Resveratrol—A Promising Therapeutic Agent with Problematic Properties
Abstract
1. Introduction
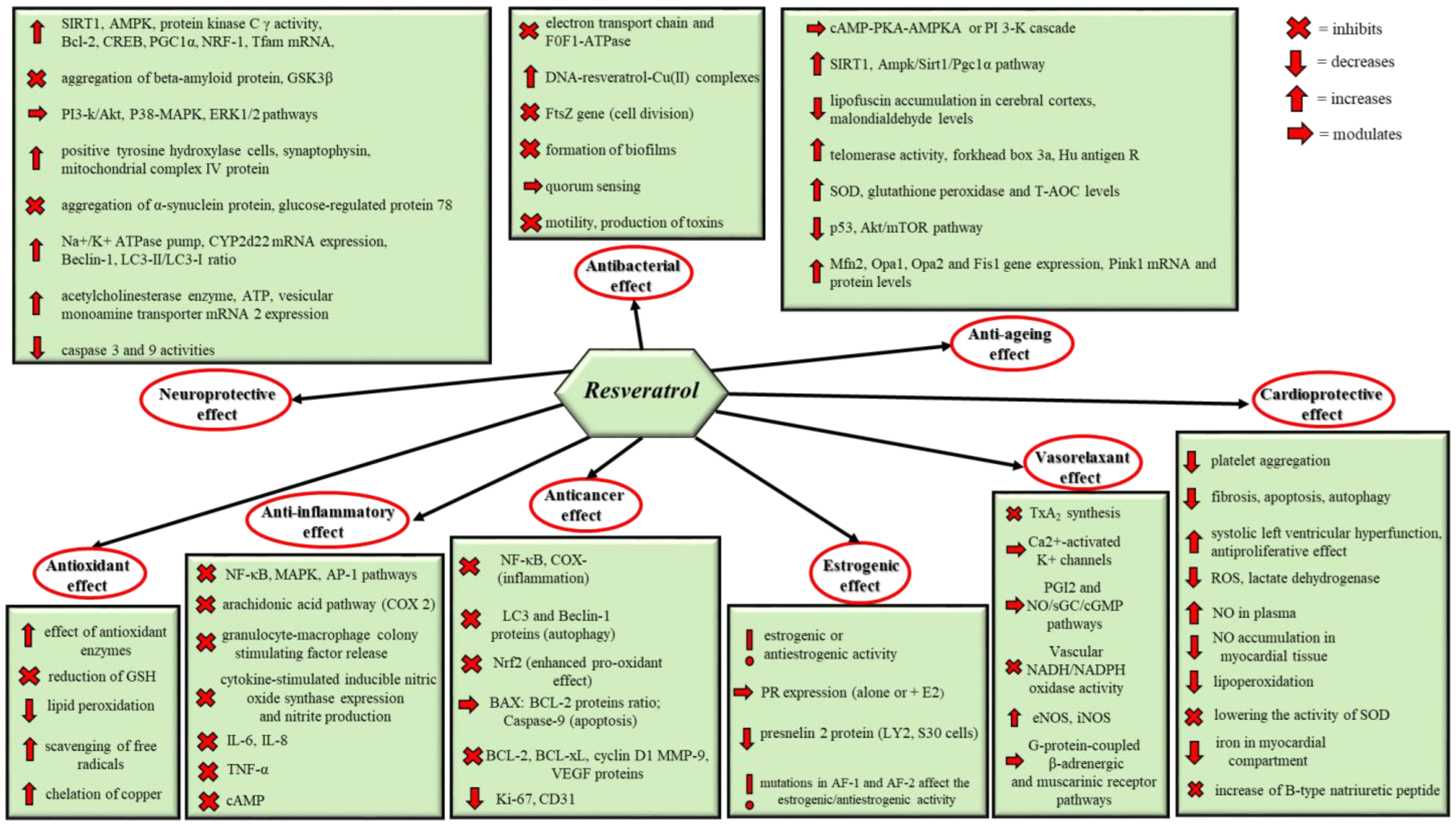
2. Biological Effects of Resveratrol
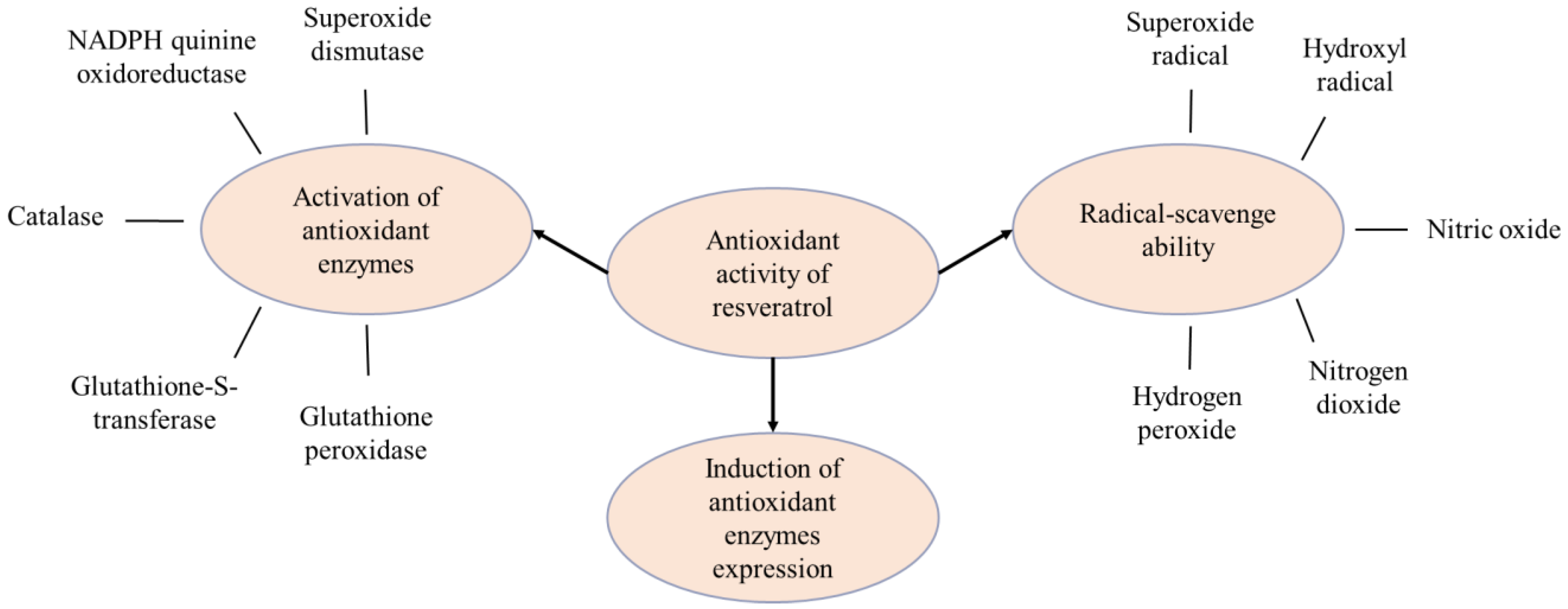
2.1. Antioxidant Effect
2.2. Anti-Inflammatory Effect
2.3. Anticancer Effect
2.4. Estrogenic Effect
2.5. Vasorelaxant Effect
2.6. Antibacterial Effect
2.7. Anti-Aging Effect
2.8. Neuroprotective Effect
2.9. Cardioprotective Effect
3. Limitations for Clinical Administration of Resveratrol
3.1. Low Aqueous Solubility
3.2. Low Stability
3.3. Low Bioavailability
4. Approaches to Improving Resveratrol’s Application
4.1. Encapsulation in Nanoparticles
4.1.1. Lipid Nanoparticles
| Type | Advantage | Reference |
|---|---|---|
| Solid lipid nanoparticles | Improved biodistribution | [74] |
| Solid lipid nanoparticles | Improved anticancer effects | [75] |
| Solid lipid nanoparticles | Alleviated insulin resistance | [76] |
| Solid lipid nanoparticles/ Nanostructured lipid carriers | Increased stability | [88] |
| Nanostructured lipid carriers | Improved pharmacokinetic properties | [77] |
| Nanostructured lipid carriers | Enhanced anticancer activity and improved pharmacokinetic properties | [78] |
| Nanostructured lipid carriers/ Liposomes | Increased solubility and cutaneous permeability | [79] |
| Liposomes | Improved anticancer effect and decreased organ toxicity | [80] |
| Liposomes | Improved anticancer effect and pharmacokinetic properties | [81] |
| Liposomes | Enhanced chemical stability and improved transdermal transport | [82] |
| Niosomes | Enhanced chemical stability | [83,84] |
4.1.2. Polymeric Nanoparticles
| Type | Advantages | Reference |
|---|---|---|
| Nanospheres | Improved biodistribution | [90] |
| Nanospheres | Improved anticancer effects | [91] |
| Nanocapsules | Alleviated insulin resistance | [92] |
| Nanocapsules | Improved pharmacokinetic properties | [93] |
| Nanocapsules | Enhanced chemical stability | [94] |
| Micelles | Increased solubility and enhanced anti-inflammatory effect | [95] |
| Micelles | Improved pharmacokinetic properties and brain distribution | [96] |
| Micelles | Increased stability Improved permeation Enhanced wound healing | [98] |
| Dendrimer | Increased solubility Enhanced antioxidant effects and cellular uptake | [99] |
| Dendrimer | Improved storage stability Enhanced topical permeability Improved bioavailability | [100] |
| Nanogel | Increased solubility Enhanced protection against oxidative stress | [101] |
| Nanogel | Increased photostability | [102] |
| Nanogel | Increased stability and antioxidant effect | [103] |
| Nanogel | Enhanced anticancer effect | [104] |
4.1.3. Inorganic and Metal Nanoparticles
4.2. Formation of Nanocrystals
4.3. Complexation
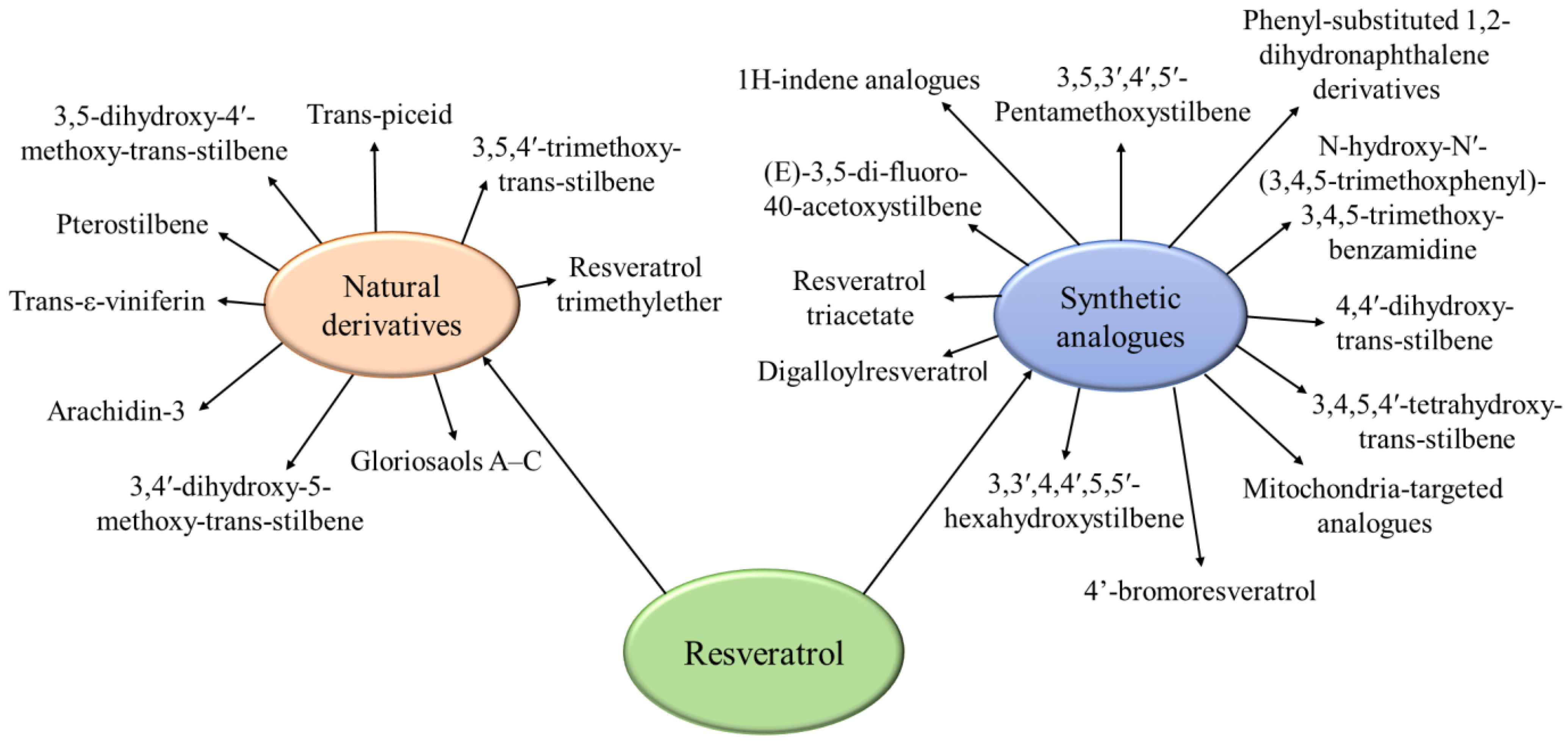
4.4. Derivatives and Analogues of Resveratrol
4.4.1. Derivatives
4.4.2. Analogues
4.5. Prodrugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anisimova, N.Y.; Kiselevsky, M.V.; Sosnov, A.V.; Sadovnikov, S.V.; Stankov, I.N.; Gakh, A.A. Trans-, Cis-, and Dihydro-Resveratrol: A Comparative Study. Chem. Cent. J. 2011, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R.J. The Production of Resveratrol by Vitis Vinifera and Other Members of the Vitaceae as a Response to Infection or Injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective Action of Resveratrol. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and Related Stilbenes: Their Anti-Aging and Anti-Angiogenic Properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- López-Hernández, J.; Paseiro-Losada, P.; Sanches-Silva, A.T.; Lage-Yusty, M.A. Study of the Changes of Trans-Resveratrol Caused by Ultraviolet Light and Determination of Trans- and Cis-Resveratrol in Spanish White Wines. Eur. Food Res. Technol. 2007, 225, 789–796. [Google Scholar] [CrossRef]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Sri Undru, S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an Antioxidant and Pro-Oxidant Agent: Mechanisms and Clinical Implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Wang, N.; Wu, T.; Ge, Q.; Chen, L. Antioxidative Stress Mechanisms behind Resveratrol: A Multidimensional Analysis. J. Food Qual. 2021, 2021, e5571733. [Google Scholar] [CrossRef]
