Comparison of Two Synthesis Methods for 3D PLA-Ibuprofen Nanofibrillar Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Morphological Characterization
2.3. Physicochemical Characterization
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Thermogravimetric Analysis (TGA) and Differential Thermogravimetric Analysis (DTG)
2.3.3. Transform Infrared Spectroscopy (FTIR)
2.3.4. Ultraviolet-Visible Spectroscopy (UV-VIS)
2.4. Cell Proliferation Assay
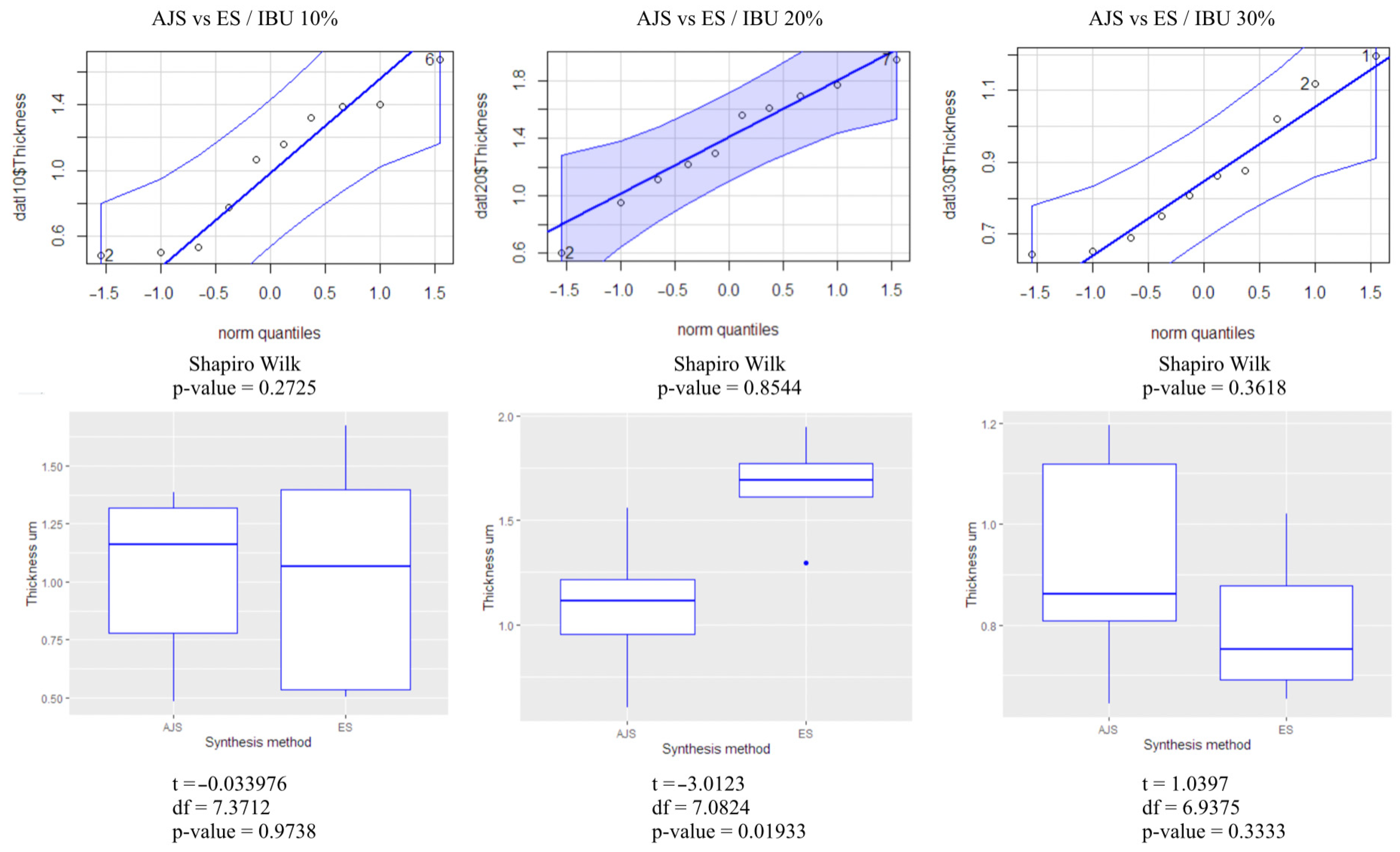
2.5. Statistical Analysis
3. Results
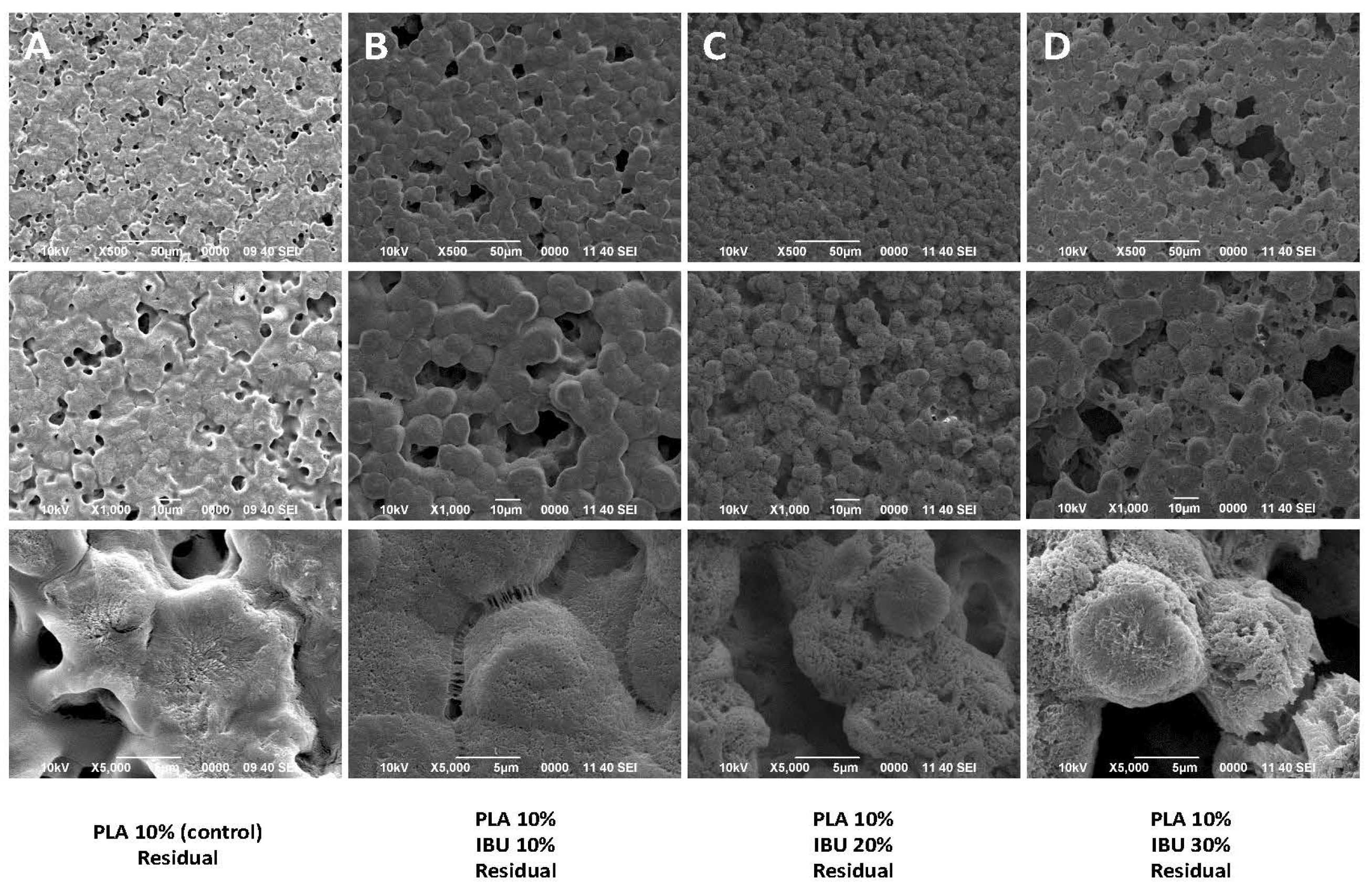
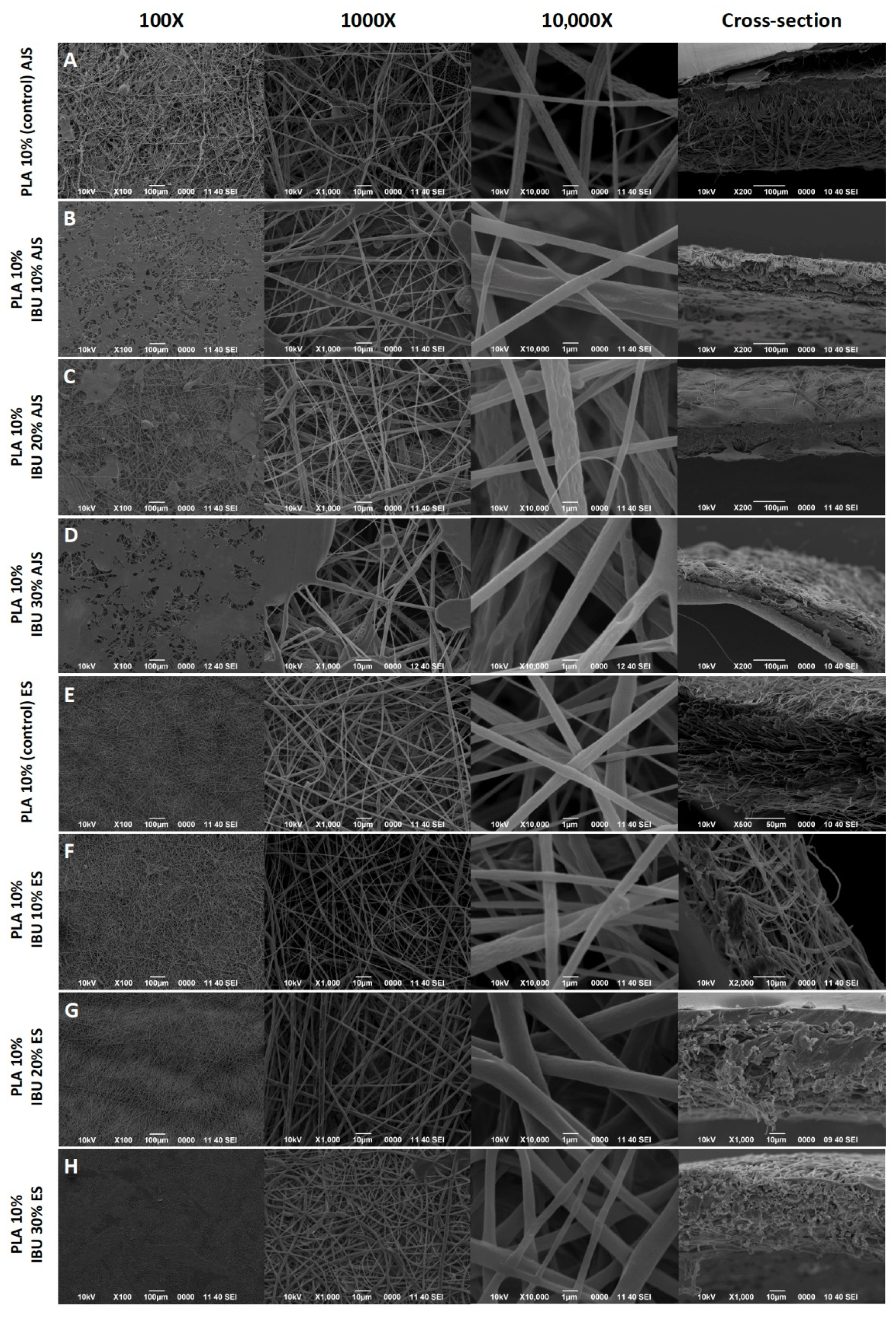
3.1. Morphological Characterization
3.2. Physicochemical Characterization
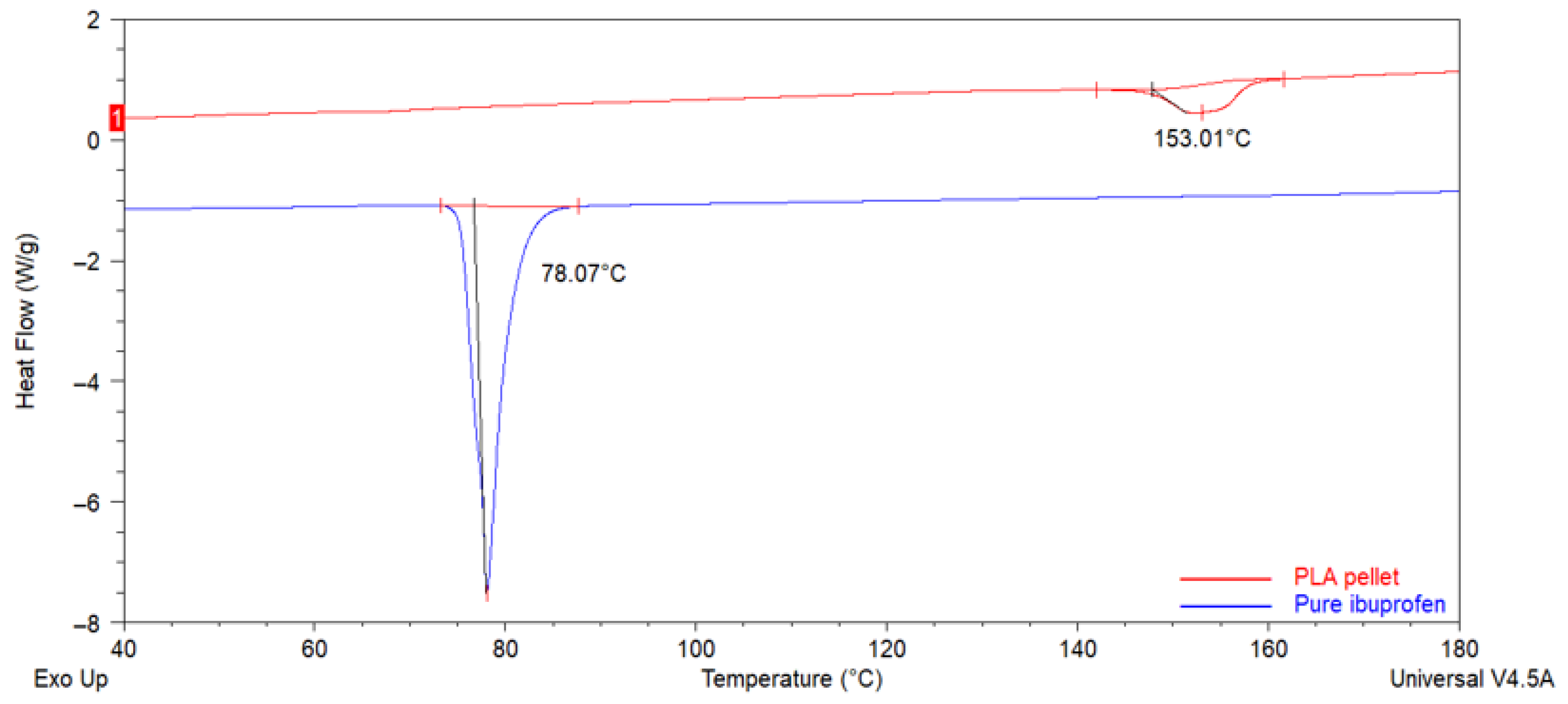
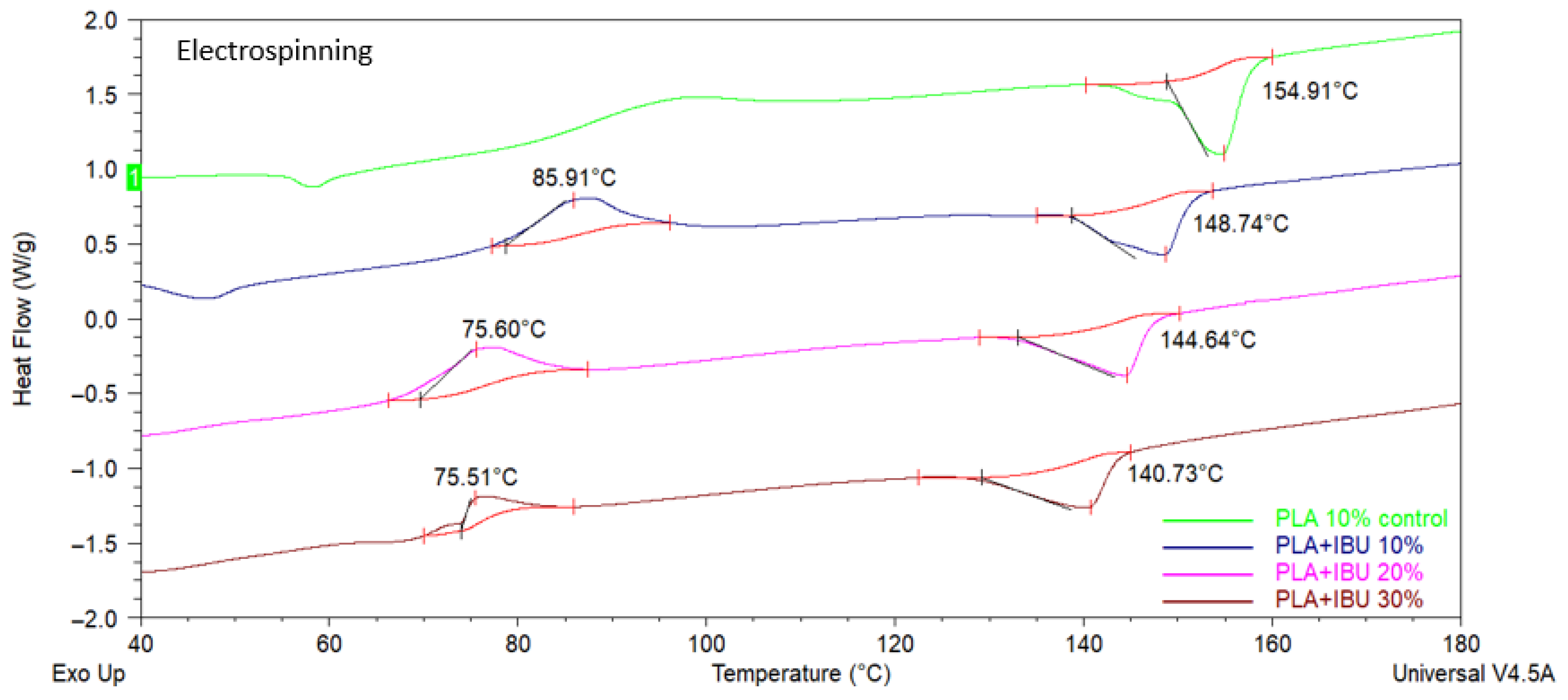
3.2.1. Differential Scanning Calorimetry (DSC)
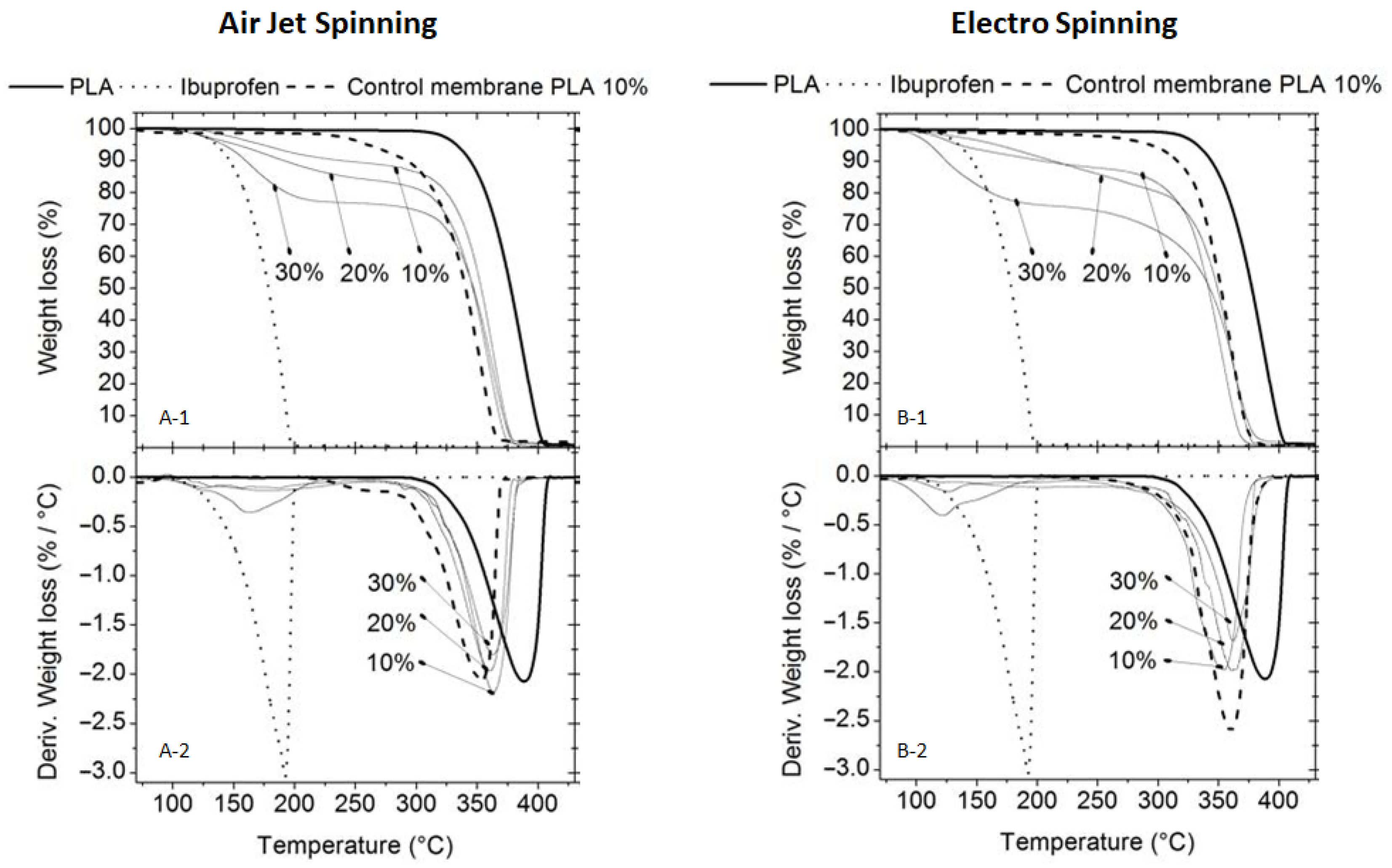
3.2.2. Thermogravimetric Analysis (TGA)
3.2.3. Transform Infrared Spectroscopy (FTIR)
3.2.4. Ultraviolet-Visible Spectroscopy (UV-VIS)
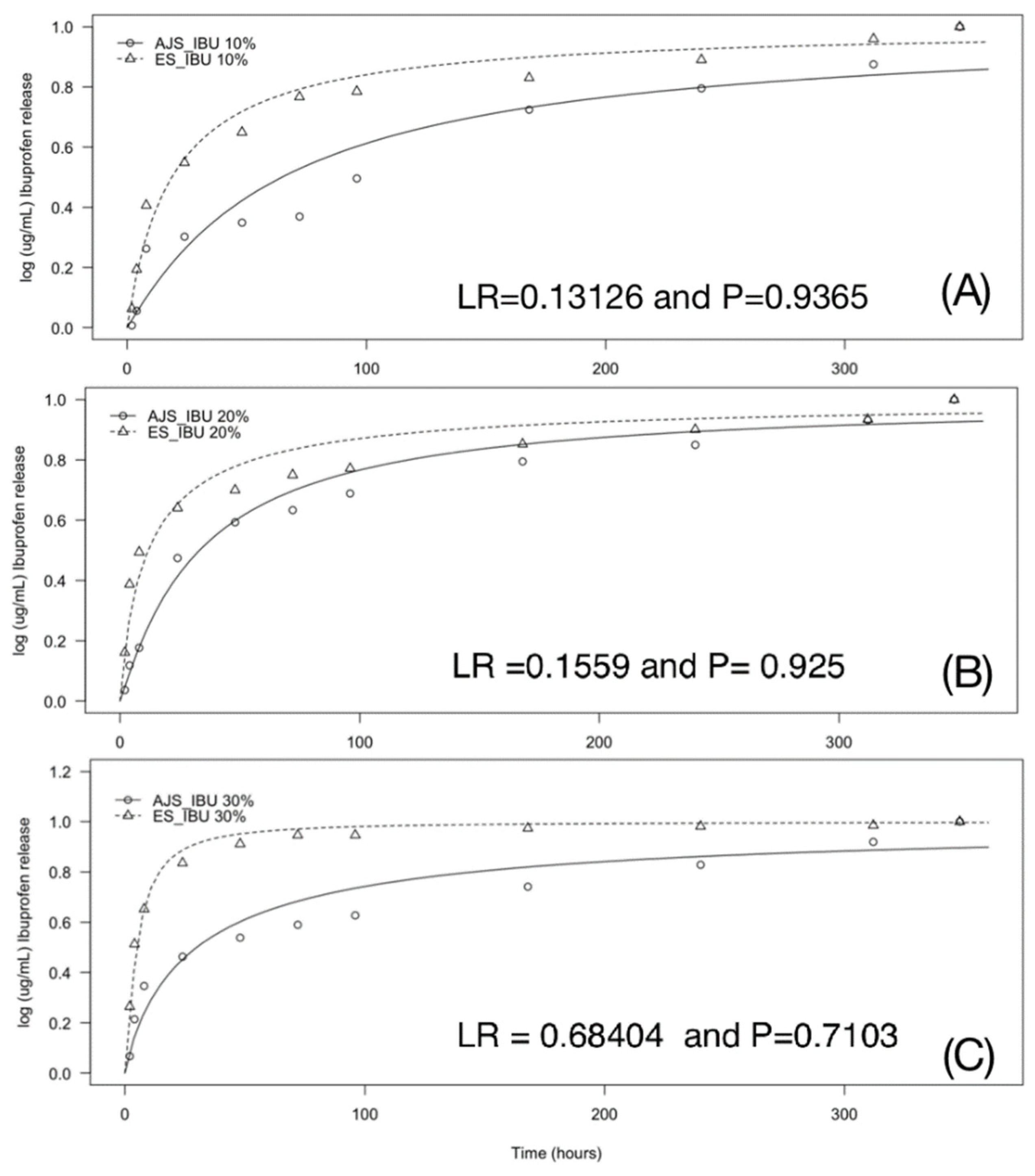
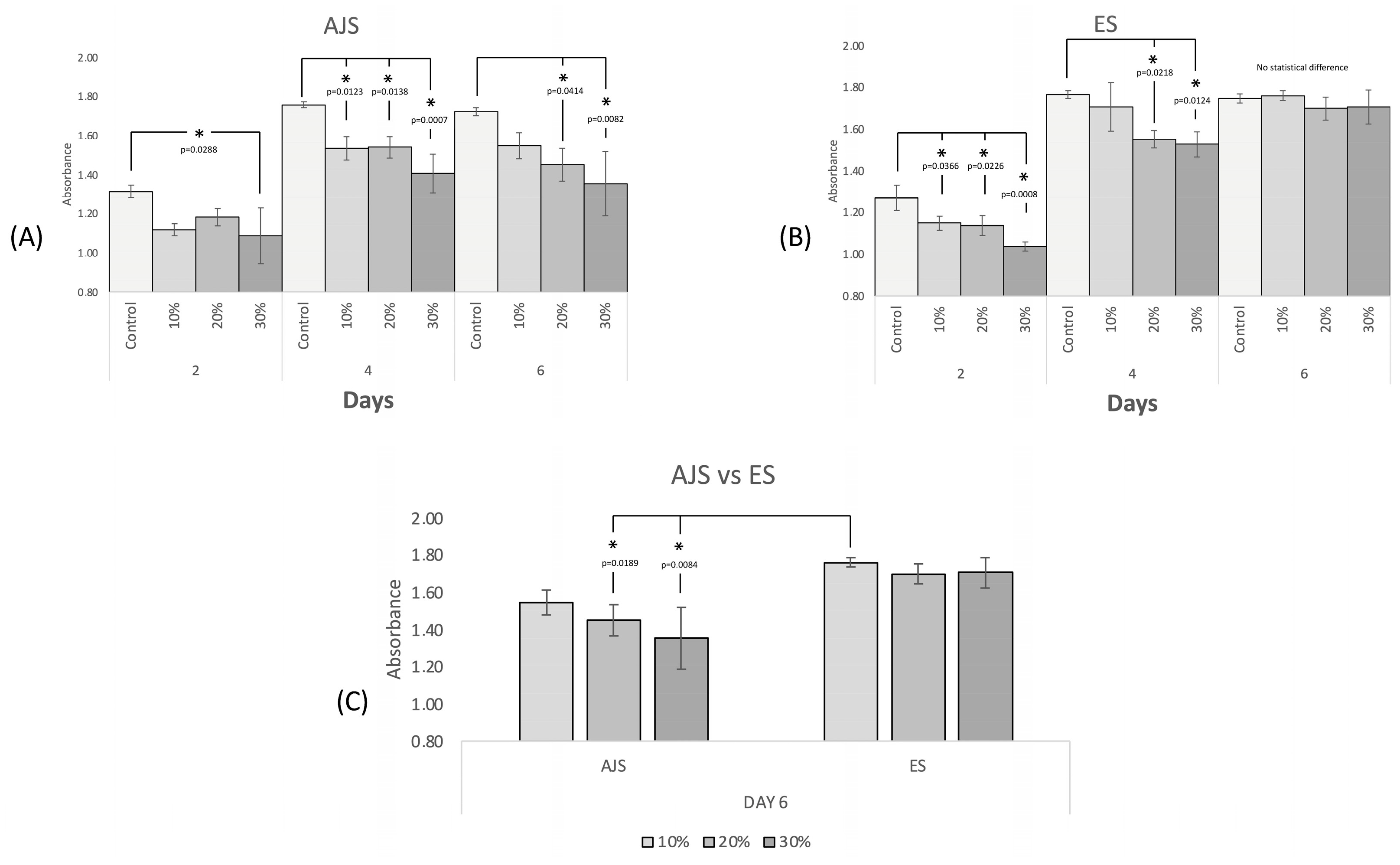
3.3. In Vitro Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chou, P.Y.; Lee, D.; Weng, C.C.; Wu, R.C.; Liao, C.T.; Liu, S.J. Bone morphogenetic protein-, antimicrobial agent-, and analgesic-incorporated nanofibrous scaffolds for the therapy of alveolar clefts. Pharmaceutics 2022, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell-scaffold interactions in tissue engineering for oral and craniofacial reconstruction. Bioact. Mater. 2023, 23, 16–44. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.C.; Chavarria, D.; Ortiz, M.; Guarino, V.; Alvarez, M.A. 3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications. Bioengineering 2022, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Munguia, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef] [PubMed]
- Cantón, I.; Mckean, R.; Charnley, M.; Blackwood, K.A.; Fiorica, C.; Ryan, A.J.; MacNeil, S. Development of an Ibuprofen-releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration. Biotechnol. Bioeng. 2010, 105, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 21. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. Biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Inamuddin, A.M., Mohammad, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–37. [Google Scholar]
