[99mTc]Technetium and Rhenium Dithiocarbazate Complexes: Chemical Synthesis and Biological Assessment
Abstract
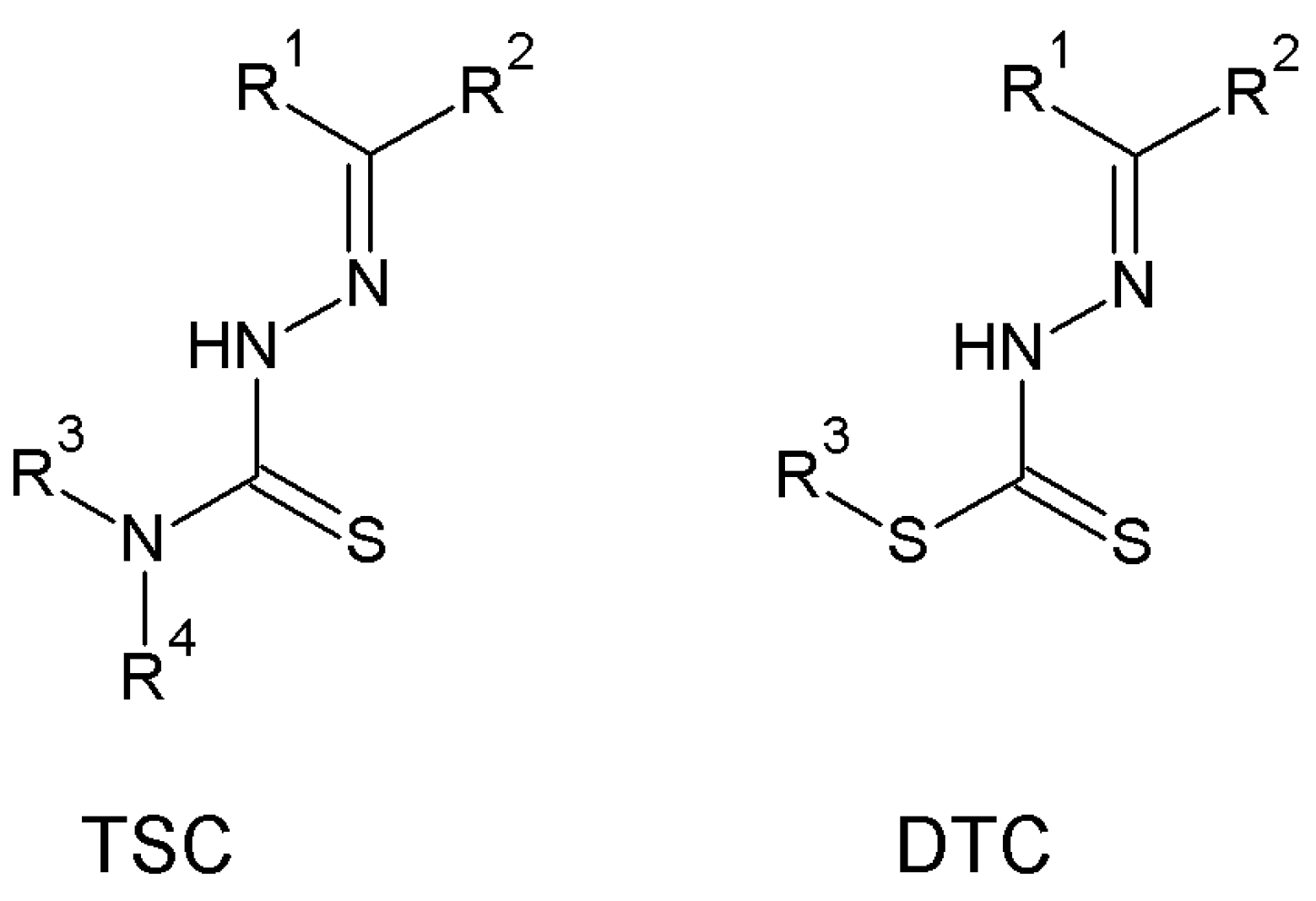
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Animal Handling
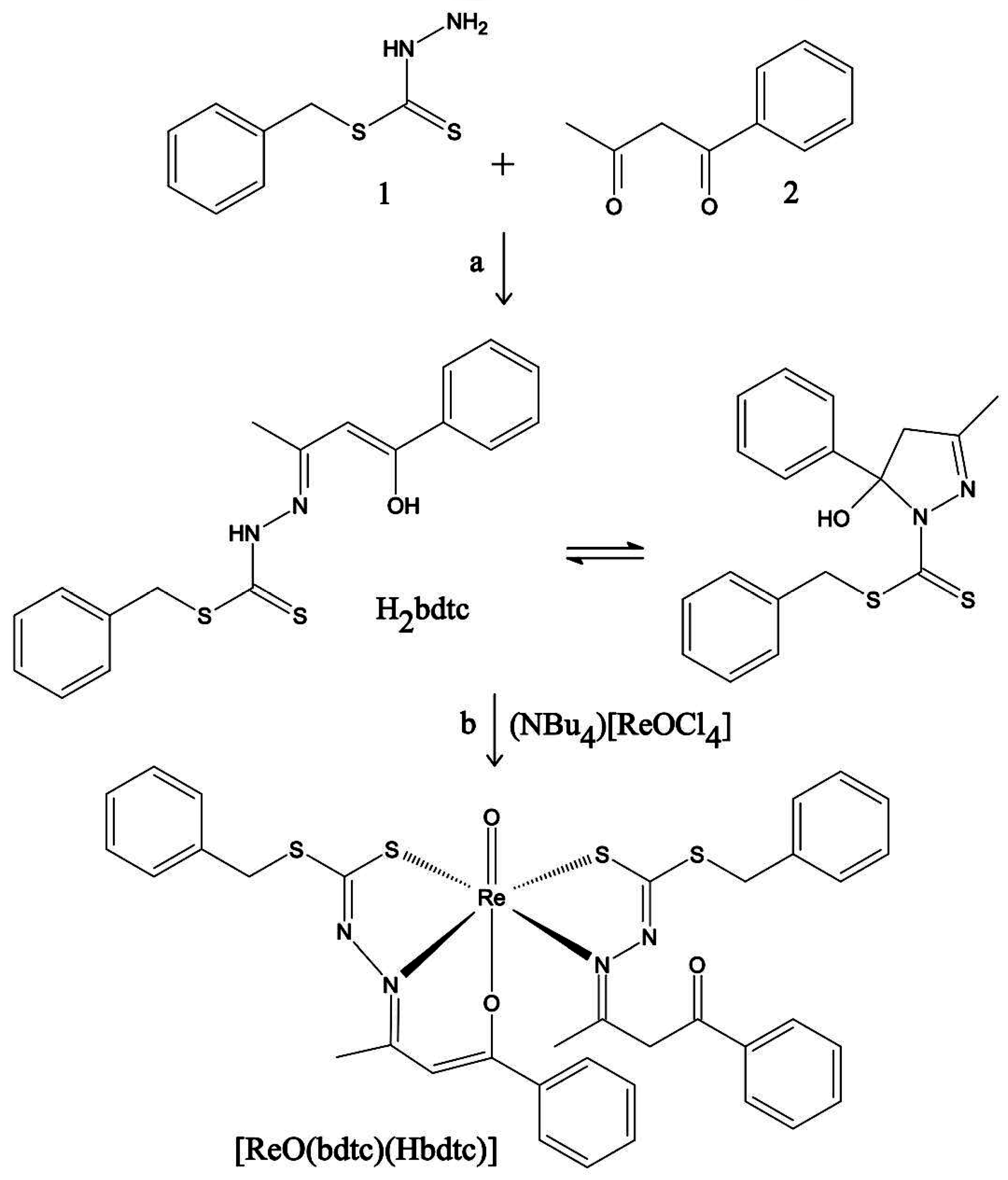
2.4. Synthesis of [ReO(bdtc)(Hbdtc)] and Chemical Characterization
2.5. Radiolabeling and Quality Control
2.5.1. Preparation of [[99mTc]TcO(bdtc)(Hbdtc)]
2.5.2. Radiochemical Purity
2.5.3. Partition Coefficient
2.5.4. Complex Stability
2.6. Biological Assay
2.6.1. In Vitro Cell Uptake
2.6.2. Ex Vivo Biodistribution
2.6.3. In Vivo Biodistribution
3. Results
3.1. Synthesis of [ReO(bdtc)(Hbdtc)]
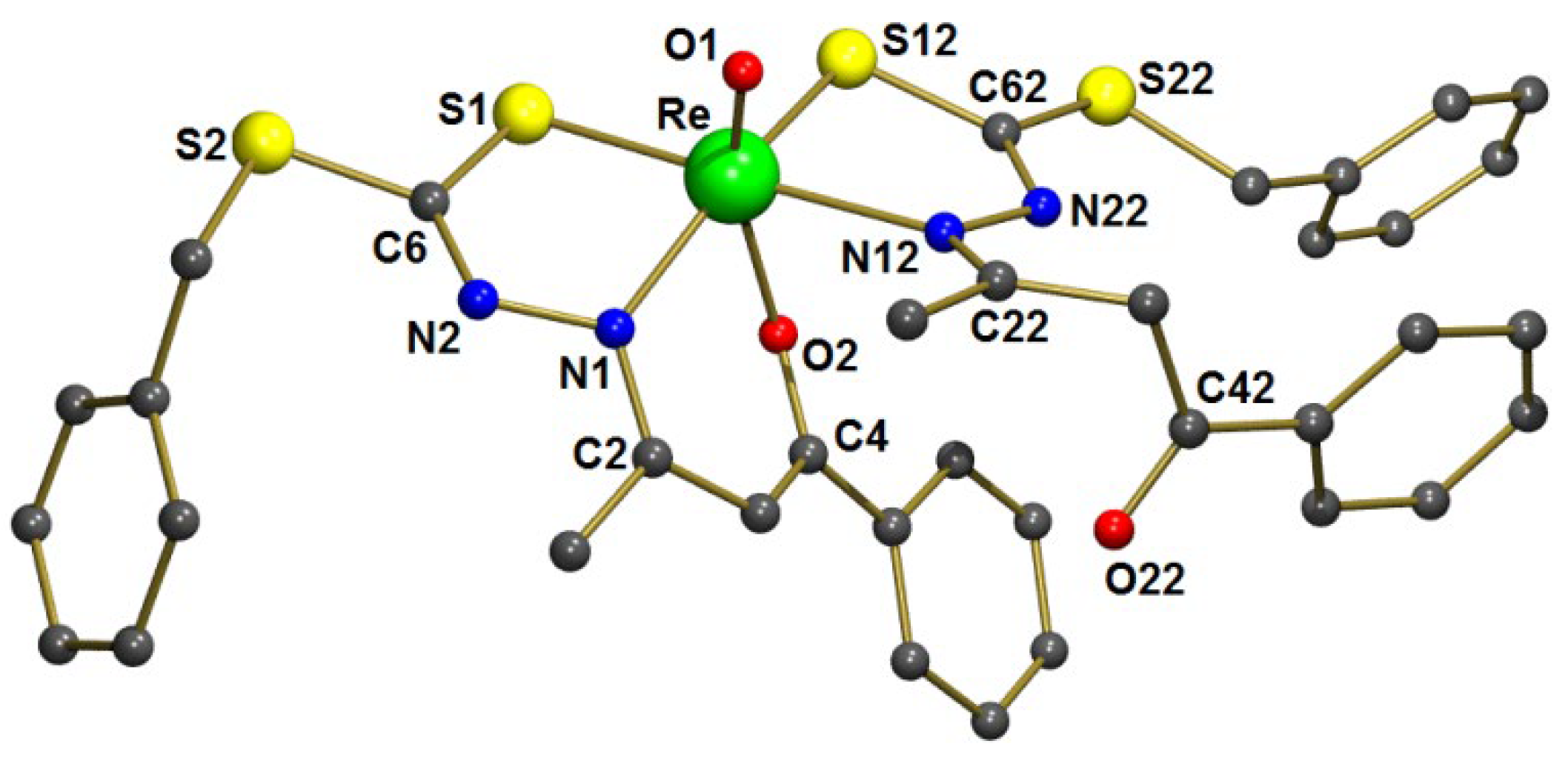
3.2. Complex Characteristics
3.2.1. Radiochemical Purity and Partition Coefficient
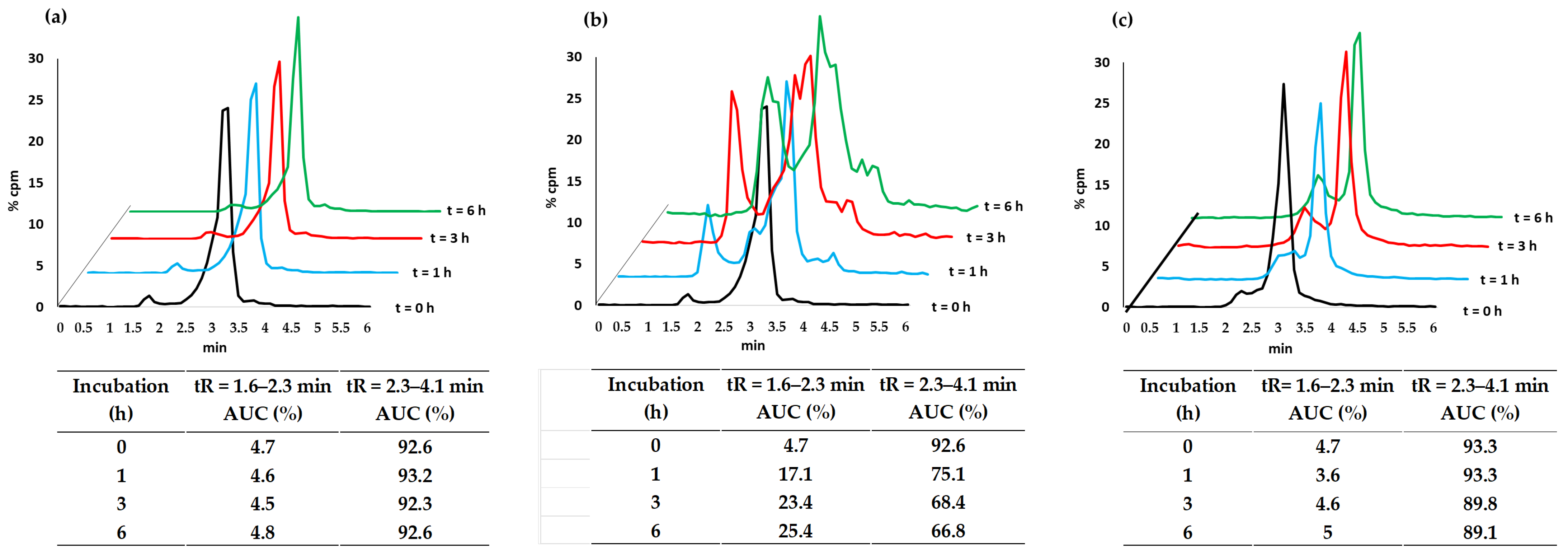
