Daunomycin Nanocarriers with High Therapeutic Payload for the Treatment of Childhood Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of N,N’-[1,3-Phenylenebis(methylene))bis[N,N’-Dimethyl-N-(1-Hexadecyl)]-Ammonium Dibromide, 16-Ph-16
2.1.2. Synthesis of Au@16-Ph-16 Precursor Gold Nanoparticles
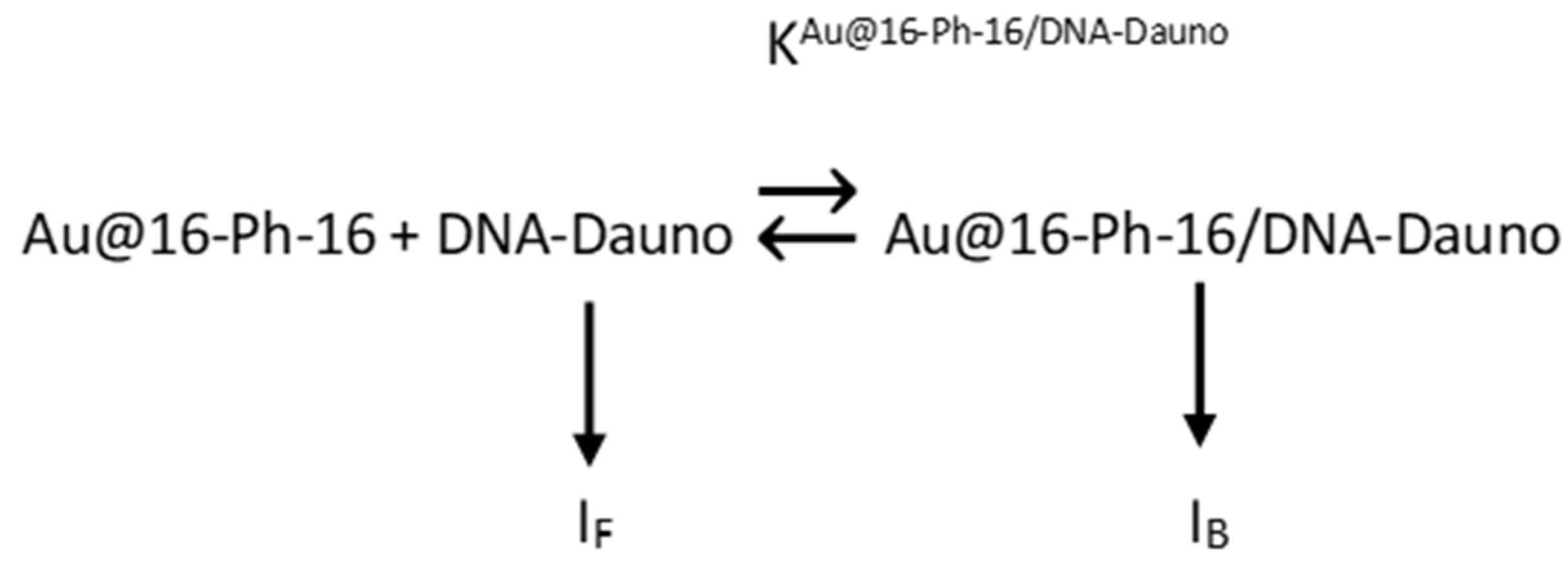
2.1.3. Synthesis of Au@16-Ph-16/DNA–Dauno Gold Nanosystem
2.1.4. Cell Lines and Culture Conditions
2.2. Methods
2.2.1. Cell Viability Assays
2.2.2. UV/Vis Spectroscopy
2.2.3. Fluorescence Spectroscopy
2.2.4. Circular Dichroism (CD) Spectroscopy
2.2.5. Atomic Force Microscopy Experiments
2.2.6. Dynamic Light Scattering (DLS), Polydispersity Index (PDI), and Zeta Potential Measurements
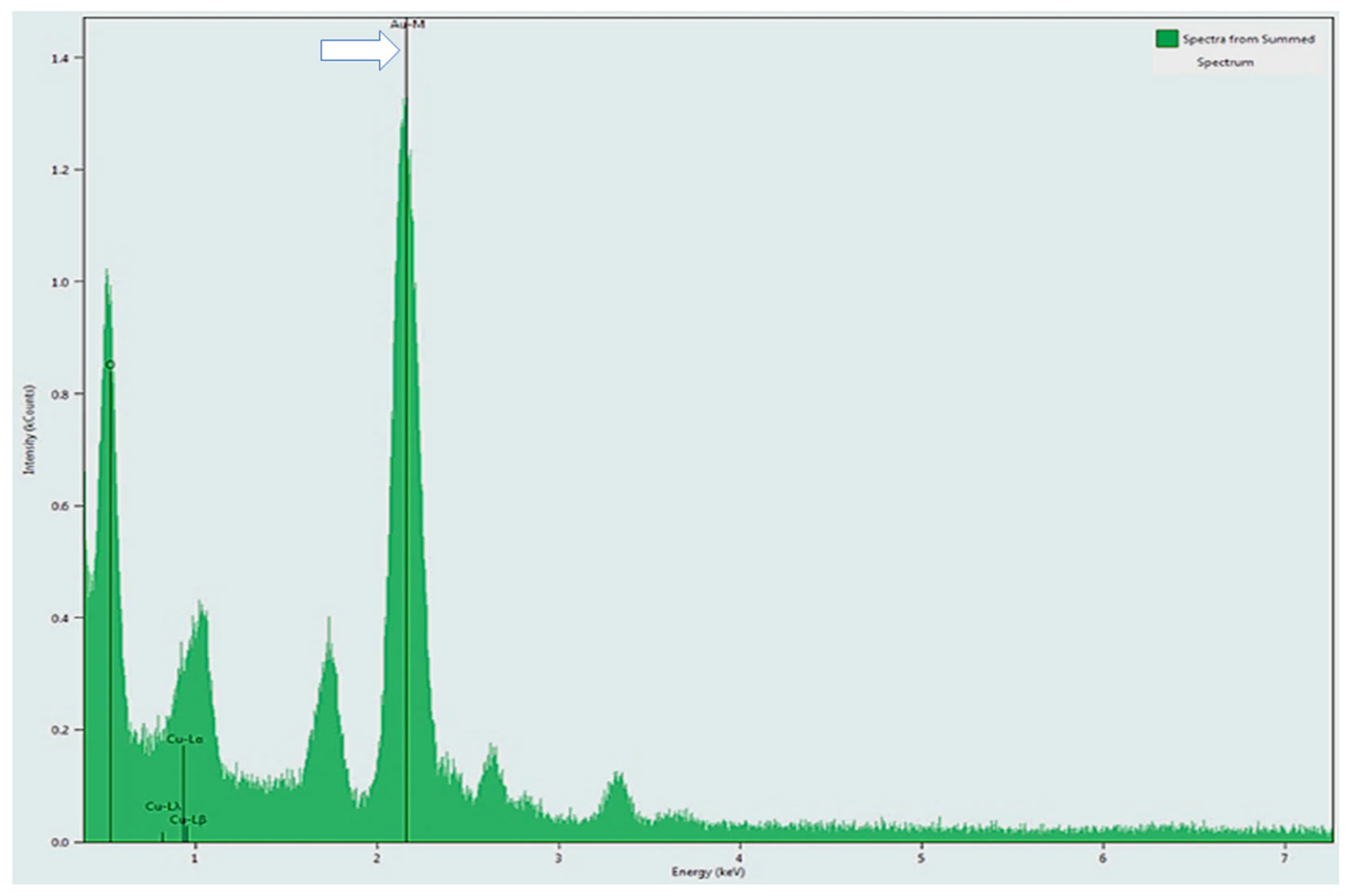
2.2.7. TEM and EDS Measurements
2.2.8. Confocal Microscope
3. Results and Discussion
3.1. Studies of the Charge, Size and Stability of Au@16-Ph-16 and Au@16-Ph-16/DNA–Dauno Nanosystems
3.2. Fluorescence Spectroscopy: DNA/Dauno and Au@16-Ph-16/DNA–Dauno Binding Studies
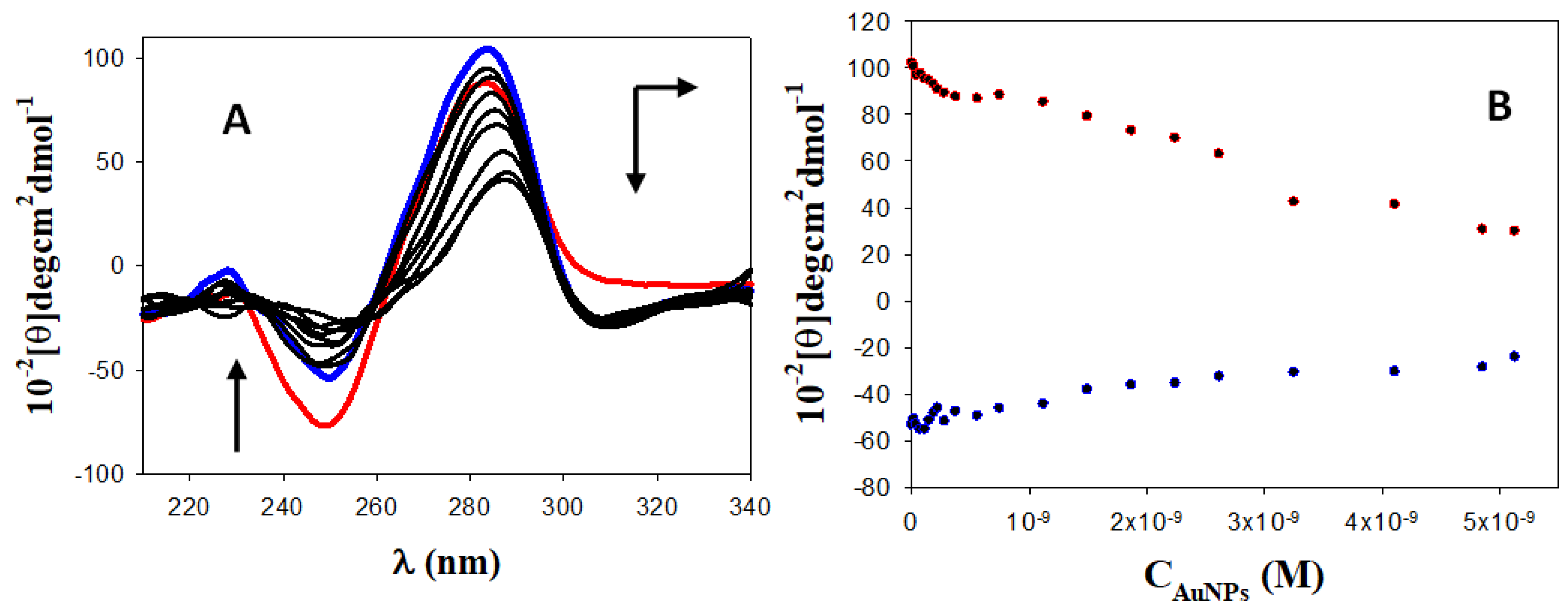
3.3. Circular Dichroism (CD) Spectroscopy: DNA/Dauno and Au@16-pH-16/DNA–Dauno Study of Conformational Changes
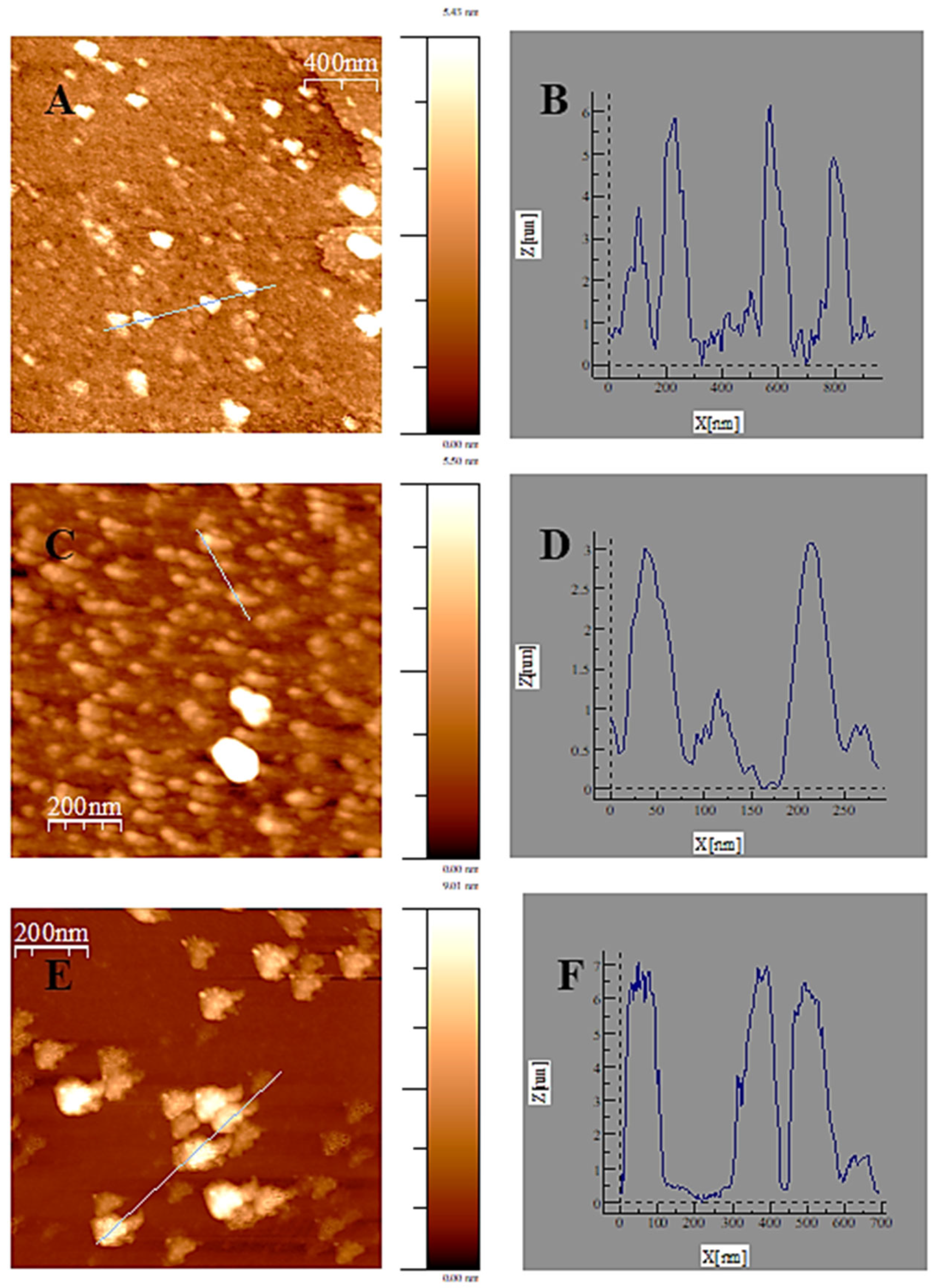
3.4. Atomic Force Microscopy (AFM) and DLS Experiments
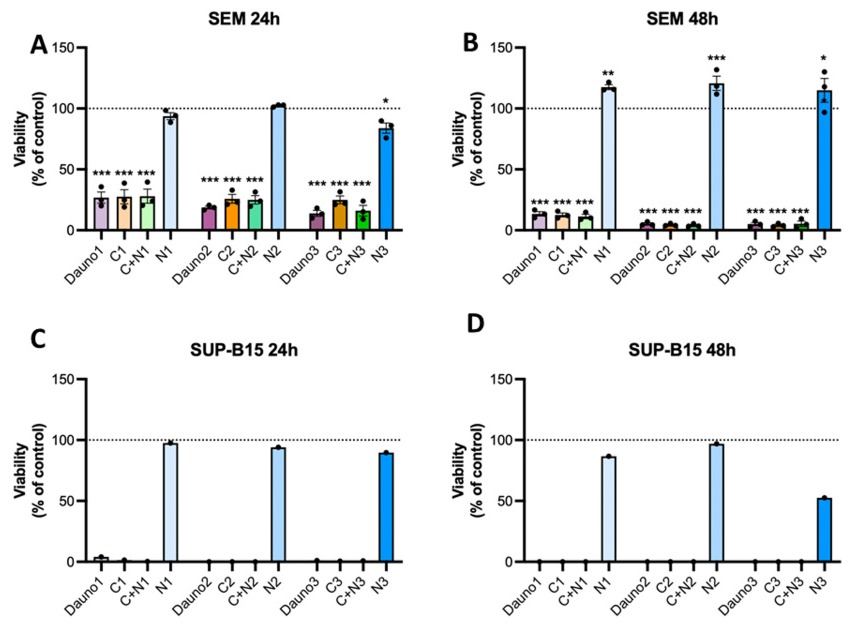
3.5. In Vitro Biocompatibility of AuNPs and Nanosystems Viability Assay
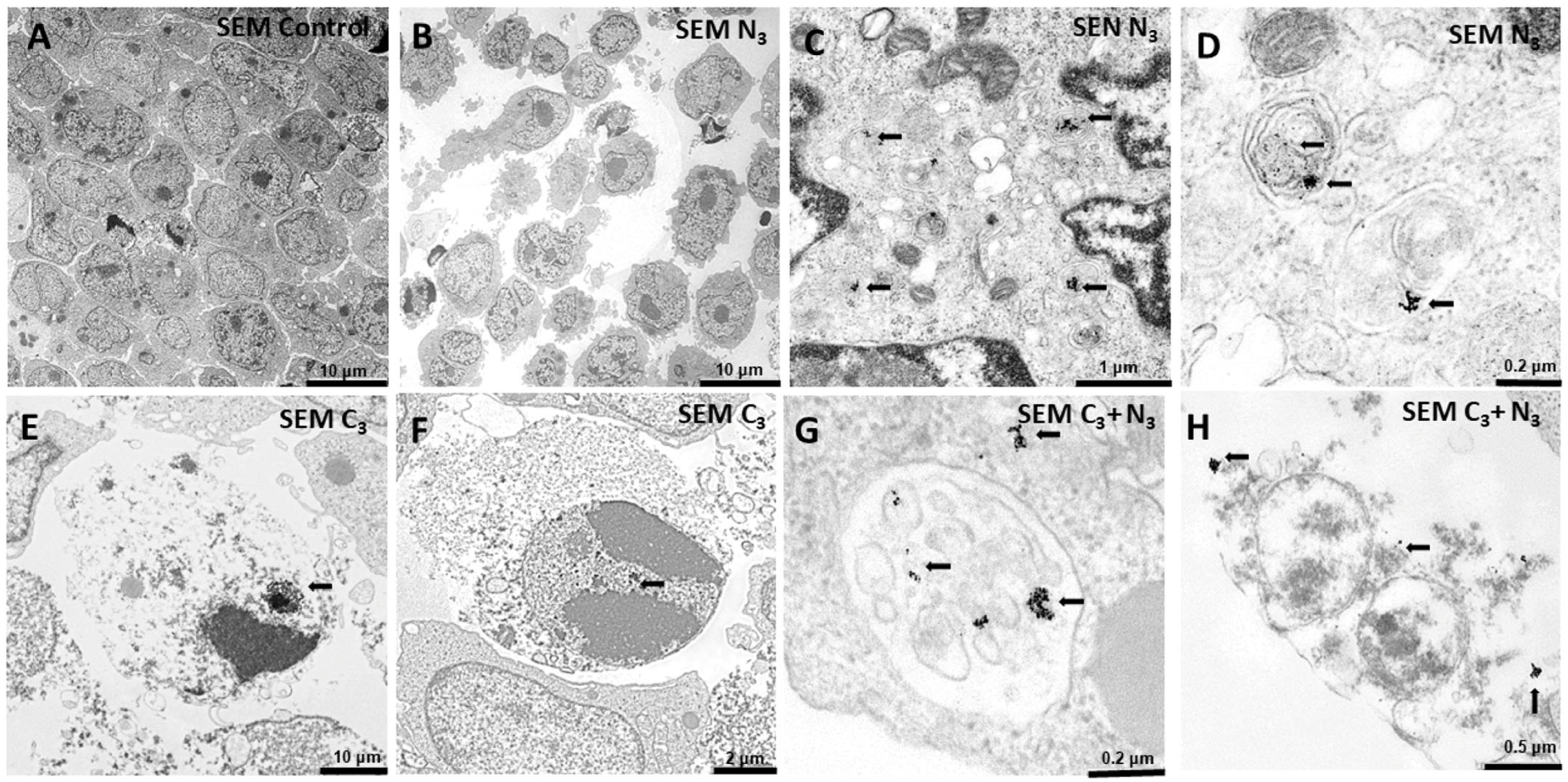
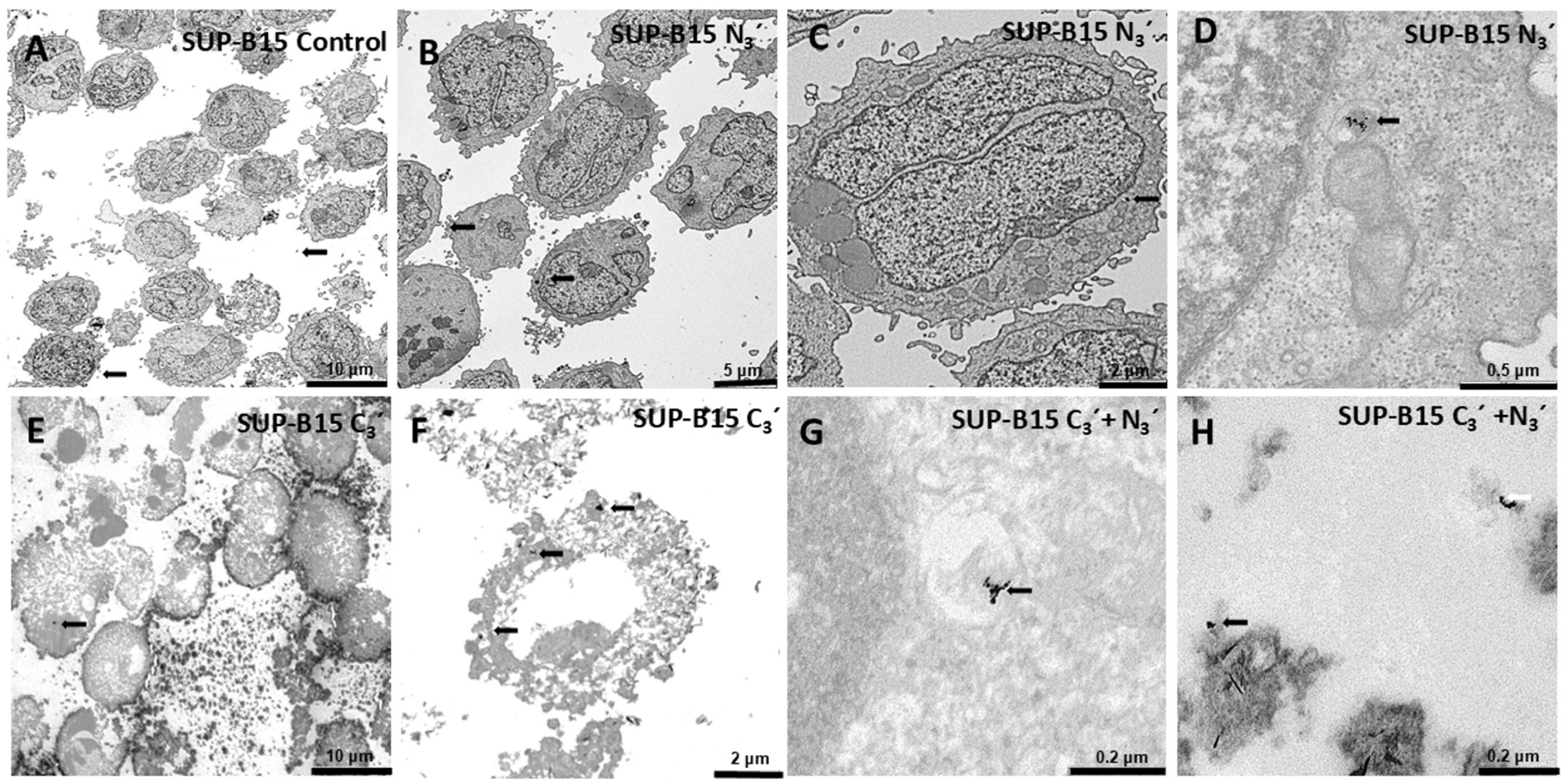
