Production of Hydrophobic Microparticles at Safe-To-Inject Sizes for Intravascular Administration
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
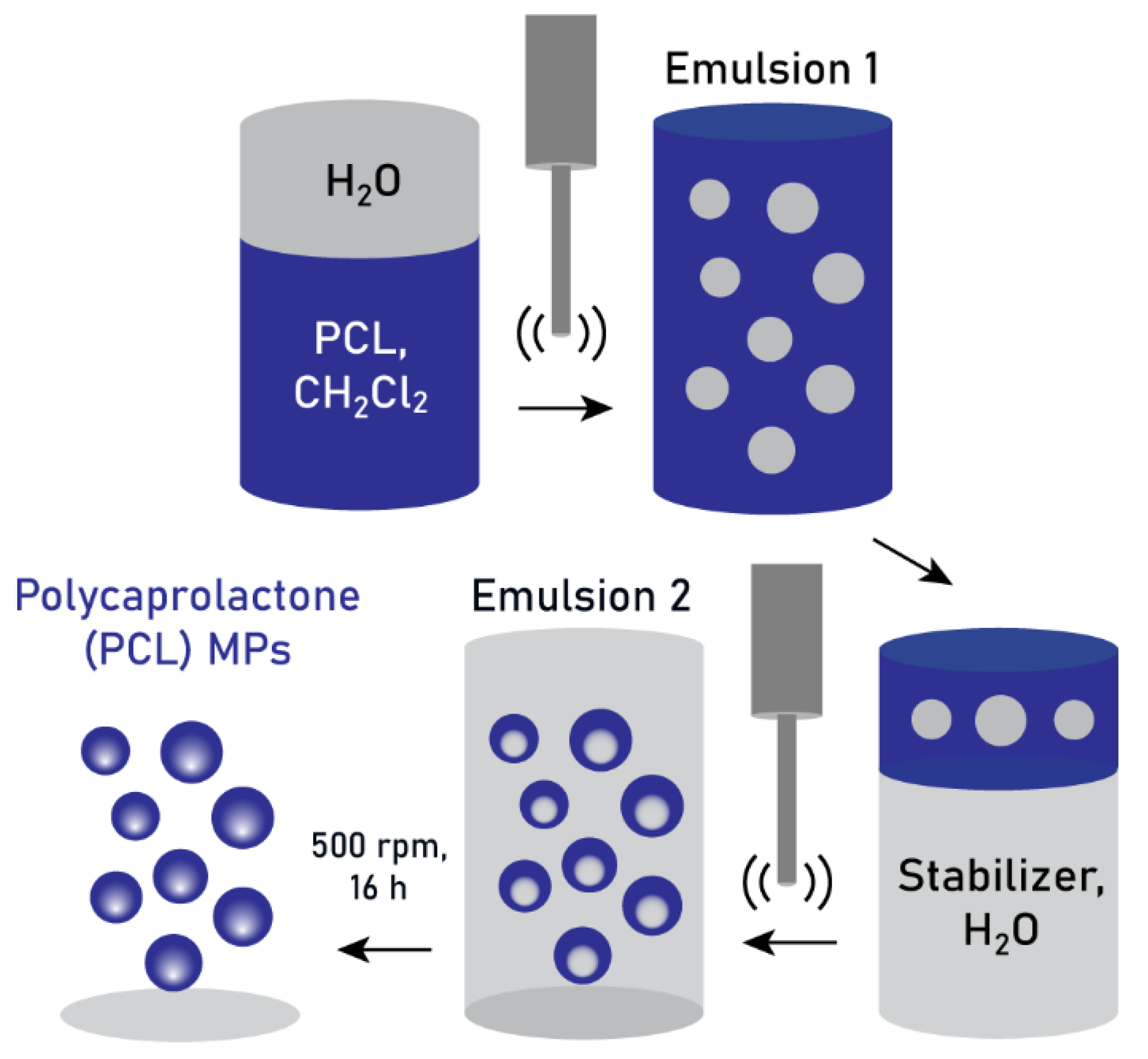
2.2. Double-Emulsification Method
2.3. Dynamic Light Scattering and Zeta Potential
2.4. Scanning Electron Microscopy
2.5. Particle Size Analysis
2.6. Flowability Assay
3. Results
3.1. Production of PCL MPs Through Double Emulsification
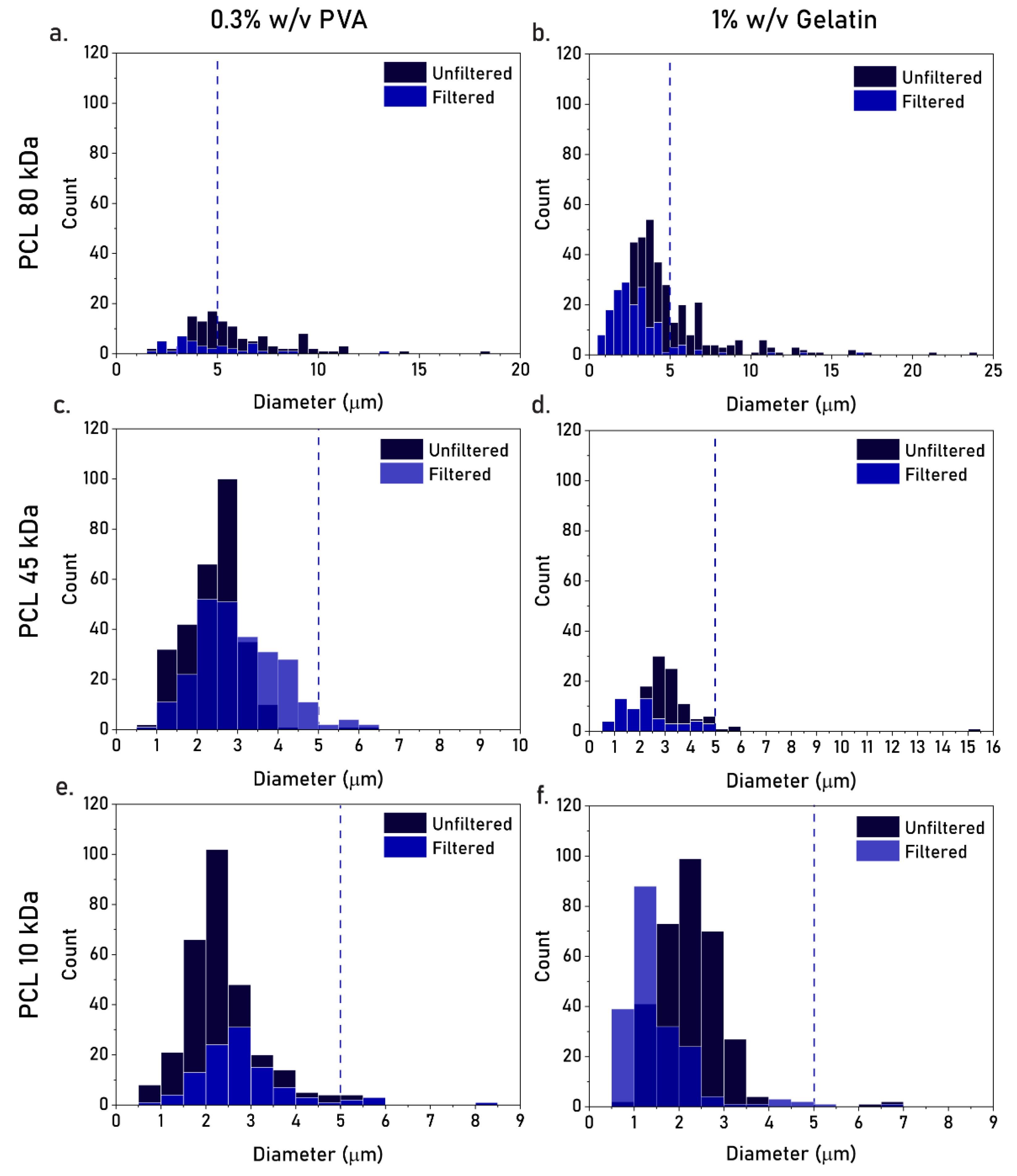
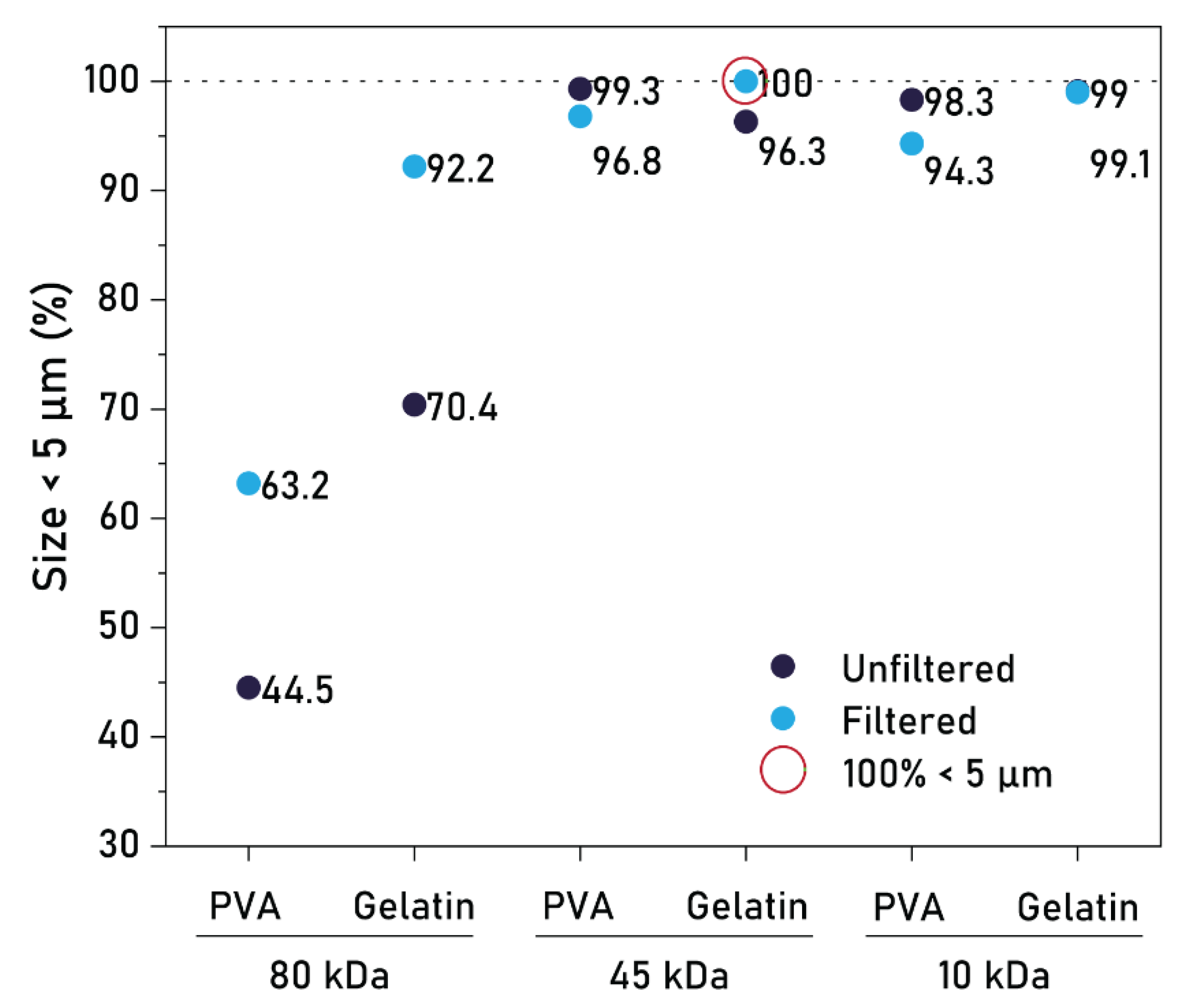
3.2. Effect of PCL MW, Type of Stabilizer, and Filtration
3.3. Effect of PCL Concentration
3.4. Flowability Tests
4. Discussion
4.1. Effect of Production Parameters on Particle Size
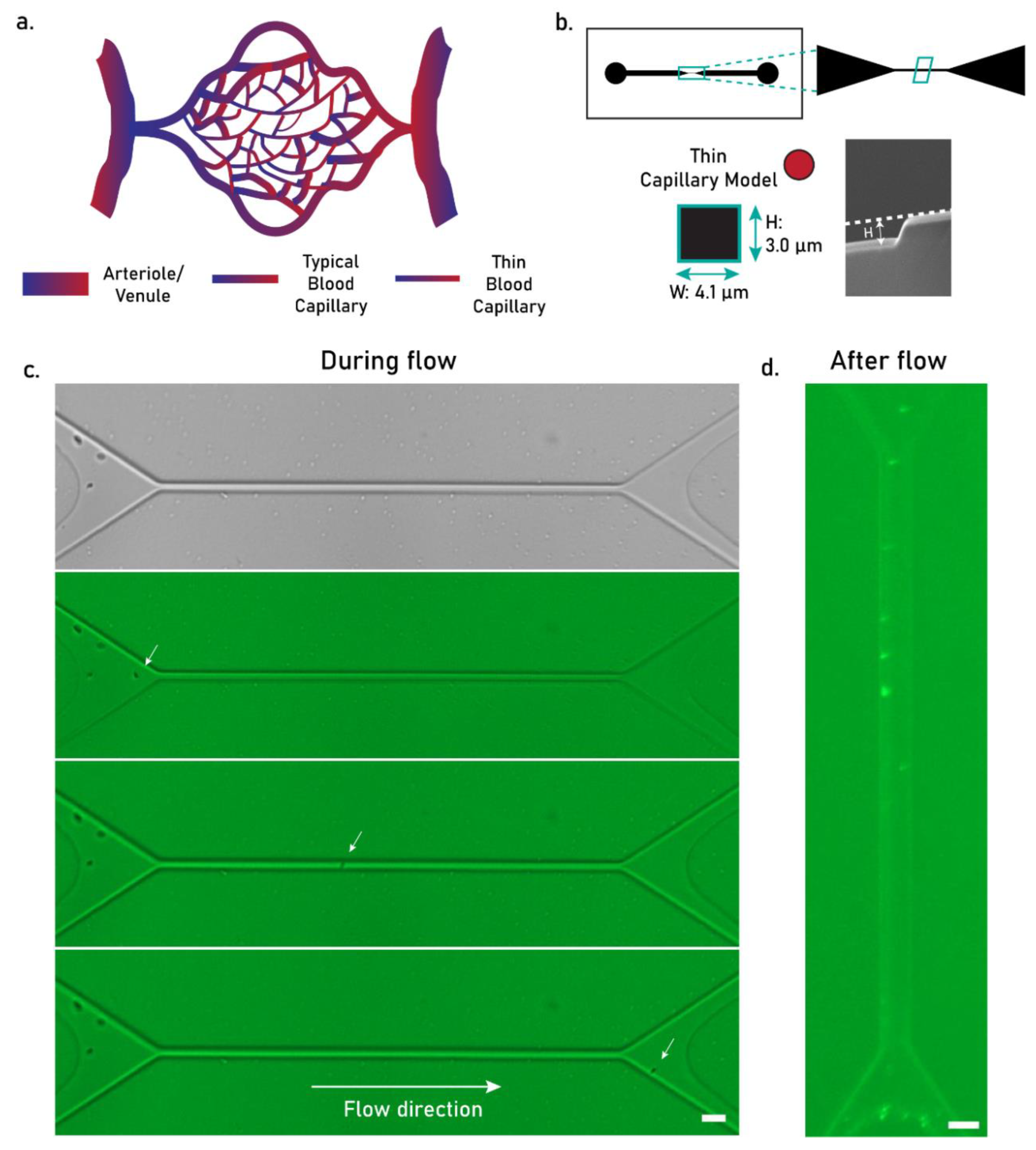
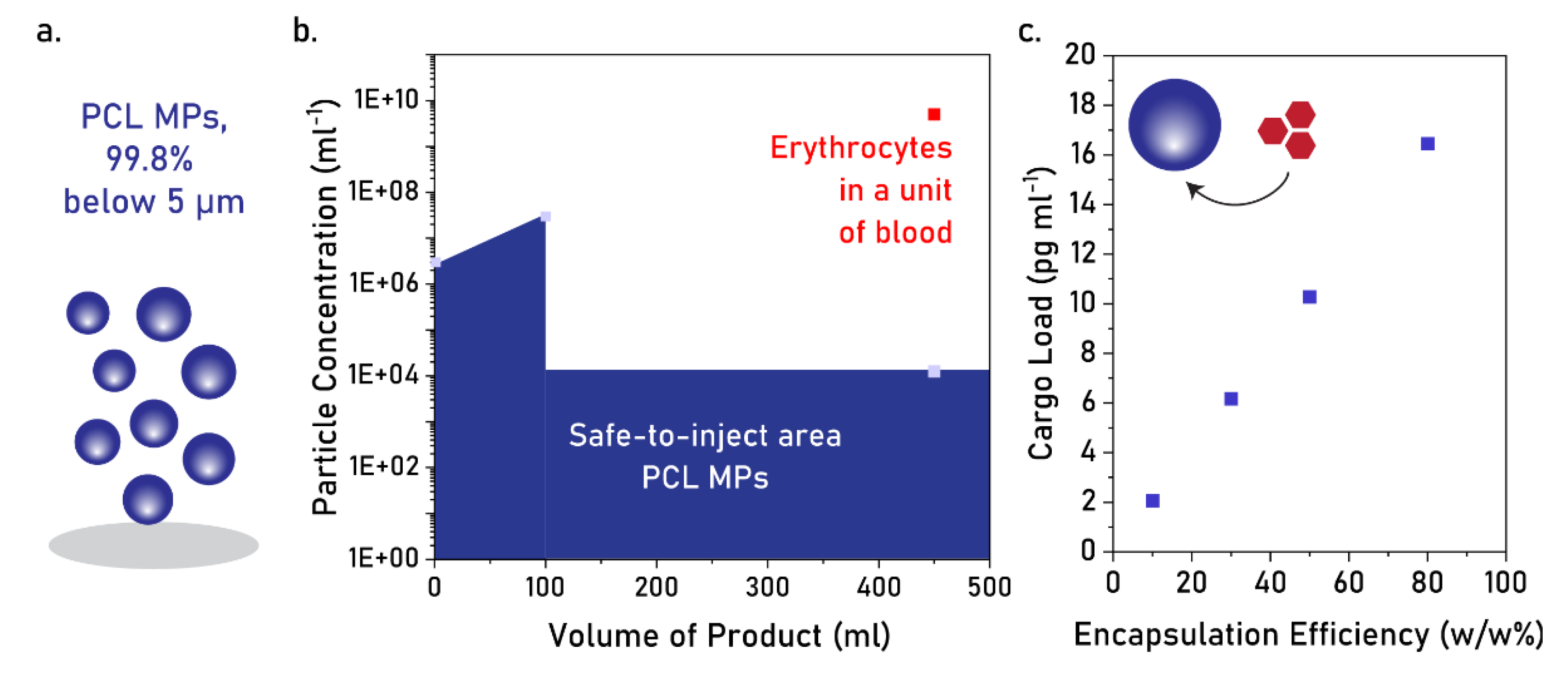
4.2. Applicability of PCL MPs as Intravascular Injectable Particles
4.3. Challenges in Manufacturing Hydrophobic Microparticles as Intravascular Injectables
4.4. Potential Solutions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stark, L.E.; Gross, A.T. Hydrophobic Protein Microparticles and Preparation Thereof. U.S. Patent US5021248A, 4 June 1991. [Google Scholar]
- Iqbal, M.; Valour, J.-P.; Fessi, H.; Elaissari, A. Preparation of biodegradable PCL particles via double emulsion evaporation method using ultrasound technique. Colloid Polym. Sci. 2015, 293, 861–873. [Google Scholar] [CrossRef]
- Grizić, D.; Lamprecht, A. Microparticle preparation by a propylene carbonate emulsification-extraction method. Int. J. Pharm. 