- Zhang, J.; Wehrle, E.; Rubert, M.; Muller, R. 3D bioprinting of human tissues: Biofabrication, bioinks, and bioreactors. Int. J. Mol. Sci. 2021, 22, 3971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Kang, S.; Sun, D.; Liu, Y.; Wang, X.; Lu, L. Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds. Front. Bioeng. Biotechnol. 2024, 12, 1345163. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, J.; Dang, X.; Yuan, Y.; Wang, Y.; He, X.; Bai, R.; Ye, X.Y.; Xie, T. Design, synthesis and biological evaluation of novel histone deacetylase (HDAC) inhibitors derived from β-elemene scaffold. J. Enzyme Inhib. Med. Chem. 2023, 38, 2195991. [Google Scholar] [CrossRef]
- Che, D.; Feng, Y.; Wei, C.; Zhou, X.; Zhang, J.; Shi, Y.; Wang, L. Research in tissue engineering scaffold materials for alveolar bone repair. Crit. Rev. Biomed. Eng. 2021, 49, 29–52. [Google Scholar] [CrossRef]
- Turk, S.; Altinsoy, I.; Celebi, G.; Ipek, M.; Ozacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.N.; Thomas, L.; Bugueno, I.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a novel electrospun polycaprolactone scaffold functionalized with ibuprofen for periodontal regeneration: An in vitro and In vivo study. Materials 2018, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Albanés-Ojeda, E.A.; Calderón-Olvera, R.M.; García-Hipólito, M.; Chavarría-Bolaños, D.; Vega-Baudrit, R.; Álvarez-Pérez, M.A.; Alvarez-Fregoso, O. Physical and chemical characterization of PLA nanofibres and PLA/ZrO2 mesoporous composites synthesized by air-jet spinning. Indian J. Fibre Text. Res. 2020, 45, 57–64. [Google Scholar]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Vazquez-Vazquez, F.C.; Chanes-Cuevas, O.A.; Masuoka, D.; Alatorre, J.A.; Chavarria-Bolaños, D.; Vega-Baudrit, J.R.; Serrano-Bello, J.; Álvarez-Pérez, M.A. Biocompatibility of developing 3D-printed tubular scaffold coated with nanofibers for bone applications. J. Nanomater. 2019, 2019, 6105818. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.S.; Karbasi, S. A new path in bone tissue engineering: Polymer-based 3D-printed magnetic scaffolds (a comprehensive review of in vitro and in vivo studies). J. Biomater. Sci. Polym. 2024, 23, 1–21, Online ahead of print. [Google Scholar] [CrossRef]
- Polak, M.; Karbowniczek, J.E.; Stachewicz, U. Strategies in Electrospun Polymer and Hybrid Scaffolds for Enhanced Cell Integration and Vascularization for Bone Tissue Engineering and Organoids. Rev. Nanomed. Nanobiotechnol. 2024, 16, e2022. [Google Scholar] [CrossRef]
- Venkata Prathyusha, E.; Gomte, S.S.; Ahmed, H.; Prabakaran, A.; Agrawal, M.; Chella, N.; Alexander, A. Nanostructured polymer composites for bone and tissue regeneration. Int. J. Biol. Macromol. 2025, 284 Pt 1, 137834. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Sanz-Fraile, H.; Ulldemolins, A.; Tejo-Otero, A.; Domínguez-Fernández, A.; Almendros, I.; Otero, J. Characterization of 3D printed metal-PLA composite scaffolds for biomedical applications. Polymers 2022, 14, 2754. [Google Scholar] [CrossRef] [PubMed]
