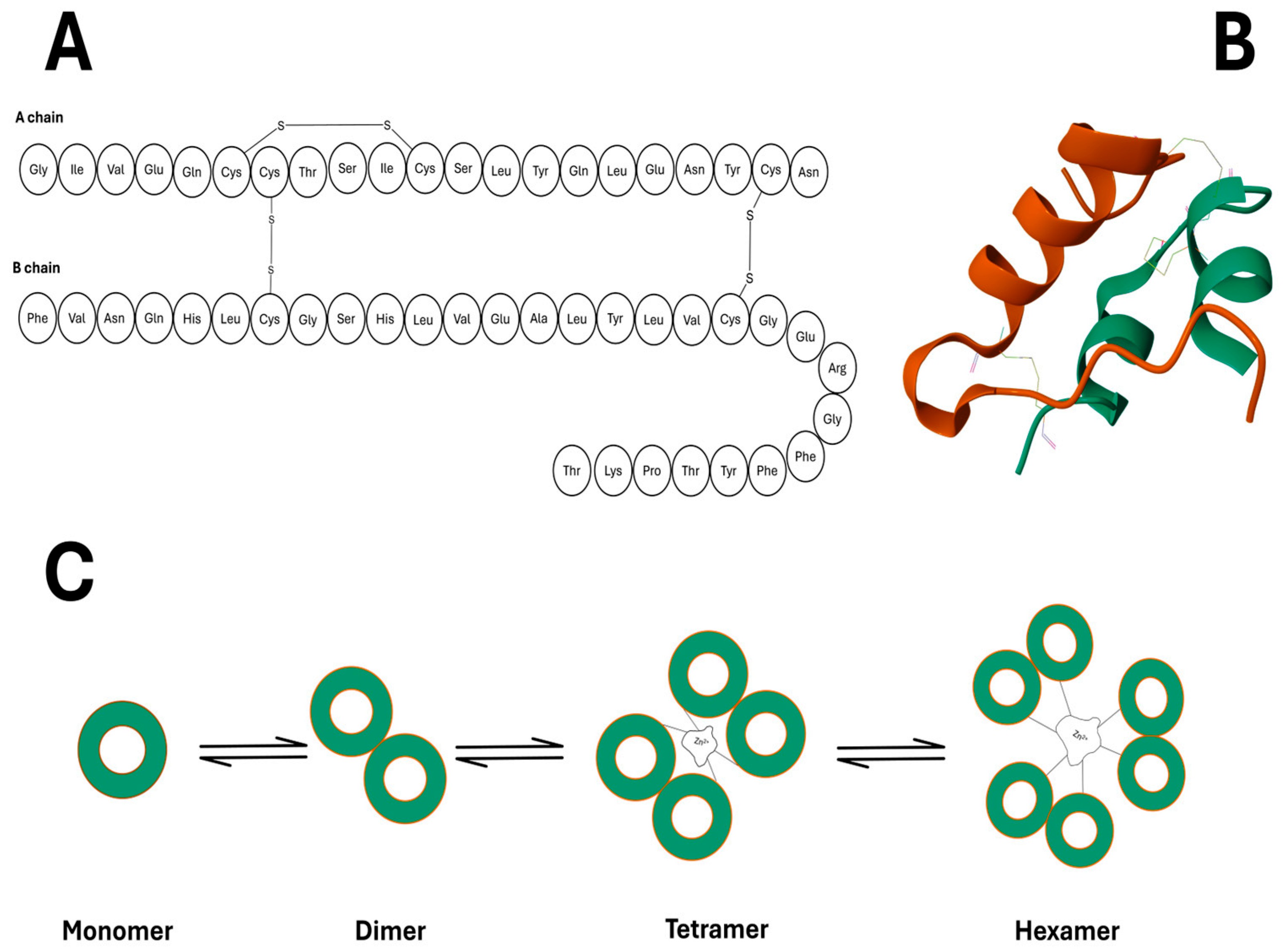
3.2. Insulin’s Architecture
3.3. Conventional Insulin Analogues
4. Emerging Insulin Analogues
4.1. Ultra-Acting Insulin Analogues
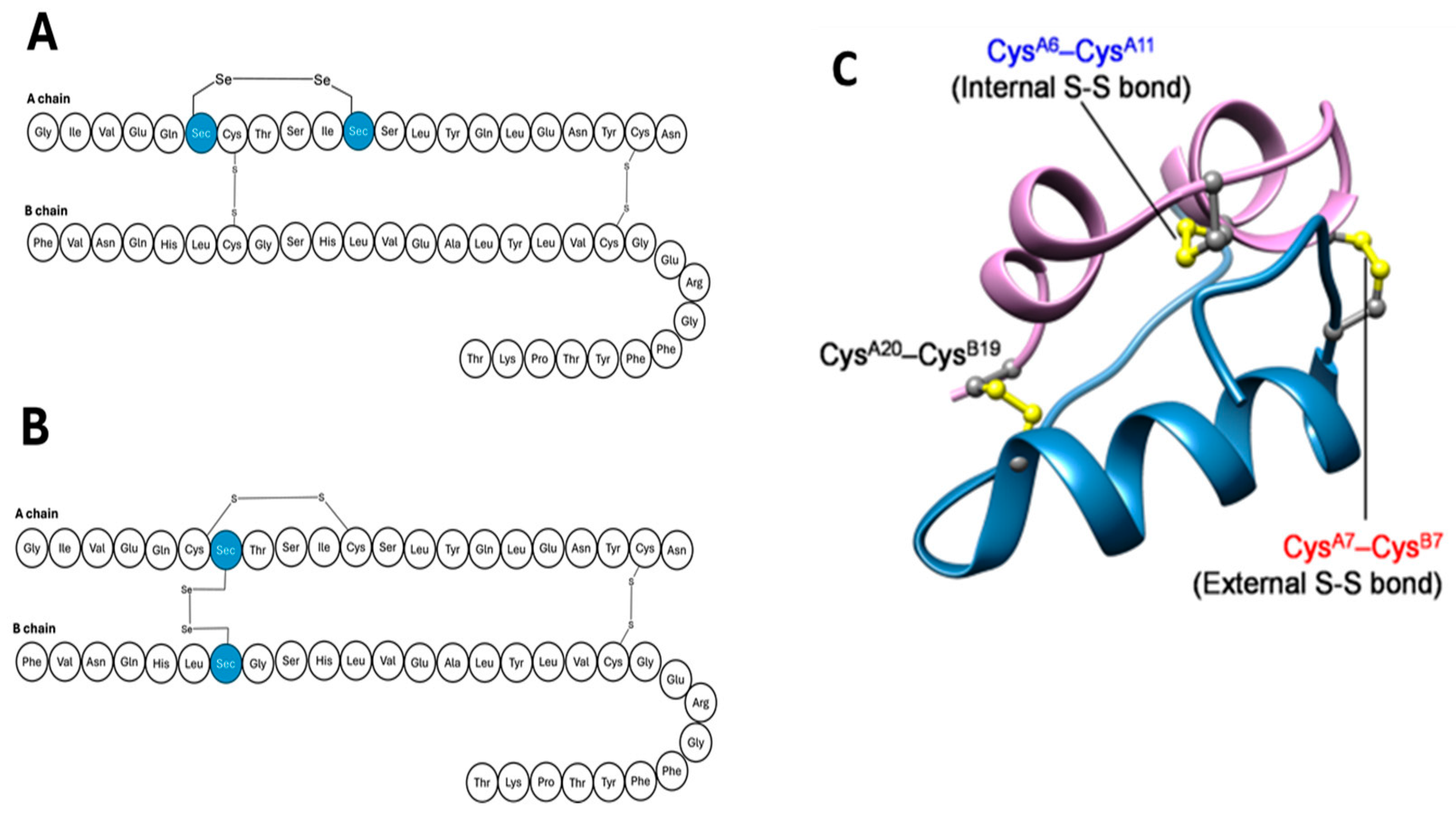
4.1.1. Seleno-Insulin
4.1.2. Degludec-like Insulins
4.2. Thermostable Insulin
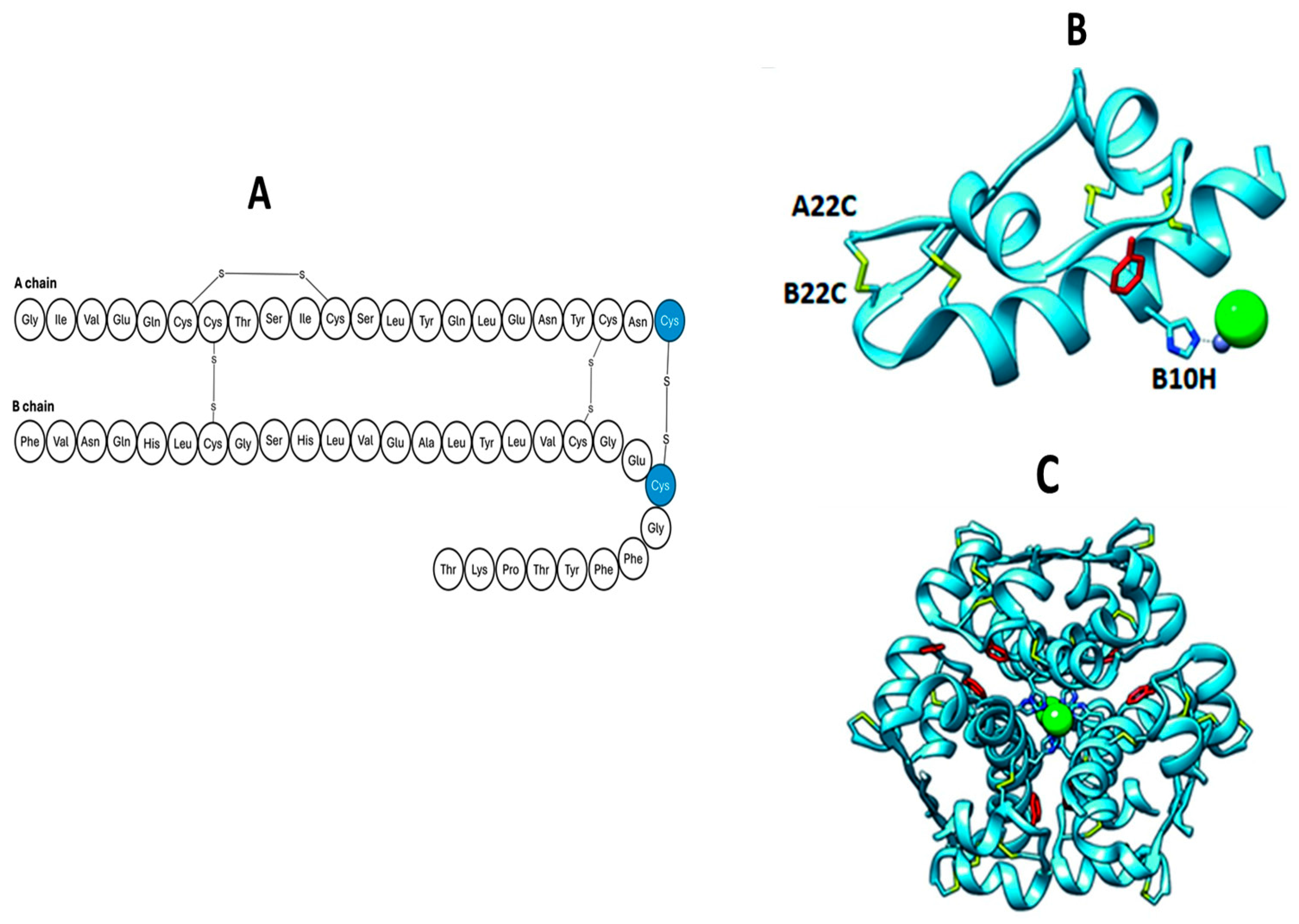
4.2.1. Additional Disulphide Bonds
4.2.2. Carbon-Based Linkers
4.2.3. Single-Chain Insulin
4.3. Glucose-Responsive Insulins
4.3.1. Saccharide Linked Insulins
4.3.2. Phenylboronic Acid Insulin
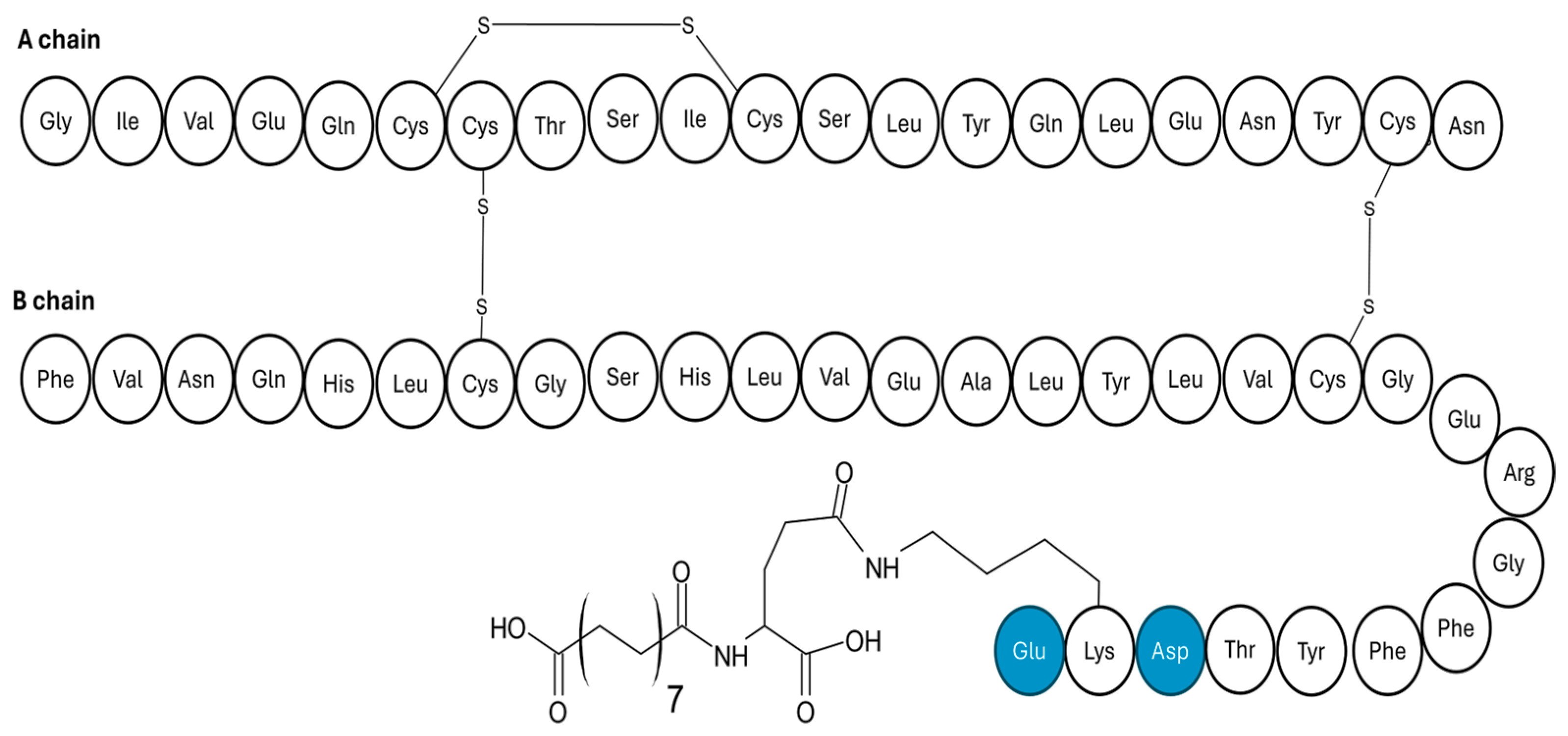
4.4. Hepato-Preferential Insulin Analogues
4.5. Once-Weekly Insulin Analogues on the Verge of Approval
5. Author Perspective and Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLUT 4 | Glucose transporter 4 |
| ER | Endoplasmic reticulum |
| IDE | Insulin degrading enzyme |
| SCI | Single-chain insulin |
References
- Goldman-Levine, J.D.; Lee, K.W. Insulin Detemir—A New Basal Insulin Analog. Ann. Pharmacother. 2005, 39, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, D.S.; Scott, M.; Farmer, B.; Williams, P.E.; Madsen, P.; Kjeldsen, T.; Brand, C.L.; Fledelius, C.; Nishimura, E.; Cherrington, A.D. Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery. JCI Insight 2019, 4, e126974. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2018, 61, 2461–2498. [Google Scholar] [CrossRef]
- American Diabetes Association. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. S1), S73–S85. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A. A Glucose-Controlled Insulin-Delivery System: Semisynthetic Insulin Bound to Lectin. Science 1979, 206, 1190–1191. [Google Scholar] [CrossRef]
- Edgerton, D.S.; Moore, M.C.; Winnick, J.J.; Scott, M.; Farmer, B.; Naver, H.; Jeppesen, C.B.; Madsen, P.; Kjeldsen, T.B.; Nishimura, E.; et al. Changes in Glucose and Fat Metabolism in Response to the Administration of a Hepato-Preferential Insulin Analog. Diabetes 2014, 63, 3946–3954. [Google Scholar] [CrossRef]
- Sen, S.; Ali, R.; Onkar, A.; Ganesh, S.; Verma, S. Strategies for Interference of Insulin Fibrillogenesis: Challenges and Advances. ChemBioChem 2022, 23, e202100678. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Steiner, D.F.; Park, S.Y.; Støy, J.; Philipson, L.H.; Bell, G.I. A brief perspective on insulin production. Diabetes Obes. Metab. 2009, 11, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Steiner, D.F.; Philipson, L.H. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships; MDText.com, Inc.: Portland, OR, USA, 2014. [Google Scholar]
- Liu, M.; Weiss, M.A.; Arunagiri, A.; Yong, J.; Rege, N.; Sun, J.; Haataja, L.; Kaufman, R.J.; Arvan, P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes. Metab. 2018, 20, 28–50. [Google Scholar] [CrossRef]
- Huang, X.F.; Arvan, P. Intracellular transport of proinsulin in pancreatic β-cells: Structural maturation probed by disulfide accessibility. J. Biol. Chem. 1995, 270, 20417–20423. [Google Scholar] [CrossRef]
- Dunn, M.F. Zinc–ligand interactions modulate assembly and stability of the insulin hexamer—A review. Biometals 2005, 18, 295–303. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdelrahman, M.M. Role of insulin and other related hormones in energy metabolism—A review. Cogent Food Agric. 2016, 2, 1267691. [Google Scholar] [CrossRef]
- Menting, J.G.; Whittaker, J.; Margetts, M.B.; Whittaker, L.J.; Kong, G.K.W.; Smith, B.J.; Watson, C.J.; Žáková, L.; Kletvíková, E.; Jiráček, J.; et al. How insulin engages its primary binding site on the insulin receptor. Nature 2013, 493, 241–245. [Google Scholar] [CrossRef]
- Lizcano, J.M.; Alessi, D.R. The insulin signalling pathway. Curr. Biol. 2002, 12, R236–R238. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Kuretu, A.; Mothibe, M.; Ngubane, P.; Sibiya, N. Elucidating the effect of drug-induced mitochondrial dysfunction on insulin signaling and glucose handling in skeletal muscle cell line (C2C12) in vitro. PLoS ONE 2024, 19, e0310406. [Google Scholar] [CrossRef] [PubMed]