2018, 544, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Bioinstructive microparticles for self-assembly of mesenchymal stem Cell-3D tumor spheroids. Biomaterials 2018, 185, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Hassan, S.; Moreira Teixeira, L.S.; Gurian, M.; Crispim, J.F.; Manhas, V.; Carlier, A.; Bae, H.; Geris, L.; Noshadi, I.; et al. Self-Oxygenation of Tissues Orchestrates Full-Thickness Vascularization of Living Implants. Adv. Funct. Mater. 2021, 31, 2100850. [Google Scholar] [CrossRef]
- Yadavali, S.; Lee, D.; Issadore, D. Robust Microfabrication of Highly Parallelized Three-Dimensional Microfluidics on Silicon. Sci. Rep. 2019, 9, 12213. [Google Scholar] [CrossRef]
- Amoyav, B.; Benny, O. Microfluidic Based Fabrication and Characterization of Highly Porous Polymeric Microspheres. Polymers 2019, 11, 419. [Google Scholar] [CrossRef]
- Qasim, M.; Le, N.X.T.; Nguyen, T.P.T.; Chae, D.S.; Park, S.-J.; Lee, N.Y. Nanohybrid biodegradable scaffolds for TGF-β3 release for the chondrogenic differentiation of human mesenchymal stem cells. Int. J. Pharm. 2020, 581, 119248. [Google Scholar] [CrossRef]
- Nguyen, P.P.T.; An, S.; Jeong, H.-H. Microfluidic formation of biodegradable PCLDA microparticles as sustainable sorbents for treatment of organic contaminants in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130409. [Google Scholar] [CrossRef]
- Heo, Y.; Shin, S.-W.; Kim, D.-S.; Lee, S.; Park, S.-Y.; Baek, S.-W.; Lee, J.-K.; Kim, J.H.; Han, D.K. Bioactive PCL microspheres with enhanced biocompatibility and collagen production for functional hyaluronic acid dermal fillers. Biomater. Sci. 2022, 10, 947–959. [Google Scholar] [CrossRef]