- Carriles, J.; Nguewa, P.; González-Gaitano, G. Advances in Biomedical applications of Solution Blow Spinning. Int. J. Mol. Sci. 2023, 24, 14757. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Khaliq, J.; Combrinck, M.; Hassanin, A.H.; Shehata, N.; Elnabawy, E.; Shyha, I. Solution blow spinning of polyvinylidene fluoride based fibers for energy harvesting applications: A review. Polymers 2020, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Kramar, A.; Luxbacher, T.; González-Benito, J. Solution blow co-spinning of cellulose acetate with poly(ethylene oxide). Structure, morphology, and properties of nanofibers. Carbohydr. Polym. 2023, 320, 121225. [Google Scholar] [CrossRef] [PubMed]
- Molfino, H.; Gonzales, M.; Alcalde-Yañez, A.; Valverde-Morón, V.; Villanueva-Salvatierra, D. Electrospinning: Advances and applications in the field of biomedicine. Rev. Fac. Med. Hum. 2020, 20, 706–713. [Google Scholar] [CrossRef]
- Madruga, L.Y.C.; Kipper, M.J. Expanding the Repertoire of Electrospinning: New and Emerging Biopolymers, Techniques, and Applications. Adv. Healthc. Mater. 2022, 11, 2101979. [Google Scholar] [CrossRef] [PubMed]
- Sabzekar, M.; Pourafshari, M.; Khayet, M.; García-Payo, C.; Mortazavi, S.M.; Golmohammadi, M. Development of Novel Electrospun Fibers Based on Cyclic Olefin Polymer. Nanomaterials 2023, 13, 2412. [Google Scholar] [CrossRef] [PubMed]
- Korat, P.S.; Kapupara, P.P. Local infiltration of the surgical wound with levobupivacaine, ibuprofen, and epinephrine in postoperative pain: An experimental study. Biomed. Pharmacother. 2017, 96, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; AbuRezeq, A.; Tarawneh, O. Development of Hydrogels, Oleogels and Bigels as Local Drug Delivery Systems for Periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Saha, S.; Murphy, S.V.; Gracz, H.; Pourdeyhimi, B.; Atala, A.; Loboa, E.G. Ibuprofen loaded PLA nanofibrous scaffolds increase proliferation of human skin cells in vitro and promote healing of full thickness incision wounds in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 327–339. [Google Scholar] [CrossRef]
- Romsing, J.; Moiniche, S.; Ostergaard, D.; Dahl, J.B. Local infiltration with NSAIDs for postoperative analgesia: Evidence for a peripheral analgesic action. Acta Anaesthesiol. Scand. 