Evaluation of Five Ready-to-Use Bases for the Topical Administration of Propranolol Hydrochloride to Treat Infantile Hemangioma
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Topical PRP-HCl Formulations
2.2. HPLC Method
2.3. Accelerated and Real-Tme Physical Stability Studies
2.4. Chemical Stability Study
2.5. In Vitro Release and Permeation Studies
2.6. Mathematical Models and Kinetic Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Accelerated and Real-Time Physical Stability Studies
3.2. Chemical Stability Study
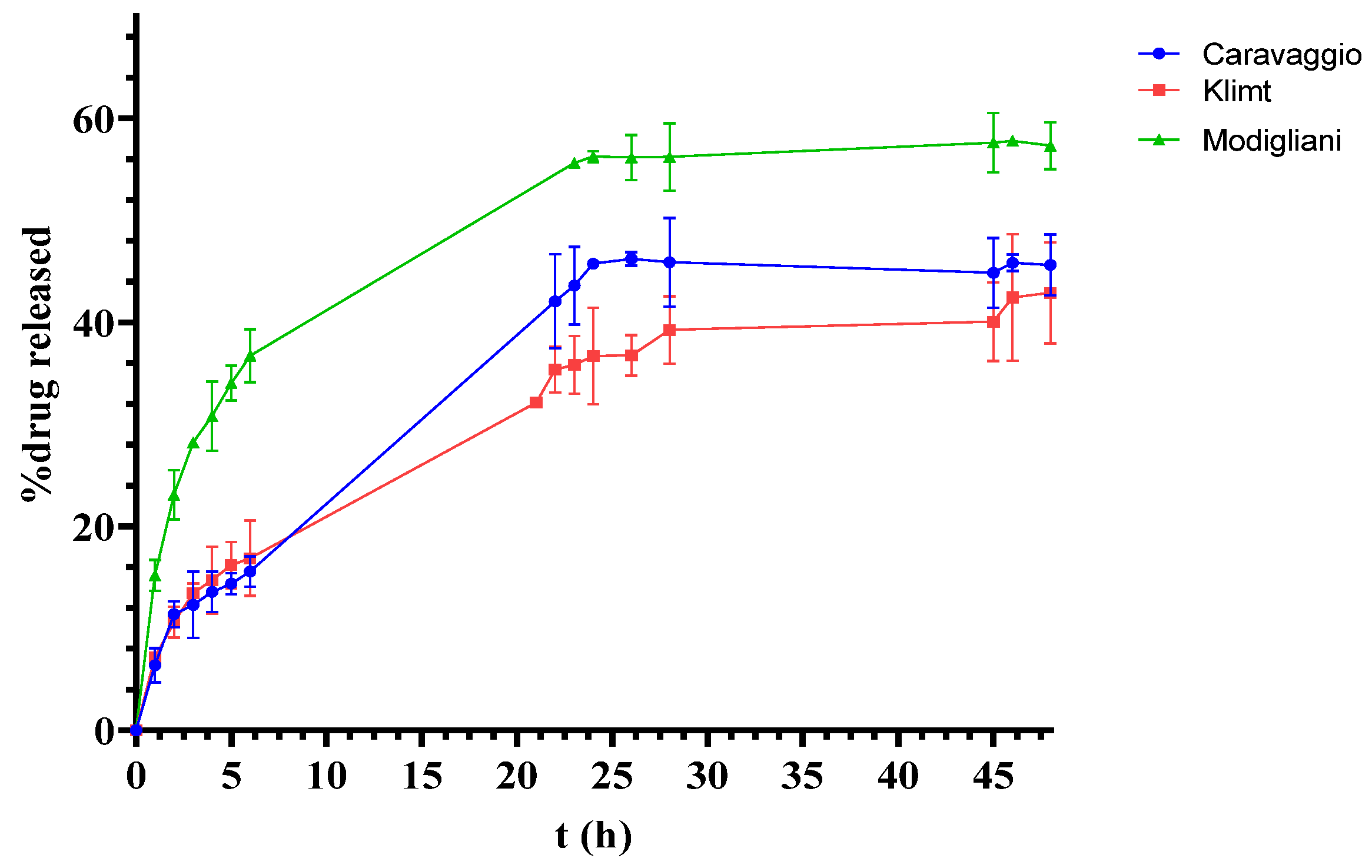
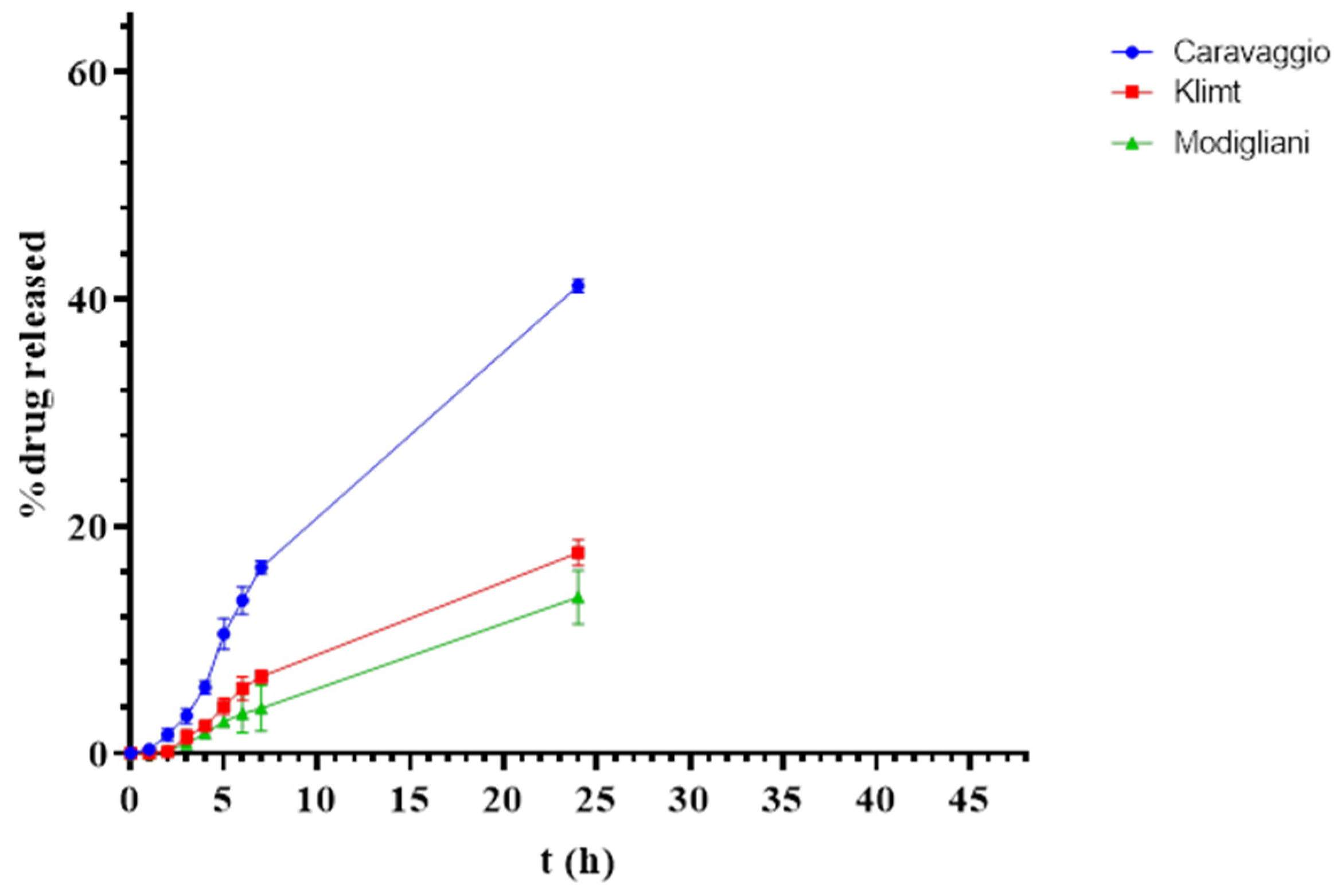
3.3. In Vitro Release and Permeation Studies
3.4. Mathematical Models and Kinetic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoeger, P.H.; Harper, J.I.; Baselga, E.; Bonnet, D.; Boon, L.M.; Atti, M.C.D.; El Hachem, M.; Oranje, A.P.; Rubin, A.T.; Weibel, L.; et al. Treatment of Infantile Haemangiomas: Recommendations of a European Expert Group. Eur. J. Pediatr. 2015, 174, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; Harper, J.I.; Hoeger, P.H. Infantile Haemangioma. Lancet 2017, 390, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Holland, K.E.; Drolet, B.A. Infantile Hemangioma. Pediatr. Clin. N. Am. 2010, 57, 1069–1083. [Google Scholar] [CrossRef]
- Rodríguez Bandera, A.I.; Sebaratnam, D.F.; Wargon, O.; Wong, L.C.F. Infantile Hemangioma. Part 1: Epidemiology, Pathogenesis, Clinical Presentation and Assessment. J. Am. Acad. Dermatol. 2021, 85, 1379–1392. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Wang, Q.