3.6. Internalization of Au@16-Ph-16 and Au@16-Ph-16/DNA–Dauno Nanosystems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International Incidence of Childhood Cancer, 2001–2010: A Population-Based Registry Study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- World Health Organization. Assessing National Capacity for the Prevention and Control of Noncommunicable Diseases: Report of the 2019 Global Survey; Electronic Version; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000231-9. [Google Scholar]
- World Health Organization. CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives; Electronic Version; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240025271. [Google Scholar]
- Lam, C.G.; Howard, S.C.; Bouffet, E.; Pritchard-Jones, K. Science and Health for All Children with Cancer. Science 2019, 363, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Walsh, M.F.; Wu, G.; Edmonson, M.N.; Gruber, T.A.; Easton, J.; Hedges, D.; Ma, X.; Zhou, X.; Yergeau, D.A.; et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2015, 373, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Early Diagnosis of Childhood Cancer; Pan American Health Organization: Washington, DC, USA, 2014; ISBN 978-92-75-11846-7. [Google Scholar]
- Vakiti, A.; Reynolds, S.B.; Mewawalla, P. Acute Myeloid Leukemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Appelbaum, F.R. Acute Leukemias in Adults. In Abeloff’s Clinical Oncology; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1783–1797.e1. ISBN 9780323476744. [Google Scholar]
- Bain, B.J.; Béné, M.C. Morphological and Immunophenotypic Clues to the WHO Categories of Acute Myeloid Leukaemia. Acta Haematol. 2019, 141, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, L.; Marcellino, B.; Mascarenhas, J. Eosinophilia in Acute Myeloid Leukemia: Overlooked and Underexamined. Blood Rev. 2019, 36, 23–31. [Google Scholar] [CrossRef]