- Zhang, Z.; Ekanem, E.E.; Nakajima, M.; Bolognesi, G.; Vladisavljević, G.T. Monodispersed Sirolimus-Loaded PLGA Microspheres with a Controlled Degree of Drug–Polymer Phase Separation for Drug-Coated Implantable Medical Devices and Subcutaneous Injection. ACS Appl. Bio Mater. 2022, 5, 3766–3777. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Simon, S.; Guven, N.; Borges, K.; Youan, B.-B.C. Formulation of Spray-Dried Phenytoin Loaded Poly(ε-Caprolactone) Microcarrier Intended for Brain Delivery to Treat Epilepsy. J. Pharm. Sci. 2007, 96, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, D.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Optimising the shell thickness-to-radius ratio for the fabrication of oil-encapsulated polymeric microspheres. Chem. Eng. J. 2016, 284, 963–971. [Google Scholar] [CrossRef]
- Ali, S.W.; Mangrio, F.A.; Li, F.; Dwivedi, P.; Rajput, M.U.; Ali, R.; Khan, M.I.; Ding, W.; Xu, R.X. Co-delivery of artemether and piperine via core-shell microparticles for enhanced sustained release. J. Drug Deliv. Sci. Technol. 2021, 63, 102505. [Google Scholar] [CrossRef]
- Ajiboye, A.L.; Trivedi, V.; Mitchell, J.C. Preparation of polycaprolactone nanoparticles via supercritical carbon dioxide extraction of emulsions. Drug Deliv. Transl. Res. 2018, 8, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Pereswetoff-Morath, L. Microspheres as nasal drug delivery systems. Adv. Drug Deliv. Rev. 1998, 29, 185–194. [Google Scholar] [CrossRef]
- Ahadian, S.; Finbloom, J.A.; Mofidfar, M.; Diltemiz, S.E.; Nasrollahi, F.; Davoodi, E.; Hosseini, V.; Mylonaki, I.; Sangabathuni, S.; Montazerian, H.; et al. Micro and nanoscale technologies in oral drug delivery. Adv. Drug Deliv. Rev. 2020, 157, 37–62. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef]
- Kenley, R.; Marden, L.; Turek, T.; Jin, L.; Ron, E.; Hollinger, J.O. Osseous regeneration in the rat calvarium using novel delivery systems for recombinant human bone morphogenetic protein-2 (rhBMP-). J. Biomed. Mater. Res. 1994, 28, 1139–1147. [Google Scholar] [CrossRef]
- Luciani, A.; Coccoli, V.; Orsi, S.; Ambrosio, L.; Netti, P.A. PCL microspheres based functional scaffolds by bottom-up approach with predefined microstructural properties and release profiles. Biomaterials 2008, 29, 4800–4807. [Google Scholar] [CrossRef]
- Ding, S.-L.; Liu, X.; Zhao, X.-Y.; Wang, K.-T.; Xiong, W.; Gao, Z.-L.; Sun, C.-Y.; Jia, M.-X.; Li, C.; Gu, Q.; et al. Microcarriers in application for cartilage tissue engineering: Recent progress and challenges. Bioact. Mater. 2022, 17, 81–108. [Google Scholar] [CrossRef]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Willemen, N.G.A.; Hassan, S.; Gurian, M.; Jasso-Salazar, M.F.; Fan, K.; Wang, H.; Becker, M.; Allijn, I.E.; Bal-Öztürk, A.; Leijten, J.; et al. Enzyme-Mediated Alleviation of Peroxide Toxicity in Self-Oxygenating Biomaterials. Adv. Healthc. Mater. 2022, 11, 2102697. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I. Zein Microparticles and Nanoparticles as Drug Delivery Systems. Polymers 2022, 14, 2172. [Google Scholar] [CrossRef]
- Gnocchi, C.; Lenzuni, M.; Fiorentini, F.; Merino, D.; Summa, M.; Golub, L.; Lee, H.-M.; Johnson, F.; Bertorelli, R.; Suarato, G.; et al. Zein spray-dried microparticles loaded with chemically modified curcumin for active wound healing. J. Drug Deliv. Sci. Technol. 2024, 101, 106155. [Google Scholar] [CrossRef]
- Du, J.; Chen, G.; Yuan, X.; Yuan, J.; Li, L. Multi-stimuli responsive Cu-MOFs@Keratin drug delivery system for chemodynamic therapy. Front. Bioeng. Biotechnol. 2023, 11, 1125348. [Google Scholar] [CrossRef]