-N.; Zhu, Y.-H.; Zhou, L.-Y.; Xu, T.; He, Z.-Y.; Yang, Y. Progress in the Treatment of Infantile Hemangioma. Ann. Transl. Med. 2019, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.M.; Gallardo-Vara, E.; Casado-Vela, J.; Recio-Poveda, L.; Botella, L.-M.; Albiñana, V. The Role of Propranolol as a Repurposed Drug in Rare Vascular Diseases. Int. J. Mol. Sci. 2022, 23, 4217. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.-C.; et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.-K.M.; Joachim, S.; Siegel, D.H.; Drolet, B.A.; Holland, K. Retrospective Review of Adverse Effects From Propranolol in Infants. JAMA Dermatol. 2013, 149, 484. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Lv, R.; Zhao, Z.; Huo, R. Topical Propranolol for Treatment of Superficial Infantile Hemangiomas. J. Am. Acad. Dermatol. 2012, 67, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Kunzi-Rapp, K. Topical Propranolol Therapy for Infantile Hemangiomas. Pediatr. Dermatol. 2012, 29, 154–159. [Google Scholar] [CrossRef]
- Price, A.; Rai, S.; Mcleod, R.W.J.; Birchall, J.C.; Elhassan, H.A. Topical Propranolol for Infantile Haemangiomas: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Piraccini, B.M.; Alessandrini, A.; Dika, E.; Starace, M.; Patrizi, A.; Neri, I. Topical Propranolol 1% Cream for Pyogenic Granulomas of the Nail: Open-label Study in 10 Patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Musazzi, U.M.; Rocco, P.; Franzè, S.; Minghetti, P. Topical Treatment of Infantile Haemangiomas: A Comparative Study on the Selection of a Semi-Solid Vehicle. Ski. Pharmacol. Physiol. 2016, 29, 210–219. [Google Scholar] [CrossRef]