3.2.2. Complex Stability
3.3. Biological Characteristics
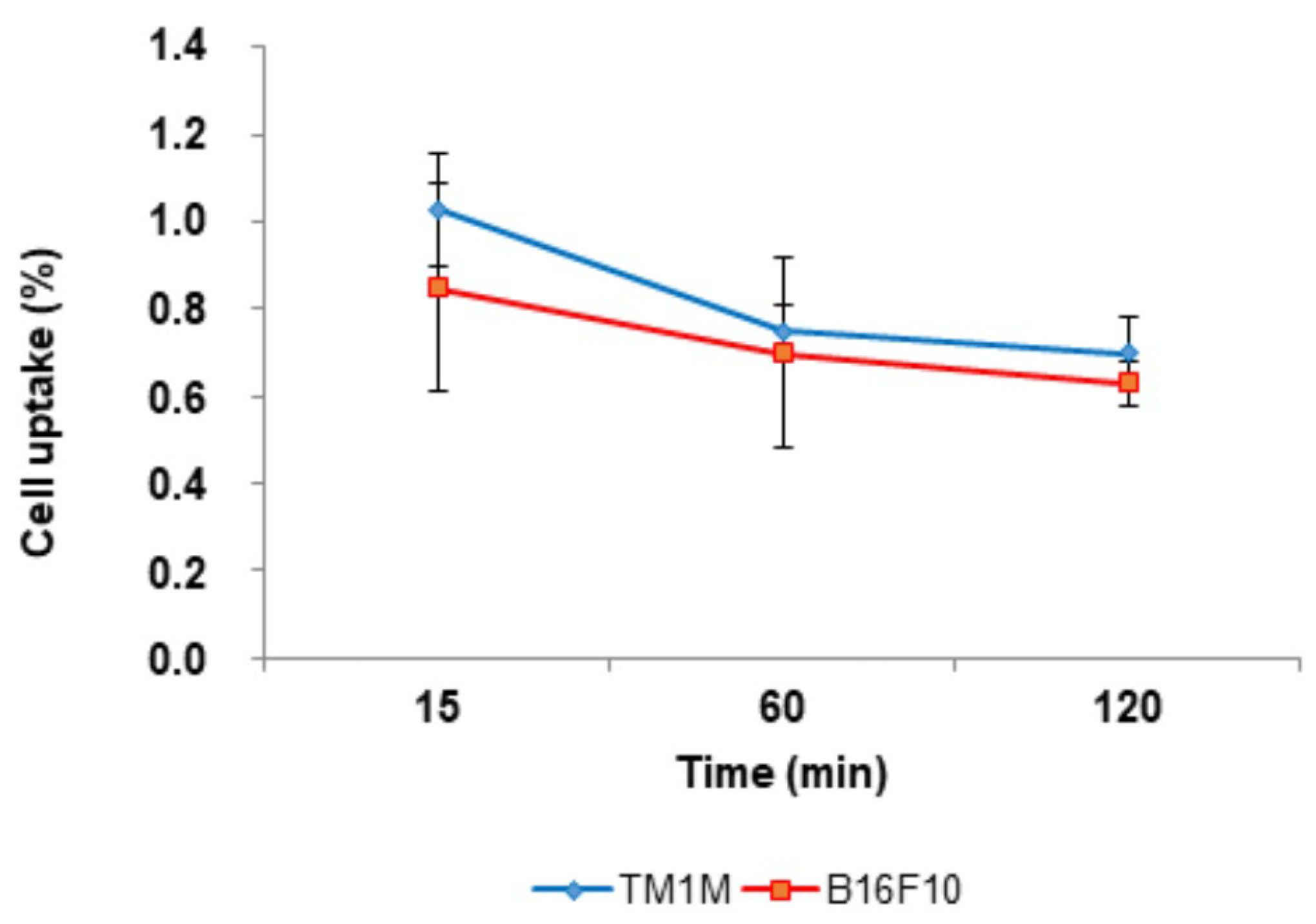
3.3.1. In Vitro Cellular Uptake
3.3.2. Ex Vivo Biodistribution
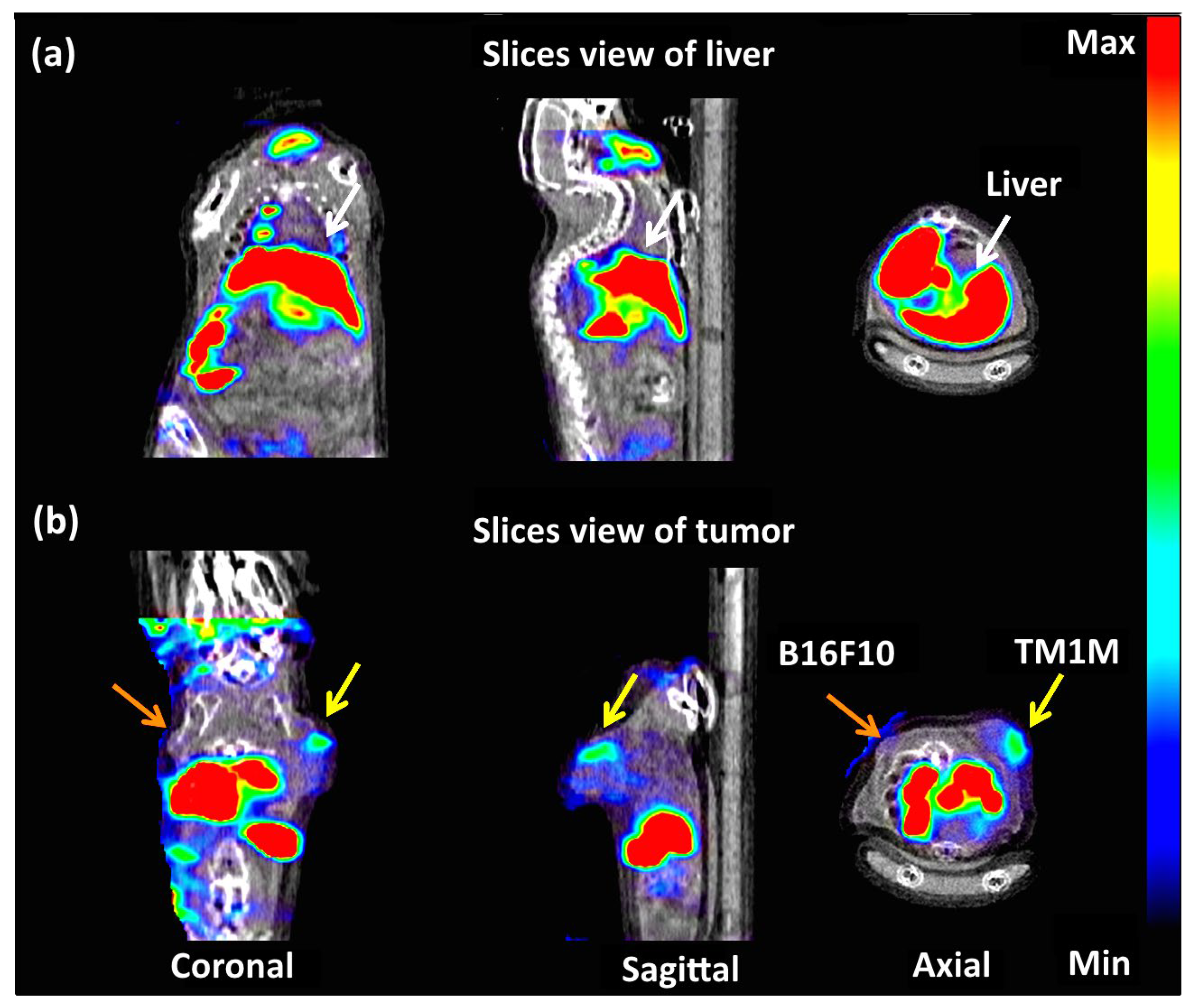
3.3.3. In Vivo Biodistribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- da Maia, P.I.S.; de Fernandes, A.G.A.; Silva, J.J.N.; Andricopulo, A.D.; Lemos, S.S.; Lang, E.S.; Abram, U.; Deflon, V.M. Dithiocarbazate complexes with the [M(PPh3)]2+ (M═Pd or Pt) moiety: Synthesis, characterization and anti-Tripanosoma cruzi activity. J. Inorg. Biochem. 2010, 104, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Bin Break, M.K.; Fung, T.Y.; Koh, M.Z.; Ho, W.Y.; Tahir, M.I.M.; Elfar, O.A.; Syed, R.U.; Khojali, W.M.A.; Alluhaibi, T.M.; Huwaimel, B.; et al. Synthesis, Crystal