- Wittlin, S.D.; Woehrle, H.J.; Gerich, J.E. Insulin Pharmacokinetics. Insulin Therapy; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 73–85. [Google Scholar]
- Amata, O.; Marino, T.; Russo, N.; Toscano, M. Human insulin-degrading enzyme working mechanism. J. Am. Chem. Soc. 2009, 131, 14804–14811. [Google Scholar] [CrossRef]
- Huang, K.; Xu, B.; Hu, S.Q.; Chu, Y.C.; Hua, Q.X.; Qu, Y.; Li, B.; Wang, S.; Wang, R.Y.; Nakagawa, S.H.; et al. How insulin binds: The B-chain α-helix contacts the L1 β-helix of the insulin receptor. J. Mol. Biol. 2004, 341, 529–550. [Google Scholar] [CrossRef]
- Davies, K.J.; Delsignore, M.E.; Lin, S.W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 1987, 262, 9902–9907. [Google Scholar] [CrossRef]
- Sanger, F. Chemistry of Insulin. Science 1959, 129, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Blundell, T.; Dodson, G.; Hodgkin, D.; Mercola, D. Insulin: The structure in the crystal and its reflection in chemistry and biology. Adv. Protein Chem. 1972, 26, 279–402. [Google Scholar]
- Brange, J.; Vølund’novo, V. Insulin analogs with improved pharmacokinetic profiles. Adv. Drug Deliv. Rev. 1999, 35, 307–335. [Google Scholar] [CrossRef]
- Ciszak, E.; Beals, J.M.; Frank, B.H.; Baker, J.C.; Carter, N.D.; David Smith, G. Role of C-terminal B-chain residues in insulin assembly: The structure of hexameric LysB 28 ProB 29-human insulin. Structure 1995, 3, 615–622. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.H.; Tang, J.G. Intra-A chain disulphide bond forms first during insulin precursor folding. Biochem. J. 1999, 343, 139–144. [Google Scholar] [CrossRef]
- Cecil, R.; Weitzman, P.D.J. The electroreduction of the disulphide bonds of insulin and other proteins. Biochem. J. 1964, 93, 1–11. [Google Scholar] [CrossRef]
- Akbarian, M.; Ghasemi, Y.; Uversky, V.N.; Yousefi, R. Chemical modifications of insulin: Finding a compromise between stability and pharmaceutical performance. Int. J. Pharm. 2018, 547, 450–468. [Google Scholar] [CrossRef]
- Baker, E.N.; Blundell, T.L.; Cutfield, J.F.; Cutfield, S.M.; Dodson, E.J.; Dodson, G.G.; Hodgkin, D.M.C.; Hubbard, R.E.; Isaacs, N.W.; Reynolds, C.D.; et al. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988, 319, 369–456. [Google Scholar] [PubMed]
- Ohkuri, T.; Yamagishi, A. Increased thermal stability against irreversible inactivation of 3-isopropylmalate dehydrogenase induced by decreased van der Waals volume at the subunit interface. Protein Eng. 2003, 16, 615–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hua, Q. Insulin: A small protein with a long journey. Protein Cell 2010, 1, 537–551. [Google Scholar] [CrossRef]
- Jarosinski, M.A.; Dhayalan, B.; Chen, Y.S.; Chatterjee, D.; Varas, N.; Weiss, M.A. Structural principles of insulin formulation and analog design: A century of innovation. Mol. Metab. 2021, 52, 101325. [Google Scholar] [CrossRef] [PubMed]
- Joslin, E.P. The Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1916, 6, 673–684. [Google Scholar]
- De Meyts, P. Insulin and its receptor: Structure, function and evolution. BioEssays 2004, 26, 1351–1362. [Google Scholar] [CrossRef]
- Mathieu, C.; Gillard, P.; Benhalima, K. Insulin analogues in type 1 diabetes mellitus: Getting better all the time. Nat. Rev. Endocrinol. 2017, 13, 385–399. [Google Scholar] [CrossRef]
- Porcellati, F.; Lucidi, P.; Candeloro, P.; Cioli, P.; Andreoli, A.M.; Curti, G.; Bolli, G.B.; Fanelli, C.G. Pharmacokinetics, Pharmacodynamics, and Modulation of Hepatic Glucose Production with Insulin Glargine U300 and Glargine U100 at Steady State with Individualised Clinical Doses in Type 1 Diabetes. Diabetes Care 2018, 42, 85–92. [Google Scholar] [CrossRef]
- Levien, T.L.; Baker, D.E.; White, J.R.; Campbell, R.K. Insulin Glargine: A new basal insulin. Ann. Pharmacother. 2002, 36, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.P.; Zhang, F.; Dimarchi, R.D. Insulin Structure and Function. Pept. Sci. 2007, 88, 687–713. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.S.; Eaton, R.P.; Delqngo, J.; Saland, L.C.; Ladman, A.J.; Carlson, G.A. Electron Microscopy of Insulin Precipitates. Diabetes Care 1982, 5, 25–30. [Google Scholar] [CrossRef]