- Spennacchio, A.; Lopalco, A.; Racaniello, G.F.; Cutrignelli, A.; la Forgia, F.M.; Fontana, S.; Cristofori, F.; Francavilla, R.; Lopedota, A.A.; Denora, N. Mucoadhesive Budesonide Solution for the Treatment of Pediatric Eosinophilic Esophagitis. Pharmaceuticals 2024, 17, 550. [Google Scholar] [CrossRef] [PubMed]
- Lacassia, C.; Spennacchio, A.; Lopedota, A.A.; Denora, N.; Lopalco, A.; Maria La Forgia, F.; Fontana, S. Physical-Chemical Stability of the Extemporaneous Paracetamol Oral Suspension in Puccini Base. Int. J. Pharm. Compd. 2024, 28, 146–150. [Google Scholar] [PubMed]
- Lacassia, C.; Spennacchio, A.; Lopalco, A.; Lopedota, A.A.; la Forgia, F.; Fontana, S.; Franco, M.; Denora, N. Lipophilic Ready to Use Vehicles Compounded Topical Medications. Int. J. Pharm. Compd. 2024, 28, 146–150. [Google Scholar]
- Spennacchio, A.; Lacassia, C.; Lopalco, A.; Lopedota, A.A.; la Forgia, F.; Fontana, S.; Franco, M.; Denora, N. Hydrophilic Ready-to-Use Vehicles and Compounded Topical Medications. Int. J. Pharm. Compd. 2024, 28, 138–145. [Google Scholar]
- Procedure per Unguenti Secondo Le Norme Di Buona Preparazione. Available online: https://www.sifap.org/procedure-norme-di-buona-preparazione/procedure-unguenti (accessed on 30 December 2024).
- Procedure per Creme Secondo Le Norme Di Buona Preparazione. Available online: https://www.sifap.org/procedure-norme-di-buona-preparazione/procedure-creme (accessed on 30 December 2024).