Structure, Antibacterial and In Vitro Anticancer Activity of Novel Macroacyclic Schiff Bases and Their Cu (II) Complexes Derived from S-Methyl and S-Benzyl Dithiocarbazate. Molecules 2023, 28, 5009. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Ahmed, T.; Hossain, M.S.; Chaki, B.M.; Abdou, A.; Zahan, K.E. Synthesis, Spectroscopic Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis of Ni(II), Zn(II), Sb(III), and U(VI) Metal Complexes Derived from a Nitrogen-Sulfur Schiff Base. Russ. J. Gen. Chem. 2023, 93, 389–397. [Google Scholar] [CrossRef]
- Malik, M.A.; Lone, S.A.; Wani, M.Y.; Talukdar, M.I.A.; Dar, O.A.; Ahmad, A.; Hashmi, A.A. S-benzyldithiocarbazate imine coordinated metal complexes kill Candida albicans by causing cellular apoptosis and necrosis. Bioorg. Chem. 2020, 98, 103771. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Mir, I.A.; Ain, Q.U.; Qadir, T.; Malik, A.Q.; Jan, S.; Shahverdi, S.; Nabi, S.A. A review of semicarbazone-derived metal complexes for application in biomedicine and related fields. J. Mol. Struct. 2024, 1295, 136216. [Google Scholar] [CrossRef]
- Siqueira, L.R.P.; de Moraes Gomes, P.A.T.; Ferreira, L.P.L.; de Melo Rêgo, M.J.B.; Leite, A.C.L. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019, 170, 237–260. [Google Scholar] [CrossRef]
- Bai, X.-G.; Zheng, Y.; Qi, J. Advances in thiosemicarbazone metal complexes as anti-lung cancer agents. Front. Pharmacol. 2022, 13, 1018951. [Google Scholar] [CrossRef]
- Jiang, X.; Fielding, L.A.; Davis, H.; Carroll, W.; Lisic, E.C.; Deweese, J.E. Inhibition of Topoisomerases by Metal Thiosemicarbazone Complexes. Int. J. Mol. Sci. 2023, 24, 12010. [Google Scholar] [CrossRef]