- Medeiros, B.C.; Chan, S.M.; Daver, N.G.; Jonas, B.A.; Pollyea, D.A. Optimizing Survival Outcomes with Post-remission Therapy in Acute Myeloid Leukemia. Am. J. Hematol. 2019, 94, 803–811. [Google Scholar] [CrossRef]
- Puckett, Y.; Chan, O. Acute Lymphocytic Leukemia; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Brown, J.R. Chronic Lymphocytic Leukemia. In Goldman-Cecil Medicine; Goldman, L., Cooney, K.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 2, ISBN 9780323930383. [Google Scholar]
- Roberts, K.G. Genetics and Prognosis of ALL in Children vs Adults. Hematology 2018, 2018, 137–145. [Google Scholar] [CrossRef]
- Jain, T.; Litzow, M.R. No Free Rides: Management of Toxicities of Novel Immunotherapies in ALL, Including Financial. Hematology 2018, 2018, 25–34. [Google Scholar] [CrossRef]
- Dinner, S.; Liedtke, M. Antibody-Based Therapies in Patients with Acute Lymphoblastic Leukemia. Hematology 2018, 2018, 9–15. [Google Scholar] [CrossRef]
- Hunger, S.P.; Teachey, D.T.; Grupp, S.; Aplenc, R. Childhood Leukemia. In Abeloff’s Clinical Oncology; Niederhuber, J.E., Armitage, J.O., Kastan, M.B., Doroshow, J.H., Tepper, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1748–1764.e4. ISBN 978-0-323-47674-4. [Google Scholar]
- Ammar, M.M.; Ali, R.; Abd Elaziz, N.A.; Habib, H.; Abbas, F.M.; Yassin, M.T.; Maniah, K.; Abdelaziz, R. Nanotechnology in Oncology: Advances in Biosynthesis, Drug Delivery, and Theranostics. Discov. Oncol. 2025, 16, 1172. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Al-Sahli, S.A.; El-Wetidy, M.S.; Mohamed, S. Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities. Cancers 2024, 16, 4234. [Google Scholar] [CrossRef]