2000, 44, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Joshi, S.J.; Prasad, R.; Jampilek, J. Biobased Nanotechnology for Green Applications, 1st ed.; Springer Nature: Cham, Switzerland, 2021; pp. 289–307. [Google Scholar]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Ibuprofen-Loaded CTS/nHA/nBG Scaffolds for the Applications of Hard Tissue Engineering. Iran Biomed. J. 2019, 23, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hersh, E.V.; Moore, P.A.; Grosser, T.; Polomano, R.C.; Farrar, J.T.; Saraghi, M.; Juska, S.A.; Mitchell, C.H.; Theken, K.N. Nonsteroidal anti-inflammatory drugs and opioids in postsurgical dental pain. J. Dent. Res. 2020, 99, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Sharma, K.; Kumar, A.; Kaur, A.; Singh, T.G. Therapeutic implications of cyclooxygenase (COX) inhibitors in ischemic injury. Inflamm. Res. 2022, 71, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Keb, C.A.F. Mechanism of NSAIDs and derived drugs for pain and inflammation control. Use of anti-inflammatories in odontology. Rev. ADM 2022, 79, 38–47. [Google Scholar] [CrossRef]
- Radi, Z.A.; Khan, K.N. Cardio-renal safety of non-steroidal anti-inflammatory drugs. J. Toxicol. Sci. 2019, 44, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef] [PubMed]
- Grzybowska, K.; Grzybowski, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Woyna-Orlewicz, K.; Szafraniec-Szczƒôsny, J.; Antosik-Rogóż, A.; Jachowicz, R.; Kowalska-Szojda, K.; Lodowski, P.; et al. Molecular dynamics and physical stability of ibuprofen in binary mixtures with an acetylated derivative of maltose. Mol. Pharm. 2020, 17, 3087–3105. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.H. Determination of Ibuprofen in Aqueaus Solutions and Pharmacetical Preparations by UV-VIS Spectrophotometric. Al-Nahrain J. Sci. 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Blanca-López, N.; Soriano, V.; Garcia, E.M.; Canto, G.; Blanca, M. NSAID-induced reactions: Classification, prevalence, impact, and management strategies. J. Asthma Allergy 2019, 12, 217–233. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, S.; Kim, I.W. Effects of humidity and surfaces on the melt crystallization of ibuprofen. Int. J. Mol. Sci. 2012, 13, 10296–10304. [Google Scholar] [CrossRef]
- Koperwas, K.; Tu, W.; Affouard, F.; Adrjanowicz, K.; Kaskosz, F.; Paluch, M. Pressure dependence of the crystallization rate for the S-enantiomer and a racemic mixture of ibuprofen. Cryst. Growth Des. 2021, 21, 7075–7086. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M-membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Uysal, İ.; Eratilla, V.; Topbaş, C.; Ergül, İ.; Çelik, Y. Comparison of local and systemic ibuprofen for relief of postoperative pain in symptomatic teeth with apical periodontitis. Med. Sci. Monit. 2022, 28, e937339-1. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.G.; Crawford, G.M.; Young, D.; Corson, S.; Brown, C. Comparison of diclofenac gel, ibuprofen gel, and ibuprofen gel with levomenthol for the topical treatment of pain associated with musculoskeletal injuries. J. Int. Med. Res. 2019, 47, 4454–4468. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zou, L.; Xu, Y.; Wang, Y. Ibuprofen-loaded poly(lactic acid) electrospun mats: The morphology, physicochemical performance, and in vitro drug release behavior. Macromol. Mater. Eng. 2020, 305, 2000457. [Google Scholar] [CrossRef]
- Serrano-Garcia, W.; Bonadies, I.; Thomas, S.W.; Guarino, V. New Insights to Design Electrospun Fibers with Tunable Electrical Conductive-Semiconductive Properties. Sensors 2023, 23, 1606. [Google Scholar] [CrossRef]
- Salama, A.; Tolba, E.; Saleh, A.K.; Cruz-Maya, I.; Alvarez-Perez, M.A.; Guarino, V. Biomineralization of Polyelectrolyte-Functionalized Electrospun Fibers: Optimization and In Vitro Validation for Bone Applications. Biomimetics 2024, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Chavarría, D.; Vega, J.; Cerda, B.; Pozos, A.; Montero, M. Translation of Tissue Engineering Aproach from Clinics. In Current Advances in Oral and Craniofacial Tissue Engineering, 1st ed.; Guarino, V., Ålvarez, M., Eds.; Taylor & Francis Group: Abingdon, UK, 2021; Chapter 2. [Google Scholar]
- Belmessaoud, N.B.; Bouslah, N.; Haddadine, N. Clay/(PEG-CMC) biocomposites as a novel delivery system for ibuprofen. J. Polym. Sci. Eng. 2020, 40, 350–359. [Google Scholar] [CrossRef]
- Livecchi, L.; McAuley, W.J.; Kerai-Varsani, L. The use of optical differential scanning calorimetry to investigate ibuprofen miscibility in polymeric films for topical drug delivery. Eur. J. Pharm. Biopharm. 2021, 169, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.W.; Paek, S.M. Recent Advances in the Synthesis of Ibuprofen and Naproxen. Molecules 2021, 26, 4792. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Solarz, D.; Witko, T.; Karcz, R.; Malagurski, I.; Ponjavic, M.; Levic, S.; Nesic, A.; Guzik, M.; Savicg, S.; Nikodinovic-Runic, J. Biological and physiochemical studies of electrospun polylactid/polyhydroxyoctanoate PLA/P(3HO) scaffolds for tissue engineering applications. RSC Adv. 2023, 13, 24112–24128. [Google Scholar] [CrossRef] [PubMed]
- Granados-Hernández, M.V.; Serrano-Bello, J.; Montesinos, J.J.; Alvarez-Gayosso, C.; Medina-Velázquez, L.A.; Alvarez Fregoso, O.; Alvarez-Perez, M.A. In vitro and in vivo biological characterization of poly(lactic acid) fiber scaffolds synthesized by air jet spinning. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Laffite, F.M. Diseño de Membranas de Ácido Poliláctico para Liberación Controlada de Tramadol; Ciudad Universitaria Rodrigo Facio: San Pedro, Costa Rica, 2023; p. 24. [Google Scholar]