- Whittingham, J.L.; Edwards, D.J.; Antson, A.A.; Clarkson, J.M.; Dodson, G.G. Interactions of phenol and m-cresol in the insulin hexamer, and their effect on the association properties of B28 Pro → Asp insulin analogues. Biochemistry 1998, 37, 11516–11523. [Google Scholar] [CrossRef]
- Ruiatkina, L.A.; Sorokin, M.I. Detemir (Levemir): Modern paradigms of insulin therapy. Probl. Endocrinol. 2013, 59, 56–64. [Google Scholar] [CrossRef][Green Version]
- Vora, J.; Cariou, B.; Evans, M.; Gross, J.L.; Harris, S.; Landstedt-Hallin, L.; Mithal, A.; Rodriguez, M.R.; Meneghini, L. Clinical use of insulin degludec. Diabetes Res. Clin. Pract. 2015, 109, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Havelund, S.; Plum, A.; Ribel, U.; Jonassen, I.; Vølund, A.; Markussen, J.; Kurtzhals, P. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm. Res. 2004, 21, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Steensgaard, D.B.; Schluckebier, G.; Strauss, H.M.; Norrman, M.; Thomsen, J.K.; Friderichsen, A.V.; Havelund, S.; Jonassen, I. Ligand-Controlled Assembly of Hexamers, Dihexamers, and Linear Multihexamer Structures by the Engineered Acylated Insulin Degludec. Biochemistry 2012, 52, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Okumura, M.; Lee, Y.H.; Katayama, H.; Mizutani, K.; Lin, Y.; Park, S.Y.; Sawada, K.; Toyoda, M.; Hojo, H.; et al. Diselenide-bond replacement of the external disulfide bond of insulin increases its oligomerisation leading to sustained activity. Commun. Chem. 2023, 6, 258. [Google Scholar] [CrossRef]
- Mousa, R.; Notis Dardashti, R.; Metanis, N. Selen und Selenocystein in der Proteinchemie. Angew. Chem. 2017, 129, 16027–16037. [Google Scholar] [CrossRef]
- Pan, K.; Shi, X.; Liu, K.; Wang, J.; Chen, Y. Efficacy, pharmacokinetics, biodistribution and excretion of a novel acylated long-acting insulin analogue ins061 in rats. Drug Des. Dev. Ther. 2021, 15, 3487–3498. [Google Scholar] [CrossRef]
- Akbarian, M.; Yousefi, R.; Farjadian, F.; Uversky, V.N. Insulin fibrillation: Toward strategies for attenuating the process. Chem. Commun. 2020, 56, 11354–11373. [Google Scholar] [CrossRef]
- Groenning, M.; Frokjaer, S.; Vestergaard, B.J. Formation mechanism of insulin fibrils and structural aspects of the insulin fibrillation process. Curr. Protein Pept. Sci. 2009, 10, 509–528. [Google Scholar] [CrossRef]
- Liu, F.; Zaykov, A.N.; Levy, J.J.; DiMarchi, R.D.; Mayer, J.P. Chemical synthesis of peptides within the insulin superfamily. J. Pept. Sci. 2016, 22, 260–270. [Google Scholar] [CrossRef]
- Hidaka, K.; Kobayashi, D.; Hayashi, J.; Denda, M.; Otaka, A. Advanced Insulin Synthesis by One-pot/Stepwise Disulfide Bond Formation Enabled by S-Protected Cysteine Sulfoxide. Chem. Eur. J. 2024, 30, e202401003. [Google Scholar] [CrossRef]
- Xiong, X.; Blakely, A.; Karra, P.; VandenBerg, M.A.; Ghabash, G.; Whitby, F.; Zhang, Y.W.; Webber, M.J.; Holland, W.L.; Hill, C.P.; et al. Novel four-disulfide insulin analog with high aggregation stability and potency. Chem. Sci. 2020, 11, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Karra, P.; VandenBerg, M.A.; Kim, J.H.; Webber, M.J.; Holland, W.L.; Chou, D.H. Synthesis and characterisation of an A6-A11 methylene thioacetal human insulin analogue with enhanced stability. J. Med. Chem. 2019, 62, 11437–11443. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.P.; Ahmad, A.; Fink, A.L. Fibrillation of human insulin A and B chains. Biochemistry 2006, 45, 9342–9353. [Google Scholar] [CrossRef] [PubMed]
- Perillo, M.; Arnone, M.I. Characterisation of insulin-like peptides (ILPs) in the sea urchin Strongylocentrotus purpuratus: Insights on the evolution of the insulin family. Gen. Comp. Endocrinol. 2014, 205, 68–79. [Google Scholar] [CrossRef]
- Delgado, A.; Sozanski, K.S.; Daly, M. Towards the Exploration and Evolution of Insulin-like Venoms in Actiniaria (Sea anemones). Mar. Drugs 2024, 22, 136. [Google Scholar] [CrossRef]