- Giráldez-Pérez, R.M.; Grueso, E.; Domínguez, I.; Pastor, N.; Kuliszewska, E.; Prado-Gotor, R.; Requena-Domenech, F. Biocompatible DNA/5-Fluorouracil-Gemini Surfactant-Functionalized Gold Nanoparticles as Promising Vectors in Lung Cancer Therapy. Pharmaceutics 2021, 13, 423. [Google Scholar] [CrossRef]
- Giráldez-Pérez, R.M.; Grueso, E.; Montero-Hidalgo, A.J.; Luque, R.M.; Carnerero, J.M.; Kuliszewska, E.; Prado-Gotor, R. Gold Nanosystems Covered with Doxorubicin/DNA Complexes: A Therapeutic Target for Prostate and Liver Cancer. Int. J. Mol. Sci. 2022, 23, 15575. [Google Scholar] [CrossRef]
- Miklán, Z.; Orbán, E.; Csík, G.; Schlosser, G.; Magyar, A.; Hudecz, F. New Daunomycin–Oligoarginine Conjugates: Synthesis, Characterization, and Effect on Human Leukemia and Human Hepatoma Cells. Pept. Sci. 2009, 92, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Kadia, T.M.; DiNardo, C.D.; Welch, M.A.; Ravandi, F. Acute Myeloid Leukemia: Treatment and Research Outlook for 2021 and the MD Anderson Approach. Cancer 2021, 127, 1186–1207. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (Cytarabine and Daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients with Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Berman, E.; Heller, G.; Santorsa, J.; McKenzie, S.; Gee, T.; Kempin, S.; Gulati, S.; Andreeff, M.; Kolitz, J.; Gabrilove, J. Results of a Randomized Trial Comparing Idarubicin and Cytosine Arabinoside with Daunorubicin and Cytosine Arabinoside in Adult Patients with Newly Diagnosed Acute Myelogenous Leukemia. Blood 1991, 77, 1666–1674. [Google Scholar] [CrossRef][Green Version]