- Inglés, M.; Gambini, J.; Miguel, M.G.; Bonet-Costa, V.; Abdelaziz, K.M.; El Alami, M.; Viña, J.; Borrás, C. PTEN Mediates the Antioxidant Effect of Resveratrol at Nutritionally Relevant Concentrations. BioMed Res. Int. 2014, 2014, 580852. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, Prooxidant and Cytotoxic Activity of Hydroxylated Resveratrol Analogues: Structure–Activity Relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Stojanović, S.; Sprinz, H.; Brede, O. Efficiency and Mechanism of the Antioxidant Action of Trans-Resveratrol and Its Analogues in the Radical Liposome Oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef]
- Mikstacka, R.; Rimando, A.M.; Ignatowicz, E. Antioxidant Effect of Trans-Resveratrol, Pterostilbene, Quercetin and Their Combinations in Human Erythrocytes In Vitro. Plant Foods Hum. Nutr. 2010, 65, 57–63. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Protective Effect of Resveratrol on Markers of Oxidative Stress in Human Erythrocytes Subjected to in Vitro Oxidative Insult. Phytother. Res. 2010, 24, S11–S14. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.F.; Ito, K.; Russell, R.E.K.; Barnes, P.J. Anti-Inflammatory Effects of Resveratrol in Lung Epithelial Cells: Molecular Mechanisms. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.-H.; Jang, H.-J.; Chae, H.-S.; Oh, Y.-C.; Choi, J.-G.; Lee, Y.-S.; Kim, J.-H.; Kim, Y.C.; Sohn, D.H.; Park, H.; et al. Anti-Inflammatory Mechanisms of Resveratrol in Activated HMC-1 Cells: Pivotal Roles of NF-κB and MAPK. Pharmacol. Res. 2009, 59, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An Overview of Its Anti-Cancer Mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Matsuoka, A.; Takeshita, K.; Furuta, A.; Ozaki, M.; Fukuhara, K.; Miyata, N. The 4′-Hydroxy Group Is Responsible for the in Vitro Cytogenetic Activity of Resveratrol. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 521, 29–35. [Google Scholar] [CrossRef]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol Improves the Anticancer Effects of Doxorubicin in Vitro and in Vivo Models: A Mechanistic Insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a Multitargeted Agent, Can Enhance Antitumor Activity of Gemcitabine in Vitro and in Orthotopic Mouse Model of Human Pancreatic Cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol Acts as a Mixed Agonist/Antagonist for Estrogen Receptors α and Β*. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Lantvit, D.; Christov, K.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Estrogenic and Antiestrogenic Properties of Resveratrol in Mammary Tumor Models1. Cancer Res. 2001, 61, 7456–7463. [Google Scholar]
- Gehm, B.D.; Levenson, A.S.; Liu, H.; Lee, E.-J.; Amundsen, B.M.; Cushman, M.; Jordan, V.C.; Jameson, J.L. Estrogenic Effects of Resveratrol in Breast Cancer Cells Expressing Mutant and Wild-Type Estrogen Receptors: Role of AF-1 and AF-2. J. Steroid Biochem. Mol. Biol. 2004, 88, 223–234. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- NADERALI, E.K.; DOYLE, P.J.; WILLIAMS, G. Resveratrol Induces Vasorelaxation of Mesenteric and Uterine Arteries from Female Guinea-Pigs. Clin. Sci. 2000, 98, 537–543. [Google Scholar] [CrossRef]
- Tan, C.S.; Loh, Y.C.; Tew, W.Y.; Yam, M.F. Vasorelaxant Effect of 3,5,4′-Trihydroxy-Trans-Stilbene (Resveratrol) and Its Underlying Mechanism. Inflammopharmacology 2020, 28, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Resveratrol—Potential Antibacterial Agent against Foodborne Pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial Activity of Resveratrol Structural Analogues: A Mechanistic Evaluation of the Structure-Activity Relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef]
- Ruan, X.; Deng, X.; Tan, M.; Yu, C.; Zhang, M.; Sun, Y.; Jiang, N. In Vitro Antibiofilm Activity of Resveratrol against Avian Pathogenic Escherichia Coli. BMC Vet. Res. 2021, 17, 249. [Google Scholar] [CrossRef]
- Qin, T.; Chen, K.; Xi, B.; Pan, L.; Xie, J.; Lu, L.; Liu, K. In Vitro Antibiofilm Activity of Resveratrol against Aeromonas Hydrophila. Antibiotics 2023, 12, 686. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Lin, Y.; Cao, J.; Xu, C.; Chen, L.; Wang, Y.; Sun, Y.; Zheng, X.; Liu, Y.; et al. Resveratrol Increases Sensitivity of Clinical Colistin-Resistant Pseudomonas Aeruginosa to Colistin In Vitro and In Vivo. Microbiol. Spectr. 2022, 11, e01992-22. [Google Scholar] [CrossRef]
- Alarcón de la Lastra, C.; Villegas, I. Resveratrol as an Anti-Inflammatory and Anti-Aging Agent: Mechanisms and Clinical Implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, e9932218. [Google Scholar] [CrossRef]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The Human Sirtuin Family: Evolutionary Divergences and Functions. Hum. Genom. 2011, 5, 485–496. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.-X.; Liu, Y.-M.; Chen, K.-L.; Chen, G. A Comparative Study of Anti-Aging Properties and Mechanism: Resveratrol and Caloric Restriction. Oncotarget 2017, 8, 65717–65729. [Google Scholar] [CrossRef]