- Suarez-Franco, J.L.; Vázquez-Vázquez, F.C.; Pozos-Guillen, A.; Montesinos, J.J.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. Influence of diameter of fiber membrane scaffolds on the biocompatibility of hPDL mesenchymal stromal cells. Dent. Mater. J. 2018, 37, 465–473. [Google Scholar] [CrossRef] [PubMed]
- González, I. Cristalización, Caracterización Estructural y Estudio de las Interacciones del Cocristal Ibuprofeno-Nicotinamida; Universidad de Oviedo: Oviedo, Spain, 2022; p. 13. [Google Scholar]
- Gómez-Pachón, E.; Graziano, V.; Montiel, R.; Cardenas-Aguazaco, W.; Ochica, A. Effects of heat treatment and the method of collection on the crystal structure of the poly lactic acid electrospinned nanofibers. Rev. Chil. Ing. 2019, 27, 586–599. [Google Scholar] [CrossRef]
- Posada, E. Validación de la Metodología de Contenido Químico de Ibuprofeno en Tabletas por Calorimetría Diferencial de Barrido; Universidad de El Salvador: San Salvador, El Salvador, 2014; pp. 158–162. [Google Scholar]
- Jiménez-Minota, J. Evaluación de la Cinética de Liberación de un Fármaco Modelo con Clasificación Biofarmacéutica Clase ii, Desde Matrices Comprimidas Compuestas por Materiales Poliméricos Aniónicos; Universidad ICESI: Cali, Colombia, 2017. [Google Scholar]
- Bonillo-Martinez, A. Desarrollo de Comprimidos de Liberaci√≥n Controlada con una Poliesteramida Derivada de L-Alanina, PADAS; Universidad de Granada: Granada, Spain, 2017; pp. 130–133. [Google Scholar]
- Rabelo, L.H.; Munhoz, R.A.; Marini, J.; Maestrelli, S.C. Development and Characterization of PLA Composites with High Contents of a Brazilian Refractory Clay and Improved Fire Performance. Mater. Res. 2022, 25, e20210444. [Google Scholar] [CrossRef]
- Namazi, Z.; Jafarzadeh-Kashi, T.S.; Erfan, M.; Najafi, F.; Bakhtiari, L.; Ghodsi, S.R.; Farhadnejad, H. Synthesis and Characterization of Ibuprofen-mesoporous Hydroxyapatite Nanohybrid as a Sustained Drug Delivery System. Iran J. Pharm. Res. 2019, 18, 1196–1211. [Google Scholar] [CrossRef]
- Khaskheli, A.R.; Sirajuddin, S.T.; Mahesar, S.A.; Kandhro, A.A.; Kalwar, N.H.; Mallah, M.A. Estimation of ibuprofen in urine and tablet formulations by transmission Fourier Transform Infrared spectroscopy by partial least square. Spectrochim Acta A Mol. Biomol. Spectrosc. 2013, 102, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Ramukutty, S.; Ramachandran, E. Growth, spectral and thermal studies of ibuprofen crystals. Cryst. Res. Technol. 2011, 47, 31–38. [Google Scholar] [CrossRef]