- Wiernik, P.; Banks, P.; Case, D.J.; Arlin, Z.; Periman, P.; Todd, M.; Ritch, P.; Enck, R.; Weitberg, A. Cytarabine plus Idarubicin or Daunorubicin as Induction and Consolidation Therapy for Previously Untreated Adult Patients with Acute Myeloid Leukemia. Blood 1992, 79, 313–319. [Google Scholar] [CrossRef]
- Wolff, S.N.; Herzig, R.H.; Fay, J.W.; Phillips, G.L.; Lazarus, H.M.; Flexner, J.M.; Stein, R.S.; Greer, J.P.; Cooper, B.; Herzig, G.P. High-Dose Cytarabine and Daunorubicin as Consolidation Therapy for Acute Myeloid Leukemia in First Remission: Long-Term Follow-up and Results. J. Clin. Oncol. 1989, 7, 1260–1267. [Google Scholar] [CrossRef]
- Champlin, R.; Gajewski, J.; Nimer, S.; Vollset, S.; Landaw, E.; Winston, D.; Schiller, G.; Ho, W. Postremission Chemotherapy for Adults with Acute Myelogenous Leukemia: Improved Survival with High-Dose Cytarabine and Daunorubicin Consolidation Treatment. J. Clin. Oncol. 1990, 8, 1199–1206. [Google Scholar] [CrossRef]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute Myeloid Leukemia: A Comprehensive Review and 2016 Update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.-H.; Wang, Z.-D.; Wang, D. Efficacy and Safety of Induction Chemotherapy with Daunorubicin or Idarubicin in the Treatment of an Adult with Acute Lymphoblastic Leukemia. Tumori J. 2022, 108, 182–188. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Informe de Posicionamiento de La Formulación de Daunorubicina y con Relación Molar 1:5 (Vyxeos®). Available online: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/2021/IPT_10-2021-Vyxeos.pdf (accessed on 7 September 2025).