- Shabanpoor, F.; Separovic, F.; Wade, J.D. The human insulin superfamily of polypeptide hormones. Vitam. Horm. 2009, 80, 1–31. [Google Scholar]
- Nakagawa, S.H.; Tager, H.S. Perturbation of insulin-receptor interactions by intramolecular hormone cross-linking. J. Biol. Chem. 1989, 264, 272–279. [Google Scholar] [CrossRef]
- Freychet, P.; Brandenburg, D.; Wollmer, A. Receptor-Binding Assay of Chemically Modified Insulins Comparison with in-vitro and in-vivo Bioassays. Diabetologia 1974, 10, 1–5. [Google Scholar] [CrossRef]
- Brems, D.N.; Brown, P.L.; Nakagawa, S.H.; Tager, H.S. The Conformational Stability and Flexibility of Insulin with an Additional Intramolecular Cross-link. J. Biol. Chem. 1991, 266, 1611–1615. [Google Scholar] [CrossRef]
- Gross, D.J.; Villa-Komaroffll, L.; Kahn, C.R.; Weir, G.C.; Halban, P.A.; Jeantet, L. Deletion of a Highly Conserved Tetrapeptide Sequence of the Proinsulin Connecting Peptide (C-peptide) Inhibits Proinsulin to Insulin Conversion by Transfected Pituitary Corticotroph (AtT20) Cells. J. Biol. Chem. 1989, 264, 21486–21490. [Google Scholar] [CrossRef] [PubMed]
- Heath, W.F.; Belagaje, R.M.; Brooke, G.S.; Chance, R.E.; Hoffmann, J.A.; Long, H.B.; Reams, S.G.; Roundtree, C.; Shaw, W.N.; Slieker, L.J.; et al. (A-C-B) human proinsulin, a novel insulin agonist and intermediate in the synthesis of biosynthetic human insulin. J. Biol. Chem. 1992, 267, 419–425. [Google Scholar] [CrossRef]
- Markussen, J.; Jørgensen, K.H.; Sørensen, A.R.; Thim, L. Single chain des-(B30) insulin: Intramolecular crosslinking of insulin by trypsin catalysed transpeptidation. Int. J. Pept. Protein Res. 1985, 26, 70–77. [Google Scholar] [CrossRef]
- Kaur, Z.P.; Ochman, A.R.; Mayer, J.P.; Gelfanov, V.M.; Dimarchi, R.D. Discovery of high potency, single-chain insulin analogs with a shortened B-chain and nonpeptide linker. ACS Chem. Biol. 2013, 8, 1822–1829. [Google Scholar] [CrossRef]
- Mao, R.; Chen, Y.; Chi, Z.; Wang, Y. Insulin and its single-chain analogue. Appl. Microbiol. Biotechnol. 2019, 103, 8737–8751. [Google Scholar] [CrossRef]
- Mathieu, C. Minimising hypoglycaemia in the real world: The challenge of insulin. Diabetologia 2021, 64, 978–984. [Google Scholar] [CrossRef]
- MacLeod, K.M.; Hepburn, D.A.; Frier, B.M. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabet. Med. 1993, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Hurwitz, S.; Turchin, A.; Trivedi, A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalised patients. Diabetes Care 2013, 36, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Lauritzen, T.; Faber, O.; Pramming, S. Insulin pharmacokinetics. Diabetes Care 1984, 7, 188–199. [Google Scholar] [CrossRef]
- Heinemann, L. Variability of insulin absorption and insulin action. Diabetes Technol. Ther. 2002, 4, 673–682. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, G.S. Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine. J. Nanoparticle Res. 2022, 24, 228. [Google Scholar] [CrossRef]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human lectins, their carbohydrate affinities and where to find them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef]
- Kaarsholm, N.C.; Lin, S.; Yan, L.; Kelly, T.; van Heek, M.; Mu, J.; Wu, M.; Dai, G.; Cui, Y.; Zhu, Y.; et al. Engineering glucose responsiveness into insulin. Diabetes 2018, 67, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Agrawal, R.; Chen, D.; Zheng, N.; Durupt, G.; Kim, J.H.; Fisher, S.J.; Chou, D.H. Long-Lasting Designer Insulin with Glucose-Dependent Solubility Markedly Reduces Risk of Hypoglycemia. Adv. Ther. 2019, 2, 1900128. [Google Scholar] [CrossRef]
- Lin, N.P.; Zheng, N.; Purushottam, L.; Zhang, Y.W.; Chou, D.H.C. Synthesis and characterisation of phenylboronic acid-modified insulin with glucose-dependent solubility. Front. Chem. 2022, 10, 859133. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, M.; Williams, M. Toxicological Profile for Boron; US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2010.