- Lopalco, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Franco, M.; Robota, M.; Hauschildt, N.; Mondelli, F.; Arduino, I.; Lopedota, A. Taste Masking of Propranolol Hydrochloride by Microbeads of EUDRAGIT® E PO Obtained with Prilling Technique for Paediatric Oral Administration. Int. J. Pharm. 2020, 574, 118922. [Google Scholar] [CrossRef]
- Sopyan, I.; Gozali, D.; Tiassetiana, S. Formulation of Tomato Extracts (Solanum lycopersicum L.) as a Sunscreen Lotion. Natl. J. Physiol. Pharm. Pharmacol. 2017, 8, 453–458. [Google Scholar] [CrossRef]
- SCHEDA DATI DI SICUREZZA PROPRANOLOLO CLORIDRATO. Available online: https://materie-prime.farmalabor.it/sds_completa/01907_it.pdf (accessed on 28 November 2012).
- Racaniello, G.F.; Balenzano, G.; Arduino, I.; Iacobazzi, R.M.; Lopalco, A.; Lopedota, A.A.; Sigurdsson, H.H.; Denora, N. Chitosan and Anionic Solubility Enhancer Sulfobutylether-β-Cyclodextrin-Based Nanoparticles as Dexamethasone Ophthalmic Delivery System for Anti-Inflammatory Therapy. Pharmaceutics 2024, 16, 277. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Miastkowska, M.; Sapuła, P.; Sycz, A.; Pluta, K.; Malina, D.; Chwastowski, J. Kinetic Analysis of in Vitro Release Profiles of Salicylic Acid and Fluocinolone Acetonide from Dual Delivery Systems Composed of Polymeric Nanocarriers and a Hydrogel Matrix. J. Drug Deliv. Sci. Technol. 2024, 92, 105355. [Google Scholar] [CrossRef]

| Base | pH and Viscosity (cP) | Composition |
|---|---|---|
| Burri Cream base | 3.5 ± 0.5; 4000–30,000 | Aqua q.s. to 100%; Glyceryl stearate SE 8%; Cetyl Alcohol/Stearyl Alcohol 2%; Oryza sativa brain oil 5%; Ethylhexyl Palmitate 7%; Tocopheryl acetate 0.25%; Sodium Cetearyl sulfate 1.5%; Glycerin 2%; Benzyl alcohol 0.5%; Xanthan gum 0.3%; Lecithin 0.2; Tocopherol 0.2%; Ascorbyl Palmitate 0.1; Citric Acid 0.05%; Tartaric acid q.s. to the required pH. |
| Caravaggio Amphiphilic cream base | 5.5 ± 0.5; 20,000–100,000 | Aqua q.s. to 100%; Petrolatum 25.5%; Propylene Glycol 10%; Caprilyc/Capric Triglyceride 7.5%; PEG-40 stearate 7%; Cetearyl Alcohol 6%; GlycerylStearate 4%; Phenoxyethanol 0.5%; Sodium Benzoate 0.4%; Potassium Sorbate 0.3%; Citric Acid q.s. to the required pH. |
| Klimt Cetomacrogol cream base | 5.5 ± 0.5; 4000–40,000 | Aqua q.s. to 100%; Petrolatum 15%; Cetearyl Alcohol 50/50 7.2%; Paraffinum Liquidum 6%; Ceteareth-20 1.8%; Benzyl Alcohol 0.5%; Sodium Benzoate 0.4%; Potassium Sorbate 0.3%; Disodium EDTA 0.1%; Citric Acid q.s. to the required pH. |
| Modigliani Antioxidant cream base | 5.5 ± 0.5; 20,000–100,000 | Aqua q.s.to 100%; Petrolatum 25.5%; Propylene Glycol 10%; Caprilyc/Capric Triglyceride 7.5%; PEG-40 stearate 7%; Glyceryl Stearate 4%; Cetyl Alcohol 3%; Stearyl alcohol 3%; Phenoxyethanol 0.5%; Sodium Benzoate 0.4%; Sodium Metabisulfite 0.4%; Potassium Sorbate 0.3%; Disodium EDTA 0.1%; Citric Acid q.s. to pH. |
| Warhol Petrolatum-lanolin base | - | Petrolatum q.b. a 100%; Lanolin 5%; Cetyl alcohol 2.5%; Stearyl alcohol 2.5%. |
| Sample | Base (g) | API (mg) | Solvent (µL) |
| Modigliani 1 | 9.745 | 100.01 | 200 |