- Martella, E.; Ferroni, C.; Guerrini, A.; Ballestri, M.; Columbaro, M.; Santi, S.; Sotgiu, G.; Serra, M.; Donati, D.M.; Lucarelli, E.; et al. Functionalized Keratin as Nanotechnology-Based Drug Delivery System for the Pharmacological Treatment of Osteosarcoma. Int. J. Mol. Sci. 2018, 19, 3670. [Google Scholar] [CrossRef]
- Müller, B.; Lang, S.; Dominietto, M.; Rudin, M.; Schulz, G.; Deyhle, H.; Germann, M.; Pfeiffer, F.; David, C.; Weitkamp, T. High-Resolution Tomographic Imaging of Microvessels; SPIE: Bellingham, WA, USA, 2008; Volume 7078. [Google Scholar]
- Anand-Apte, B.; Hollyfield, J.G. Developmental Anatomy of the Retinal and Choroidal Vasculature. In Encyclopedia of the Eye; Dartt, D.A., Ed.; Academic Press: Oxford, UK, 2010; pp. 9–15. [Google Scholar]
- Fu, X.; Ohta, S.; Kawakatsu, T.; Kamihira, M.; Sakai, Y.; Ito, T. Bioinspired Perfluorocarbon-Based Oxygen Carriers with Concave Shape and Deformable Shell. Adv. Mater. Technol. 2022, 7, 2100573. [Google Scholar] [CrossRef]
- Zhou, S.; Giannetto, M.; DeCourcey, J.; Kang, H.; Kang, N.; Li, Y.; Zheng, S.; Zhao, H.; Simmons, W.R.; Wei, H.S.; et al. Oxygen tension–mediated erythrocyte membrane interactions regulate cerebral capillary hyperemia. Sci. Adv. 2019, 5, eaaw4466. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Zhou, F.L.; Hubbard Cristinacce, P.L.; Eichhorn, S.J.; Parker, G.J. Preparation and characterization of polycaprolactone microspheres by electrospraying. Aerosol. Sci. Technol. 2016, 50, 1201–1215. [Google Scholar] [CrossRef]
- Gurler, E.B.; Ergul, N.M.; Ozbek, B.; Ekren, N.; Oktar, F.N.; Haskoylu, M.E.; Oner, E.T.; Eroglu, M.S.; Ozbeyli, D.; Korkut, V.; et al. Encapsulated melatonin in polycaprolactone (PCL) microparticles as a promising graft material. Mater. Sci. Eng. C 2019, 100, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Akbari, M.; Paul, A.; Memic, A.; Dolatshahi-Pirouz, A.; Khademhosseini, A. Gelatin-Based Biomaterials For Tissue Engineering And Stem Cell Bioengineering. In Biomaterials from Nature for Advanced Devices and Therapies; Wiley & Sons, Inc.: New York, NY, USA, 2016; pp. 37–62. [Google Scholar]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Agola, J.O.; Serda, R.; Franco, S.; Lei, Q.; Wang, L.; Minster, J.; Croissant, J.G.; Butler, K.S.; Zhu, W.; et al. Biomimetic Rebuilding of Multifunctional Red Blood Cells: Modular Design Using Functional Components. ACS Nano 2020, 14, 7847–7859. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Hombreiro Pérez, M.; Lehr, C.M.; Hoffman, M.; Maincent, P. Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int. J. Pharm. 2000, 196, 177–182. [Google Scholar] [CrossRef]
- Konan, Y.N.; Gurny, R.; Allémann, E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int. J. Pharm. 2002, 233, 239–252. [Google Scholar] [CrossRef]
- Mittal, G.; Sahana, D.K.; Bhardwaj, V.; Ravi Kumar, M.N.V. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J. Control. Release 2007, 119, 77–85. [Google Scholar] [CrossRef]
- Bukofzer, S.; Ayres, J.; Chavez, A.; Devera, M.; Miller, J.; Ross, D.; Shabushnig, J.; Vargo, S.; Watson, H.; Watson, R. Industry Perspective on the Medical Risk of Visible Particles in Injectable Drug Products. PDA J. Pharm. Sci. Technol. 2015, 69, 123–139. [Google Scholar] [CrossRef]