- Gou, Y.; Jia, X.; Hou, L.X.; Deng, J.G.; Huang, G.J.; Jiang, H.W.; Yang, F.J. Dithiocarbazate−FeIII, −CoIII, −NiII, and −ZnII Complexes: Design, Synthesis, Structure, and Anticancer Evaluation. J. Med. Chem. 2022, 65, 6677–6689. [Google Scholar] [CrossRef]
- Deng, J.G.; Peng, C.; Hou, L.X.; Wu, Y.R.; Liu, W.; Fang, G.H.; Jiang, H.W.; Qin, S.F.; Yang, F.; Huang, G.J.; et al. Dithiocarbazate-Copper Complex Loaded Thermosensitive Hydrogel for Lung Cancer Therapy via Tumor in situ Sustained-Release. Inorg. Chem. Front. 2022, 9, 6190–6201. [Google Scholar] [CrossRef]
- Sahu, G.; Patra, S.A.; Lima, S.; Das, S.; Görls, H.; Plass, W.; Dinda, R. Ruthenium(II)-Dithiocarbazates as Anticancer Agents: Synthesis, Solution Behavior, and Mitochondria-Targeted Apoptotic Cell Death. Chem. Eur. J. 2023, 29, e202202694. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.A.; Mariotti, E.; Pavlovic, D.; Shaw, K.P.; Eykyn, T.R.; Blower, P.J.; Southworth, R. 64Cu-CTS: A Promising Radiopharmaceutical for the Identification of Low-Grade Cardiac Hypoxia by PET. J. Nucl. Med. 2015, 56, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rodriguez, M.A.; Rios, C.; Carrasco-Hernandez, J.; Manrique-Arias, J.C.; Martinez-Hernandez, R.; García-Pérez, F.O.; Jalilian, A.R.; Martinez-Rodriguez, E.; Romero-Piña, M.E.; Diaz-Ruiz, A. Biodistribution and radiation dosimetry of [64Cu]copper dichloride: First-in-human study in healthy volunteers. EJNMMI Res. 2017, 7, 98. [Google Scholar] [CrossRef]