- Liu, M.; Yin, Y.; Ye, X.; Zeng, M.; Zhao, Q.; Keefe, D.L.; Liu, L. Resveratrol Protects against Age-Associated Infertility in Mice. Hum. Reprod. 2013, 28, 707–717. [Google Scholar] [CrossRef]
- dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in In Vivo and In Vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef]
- Gomes, B.A.Q.; Silva, J.P.B.; Romeiro, C.F.R.; dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.F.S.; Varela, E.L.P.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxidative Med. Cell. Longev. 2018, 2018, e8152373. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The Pleiotropic Neuroprotective Effects of Resveratrol in Cognitive Decline and Alzheimer’s Disease Pathology: From Antioxidant to Epigenetic Therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Lin, C.-H.; Nicol, C.J.B.; Cheng, Y.-C.; Yen, C.; Wang, Y.-S.; Chiang, M.-C. Neuroprotective Effects of Resveratrol against Oxygen Glucose Deprivation Induced Mitochondrial Dysfunction by Activation of AMPK in SH-SY5Y Cells with 3D Gelatin Scaffold. Brain Res. 2020, 1726, 146492. [Google Scholar] [CrossRef]
- Porquet, D.; Griñán-Ferré, C.; Ferrer, I.; Camins, A.; Sanfeliu, C.; del Valle, J.; Pallàs, M. Neuroprotective Role of Trans-Resveratrol in a Murine Model of Familial Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1209–1220. [Google Scholar] [CrossRef]
- Fonseca-Kelly, Z.; Nassrallah, M.; Uribe, J.; Khan, R.; Dine, K.; Dutt, M.; Shindler, K. Resveratrol Neuroprotection in a Chronic Mouse Model of Multiple Sclerosis. Front. Neurol. 2012, 3, 84. [Google Scholar] [CrossRef]
- Meng, H.-Y.; Shao, D.-C.; Li, H.; Huang, X.-D.; Yang, G.; Xu, B.; Niu, H.-Y. Resveratrol Improves Neurological Outcome and Neuroinflammation Following Spinal Cord Injury through Enhancing Autophagy Involving the AMPK/mTOR Pathway. Mol. Med. Rep. 2018, 18, 2237–2244. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.-R.; Hsieh, T.-C.; Bruder, J.; Zou, J.-G.; Huang, Y.-Z. Mechanism of Cardioprotection by Resveratrol, a Phenolic Antioxidant Present in Red Wine (Review). Int. J. Mol. Med. 2001, 8, 3–17. [Google Scholar] [CrossRef]
- Hung, L.-M.; Chen, J.-K.; Huang, S.-S.; Lee, R.-S.; Su, M.-J. Cardioprotective Effect of Resveratrol, a Natural Antioxidant Derived from Grapes. Cardiovasc. Res. 2000, 47, 549–555. [Google Scholar] [CrossRef]
- Sebai, H.; Sani, M.; Aouani, E.; Ghanem-Boughanmi, N. Cardioprotective Effect of Resveratrol on Lipopolysaccharide-Induced Oxidative Stress in Rat. Drug Chem. Toxicol. 2011, 34, 146–150. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxidative Med. Cell. Longev. 2017, 2017, e6819281. [Google Scholar] [CrossRef]
- Grujić-Milanović, J.; Jaćević, V.; Miloradović, Z.; Jovović, D.; Milosavljević, I.; Milanović, S.D.; Mihailović-Stanojević, N. Resveratrol Protects Cardiac Tissue in Experimental Malignant Hypertension Due to Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef]
- Osman, A.-M.M.; Al-Harthi, S.E.; AlArabi, O.M.; Elshal, M.F.; Ramadan, W.S.; Alaama, M.N.; Al-Kreathy, H.M.; Damanhouri, Z.A.; Osman, O.H. Chemosensetizing and Cardioprotective Effects of Resveratrol in Doxorubicin- Treated Animals. Cancer Cell Int. 2013, 13, 52. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Zhang, D. Resveratrol, a Polyphenol Phytoalexin, Protects against Doxorubicin-Induced Cardiotoxicity. J. Cell. Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double Encapsulation of Resveratrol and Doxorubicin in Composite Nanogel—An Opportunity to Reduce Cardio- and Neurotoxicity of Doxorubicin. Gels 2024, 10, 699. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceutics 2023, 15, 1287. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]
- Hung, C.-F.; Chen, J.-K.; Liao, M.-H.; Lo, H.-M.; Fang, J.-Y. Development and Evaluation of Emulsion-Liposome Blends for Resveratrol Delivery. J. Nanosci. Nanotechnol. 2006, 6, 2950–2958. [Google Scholar] [CrossRef]
- Das, S.; Lin, H.-S.; Ho, P.C.; Ng, K.-Y. The Impact of Aqueous Solubility and Dose on the Pharmacokinetic Profiles of Resveratrol. Pharm. Res. 2008, 25, 2593–2600. [Google Scholar] [CrossRef]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and Solubility of Trans-Resveratrol Are Strongly Influenced by pH and Temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Silva, C.G.; Monteiro, J.; Marques, R.R.N.; Silva, A.M.T.; Martínez, C.; Canle, L.M.; Faria, J.L. Photochemical and Photocatalytic Degradation of Trans-Resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef]
- Corduneanu, O.; Janeiro, P.; Brett, A.M.O. On the Electrochemical Oxidation of Resveratrol. Electroanalysis 2006, 18, 757–762. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Vitaglione, P.; Sforza, S.; Galaverna, G.; Ghidini, C.; Caporaso, N.; Vescovi, P.P.; Fogliano, V.; Marchelli, R. Bioavailability of Trans-Resveratrol from Red Wine in Humans. Mol. Nutr. Food Res. 2005, 49, 495–504. [Google Scholar] [CrossRef]
- Yu, C.; Shin, Y.G.; Chow, A.; Li, Y.; Kosmeder, J.W.; Lee, Y.S.; Hirschelman, W.H.; Pezzuto, J.M.; Mehta, R.G.; van Breemen, R.B. Human, Rat, and Mouse Metabolism of Resveratrol. Pharm. Res. 2002, 19, 1907–1914. [Google Scholar] [CrossRef]
- Wang, L.-X.; Heredia, A.; Song, H.; Zhang, Z.; Yu, B.; Davis, C.; Redfield, R. Resveratrol Glucuronides as the Metabolites of Resveratrol in Humans: Characterization, Synthesis, and Anti-HIV Activity. J. Pharm. Sci. 2004, 93, 2448–2457. [Google Scholar] [CrossRef]
- Ruotolo, R.; Calani, L.; Fietta, E.; Brighenti, F.; Crozier, A.; Meda, C.; Maggi, A.; Ottonello, S.; Del Rio, D. Anti-Estrogenic Activity of a Human Resveratrol Metabolite. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1086–1092. [Google Scholar] [CrossRef]
- Miksits, M.; Wlcek, K.; Svoboda, M.; Kunert, O.; Haslinger, E.; Thalhammer, T.; Szekeres, T.; Jäger, W. Antitumor Activity of Resveratrol and Its Sulfated Metabolites against Human Breast Cancer Cells. Planta Med. 2009, 75, 1227–1230. [Google Scholar] [CrossRef]
- Hoshino, J.; Park, E.-J.; Kondratyuk, T.P.; Marler, L.; Pezzuto, J.M.; van Breemen, R.B.; Mo, S.; Li, Y.; Cushman, M. Selective Synthesis and Biological Evaluation of Sulfate-Conjugated Resveratrol Metabolites. J. Med. Chem. 2010, 53, 5033–5043. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Sharma, A.N.; Upadhyay, P.K.; Dewangan, H.K. Development, Evaluation, Pharmacokinetic and Biodistribution Estimation of Resveratrol-Loaded Solid Lipid Nanoparticles for Prostate Cancer Targeting. J. Microencapsul. 2022, 39, 563–574. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Xu, Y.; Peng, W.; Zhang, S.; Li, R.; Zhang, H.; Zhang, H.; Cheng, S.; Wang, Y.; et al. Resveratrol-Loaded TPGS-Resveratrol-Solid Lipid Nanoparticles for Multidrug-Resistant Therapy of Breast Cancer: In Vivo and In Vitro Study. Front. Bioeng. Biotechnol. 2021, 9, 762489. [Google Scholar] [CrossRef]
- Mohseni, R.; ArabSadeghabadi, Z.; Ziamajidi, N.; Abbasalipourkabir, R.; RezaeiFarimani, A. Oral Administration of Resveratrol-Loaded Solid Lipid Nanoparticle Improves Insulin Resistance Through Targeting Expression of SNARE Proteins in Adipose and Muscle Tissue in Rats with Type 2 Diabetes. Nanoscale Res. Lett. 2019, 14, 227. [Google Scholar] [CrossRef]
- Poonia, N.; Kaur Narang, J.; Lather, V.; Beg, S.; Sharma, T.; Singh, B.; Pandita, D. Resveratrol Loaded Functionalized Nanostructured Lipid Carriers for Breast Cancer Targeting: Systematic Development, Characterization and Pharmacokinetic Evaluation. Colloids Surf. B Biointerfaces 2019, 181, 756–766. [Google Scholar] [CrossRef]
- Gadag, S.; Narayan, R.; Nayak, A.S.; Catalina Ardila, D.; Sant, S.; Nayak, Y.; Garg, S.; Nayak, U.Y. Development and Preclinical Evaluation of Microneedle-Assisted Resveratrol Loaded Nanostructured Lipid Carriers for Localized Delivery to Breast Cancer Therapy. Int. J. Pharm. 2021, 606, 120877. [Google Scholar] [CrossRef]
- Casamonti, M.; Piazzini, V.; Bilia, A.R.; Bergonzi, M.C. Evaluation of Skin Permeability of Resveratrol Loaded Liposomes and Nanostructured Lipid Carriers Using a Skin Mimic Artificial Membrane (Skin-PAMPA). Drug Deliv. Lett. 2019, 9, 134–145. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-Breast Cancer Activity of Resveratrol Encapsulated in Liposomes. J. Mater. Chem. B 2020, 8, 27–37. [Google Scholar] [CrossRef]
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984. [Google Scholar] [CrossRef] [PubMed]
- Lafarge, E.; Villette, S.; Cario-André, M.; Lecomte, S.; Faure, C. Transdermal Diffusion of Resveratrol by Multilamellar Liposomes: Effect of Encapsulation on Its Stability. J. Drug Deliv. Sci. Technol. 2022, 76, 103742. [Google Scholar] [CrossRef]
- Machado, N.D.; Gutiérrez, G.; Matos, M.; Fernández, M.A. Preservation of the Antioxidant Capacity of Resveratrol via Encapsulation in Niosomes. Foods 2021, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.D.; Fernández, M.A.; Häring, M.; Saldías, C.; Díaz, D.D. Niosomes Encapsulated in Biohydrogels for Tunable Delivery of Phytoalexin Resveratrol. RSC Adv. 2019, 9, 7601–7609. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Uhumwangho, M.; Okor, R. Current Trends in the Production and Biomedical Applications of Liposomes: A Review. J. Med. Biomed. Res. 2009, 4, 9–21. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288. [Google Scholar] [CrossRef]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C.; Reis, S. Novel Resveratrol Nanodelivery Systems Based on Lipid Nanoparticles to Enhance Its Oral Bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef]
- de Castro, K.C.; Costa, J.M.; Campos, M.G.N. Drug-Loaded Polymeric Nanoparticles: A Review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Pujara, N.; Wong, K.Y.; Qu, Z.; Wang, R.; Moniruzzaman; Rewatkar, P.; Kumeria, T.; Ross, B.P.; McGuckin, M.; Popat, A. Oral Delivery of β-Lactoglobulin-Nanosphere-Encapsulated Resveratrol Alleviates Inflammation in Winnie Mice with Spontaneous Ulcerative Colitis. Mol. Pharm. 2021, 18, 627–640. [Google Scholar] [CrossRef]
- Cassano, R.; De Amicis, F.; Servidio, C.; Curcio, F.; Trombino, S. Preparation, Characterization and in Vitro Evaluation of Resveratrol-Loaded Nanospheres Potentially Useful for Human Breast Carcinoma. J. Drug Deliv. Sci. Technol. 2020, 57, 101748. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A. Ultrasonicated Resveratrol Loaded Starch Nanocapsules: Characterization, Bioactivity and Release Behaviour under in-Vitro Digestion. Carbohydr. Polym. 2021, 251, 117111. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Julian McClements, D.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-Loaded Core-Shell Nanostructured Delivery Systems: Cyclodextrin-Based Metal-Organic Nanocapsules Prepared by Ionic Gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in Storage Stability and Resveratrol Retention by Fabrication of Hollow Zein-Chitosan Composite Particles. Food Hydrocoll. 2021, 113, 106477. [Google Scholar] [CrossRef]
- Kamenova, K.; Radeva, L.; Konstantinov, S.; Petrov, P.D.; Yoncheva, K. Copolymeric Micelles of Poly(ε-Caprolactone) and Poly(Methacrylic Acid) as Carriers for the Oral Delivery of Resveratrol. Polymers 2023, 15, 3769. [Google Scholar] [CrossRef]
- Katekar, R.; Thombre, G.; Riyazuddin, M.; Husain, A.; Rani, H.; Praveena, K.S.; Gayen, J.R. Pharmacokinetics and Brain Targeting of Trans-Resveratrol Loaded Mixed Micelles in Rats Following Intravenous Administration. Pharm. Dev. Technol. 2020, 25, 300–307. [Google Scholar] [CrossRef]
- Lazarova, M.; Stefanova, M.; Tsvetanova, E.; Georgieva, A.; Tasheva, K.; Radeva, L.; Yoncheva, K. Resveratrol-Loaded Pluronic Micelles Ameliorate Scopolamine-Induced Cognitive Dysfunction Targeting Acetylcholinesterase Activity and Programmed Cell Death. Int. J. Mol. Sci. 2024, 25, 12777. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Li, R.; Yan, M. New Resveratrol Micelle Formulation for Ocular Delivery: Characterization and in Vitro/in Vivo Evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1960–1970. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Lu, K.; Hui, Q.; Miao, M. Characterizations and Bioavailability of Dendrimer-like Glucan Nanoparticulate System Containing Resveratrol. J. Agric. Food Chem. 2020, 68, 6420–6429. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, S.; Patil, U.K.; Chauhan, A.S. Poly(Amidoamine) Dendrimers a ‘Soft Polymer’ for Topical Application of Resveratrol: Ex-Vivo & in-Vivo Study. J. Drug Deliv. Sci. Technol. 2024, 97, 105792. [Google Scholar] [CrossRef]
- Radeva, L.; Stefanova, D.; Yordanov, Y.; Kamenova, K.; Petrov, P.D.; Marinova, M.K.; Simeonov, S.P.; Kondeva-Burdina, M.; Tzankova, V.; Yoncheva, K. Incorporation of Resveratrol in Polymeric Nanogel for Improvement of Its Protective Effects on Cellular and Microsomal Oxidative Stress Models. Gels 2023, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Yao, X.; Wang, S.; Lv, K.; Luo, G.; Wang, J.; Li, G. Intestinal Targeted Nanogel with Broad-Spectrum Autonomous ROS Scavenging Performance for Enhancing the Bioactivity of Trans-Resveratrol. Int. J. Nanomed. 2024, 19, 5995–6014. [Google Scholar] [CrossRef] [PubMed]
- Metawea, O.R.M.; Teleb, M.; Haiba, N.S.; Elzoghby, A.O.; Khafaga, A.F.; Noreldin, A.E.; Khattab, S.N.; Khalil, H.H. Folic Acid-Poly(N-Isopropylacrylamide-Maltodextrin) Nanohydrogels as Novel Thermo-/pH-Responsive Polymer for Resveratrol Breast Cancer Targeted Therapy. Eur. Polym. J. 2023, 182, 111721. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.-X. Overcoming the Barriers in Micellar Drug Delivery: Loading Efficiency, in Vivo Stability, and Micelle–Cell Interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef]
- Pawar, A.; Kamdi, V.; Alaspure, A.; Gangane, P. Recent Updates on Polymeric Micelles: A Review. Int. J. Pharm. Sci. Rev. Res. 2022, 73, 37–52. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and Inorganic Nanomaterials: Fabrication, Properties and Applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef]
- Marinheiro, D.; Ferreira, B.J.M.L.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A.L. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wen, S.; Li, Y.; An, J.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Novel Lactoferrin-Functionalized Manganese-Doped Silica Hollow Mesoporous Nanoparticles Loaded with Resveratrol for the Treatment of Ischemic Stroke. Mater. Today Adv. 2022, 15, 100262. [Google Scholar] [CrossRef]
- Gu, Y.; Fei, Z. Mesoporous Silica Nanoparticles Loaded with Resveratrol Are Used for Targeted Breast Cancer Therapy. J. Oncol. 2022, 2022, 8471331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-Gold Loaded with Resveratrol Enhance the Anti-Hepatoma Effect of Resveratrol In Vitro and In Vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gu, P.; Liu, X.; Hu, S.; Zheng, H.; Liu, T.; Li, C. Gold Nanoparticles Encapsulated Resveratrol as an Anti-Aging Agent to Delay Cataract Development. Pharmaceuticals 2023, 16, 26. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, M.; Go, E.B.; Chung, N. Resveratrol-Loaded Gold Nanoparticles Enhance Caspase-Mediated Apoptosis in PANC-1 Pancreatic Cells via Mitochondrial Intrinsic Apoptotic Pathway. Cancer Nanotechnol. 2022, 13, 34. [Google Scholar] [CrossRef]
- Iqbal, Y.; Amin, F.; Aziz, M.H.; Wahab, R. In-Situ Fabrication of Resveratrol Loaded Sodium Alginate Coated Silver Nanoparticles for in Vitro Studies of Mitochondrial-Targeted Anticancer Treatment against MCF-7 Cell Lines. Int. J. Biol. Macromol. 2024, 280, 135656. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Z.; An, J.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Chitosan-Modified Hollow Manganese Dioxide Nanoparticles Loaded with Resveratrol for the Treatment of Spinal Cord Injury. Drug Deliv. 2022, 29, 2498–2512. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Zhu, Z.; Ni, Y. Functionalization of Fe3O4/rGO Magnetic Nanoparticles with Resveratrol and in Vitro DNA Interaction. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 273, 121032. [Google Scholar] [CrossRef]
- Ghaferi, M.; Koohi Moftakhari Esfahani, M.; Raza, A.; Al Harthi, S.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Mesoporous Silica Nanoparticles: Synthesis Methods and Their Therapeutic Use-Recent Advances. J. Drug Target. 2021, 29, 131–154. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical Nanocrystals: Production by Wet Milling and Applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Gujar, K.; Wairkar, S. Nanocrystal Technology for Improving Therapeutic Efficacy of Flavonoids. Phytomedicine 2020, 71, 153240. [Google Scholar] [CrossRef] [PubMed]
- Ančić, D.; Oršolić, N.; Odeh, D.; Tomašević, M.; Pepić, I.; Ramić, S. Resveratrol and Its Nanocrystals: A Promising Approach for Cancer Therapy? Toxicol. Appl. Pharmacol. 2022, 435, 115851. [Google Scholar] [CrossRef]
- Argenziano, M.; Ansari, I.A.; Muntoni, E.; Spagnolo, R.; Scomparin, A.; Cavalli, R. Lipid-Coated Nanocrystals as a Tool for Improving the Antioxidant Activity of Resveratrol. Antioxidants 2022, 11, 1007. [Google Scholar] [CrossRef]
- Singh, S.K.; Makadia, V.; Sharma, S.; Rashid, M.; Shahi, S.; Mishra, P.R.; Wahajuddin, M.; Gayen, J.R. Preparation and In-Vitro/in-Vivo Characterization of Trans-Resveratrol Nanocrystals for Oral Administration. Drug Deliv. Transl. Res. 2017, 7, 395–407. [Google Scholar] [CrossRef]
- Xiong, S.; Liu, W.; Zhou, Y.; Mo, Y.; Liu, Y.; Chen, X.; Pan, H.; Yuan, D.; Wang, Q.; Chen, T. Enhancement of Oral Bioavailability and Anti-Parkinsonian Efficacy of Resveratrol through a Nanocrystal Formulation. Asian J. Pharm. Sci. 2020, 15, 518–528. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Wang, C.; Xie, X.; Ma, Y.; Wang, Y. Biodegradable and Dissolvable Resveratrol Nanocrystals Non-Silicon Microneedles for Transdermal Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104653. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef]
- Wan, Z.-L.; Wang, J.-M.; Wang, L.-Y.; Yuan, Y.; Yang, X.-Q. Complexation of Resveratrol with Soy Protein and Its Improvement on Oxidative Stability of Corn Oil/Water Emulsions. Food Chem. 2014, 161, 324–331. [Google Scholar] [CrossRef]
- Zhang, J.; Mi, Q.; Shen, M. Resveratrol Binding to Collagen and Its Biological Implication. Food Chem. 2012, 131, 879–884. [Google Scholar] [CrossRef]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of β-Lactoglobulin with Resveratrol and Its Biological Implications. Biomacromolecules 2008, 9, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; Pérez-Ciordia, S.; Marín-Arroyo, M.R.; McClements, D.J. Improving Resveratrol Bioaccessibility Using Biopolymer Nanoparticles and Complexes: Impact of Protein–Carbohydrate Maillard Conjugation. J. Agric. Food Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef]
- Bertacche, V.; Lorenzi, N.; Nava, D.; Pini, E.; Sinico, C. Host–Guest Interaction Study of Resveratrol With Natural and ModifiedCyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 279–287. [Google Scholar] [CrossRef]
- Duarte, A.; Martinho, A.; Luís, Â.; Figueiras, A.; Oleastro, M.; Domingues, F.C.; Silva, F. Resveratrol Encapsulation with Methyl-β-Cyclodextrin for Antibacterial and Antioxidant Delivery Applications. LWT 2015, 63, 1254–1260. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, R.; Fu, R.; Xiong, J.; Hu, Y. Cytotoxicity and Inhibition of Lipid Peroxidation Activity of Resveratrol/Cyclodextrin Inclusion Complexes. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 313–320. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tibi, I.P.-E.; Zaharieva, M.M.; Kaleva, M.; Najdenski, H.; Petrov, P.D.; Tzankova, V.; et al. Incorporation of Resveratrol-Hydroxypropyl-β-Cyclodextrin Complexes into Hydrogel Formulation for Wound Treatment. Gels 2024, 10, 346. [Google Scholar] [CrossRef]
- Krstić, L.; Jarho, P.; Ruponen, M.; Urtti, A.; González-García, M.J.; Diebold, Y. Improved Ocular Delivery of Quercetin and Resveratrol: A Comparative Study between Binary and Ternary Cyclodextrin Complexes. Int. J. Pharm. 2022, 624, 122028. [Google Scholar] [CrossRef]
- Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Parekh, H.S.; Popat, A. Enhancing Delivery and Cytotoxicity of Resveratrol through a Dual Nanoencapsulation Approach. J. Colloid Interface Sci. 2016, 462, 368–374. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kulkarni, N.S.; Muth, A.; Kunda, N.K.; Gupta, V. Inhalable Resveratrol-Cyclodextrin Complex Loaded Biodegradable Nanoparticles for Enhanced Efficacy against Non-Small Cell Lung Cancer. Int. J. Biol. Macromol. 2020, 164, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, J.; Zhang, H.; Qin, Y.; Xu, X.; Jin, Z. Novel Approach with Controlled Nucleation and Growth for Green Synthesis of Size-Controlled Cyclodextrin-Based Metal–Organic Frameworks Based on Short-Chain Starch Nanoparticles. J. Agric. Food Chem. 2018, 66, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-M.; Lee, Y.; Shin, Y.-J.; Woo, S.-H.; Kim, J.-S.; Jeong, D.-W.; Shin, S.; Jeon, S.H.; Shim, J.-H. Development of an Enzymatic Encapsulation Process for a Cycloamylose Inclusion Complex with Resveratrol. Food Chem. 2021, 345, 128777. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, Oral Bioavailability, and Metabolic Profile of Resveratrol and Its Dimethylether Analog, Pterostilbene, in Rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-Dose Pterostilbene, but Not Resveratrol, Is a Potent Neuromodulator in Aging and Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef]
- Nutakul, W.; Sobers, H.S.; Qiu, P.; Dong, P.; Decker, E.A.; McClements, D.J.; Xiao, H. Inhibitory Effects of Resveratrol and Pterostilbene on Human Colon Cancer Cells: A Side-by-Side Comparison. J. Agric. Food Chem. 2011, 59, 10964–10970. [Google Scholar] [CrossRef]
- Lin, H.-S.; Ho, P.C. A Rapid HPLC Method for the Quantification of 3,5,4′-Trimethoxy-Trans-Stilbene (TMS) in Rat Plasma and Its Application in Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2009, 49, 387–392. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Ruan, C.-C.; Cheang, W.S. Two Methoxy Derivatives of Resveratrol, 3,3′,4,5′-Tetramethoxy-Trans-Stilbene and 3,4′,5-Trimethoxy-Trans-Stilbene, Suppress Lipopolysaccharide-Induced Inflammation through Inactivation of MAPK and NF-κB Pathways in RAW 264.7 Cells. Chin. Med. 2021, 16, 69. [Google Scholar] [CrossRef]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated Resveratrol Prodrugs and Metabolites as Potential Therapeutics for Neurodegenerative Diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef]