- Shoaib, Q.; Abbas, N.; Irfan, M.; Hussain, A.; Sohail, M.; Hussain, S.; Latif, S.; Bukhari, N. Development and Evaluation of Scaffold Based Nanosponge Formulation for Controlled Drug Delivery of Naproxen and Ibuprofen. Trop. J. Pharm. Res. 2018, 17, 1465. [Google Scholar] [CrossRef]
- Lemraski, E.G.; Alibeigi, S.; Abbasi, Z. Ibuprofen @silver loaded on poly(vinyl alcohol)/chitosan co-polymer scaffold as a novel drug delivery system. Mater. Today Commun. 2022, 33, 104311. [Google Scholar] [CrossRef]
- Rodríguez, M. Preparación de Biocatalizadores Basados en Lipasa Inmovilizada. Aplicación en la Resolución de Ibuprofeno Racémico; Universidad Politécnica de Madrid: Madrid, Spain, 2018. [Google Scholar]
- Perneger, T.V. How to use likelihood ratios to interpret evidence from randomized trials. J. Clin. Epidemiol. 2021, 136, 235–242. [Google Scholar] [CrossRef]
- Elston, D.M. Likelihood ratios. J. Am. Acad. Dermatol. 2022, 86, 1229. [Google Scholar] [CrossRef]
- Riggin, C.N.; Qu, F.; Kim, D.H.; Huegel, J.; Steinberg, D.R.; Kuntz, A.F.; Bernstein, J. Electrospun PLGA Nanofiber Scaffolds Release Ibuprofen Faster and Degrade Slower After In Vivo Implantation. Ann. Biomed. Eng. 2017, 45, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.F.; Pegorin, G.S.; Miranda, M.C.R.; Cachaneski-Lopes, J.P.; Silva, W.M.; Borges, F.A.; Guerra, N.B.; Herculano, R.D.; Batagin-Neto, A. Ibuprofen-loaded biocompatible latex membrane for drug release: Characterization and molecular modeling. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211005383. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Illescas-Montes, R.; Ramos-Torrecillas, J.; García-Martínez, O.; Ruiz, C. Repercussion of nonsteroidal anti-inflammatory drugs on the gene expression of human osteoblasts. PeerJ 2018, 6, e5415. [Google Scholar] [CrossRef]
- Al-Waeli, H.; Reboucas, A.P.; Mansour, A.; Morris, M.; Tamimi, F.; Nicolás, B. Non-steroidal anti-inflammatory drugs and bone healing in animal models-a systematic review and meta-analysis. Syst. Rev. 2021, 10, 201. [Google Scholar] [CrossRef]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Manzano-Moreno, F.J.; Ruiz, C. Repercussions of NSAIDS drugs on bone tissue: The osteoblast. Life Sci. 2015, 123, 72–77. [Google Scholar] [CrossRef]
- Limami, Y.; Leger, D.Y.; Liagre, B.; Pécout, N.; Viana, M. Ibuprofen-loaded calcium phosphate granules: A new bone substitute for local relieving symptoms of osteoarthritis. Eur. J. Pharm. Sci. 2021, 1, 158. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Magnusson, P.; LeQuang, J.; Gharibo, C.; Varrassi, G. The pharmacological management of dental pain. Expert Opin. Pharmacother. 2020, 21, 591–601. [Google Scholar] [CrossRef]