- Liu, F.; Hutchinson, R. Visible particles in parenteral drug products: A review of current safety assessment practice. Curr. Res. Toxicol. 2024, 7, 100175. [Google Scholar] [CrossRef]
- FDA, U.S. Doxil Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050718s029lbl.pdf (accessed on 5 January 2025).
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent Applications of PLGA in Drug Delivery Systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Yadavali, S.; Jeong, H.-H.; Lee, D.; Issadore, D. Silicon and glass very large scale microfluidic droplet integration for terascale generation of polymer microparticles. Nat. Commun. 2018, 9, 1222. [Google Scholar] [CrossRef]
- Visser, C.W.; Kamperman, T.; Karbaat, L.P.; Lohse, D.; Karperien, M. In-air microfluidics enables rapid fabrication of emulsions, suspensions, and 3D modular (bio)materials. Sci. Adv. 2018, 4, eaao1175. [Google Scholar] [CrossRef] [PubMed]
- EmulTech BV. Available online: https://www.tue.nl/en/impact/tue-participations/spin-offs-in-tue-participations/emultech/ (accessed on 5 January 2025).
- Xu, J.; Wong, D.H.; Byrne, J.D.; Chen, K.; Bowerman, C.; DeSimone, J.M. Future of the particle replication in nonwetting templates (PRINT) technology. Angew. Chem. Int. Ed. Engl. 2013, 52, 6580–6589. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Shimizu, M.; Kukizaki, M. Particle control of emulsion by membrane emulsification and its applications. Adv. Drug Deliv. Rev. 2000, 45, 47–56. [Google Scholar] [CrossRef]
- Aquamarijn and Nanomi: Microsieve Membrane Emulsification. Available online: https://www.aquamarijn.nl/showcases/emulsification/ (accessed on 5 January 2025).
- Nanomi: Technology. Available online: https://www.nanomi.com/technology/ (accessed on 5 January 2025).