- Ader, M.A.; Bergman, R.N. Peripheral effects of insulin dominate suppression of fasting hepatic glucose production. Am. J. Physiol.-Endocrinol. Metab. 1990, 258, E1020–E1032. [Google Scholar] [CrossRef]
- Bergman, R.N.; Kabir, M.; Ader, M. The physiology of insulin clearance. Int. J. Mol. Sci. 2022, 23, 1826. [Google Scholar] [CrossRef]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin delivery methods: Past, present and future. Int. J. Pharm. Investig. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Sibiya, N.; Mbatha, B.; Ngubane, P.; Khathi, A. Celebrating a century of insulin Discovery: A critical Appraisal of the emerging alternative insulin Delivery systems. Curr. Drug Deliv. 2023, 20, 656–668. [Google Scholar] [CrossRef]
- Brand, C.L.; Bouman, S.D.; Kildegaard, J.; Kjeldsen, T.B.; Madsen, P.; Naver, H.; Jeppesen, C.B.; Fels, J.J.; Sturis, J.; Nishimura, E. Liver preferential effects of an insulin analogue demonstrated by insulin receptor phosphorylation and glucose dynamics in insulin target tissues in vivo. Diabetologia 2012, 55, S376. [Google Scholar]
- Moyers, J.S.; Hansen, R.J.; Day, J.W.; Dickinson, C.D.; Zhang, C.; Ruan, X.; Ding, L.; Brown, R.M.; Baker, H.E.; Beals, J.M. Preclinical characterisation of LY3209590, a novel weekly basal insulin fc-fusion protein. J. Pharmacol. Exp. Ther. 2022, 382, 346–355. [Google Scholar] [CrossRef]
- Philis-Tsimikas, A.; Bajaj, H.S.; Begtrup, K.; Cailleteau, R.; Gowda, A.; Lingvay, I.; Mathieu, C.; Russell-Jones, D.; Rosenstock, J. Rationale and design of the phase 3a development programme (ONWARDS 1–6 trials) investigating once-weekly insulin Icodec in diabetes. Diabetes Obes. Metab. 2023, 25, 331–341. [Google Scholar] [CrossRef]
- Kjeldsen, T.B.; Hubalek, F.; Hjørringgaard, C.U.; Tagmose, T.M.; Nishimura, E.; Stidsen, C.E.; Porsgaard, T.; Fledelius, C.; Refsgaard, H.H.; Gram-Nielsen, S.; et al. Molecular engineering of insulin Icodec, the first acylated insulin analog for once-weekly administration in humans. J. Med. Chem. 2021, 64, 8942–8950. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.; Pridal, L.; Glendorf, T.; Hansen, B.F.; Hubálek, F.; Kjeldsen, T.; Kristensen, N.R.; Lützen, A.; Lyby, K.; Madsen, P.; et al. Molecular and pharmacological characterisation of insulin Icodec: A new basal insulin analog designed for once-weekly dosing. BMJ Open Diabetes Res Care. 2021, 9, e002301. [Google Scholar] [CrossRef]
- Home, P. Making sense of weekly insulins. Lancet Diabetes Endocrinol. 2023, 11, 140–141. [Google Scholar] [CrossRef]
- Plum-Mörschel, L.; Andersen, L.R.; Hansen, S.; Hövelmann, U.; Krawietz, P.; Kristensen, N.R.; Lehrskov, L.L.; Haahr, H. Pharmacokinetic and pharmacodynamic characteristics of insulin Icodec after subcutaneous administration in the thigh, abdomen or upper arm in individuals with type 2 diabetes Mellitus. Clin. Drug Investig. 2023, 43, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Juneja, R.; Beals, J.M.; Moyers, J.S.; Ilag, L.; McCrimmon, R.J. The basis for weekly insulin therapy: Evolving evidence with insulin Icodec and insulin efsitora alfa. Endocr. Rev. 2024, 45, 379–413. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Buse, J.B.; Franek, E.; Hansen, M.V.; Koefoed, M.M.; Mathieu, C.; Pettus, J.; Stachlewska, K.; Rosenstock, J. A randomised, open-label comparison of once-weekly insulin Icodec titration strategies versus once-daily insulin Glargine U100. Diabetes Care 2021, 44, 1595–1603. [Google Scholar] [CrossRef]