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- European Medicines Agency. Vyxeos Liposomal. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/vyxeos-liposomal-epar-product-information_en.pdf (accessed on 7 September 2025).
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current Trends in Using Polymer Coated Gold Nanoparticles for Cancer Therapy. Int. J. Pharm. 2015, 484, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.M.; Carrino, J.J.; Dickler, M.N.; Leibowitz, D.; Smith, S.D.; Westbrook, C.A. Heterogeneity of Genomic Fusion of BCR and ABL in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Proc. Natl. Acad. Sci. USA 1988, 85, 2795–2799. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.S.; Clark, S.S.; Coyne, M.Y.; Smith, S.D.; Champlin, R.; Witte, O.N.; McCormick, F.P. Diagnosis of Chronic Myeloid and Acute Lymphocytic Leukemias by Detection of Leukemia-Specific MRNA Sequences Amplified in Vitro. Proc. Natl. Acad. Sci. USA 1988, 85, 5698–5702. [Google Scholar] [CrossRef] [PubMed]
- Greil, J.; Gramatzki, M.; Burger, R.; Marschalek, R.; Peltner, M.; Trautmann, U.; Hansen-Hagge, T.E.; Bartram, C.R.; Fey, G.H.; Stehr, K.; et al. The Acute Lymphoblastic Leukaemia Cell Line SEM with t(4;11) Chromosomal Rearrangement Is Biphenotypic and Responsive to Interleukin-7. Br. J. Haematol. 1994, 86, 275–283. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring, NY, USA, 1989; ISBN 0-87969-309-6. [Google Scholar]
- Felsenfeld, G.; Hirschman, S.Z. A Neighbor-Interaction Analysis of the Hypochromism and Spectra of DNA. J. Mol. Biol. 1965, 13, 407–427. [Google Scholar] [CrossRef]
- Kuliszewska, E.; Brecker, L. Gemini Surfactants Foam Formation Ability and Foam Stability Depends on Spacer Length. J. Surfactants Deterg. 2014, 17, 951–957. [Google Scholar] [CrossRef]
- Chaires, J.B.; Dattagupta, N.; Crothers, D.M. Kinetics of the Daunomycin-DNA Interaction. Biochemistry 1985, 24, 260–267. [Google Scholar] [CrossRef]
- Hormaechea-Agulla, D.; Jiménez-Vacas, J.M.; Gómez-Gómez, E.; López, F.L.; Carrasco-Valiente, J.; Valero-Rosa, J.; Moreno, M.M.; Sánchez-Sánchez, R.; Ortega-Salas, R.; Gracia-Navarro, F.; et al. The Oncogenic Role of the Spliced Somatostatin Receptor Sst5TMD4 Variant in Prostate Cancer. FASEB J. 2017, 31, 4682–4696. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-Potential Measurement Using the Smoluchowski Equation and the Slope of the Current–Time Relationship in Electroosmotic Flow. J. Colloid. Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Clayton, K.N.; Salameh, J.W.; Wereley, S.T.; Kinzer-Ursem, T.L. Physical Characterization of Nanoparticle Size and Surface Modification Using Particle Scattering Diffusometry. Biomicrofluidics 2016, 10, 054107. [Google Scholar] [CrossRef]
- Kumar Kariganaur, A.; Kumar, H.; Arun, M. Effect of Temperature on Sedimentation Stability and Flow Characteristics of Magnetorheological Fluids with Damper as the Performance Analyser. J. Magn. Magn. Mater. 2022, 555, 169342. [Google Scholar] [CrossRef]
- Templeton, A.C.; Chen, S.; Gross, S.M.; Murray, R.W. Water-Soluble, Isolable Gold Clusters Protected by Tiopronin and Coenzyme A Monolayers. Langmuir 1999, 15, 66–76. [Google Scholar] [CrossRef]
- Wuelfing, W.P.; Gross, S.M.; Miles, D.T.; Murray, R.W. Nanometer Gold Clusters Protected by Surface-Bound Monolayers of Thiolated Poly(Ethylene Glycol) Polymer Electrolyte. J. Am. Chem. Soc. 1998, 120, 12696–12697. [Google Scholar] [CrossRef]
- Yetgin, S.; Balkose, D. Calf Thymus DNA Characterization and Its Adsorption on Different Silica Surfaces. RSC Adv. 2015, 5, 57950–57959. [Google Scholar] [CrossRef]