| Modigliani 2 | 9.777 | 100.20 | 200 |
| Modigliani 3 | 9.721 | 100.39 | 200 |
| Sample | Base (g) | API (mg) | Solvent (µL) |
| Caravaggio 1 | 9.746 | 100.84 | 200 |
| Caravaggio 2 | 9.669 | 100.61 | 200 |
| Caravaggio 3 | 9.676 | 101.18 | 200 |
| Sample | Base (g) | API (mg) | Solvent (µL) |
| Burri 1 | 9.744 | 98.50 | 200 |
| Burri 2 | 9.745 | 99.95 | 200 |
| Burri 3 | 9.770 | 98.55 | 200 |
| Sample | Base (g) | API (mg) | Solvent (µL) |
| Klimt 1 | 9.681 | 98.90 | 200 |
| Klimt 2 | 9.696 | 98.29 | 200 |
| Klimt 3 | 9.736 | 98.86 | 200 |
| Sample | Base (g) | API (mg) | Solvent (µL) |
| Warhol 1 | 9.704 | 99.34 | 200 |
| Warhol 2 | 9.776 | 99.40 | 200 |
| Warhol 3 | 9.774 | 99.95 | 200 |
| Sample | PRP-HCl (%) | ||
|---|---|---|---|
| 0 | 15 Days | 30 Days | |
| Modigliani | 95.41 ± 3.81 | 95.06 ± 3.98 | 100.78 ± 2.58 |
| Caravaggio | 97.13 ± 1.22 | 96.96 ± 1.5 | 98.43 ± 5.41 |
| Burri | 99.37 ± 1.14 | 94.50 ± 6.6 | 99.64 ± 2.97 |
| Klimt | 96.62 ± 4.16 | 96.67 ± 1.5 | 97.13 ± 2.2 |
| Warhol | 88.74 ± 6.17 | 87.10 ± 4.12 | 90.74 ± 5.2 |
| Mathematical Model | Modigliani | Klimt | Caravaggio | |
|---|---|---|---|---|
| Zero Order | Equation | y = 2.0466x + 16.08 | y = 1.2196x + 6.2199 | y = 1.7843x + 6.1141 |
| R2 | 0.8575 | 0.9425 | 0.9716 | |
| First Order | Equation | y = −0.0149x + 1.9304 | y = −0.0067x + 1.9741 | y = −0.0109x + 1.9785 |
| R2 | 0.9432 | 0.9655 | 0.987 | |
| Higuchi— Connors | Equation | y = 12.178x + 3.5964 | y = 6.9394x − 0.5024 | y = 9.8998x − 3.1504 |
| R2 | 0.9858 | 0.9907 | 0.9711 | |
| Kosmeyer – Peppas | Equation | y = 0.4419x + 1.193 | y = 0.4978x + 0.8261 | y = 0.5653x + 0.8607 |
| R2 | 0.9829 | 0.9815 | 0.9612 | |
| n | 0.4419 | 0.4978 | 0.5653 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacassia, C.; Cutrignelli, A.; la Forgia, F.M.; Fontana, S.; Lopalco, A.; Denora, N.; Lopedota, A.A. Evaluation of Five Ready-to-Use Bases for the Topical Administration of Propranolol Hydrochloride to Treat Infantile Hemangioma. Pharmaceutics 2025, 17, 83. https://doi.org/10.3390/pharmaceutics17010083
Lacassia C, Cutrignelli A, la Forgia FM, Fontana S, Lopalco A, Denora N, Lopedota AA. Evaluation of Five Ready-to-Use Bases for the Topical Administration of Propranolol Hydrochloride to Treat Infantile Hemangioma. Pharmaceutics. 2025; 17(1):83. https://doi.org/10.3390/pharmaceutics17010083
Chicago/Turabian StyleLacassia, Chiara, Annalisa Cutrignelli, Flavia Maria la Forgia, Sergio Fontana, Antonio Lopalco, Nunzio Denora, and Angela Assunta Lopedota. 2025. "Evaluation of Five Ready-to-Use Bases for the Topical Administration of Propranolol Hydrochloride to Treat Infantile Hemangioma" Pharmaceutics 17, no. 1: 83. https://doi.org/10.3390/pharmaceutics17010083
APA StyleLacassia, C., Cutrignelli, A., la Forgia, F. M., Fontana, S., Lopalco, A., Denora, N., & Lopedota, A. A. (2025). Evaluation of Five Ready-to-Use Bases for the Topical Administration of Propranolol Hydrochloride to Treat Infantile Hemangioma. Pharmaceutics, 17(1), 83. https://doi.org/10.3390/pharmaceutics17010083