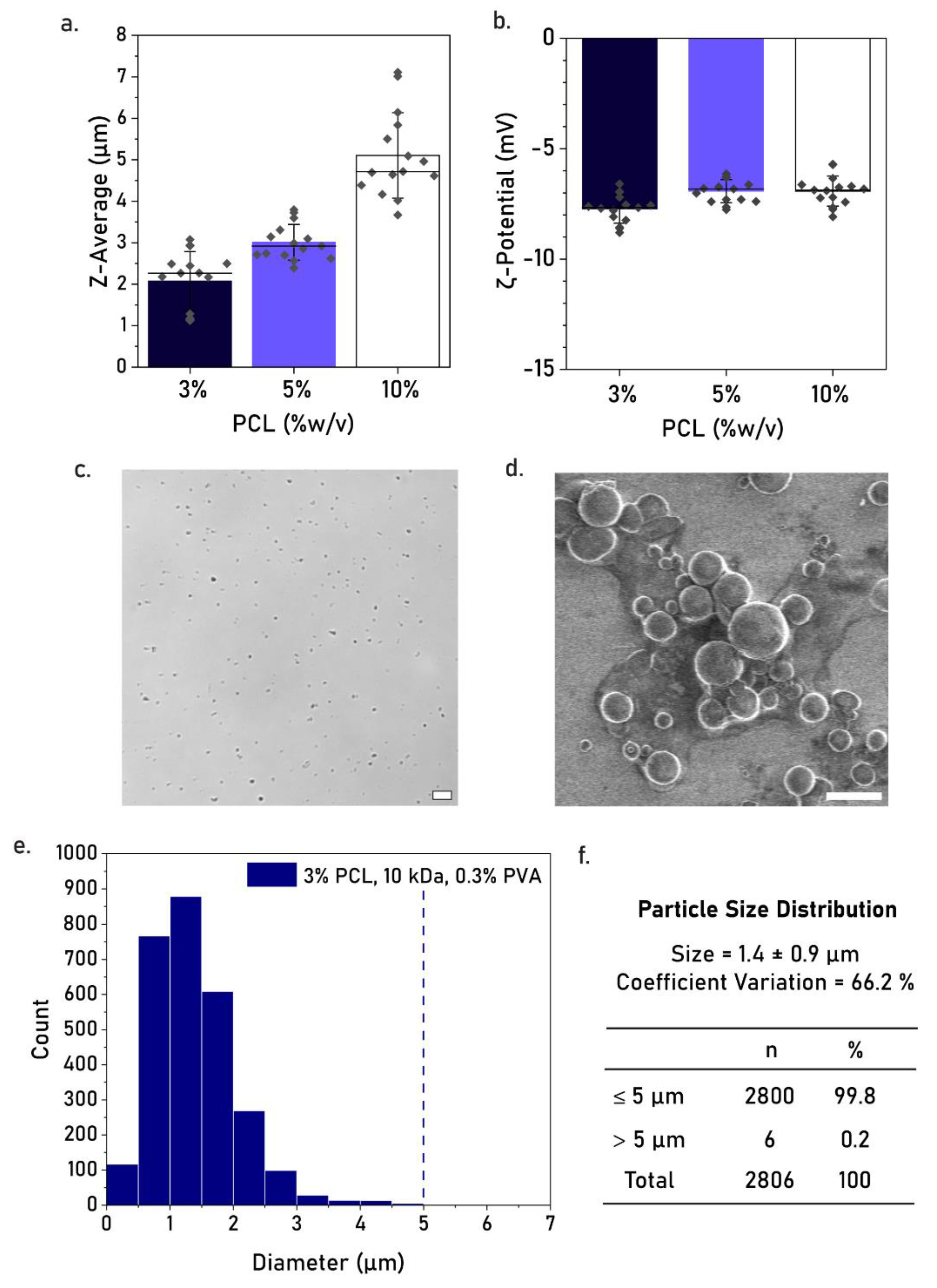
| PCL Concentration (w/v) | PCL MW | Stabilizer | Z-Average Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 3% | 10 kDa | 0.3% w/v PVA | 2275 ± 388.3 | 0.589 ± 0.062 | −7.73 ± 0.36 |
| 5% | 10 kDa | 0.3% w/v PVA | 3010 ± 145.9 | 0.867 ± 0.057 | −6.92 ± 0.40 |
| 10% | 10 kDa | 0.3% w/v PVA | 5104 ± 403.4 | 0.857 ± 0.038 | −6.92 ± 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, F.L.; Conceição, F.; Teixeira, L.M.; Leijten, J.; Jonkheijm, P. Production of Hydrophobic Microparticles at Safe-To-Inject Sizes for Intravascular Administration. Pharmaceutics 2025, 17, 64. https://doi.org/10.3390/pharmaceutics17010064
Gomes FL, Conceição F, Teixeira LM, Leijten J, Jonkheijm P. Production of Hydrophobic Microparticles at Safe-To-Inject Sizes for Intravascular Administration. Pharmaceutics. 2025; 17(1):64. https://doi.org/10.3390/pharmaceutics17010064
Chicago/Turabian StyleGomes, Francisca L., Francisco Conceição, Liliana Moreira Teixeira, Jeroen Leijten, and Pascal Jonkheijm. 2025. "Production of Hydrophobic Microparticles at Safe-To-Inject Sizes for Intravascular Administration" Pharmaceutics 17, no. 1: 64. https://doi.org/10.3390/pharmaceutics17010064
APA StyleGomes, F. L., Conceição, F., Teixeira, L. M., Leijten, J., & Jonkheijm, P. (2025). Production of Hydrophobic Microparticles at Safe-To-Inject Sizes for Intravascular Administration. Pharmaceutics, 17(1), 64. https://doi.org/10.3390/pharmaceutics17010064