- Hughes, J.M.; Budd, P.M.; Grieve, A.; Dutta, P.; Tiede, K.; Lewis, J. Highly Monodisperse, Lanthanide-containing Polystyrene Nanoparticles as Potential Standard Reference Materials for Environmental “Nano” Fate Analysis. J. Appl. Polym. Sci. 2015, 132, 42061. [Google Scholar] [CrossRef]
- Chaires, J.B. Biophysical Chemistry of the Daunomycin-DNA Interaction. Biophys. Chem. 1990, 35, 191–202. [Google Scholar] [CrossRef]
- Chaires, J.B.; Dattagupta, N.; Crothers, D.M. Studies on Interaction of Anthracycline Antibiotics and Deoxyribonucleic Acid: Equilibrium Binding Studies on the Interaction of Daunomycin with Deoxyribonucleic Acid. Biochemistry 1982, 21, 3933–3940. [Google Scholar] [CrossRef]
- Menger, F.M.; Portnoy, C.E. Chemistry of Reactions Proceeding inside Molecular Aggregates. J. Am. Chem. Soc. 1967, 89, 4698–4703. [Google Scholar] [CrossRef]
- Alkhaibari, I.S.; Horton, P.N.; Coles, S.J.; Buurma, N.J.; Pope, S.J.A. DNA-Binding Properties of Non-Intercalating Water-Soluble Organometallic Ir(III) Luminophores. Chem. Eur. J. 2025, 31, e202500290. [Google Scholar] [CrossRef] [PubMed]
- Grueso, E.; Perez-Tejeda, P.; Prado-Gotor, R.; Cerrillos, C. DNA Strand Elongation Induced by Small Gold Nanoparticles at High Ethanol Content. J. Phys. Chem. C 2014, 118, 4416–4428. [Google Scholar] [CrossRef]
- Ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Neidle, S. Nucleic Acid Structure and Recognition, 1st ed.; Oxford University Press: New York, NY, USA, 2002; ISBN 019850635X. [Google Scholar]
- Visone, V.; Szabó, I.; Perugino, G.; Hudecz, F.; Bánóczi, Z.; Valenti, A. Topoisomerases Inhibition and DNA Binding Mode of Daunomycin–Oligoarginine Conjugate. J. Enzyme Inhib. Med. Chem. 2020, 35, 1363–1371. [Google Scholar] [CrossRef]
- Airoldi, M.; Barone, G.; Gennaro, G.; Giuliani, A.M.; Giustini, M. Interaction of Doxorubicin with Polynucleotides. A Spectroscopic Study. Biochemistry 2014, 53, 2197–2207. [Google Scholar] [CrossRef]
- Chang, Y.-M.; Chen, C.K.-M.; Hou, M.-H. Conformational Changes in DNA upon Ligand Binding Monitored by Circular Dichroism. Int. J. Mol. Sci. 2012, 13, 3394–3413. [Google Scholar] [CrossRef]
- Prado-Gotor, R.; Grueso, E. A Kinetic Study of the Interaction of DNA with Gold Nanoparticles: Mechanistic Aspects of the Interaction. Phys. Chem. Chem. Phys. 2011, 13, 1479–1489. [Google Scholar] [CrossRef]
- Krishna, A.G.; Kumar, D.V.; Khan, B.M.; Rawal, S.K.; Ganesh, K.N. Taxol–DNA Interactions: Fluorescence and CD Studies of DNA Groove Binding Properties of Taxol. Biochim. Biophys. Acta BBA General. Subj. 1998, 1381, 104–112. [Google Scholar] [CrossRef]
- Kypr, J.; Kejnovska, I.; Renciuk, D.; Vorlickova, M. Circular Dichroism and Conformational Polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Main, K.H.S.; Provan, J.I.; Haynes, P.J.; Wells, G.; Hartley, J.A.; Pyne, A.L.B. Atomic Force Microscopy—A Tool for Structural and Translational DNA Research. APL Bioeng. 2021, 5, 031504. [Google Scholar] [CrossRef] [PubMed]
- Ukraintsev, E.; Kromka, A.; Kozak, H.; Reme, Z.; Rezek, B. Artifacts in Atomic Force Microscopy of Biological Samples. In Atomic Force Microscopy Investigations into Biology—From Cell to Protein; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Grueso, E.; Giráldez-Pérez, R.M.; Perez-Tejeda, P.; Roldán, E.; Prado-Gotor, R. What Controls the Unusual Melting Profiles of Small AuNPs/DNA Complexes. Phys. Chem. Chem. Phys. 2019, 21, 11019–11032. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Li, H.; Huang, N.; Jin, Q.; Ren, K.; Ji, J. Enhanced Retention and Cellular Uptake of Nanoparticles in Tumors by Controlling Their Aggregation Behavior. ACS Nano 2013, 7, 6244–6257. [Google Scholar] [CrossRef]
- Deng, M.; Guo, R.; Zang, S.; Rao, J.; Li, M.; Tang, X.; Xia, C.; Li, M.; Zhang, Z.; He, Q. PH-Triggered Copper-Free Click Reaction-Mediated Micelle Aggregation for Enhanced Tumor Retention and Elevated Immuno–Chemotherapy against Melanoma. ACS Appl. Mater. Interfaces 2021, 13, 18033–18046. [Google Scholar] [CrossRef]
- Sanvicens, N.; Marco, M.P. Multifunctional Nanoparticles—Properties and Prospects for Their Use in Human Medicine. Trends Biotechnol. 2008, 26, 425–433. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, M.; Zhu, Y.; Mao, C. Metallic Nanoclusters for Cancer Imaging and Therapy. Curr. Med. Chem. 2018, 25, 1379–1396. [Google Scholar] [CrossRef]
- Cai, F.; Luis, M.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced Cardiotoxicity in the Chemotherapy Treatment of Breast Cancer: Preventive Strategies and Treatment (Review). Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Chang, V.Y.; Wang, J.J. Pharmacogenetics of Chemotherapy-Induced Cardiotoxicity. Curr. Oncol. Rep. 2018, 20, 52. [Google Scholar] [CrossRef]


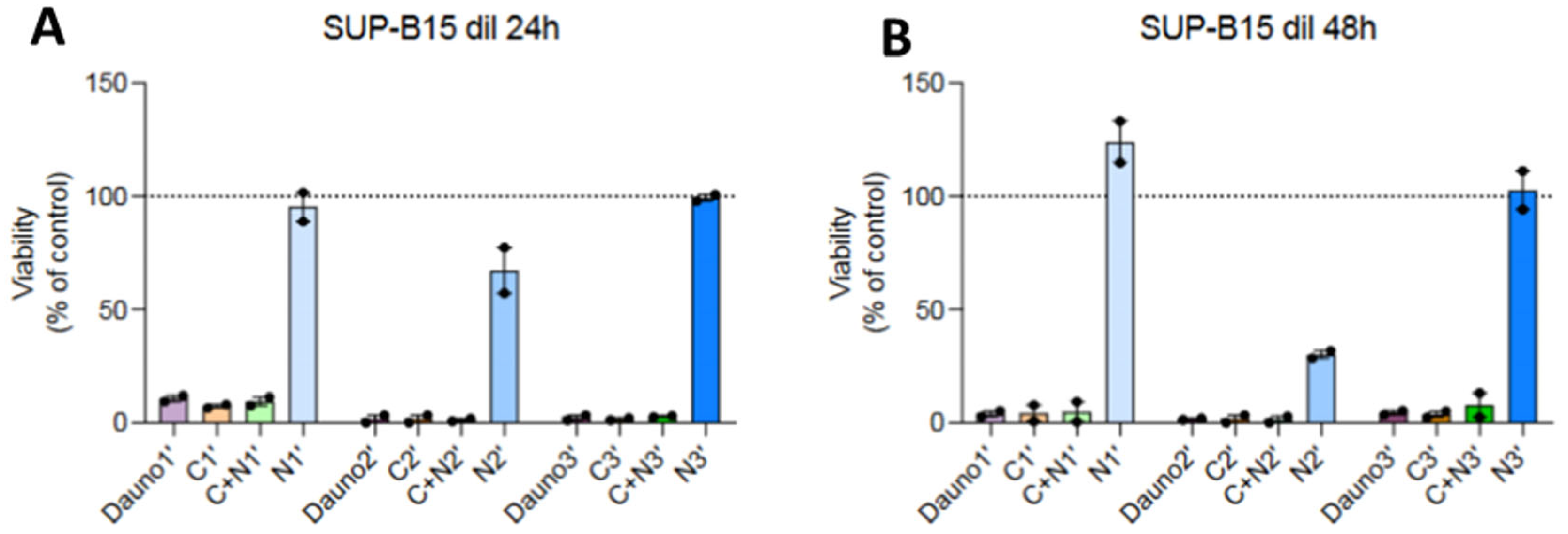



| Compound | Au@16-Ph 16 Concentration | Compound | Reactant Concentrations |
|---|---|---|---|
| N1 | CAu@16-Ph-16 = 1.19 nM | C1 | CDauno = 0.25 µM CDNA = 2.5 µM CAu@16-Ph-16 = 0.055 nM |
| N2 | CAu@16-Ph-16 = 4.78 nM | C2 | CDauno = 1.0 µM CDNA = 10.0 µM CAu@16-Ph-16 = 0.220 nM |
| N3 | CAu@16-Ph-16 = 5.60 nM | C3 | CDauno = 1.3 µM CDNA = 13.0 µM CAu@16-Ph-16 = 0.286 nM |
| Au@16-Ph-16 | Zeta Potential (mV) | Mean Size (nm) | PDI |
|---|---|---|---|
| N1 | 30.4 ± 1.5 | 24.0 ± 3.0 | 0.016 |
| N2 | 34.0 ± 3.0 | 25.3 ± 0.4 | 0.0003 |
| N3 | 38.8 ± 0.5 | 21.8 ± 0.8 | 0.0013 |
| Au@16-Ph-16/DNA–Dauno | Zeta Potential (mV) | Mean Size (nm) | PDI |
|---|---|---|---|
| C1 | −30 ± 2.0 | 74 ± 6.0 | 0.007 |
| C2 | −31 ± 7.0 | 77± 3.0 | 0.0015 |
| C3 | −33 ± 8.0 | 82 ± 1.0 | 0.033 |
| Intensity,λ | KAu@16-Ph-16/DNA–Dauno (M−1) | IF | IB |
|---|---|---|---|
| I555 nm | (5.3 ± 0.5) × 107 | 205.0 ± 2.0 | 0.17 ± 0.02 |
| I592 nm | (4.9 ± 0.7) × 107 | 152.3 ± 1.8 | 0.18 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giráldez-Pérez, R.M.; Grueso, E.M.; Montero-Hidalgo, A.J.; Muriana-Fernández, C.; Kuliszewska, E.; Luque, R.M.; Prado-Gotor, R. Daunomycin Nanocarriers with High Therapeutic Payload for the Treatment of Childhood Leukemia. Pharmaceutics 2025, 17, 1236. https://doi.org/10.3390/pharmaceutics17091236
Giráldez-Pérez RM, Grueso EM, Montero-Hidalgo AJ, Muriana-Fernández C, Kuliszewska E, Luque RM, Prado-Gotor R. Daunomycin Nanocarriers with High Therapeutic Payload for the Treatment of Childhood Leukemia. Pharmaceutics. 2025; 17(9):1236. https://doi.org/10.3390/pharmaceutics17091236
Chicago/Turabian StyleGiráldez-Pérez, Rosa M., Elia M. Grueso, Antonio J. Montero-Hidalgo, Cristina Muriana-Fernández, Edyta Kuliszewska, Raúl M. Luque, and Rafael Prado-Gotor. 2025. "Daunomycin Nanocarriers with High Therapeutic Payload for the Treatment of Childhood Leukemia" Pharmaceutics 17, no. 9: 1236. https://doi.org/10.3390/pharmaceutics17091236
APA StyleGiráldez-Pérez, R. M., Grueso, E. M., Montero-Hidalgo, A. J., Muriana-Fernández, C., Kuliszewska, E., Luque, R. M., & Prado-Gotor, R. (2025). Daunomycin Nanocarriers with High Therapeutic Payload for the Treatment of Childhood Leukemia. Pharmaceutics, 17(9), 1236. https://doi.org/10.3390/pharmaceutics17091236