- Pell, V.R.; Baark, F.; Mota, F.; Clark, J.E.; Southworth, R. PET Imaging of Cardiac Hypoxia: Hitting Hypoxia Where It Hurts. Curr. Cardiovasc. Imaging Rep. 2018, 11, 7. [Google Scholar] [CrossRef]
- Hueting, R.; Kersemans, V.; Cornelissen, B.; Tredwell, M.; Hussien, K.; Christlieb, M.; Gee, A.D.; Passchier, J.; Smart, S.C.; Dilworth, J.R.; et al. A Comparison of the Behavior of 64Cu-Acetate and 64Cu-ATSM In Vitro and In Vivo. J. Nucl. Med. 2014, 55, 128–134. [Google Scholar] [CrossRef]
- Pérès, E.A.; Toutain, J.; Paty, L.P.; Divoux, D.; Ibazizène, M.; Guillouet, S.; Barré, L.; Vidal, A.; Cherel, M.; Bourgeois, M.; et al. 64Cu-ATSM/64Cu-Cl2 and their relationship to hypoxia in glioblastoma: A preclinical study. EJNMMI Res. 2019, 9, 114. [Google Scholar] [CrossRef]
- Kurihara, H.; Narita, Y.; Ito, K.; Sato, H.; Miyakita, Y.; Takahashi, M.; Yanagisawa, S.; Okita, N.T.; