Monitoring M-Protein, Therapeutic Antibodies, and Polyclonal Antibodies in a Multiparametric Mass Spectrometry Assay Provides Insight into Therapy Response Kinetics in Patients with Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Sample Preparation and Digestion
2.3. LC-MS/MS Acquisition and Data Processing
2.4. M-Protein Sequencing and Clonotypic Peptide Selection
2.5. MS-MRD Assay Sensitivity
2.6. Peptide Selection for the Monitoring of Polyclonal Immunoglobulins
2.7. M-Protein, t-Ab, and Polyclonal Immunoglobulin Quantification in Follow-Up Sera and CSF
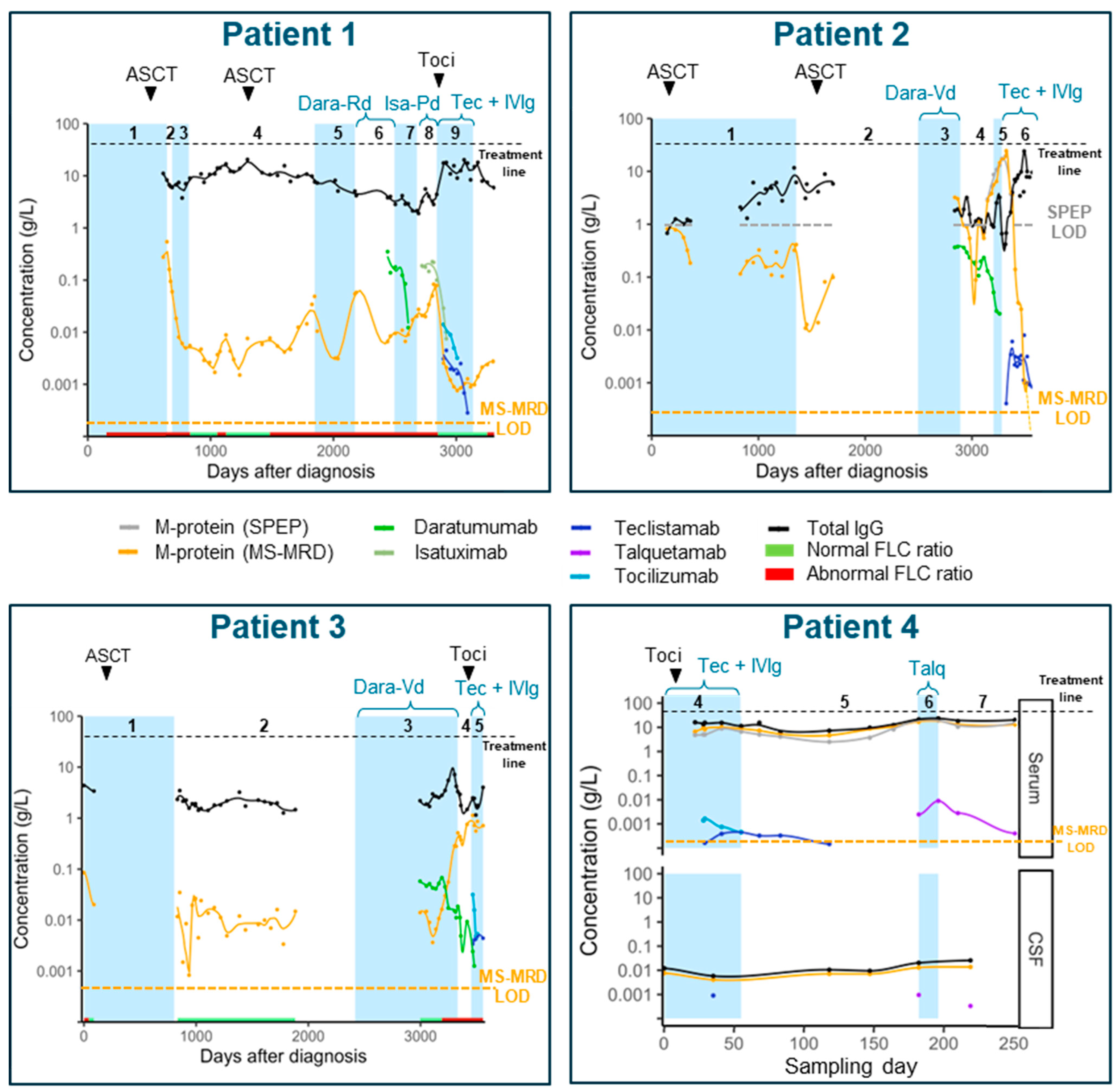
3. Results
3.1. Patient Cohort and Therapy Response
3.2. Clonotypic Peptide Selection of M-Proteins, t-Abs, and s-Abs
3.3. M-Protein Monitoring
3.4. M-Protein Kinetics
3.5. Monitoring Therapeutic and Supportive Antibodies
3.6. Half-Life of Therapeutic Antibodies and Supportive Antibodies
3.7. Monitoring Polyclonal Immunoglobulins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van de Donk, N.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Laubach, J.P.; Kaufman, J.L.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.W.; Costa, L.J.; Anderson, L.D., Jr.; Nathwani, N.; et al. Daratumumab (DARA) Plus Lenalidomide, Bortezomib, and Dexamethasone (RVd) in Patients (Pts) with Transplant-Eligible Newly Diagnosed Multiple Myeloma (NDMM): Updated Analysis of Griffin after 24 Months of Maintenance. Blood 2021, 138, 79. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Perrot, A.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Zweegman, S.; Caillon, H.; Caillot, D.; et al. Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.W.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, K.; Powers, G.; Luistro, L.; Babich, A.; Baldwin, E.; Li, Y.; Zhang, X.; Mendonca, M.; Majewski, N.; Nanjunda, R.; et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020, 4, 4538–4549. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.; Rodriguez-Otero, P.; Askari, E.; Mateos, M.V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef]
- Mohan, M.; Monge, J.; Shah, N.; Luan, D.; Forsberg, M.; Bhatlapenumarthi, V.; Balev, M.; Patwari, A.; Cheruvalath, H.; Bhutani, D.; et al. Teclistamab in relapsed refractory multiple myeloma: Multi-institutional real-world study. Blood Cancer J. 2024, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Ludwig, H.; Terpos, E.; van de Donk, N.; Mateos, M.V.; Moreau, P.; Dimopoulos, M.A.; Delforge, M.; Rodriguez-Otero, P.; San-Miguel, J.; Yong, K.; et al. Prevention and management of adverse events during treatment with bispecific antibodies and CAR T cells in multiple myeloma: A consensus report of the European Myeloma Network. Lancet Oncol. 2023, 24, e255–e269. [Google Scholar] [CrossRef]
- Frerichs, K.A.; Verkleij, C.P.M.; Mateos, M.V.; Martin, T.G.; Rodriguez, C.; Nooka, A.; Banerjee, A.; Chastain, K.; Perales-Puchalt, A.; Stephenson, T.; et al. Teclistamab impairs humoral immunity in patients with heavily pretreated myeloma: Importance of immunoglobulin supplementation. Blood Adv. 2024, 8, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Yan, X.; Puchalski, T.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Plesner, T.; Liu, K.; Khan, I.; Jansson, R.; et al. Clinical implications of complex pharmacokinetics for daratumumab dose regimen in patients with relapsed/refractory multiple myeloma. Clin. Pharmacol. Ther. 2017, 101, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, M.C.; García-Sanz, R.; Puig, N.; Pérez-Surribas, D.; Flores-Montero, J.; Ortiz-Espejo, M.; De la Rubia, J.; Cruz-Iglesias, E. Recommendations for the study of monoclonal gammopathies in the clinical laboratory. A consensus of the Spanish Society of Laboratory Medicine and the Spanish Society of Hematology and Hemotherapy. Part II: Methodological and clinical recommendations for the diagnosis and follow-up of monoclonal gammopathies. Clin. Chem. Lab. Med. 2023, 61, 2131–2142. [Google Scholar] [CrossRef]
- More, S.; Corvatta, L.; Manieri, V.M.; Morsia, E.; Offidani, M. The challenging approach to multiple myeloma: From disease diagnosis and monitoring to complications management. Cancers 2024, 16, 2263. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.F.M.; Turner, K.A.; Graziani, M.S.; Frinack, J.L.; Ettore, M.W.; Tate, J.R.; Booth, R.A.; McCudden, C.R.; Keren, D.F.; Delgado, J.C.; et al. An international multi-center serum protein electrophoresis accuracy and M-protein isotyping study. Part II: Limit of detection and follow-up of patients with small M-proteins. Clin. Chem. Lab. Med. 2020, 58, 547–559. [Google Scholar] [CrossRef] [PubMed]
- McCudden, C.; Axel, A.E.; Slaets, D.; Dejoie, T.; Clemens, P.L.; Frans, S.; Bald, J.; Plesner, T.; Jacobs, J.F.; van de Donk, N.W.; et al. Monitoring multiple myeloma patients treated with daratumumab: Teasing out monoclonal antibody interference. Clin. Chem. Lab. Med. 2016, 54, 1095–1104. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Otten, H.G.; El Haddad, O.; Axel, A.; Sasser, A.K.; Croockewit, S.; Jacobs, J.F. Interference of daratumumab in monitoring multiple myeloma patients using serum immunofixation electrophoresis can be abrogated using the daratumumab IFE reflex assay (DIRA). Clin. Chem. Lab. Med. 2016, 54, 1105–1109. [Google Scholar] [CrossRef]
- McCudden, C.R.; Jacobs, J.F.M.; Keren, D.; Caillon, H.; Dejoie, T.; Andersen, K. Recognition and management of common, rare, and novel serum protein electrophoresis and immunofixation interferences. Clin. Biochem. 2018, 51, 72–79. [Google Scholar] [CrossRef]
- Shirouchi, Y.; Kaihara, K.; Sekita, T.; Amano, N.; Nakayama, K.; Miyake, K.; Abe, H.; Oinuma, H.; Maruyama, D. Impact of M-protein detection on the response evaluations of patients undergoing treatment with the IgG-kappa monoclonal antibodies daratumumab or isatuximab, and discrepancies between immunofixation electrophoresis (IFE) systems and reagents. Cancer Med. 2024, 13, e70128. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.R.; Kohlhagen, M.C.; Willrich, M.A.; Kourelis, T.; Dispenzieri, A.; Murray, D.L. A universal solution for eliminating false positives in myeloma due to therapeutic monoclonal antibody interference. Blood 2018, 132, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Wijnands, C.; Langerhorst, P.; Bonifay, V.; Stingl, C.; Touzeau, C.; Corre, J.; Perrot, A.; Moreau, P.; Caillon, H.; et al. Dynamic monitoring of myeloma minimal residual disease with targeted mass spectrometry. Blood Cancer J. 2023, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, C.; Armony, G.; Noori, S.; Gloerich, J.; Bonifay, V.; Caillon, H.; Luider, T.M.; Brehmer, S.; Pfennig, L.; Srikumar, T.; et al. An automated workflow based on data independent acquisition for practical and high-throughput personalized assay development and minimal residual disease monitoring in multiple myeloma patients. Clin. Chem. Lab. Med. 2024, 62, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, C.; Langerhorst, P.; Noori, S.; Keizer-Garritsen, J.; Wessels, H.J.C.T.; Gloerich, J.; Bonifay, V.; Caillon, H.; Luider, T.M.; van Gool, A.J.; et al. M-protein diagnostics in multiple myeloma patients using ultra-sensitive targeted mass spectrometry and an off-the-shelf calibrator. Clin. Chem. Lab. Med. 2024, 62, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Verkleij, C.P.M.; Zajec, M.; Langerhorst, P.; Bosman, P.W.C.; de Rijke, Y.B.; Zweegman, S.; VanDuijn, M.; Luider, T.; van de Donk, N.; et al. Monitoring the M-protein of multiple myeloma patients treated with a combination of monoclonal antibodies: The laboratory solution to eliminate interference. Clin. Chem. Lab. Med. 2021, 59, 1963–1971. [Google Scholar] [CrossRef]
- Xu, T.; Park, S.K.; Venable, J.D.; Wohlschlegel, J.A.; Diedrich, J.K.; Cociorva, D.; Lu, B.; Liao, L.; Hewel, J.; Han, X.; et al. ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J. Proteom. 2015, 129, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Stefano, L.D.; Mouktadi, Z.; Vimard, V.; Moreau, S.; Mace, S.; Klippel, Z.K.; Bonifay, V.; Dubroc, C.R.; Sonigo, P. Blood-based mass spectrometry MRD tracking (M-InSight) in multiple myeloma patients from clinical trial NCT02513186. Blood 2023, 142, 3360. [Google Scholar] [CrossRef]
- Bonifay, V.; Vimard, V.; Noori, S.; Wijnands, C.; Touzeau, C.; Corre, J.; Perrot, A.; Moreau, P.; Caillon, H.; Dejoie, T.; et al. An ultra-sensitive method for sequencing and monitoring of M-protein in peripheral blood (M-inSight). Hemasphere 2023, 7, 495. [Google Scholar] [CrossRef]
- van Tetering, G.; Evers, M.; Chan, C.; Stip, M.; Leusen, J. Fc Engineering Strategies to Advance IgA Antibodies as Therapeutic Agents. Antibodies 2020, 9, 70. [Google Scholar] [CrossRef]
- Langerhorst, P.; Noori, S.; Zajec, M.; De Rijke, Y.B.; Gloerich, J.; van Gool, A.J.; Caillon, H.; Joosten, I.; Luider, T.M.; Corre, J.; et al. Multiple Myeloma Minimal Residual Disease Detection: Targeted Mass Spectrometry in Blood vs. Next-Generation Sequencing in Bone Marrow. Clin. Chem. 2021, 67, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Langerhorst, P.; Brinkman, A.B.; VanDuijn, M.M.; Wessels, H.; Groenen, P.; Joosten, I.; van Gool, A.J.; Gloerich, J.; Scheijen, B.; Jacobs, J.F.M. Clonotypic Features of Rearranged Immunoglobulin Genes Yield Personalized Biomarkers for Minimal Residual Disease Monitoring in Multiple Myeloma. Clin. Chem. 2021, 67, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Zajec, M.; Jacobs, J.F.M.; Groenen, P.; de Kat Angelino, C.M.; Stingl, C.; Luider, T.M.; De Rijke, Y.B.; VanDuijn, M.M. Development of a Targeted Mass-Spectrometry Serum Assay To Quantify M-Protein in the Presence of Therapeutic Monoclonal Antibodies. J. Proteome Res. 2018, 17, 1326–1333. [Google Scholar] [CrossRef]
- Fau, J.B.; El-Cheikh, R.; Brillac, C.; Koiwai, K.; Mace, N.; Campana, F.; Semiond, D.; Nguyen, L. Drug-Disease Interaction and Time-Dependent Population Pharmacokinetics of Isatuximab in Relapsed/Refractory Multiple Myeloma Patients. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.C.; Chang, C.W.; Lien, Z.Y.; Lin, J.A.; Chen, T.T.; Yeh, S.P. Better resolving of anti-CD38 antibody interference with blood compatibility testing by using manual polybrene method compared with dithiothreitol-pretreatment indirect antiglobulin test. J. Clin. Lab. Anal. 2023, 37, e24891. [Google Scholar] [CrossRef]
- Kim, K.; Phelps, M.A. Clinical Pharmacokinetics and Pharmacodynamics of Daratumumab. Clin. Pharmacokinet. 2023, 62, 789–806. [Google Scholar] [CrossRef]
- Miao, X.; Wu, L.S.; Lin, S.X.W.; Xu, Y.; Chen, Y.; Iwaki, Y.; Kobos, R.; Stephenson, T.; Kemmerer, K.; Uhlar, C.M.; et al. Population Pharmacokinetics and Exposure-Response with Teclistamab in Patients With Relapsed/Refractory Multiple Myeloma: Results From MajesTEC-1. Target. Oncol. 2023, 18, 667–684. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.C.; Morillo, D.; Gatt, M.E.; Sebag, M.; Kim, K.; Min, C.K.; Oriol, A.; Ocia, E.M.; Yoon, S.S.; Mateos, M.V.; et al. First results from the RedirecTT-1 study with teclistamab (tec) + talquetamab (tal) simultaneously targeting BCMA and GPRC5D in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2023, 41, 16. [Google Scholar] [CrossRef]
| Patient | M-Protein | Therapy | Therapy Line | Therapy Response |
|---|---|---|---|---|
| 1 | FLC-Kappa | VCD + HDM + ASCT | 1 | VGPR |
| Vd | 2 | VGPR | ||
| REP | 3 | PR | ||
| HDM + ASCT, R maintenance | 4 | sCR | ||
| Ixa-D | 5 | VGPR | ||
| Dara-Rd | 6 | VGPR | ||
| C-Iber-D | 7 | SD | ||
| Isa-Pd | 8 | SD | ||
| Teclistamab mono | 9 | sCR | ||
| 2 | IgA-Kappa | VCD + HDM + ASCT +VRd (consolidation), R maintenance | 1 | sCR |
| VTD + HDM + ASCT | 2 | sCR | ||
| Dara-Vd, dara mono | 3 | PR | ||
| Pom-Cd, pom mono | 4 | VGPR | ||
| Pom-C | 5 | PD | ||
| Teclistamab mono | 6 | sCR | ||
| 3 | FLC-Kappa | VCD + HDM + ASCT, R maintenance | 1 | sCR |
| KPd, Pd maintenance | 2 | sCR | ||
| Dara-Vd, dara mono, dara-R | 3 | sCR | ||
| C-Iber-D | 4 | SD | ||
| Teclistamab mono | 5 | SD | ||
| 4 | IgG-Kappa | VCD, HDM + ASCT, R maintenance | 1 | VGPR |
| Isa-Pd | 2 | PD | ||
| KCd | 3 | VGPR | ||
| Teclistamab mono | 4 | PD | ||
| Pom-C | 5 | PR | ||
| Talquetamab mono | 6 | PD | ||
| Pom-C | 7 | PD |
| Analyte | LoD (g/L) | LLoQ (g/L) | R2 | Slope |
|---|---|---|---|---|
| M-protein patient 1 | 0.0003 | 0.0009 | 0.992 | −0.884 |
| M-protein patient 2 | 0.0004 | 0.0011 | 0.995 | −0.780 |
| M-protein patient 3 | 0.0005 | 0.0014 | 0.969 | −0.801 |
| M-protein patient 4 | 0.0003 | 0.0010 | 0.985 | −0.790 |
| Daratumumab | 0.0006 | 0.0017 | 1.000 | −0.913 |
| Isatuximab | 0.0004 | 0.0013 | 0.997 | −0.877 |
| Talquetamab | 0.0006 | 0.0010 | 0.972 | −0.885 |
| Teclistamab | 0.0002 | 0.0006 | 0.972 | −0.885 |
| Tocilizumab | 0.0001 | 0.0003 | 0.998 | −0.934 |
| Treatment Line | Slope | Lowest M-Protein (g/L) |
|---|---|---|
| 1 | no data | no data |
| 2 | −8.2 | 0.0599 |
| 3 | −2.5 | 0.0050 |
| 4 | −2.2 | 0.0016 |
| 5 | −2.1 | 0.0032 |
| 6 | −1.3 | 0.0066 |
| 7 | −1.9 | 0.0067 |
| 8 | −1.5 | 0.0209 |
| 9 | −4.9 | 0.0008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijnands, C.; Karel, P.G.A.; Gloerich, J.; Armony, G.; Tzasta, A.; de Kat Angelino, C.M.; Di Stefano, L.; Bonifay, V.; Luider, T.M.; VanDuijn, M.M.; et al. Monitoring M-Protein, Therapeutic Antibodies, and Polyclonal Antibodies in a Multiparametric Mass Spectrometry Assay Provides Insight into Therapy Response Kinetics in Patients with Multiple Myeloma. Pharmaceutics 2025, 17, 135. https://doi.org/10.3390/pharmaceutics17010135
Wijnands C, Karel PGA, Gloerich J, Armony G, Tzasta A, de Kat Angelino CM, Di Stefano L, Bonifay V, Luider TM, VanDuijn MM, et al. Monitoring M-Protein, Therapeutic Antibodies, and Polyclonal Antibodies in a Multiparametric Mass Spectrometry Assay Provides Insight into Therapy Response Kinetics in Patients with Multiple Myeloma. Pharmaceutics. 2025; 17(1):135. https://doi.org/10.3390/pharmaceutics17010135
Chicago/Turabian StyleWijnands, Charissa, Peter G. A. Karel, Jolein Gloerich, Gad Armony, Anastasia Tzasta, Corrie M. de Kat Angelino, Luciano Di Stefano, Vincent Bonifay, Theo M. Luider, Martijn M. VanDuijn, and et al. 2025. "Monitoring M-Protein, Therapeutic Antibodies, and Polyclonal Antibodies in a Multiparametric Mass Spectrometry Assay Provides Insight into Therapy Response Kinetics in Patients with Multiple Myeloma" Pharmaceutics 17, no. 1: 135. https://doi.org/10.3390/pharmaceutics17010135
APA StyleWijnands, C., Karel, P. G. A., Gloerich, J., Armony, G., Tzasta, A., de Kat Angelino, C. M., Di Stefano, L., Bonifay, V., Luider, T. M., VanDuijn, M. M., Croockewit, S. J., de Kort, E. A., Castelijn, D. A. R., Stege, C. A. M., Wessels, H. J. C. T., van Gool, A. J., van de Donk, N. W. C. J., & Jacobs, J. F. M. (2025). Monitoring M-Protein, Therapeutic Antibodies, and Polyclonal Antibodies in a Multiparametric Mass Spectrometry Assay Provides Insight into Therapy Response Kinetics in Patients with Multiple Myeloma. Pharmaceutics, 17(1), 135. https://doi.org/10.3390/pharmaceutics17010135