- Bajaj, H.S.; Bergenstal, R.M.; Christoffersen, A.; Davies, M.J.; Gowda, A.; Isendahl, J.; Lingvay, I.; Senior, P.A.; Silver, R.J.; Trevisan, R.; et al. Switching to once-weekly insulin Icodec versus once-daily insulin Glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: A phase 2 randomised controlled trial. Diabetes Care 2021, 44, 1586–1594. [Google Scholar] [CrossRef]
- Silver, R.J.; Asong, M.; Begtrup, K.; Koefoed, M.M.; Heller, S.R.; Rosenstock, J. 191-OR: Similar hypoglycemia duration with once-weekly insulin icodec vs. Insulin glargine U100 in insulin naïve or experienced patients with T2D. Diabetes 2021, 70 (Suppl. S1), 191-OR. [Google Scholar] [CrossRef]
- Rosenstock, J.; Bain, S.C.; Gowda, A.; Jódar, E.; Liang, B.; Lingvay, I.; Nishida, T.; Trevisan, R.; Mosenzon, O. Weekly Icodec versus daily Glargine U100 in type 2 diabetes without previous insulin. N. Engl. J. Med. 2023, 389, 297–308. [Google Scholar] [CrossRef]
- Kurtzhals, P.; Nishimura, E.; Haahr, H.; Høeg-Jensen, T.; Johansson, E.; Madsen, P.; Sturis, J.; Kjeldsen, T. Commemorating insulin’s centennial: Engineering insulin pharmacology towards physiology. Trends Pharmacol. Sci. 2021, 42, 620–639. [Google Scholar] [CrossRef]
- Frias, J.; Chien, J.; Zhang, Q.; Chigutsa, E.; Landschulz, W.; Syring, K.; Wullenweber, P.; Haupt, A.; Kazda, C. Safety and efficacy of once-weekly basal insulin Fc in people with type 2 diabetes previously treated with basal insulin: A multicentre, open-label, randomised, phase 2 study. Lancet Diabetes Endocrinol. 2023, 11, 158–168. [Google Scholar] [CrossRef]
- Kazda, C.M.; Bue-Valleskey, J.M.; Chien, J.; Zhang, Q.; Chigutsa, E.; Landschulz, W.; Wullenweber, P.; Haupt, A.; Dahl, D. Novel once-weekly basal insulin Fc achieved similar glycemic control with a safety profile comparable to insulin degludec in patients with type 1 diabetes. Diabetes Care 2023, 46, 1052–1059. [Google Scholar] [CrossRef]
- Bue-Valleskey, J.M.; Kazda, C.M.; Ma, C.; Chien, J.; Zhang, Q.; Chigutsa, E.; Landschulz, W.; Haupt, A.; Frias, J.P. Once-Weekly basal insulin fc demonstrated similar glycemic control to once-daily insulin degludec in insulin-naive patients with type 2 diabetes: A phase 2 randomised control trial. Diabetes Care 2023, 46, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Grajower, M.M.; Fraser, C.G.; Holcombe, J.H.; Daugherty, M.L. How long should insulin be used once a vial is started? Diabetes Care 2003, 26, 2665. [Google Scholar] [CrossRef]
- Ogle, G.D.; Middlehurst, A.C.; Silink, M. The IDF Life for a Child Program Index of diabetes care for children and youth. Pediatr. Diabetes 2016, 17, 374–384. [Google Scholar] [PubMed]