- García-Ramírez, P.E.; González-Rodríguez, S.G.; Soto-Acevedo, F.; Brito-Zurita, O.R.; Cabello-Molina, R.; López-Morales, C.M. Dolor postoperatorio: Frecuencia y caracterización del manejo. Rev. Colomb. Anestesiol. 2018, 46, 98–102. [Google Scholar] [CrossRef]
- Abella-Palacios, P.; Arias-Amézquita, F.; Barsella, A.R.; Hernández-Porras, B.C.; Narazaki, D.; Salomón-Molina, P.A. Inadequate management of acute postoperative pain: Prevalence, prevention, and consequences. Review of the situation in Latin America. Rev. Mex. Anestesiol. 2021, 44, 190–199. [Google Scholar] [CrossRef]
- Al-Bayati, O.; Font, K.; Soldatos, N.; Carlson, E.; Parsons, J.; Powell, C.A. Evaluation of the need to prescribe opioid medication to control postsurgical pain of different periodontal/oral surgeries. J. Periodontol. 2021, 92, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Forget, P. Opioid-free anaesthesia. Why and how? A contextual analysis. Anaesth Crit. Care Pain Med. 2019, 38, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Malamed, S.F. Manejo del dolor después de un traumatismo dental y procedimientos quirúrgicos. Dent. Traumatol. 2023, 39, 295–303. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena-Porras, E.; Contreras-Aleman, A.; Guevara-Hidalgo, M.F.; Avendaño Soto, E.; Batista Menezes, D.; Alvarez-Perez, M.A.; Chavarría-Bolaños, D. Comparison of Two Synthesis Methods for 3D PLA-Ibuprofen Nanofibrillar Scaffolds. Pharmaceutics 2025, 17, 106. https://doi.org/10.3390/pharmaceutics17010106
Mena-Porras E, Contreras-Aleman A, Guevara-Hidalgo MF, Avendaño Soto E, Batista Menezes D, Alvarez-Perez MA, Chavarría-Bolaños D. Comparison of Two Synthesis Methods for 3D PLA-Ibuprofen Nanofibrillar Scaffolds. Pharmaceutics. 2025; 17(1):106. https://doi.org/10.3390/pharmaceutics17010106
Chicago/Turabian StyleMena-Porras, Esteban, Annaby Contreras-Aleman, María Francinie Guevara-Hidalgo, Esteban Avendaño Soto, Diego Batista Menezes, Marco Antonio Alvarez-Perez, and Daniel Chavarría-Bolaños. 2025. "Comparison of Two Synthesis Methods for 3D PLA-Ibuprofen Nanofibrillar Scaffolds" Pharmaceutics 17, no. 1: 106. https://doi.org/10.3390/pharmaceutics17010106
APA StyleMena-Porras, E., Contreras-Aleman, A., Guevara-Hidalgo, M. F., Avendaño Soto, E., Batista Menezes, D., Alvarez-Perez, M. A., & Chavarría-Bolaños, D. (2025). Comparison of Two Synthesis Methods for 3D PLA-Ibuprofen Nanofibrillar Scaffolds. Pharmaceutics, 17(1), 106. https://doi.org/10.3390/pharmaceutics17010106