- Murias, M.; Handler, N.; Erker, T.; Pleban, K.; Ecker, G.; Saiko, P.; Szekeres, T.; Jäger, W. Resveratrol Analogues as Selective Cyclooxygenase-2 Inhibitors: Synthesis and Structure–Activity Relationship. Bioorg. Med. Chem. 2004, 12, 5571–5578. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Li, C.; Wang, S.; Zhu, M.; Wang, J.; Yue, H.; Ma, X.; Zhen, Y.; Shu, X. Metabolic Profile and Structure–Activity Relationship of Resveratrol and Its Analogs in Human Bladder Cancer Cells. Cancer Manag. Res. 2019, 11, 4631–4642. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shan, W.; Wu, Y.; Ren, L.; Dong, J.; Ji, Z. Synthesis and Anti-Inflammatory Activity of Resveratrol Analogs. Chem. Pharm. Bull. 2005, 53, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, Y.; Yang, J.; Zhou, L.; Zhang, S.; Wu, X.; Chen, J.; Hu, D.; Gan, X. Novel Trans-Resveratrol Derivatives: Design, Synthesis, Antibacterial Activity, and Mechanisms. J. Agric. Food Chem. 2024, 72, 15561–15571. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Ganellin, C.R. Analogue-Based Drug Discovery. Chem. Int.-Newsmag. IUPAC 2010, 32, 12–15. [Google Scholar] [CrossRef]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and Clinical Applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Sassi, N.; Azzolini, M.; Romio, M.; Paradisi, C.; Zoratti, M. Improving the Efficacy of Plant Polyphenols. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2014, 14, 1332–1342. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability Is the Problem, What Is the Solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef]
- Mattarei, A.; Azzolini, M.; La Spina, M.; Zoratti, M.; Paradisi, C.; Biasutto, L. Amino Acid Carbamates As Prodrugs Of Resveratrol. Sci. Rep. 2015, 5, 15216. [Google Scholar] [CrossRef]
- Mattarei, A.; Azzolini, M.; Carraro, M.; Sassi, N.; Zoratti, M.; Paradisi, C.; Biasutto, L. Acetal Derivatives as Prodrugs of Resveratrol. Mol. Pharm. 2013, 10, 2781–2792. [Google Scholar] [CrossRef]
- Liang, L.; Liu, X.; Wang, Q.; Cheng, S.; Zhang, S.; Zhang, M. Pharmacokinetics, Tissue Distribution and Excretion Study of Resveratrol and Its Prodrug 3,5,4′-Tri-O-Acetylresveratrol in Rats. Phytomedicine 2013, 20, 558–563. [Google Scholar] [CrossRef]
- Belmonte-Reche, E.; Peñalver, P.; Caro-Moreno, M.; Mateos-Martín, M.L.; Adán, N.; Delgado, M.; González-Rey, E.; Morales, J.C. Silyl Resveratrol Derivatives as Potential Therapeutic Agents for Neurodegenerative and Neurological Diseases. Eur. J. Med. Chem. 2021, 223, 113655. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, K.; Gabriel, H.; Aura, R.; Erzsébet, V.; Blanka, S.S. Prodrug Strategy in Drug Development. Acta Medica Marisiensis 2016, 62, 356–362. [Google Scholar] [CrossRef]


| Type | Advantages | Reference |
|---|---|---|
| Mesoporous silica nanoparticles | Increased solubility Enhanced anticancer effect | [111] |
| Lactoferrin functionalized mesoporous manganese doped silica nanoparticles | Enhanced antioxidant and anti-inflammatory effect Improved bioavailability | [112] |
| Mesoporous silica nanoparticles | Enhanced anticancer effect | [113] |
| Metal (gold) nanoparticles | Improved antitumor effects | [114] |
| Metal (gold) nanoparticles | Enhanced antioxidant effects | [115] |
| Metal (gold) nanoparticles | Improved antitumor effects | [116] |
| Metal (silver) nanoparticles | Improved antitumor effects | [117] |
| Metal (manganese dioxide) nanoparticles | Improved antioxidant and anti-inflammatory effects | [118] |
| Magnetic (Fe3O4/rGO) nanoparticles | Improved interactions with thymus DNA | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radeva, L.; Yoncheva, K. Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics 2025, 17, 134. https://doi.org/10.3390/pharmaceutics17010134
Radeva L, Yoncheva K. Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics. 2025; 17(1):134. https://doi.org/10.3390/pharmaceutics17010134
Chicago/Turabian StyleRadeva, Lyubomira, and Krassimira Yoncheva. 2025. "Resveratrol—A Promising Therapeutic Agent with Problematic Properties" Pharmaceutics 17, no. 1: 134. https://doi.org/10.3390/pharmaceutics17010134
APA StyleRadeva, L., & Yoncheva, K. (2025). Resveratrol—A Promising Therapeutic Agent with Problematic Properties. Pharmaceutics, 17(1), 134. https://doi.org/10.3390/pharmaceutics17010134