- Ogle, G.D.; Abdullah, M.; Mason, D.; Januszewski, A.S.; Besançon, S. Insulin storage in hot climates without refrigeration: Temperature reduction efficacy of clay pots and other techniques. Diabet. Med. 2016, 33, 1544–1553. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef] [PubMed]
- Nieuwlaat, R.; Wilczynski, N.; Navarro, T.; Hobson, N.; Jeffery, R.; Keepanasseril, A.; Agoritsas, T.; Mistry, N.; Iorio, A.; Jack, S.; et al. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 2014, 11, 25412402. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, A.; Shehadeh, N. Hypoglycemia in diabetes: An update on pathophysiology, treatment, and prevention. World J. Diabetes 2021, 12, 2036–2049. [Google Scholar] [CrossRef] [PubMed]
- Pettus, J.H.; Zhou, F.L.; Shepherd, L.; Preblick, R.; Hunt, P.R.; Paranjape, S.; Miller, K.M.; Edelman, S.V. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in US adult patients with type 1 diabetes: A real-world study. Diabetes Care 2019, 42, 2220–2227. [Google Scholar] [CrossRef] [PubMed]


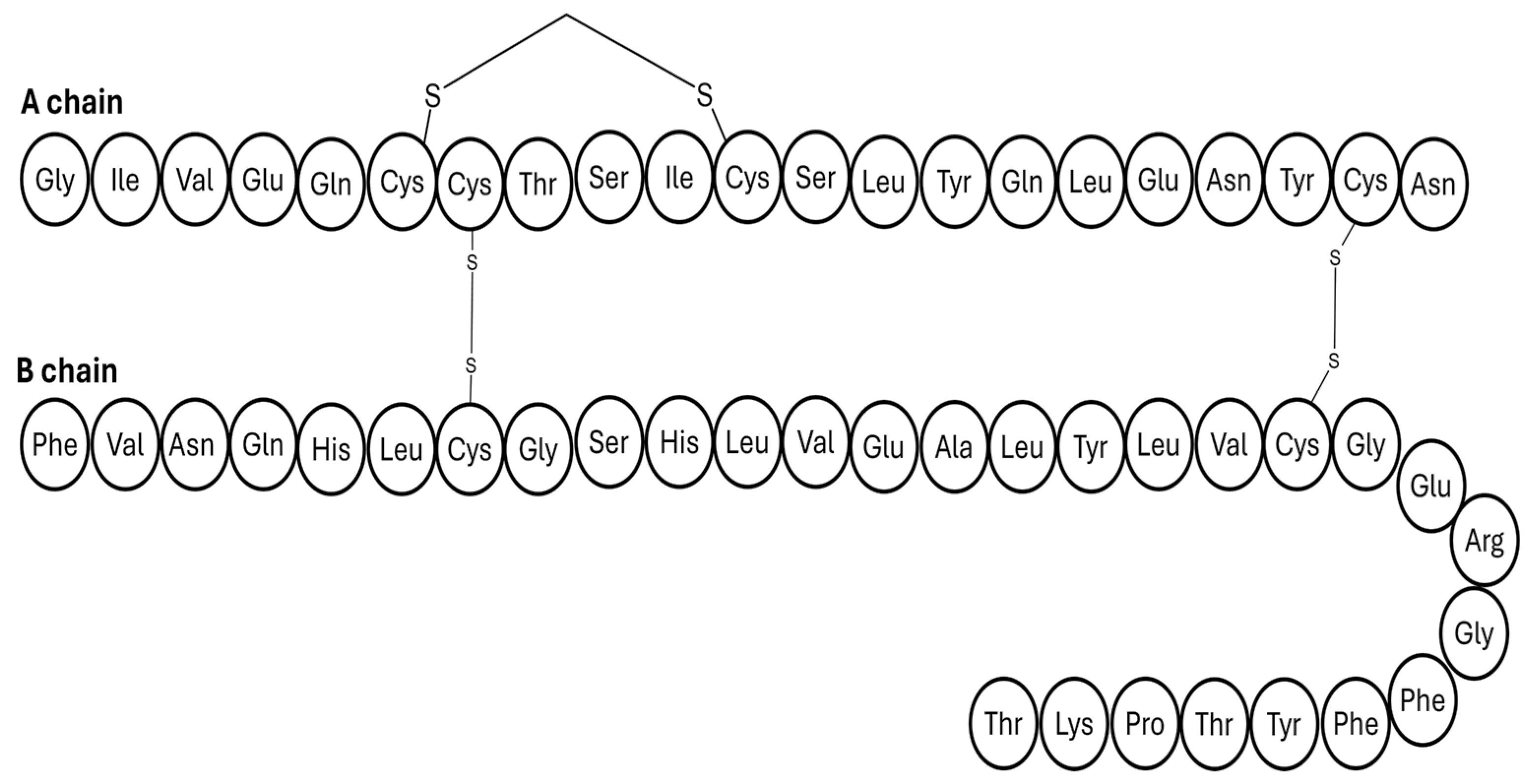
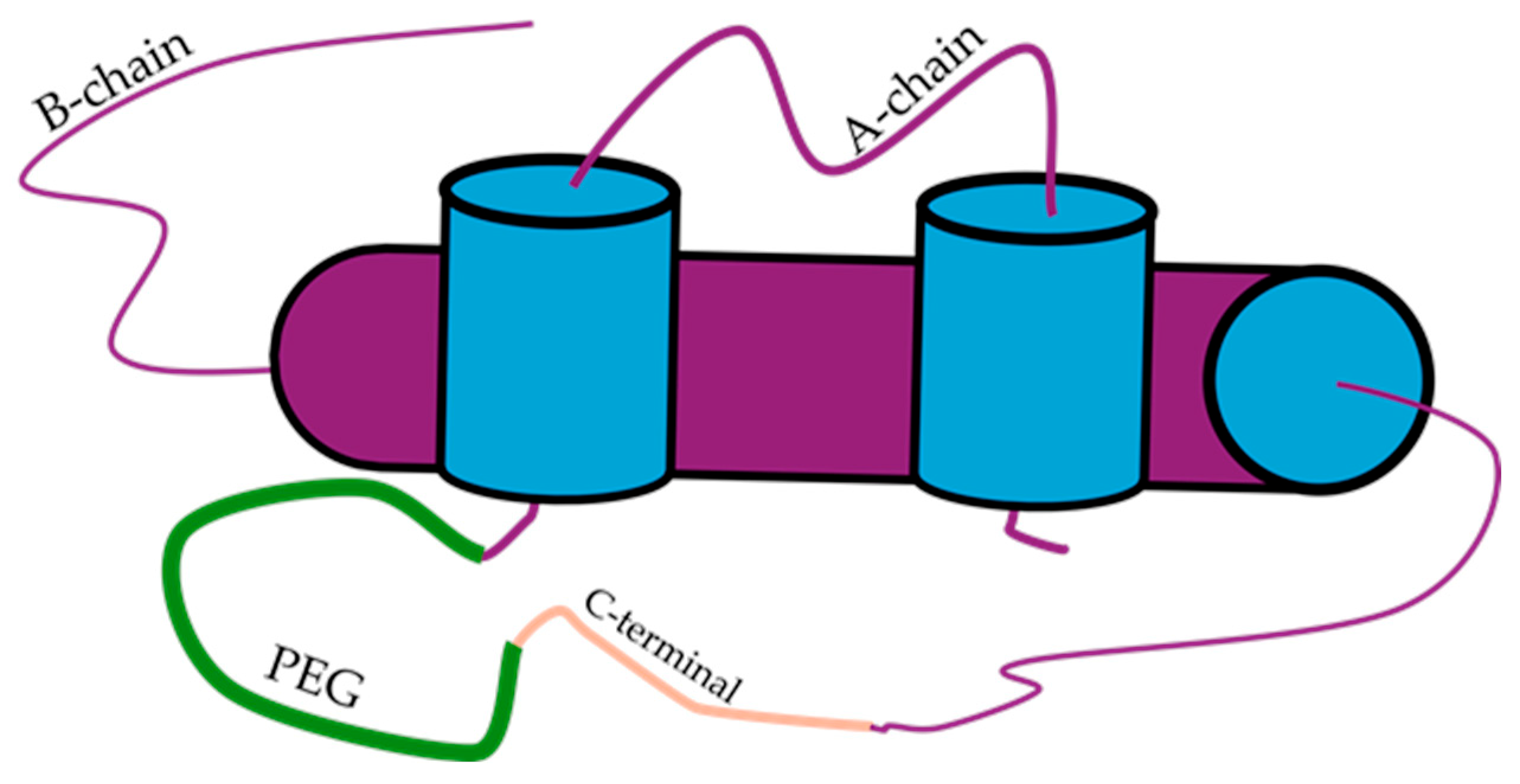
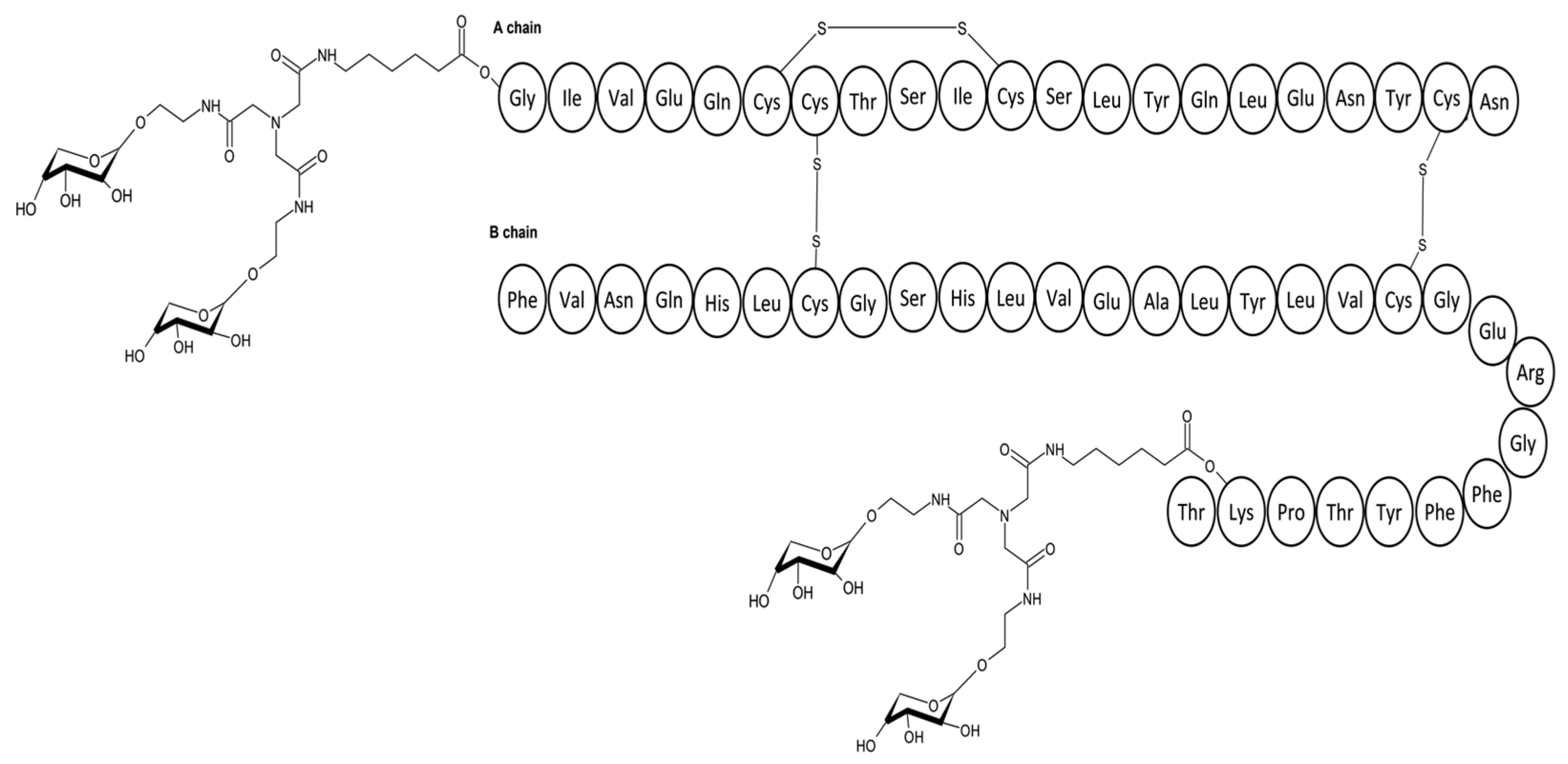
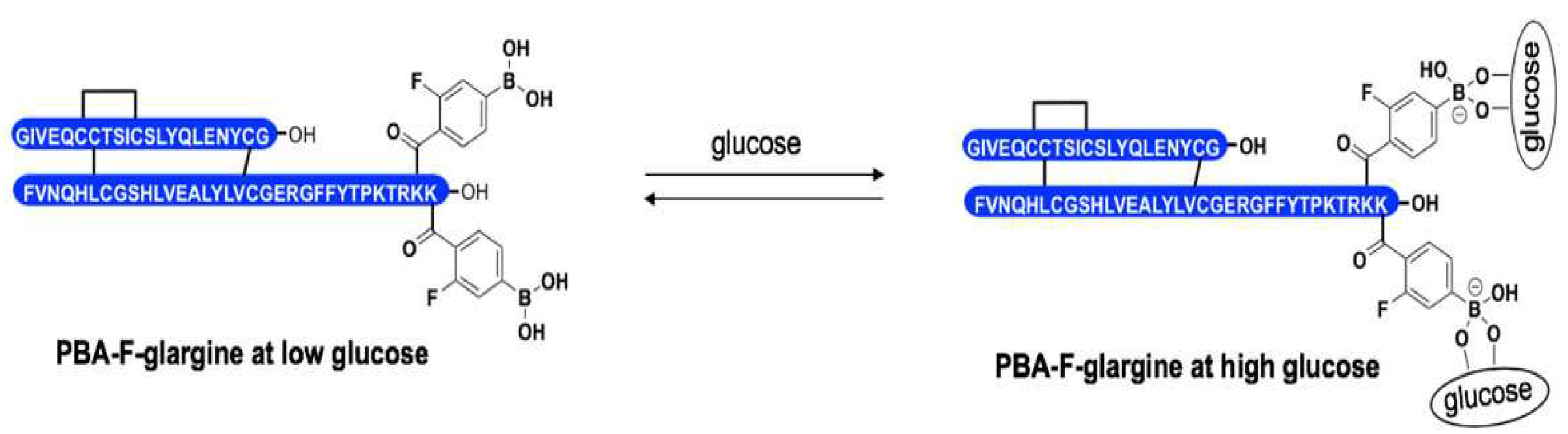
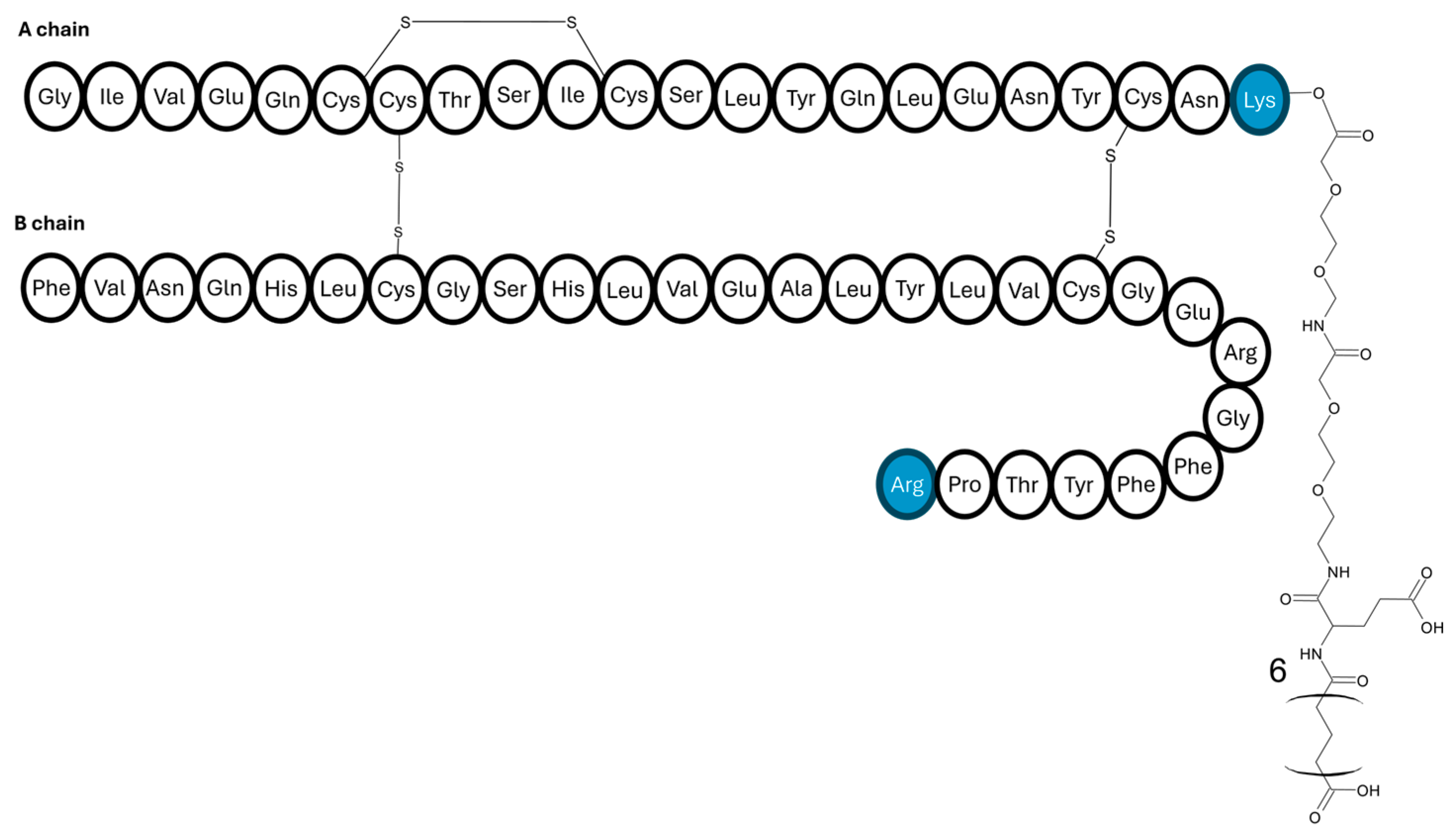

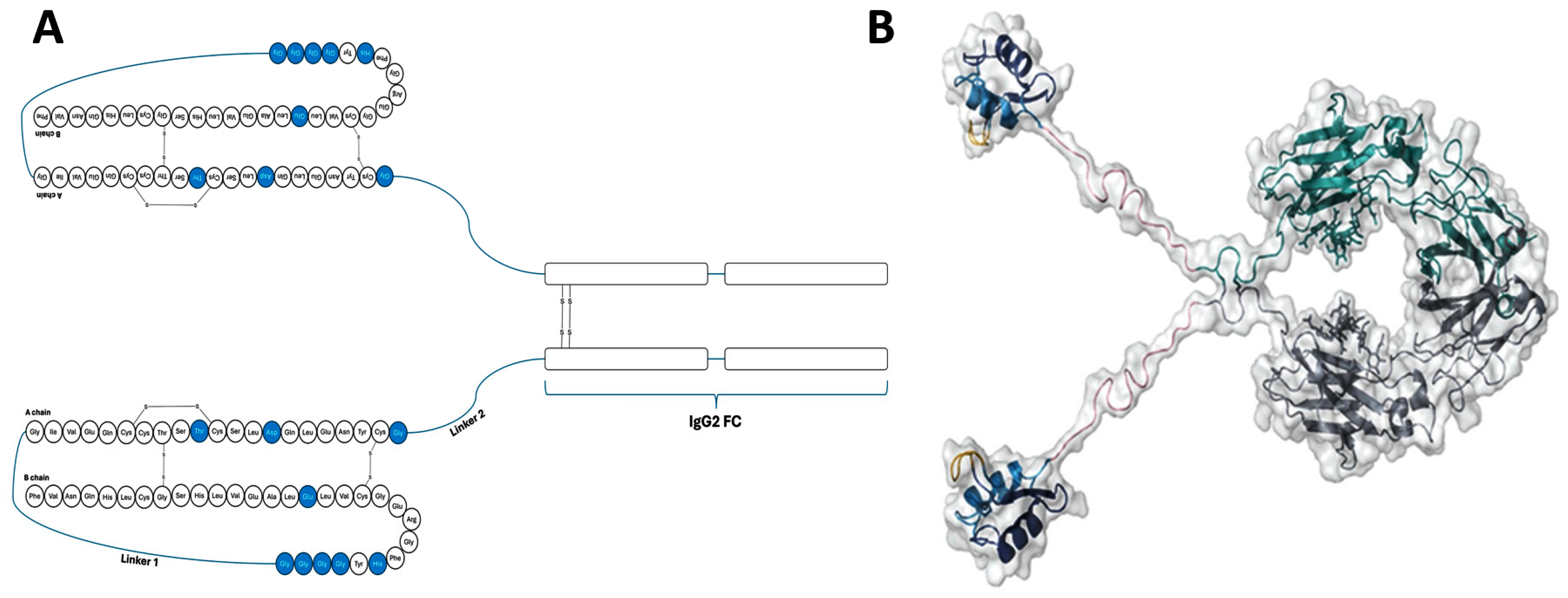
| Insulin Analogue | Design | Pharmacokinetic and Pharmacodynamic Effects |
|---|---|---|
| Seleno-insulin | Replacing the solvent-exposed disulphide bridge with the diselenide bridge at CysA7-CysB8. |
|
| Substitution of intrachain disulphide bridge to form diselenide CysA6-CysA11. |
| |
| Degludec-like insulins (INS061) | Acylation of LysB29 with hexadecenoic acid and substituting AspB28 and GluB30 with ProB28 and ThrB30. |
|
| Additional disulphide bonds | Additional disulphide bond (4th) on an extended A chain (24 amino acids) and truncated B chain (22 amino acids) (A22–B22). |
|
| Carbon-based Linkers | Replacing the intrachain (A6–A11) disulphide bond with a methylene thioacetal bond. |
|
| Single-chain insulin (SCI) | Replacing the C-peptide in proinsulin with apidic acid and combining the two chains at A1 and B29. |
|
| Linking A and B chains with a shorter than usual C domain (EEGPRR) form a 57 amino acid chain. |
| |
| Saccharide linked insulin | Coupling insulin with mannose or fructose at A1, B1 and B29. |
|
| Phenylboronic acid (PBA)-insulin | Incorporation of phenylboronic acid on Glargine to act as a glucose sensor. |
|
| Hepato-preferential insulin analogue | Attaching a 22-carbon length fatty diacid. |
|
| Insulin Icodec | Acylated by 20 carbon fatty acid. |
|
| Insulin Efsitora | Fc bound. |
|
| Trial Acronym, (NCT Number) | Duration (Weeks) | Background DM and Therapy | n | Treatment Group | % Change in HbA1c | Number of Hypoglycemic Episodes | Sponsor | |
|---|---|---|---|---|---|---|---|---|
| Š | Ćś | |||||||
| ONWARDS1 (NCT04460885) | 78 | Type 2 DM, insulin naive | 984 | Insulin Icodec Insulin Glargine | −1.55 −1.35 | 1 3 | 143 75 | Novo Nordisk A/S, Bagsværd, Denmark |
| ONWARDS 2 (NCT04770532) | 26 | Type 2DM, insulin switch | 526 | Insulin Icodec Insulin Degludec | −0.93 −0.71 | 0 1 | 113 41 | Novo Nordisk A/S |
| ONWARDS 3 (NCT04795531) | 26 | Type 2DM, insulin naive | 588 | Insulin Icodec Insulin Degludec | −1.57 −1.36 | 0 2 | 53 23 | Novo Nordisk A/S |
| ONWARDS 4 (NCT04880850) | 26 | Type 2DM, basal-bolus therapy | 582 | Insulin Icodec + Insulin Aspart Insulin Glargine + Insulin Aspart | −1.16 −1.18 | 7 3 | 937 935 | Novo Nordisk A/S |
| ONWARDS 5 (NCT04760626) | 52 | Type 2 insulin naïve | 1085 | Insulin icodec Insulin glargine or insulin degludec | −1.68 −1.31 | 0 5 | 104 76 | Novo Nordisk A/S |
| ONWARDS 6 (NCT04848480) | 52 | T1DM, basal-bolus therapy | 582 | Insulin icodec + insulin Aspart Insulin degludec + insulin Aspart | −0.37 −0.54 | 56 25 | 5047 2811 | Novo Nordisk A/S |
| Trial Acronym, (NCT Number) | Duration (Weeks) | Background DM and Therapy | n | Treatment Group | % Change in HbA1c | Hypoglycemic Event Rate (%) | Sponsor |
|---|---|---|---|---|---|---|---|
| QWINT 1 (NCT05662332) | 52 | T2DM, insulin naive | 715 | 500 U/mL Insulin Efsitora Alfa 100 U/mL Insulin Glargine | −1.19 −1.16 | 0.50 0.88 | Eli Lilly and Company, Indianapolis, IN, USA |
| QWINT 2 (NCT05362058) | 52 | T2DM, insulin naive | 921 | 500 U/mL Insulin Efsitora Alfa 100 U/mL Insulin Degludec | −1.26 −1.17 | 0.58 0.45 | Eli Lilly and Company |
| QWINT 3 (NCT05275400) | 26 | T2DM, insulin switch. | 977 | 500 U/mL Insulin Efsitora Alfa 100 U/mL Insulin Degludec | −0.81 −0.72 | 0.84 0.74 | Eli Lilly and Company |
| QWINT 4 (NCT05462756) | 26 | T2DM, basal-bolus therapy | 723 | 500 U/mL Insulin Efsitora Alfa + 100 U/mL insulin lispro 100 U/mL Insulin Glargine + 100 U/mL insulin lispro | −1.01 −1.00 | 6.58 5.94 | Eli Lilly and Company |
| QWINT 5 (NCT05463744) | 26 | T1DM, basal-bolus therapy | 689 | 500 U/mL Insulin Efsitora Alfa 100 U/mL Insulin Degludec | −0.51 −0.56 | 14.03 11.59 | Eli Lilly and Company |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibiya, N.; Dzimwasha, L.; Zvandasara, S.; Zuma, A.; Khathi, A. Emerging Insulin Analogues: A Glimpse into How Insulin Analogues May Look in the near Future. Pharmaceutics 2025, 17, 1239. https://doi.org/10.3390/pharmaceutics17101239
Sibiya N, Dzimwasha L, Zvandasara S, Zuma A, Khathi A. Emerging Insulin Analogues: A Glimpse into How Insulin Analogues May Look in the near Future. Pharmaceutics. 2025; 17(10):1239. https://doi.org/10.3390/pharmaceutics17101239
Chicago/Turabian StyleSibiya, Ntethelelo, Lorah Dzimwasha, Samarah Zvandasara, Amanda Zuma, and Andile Khathi. 2025. "Emerging Insulin Analogues: A Glimpse into How Insulin Analogues May Look in the near Future" Pharmaceutics 17, no. 10: 1239. https://doi.org/10.3390/pharmaceutics17101239
APA StyleSibiya, N., Dzimwasha, L., Zvandasara, S., Zuma, A., & Khathi, A. (2025). Emerging Insulin Analogues: A Glimpse into How Insulin Analogues May Look in the near Future. Pharmaceutics, 17(10), 1239. https://doi.org/10.3390/pharmaceutics17101239





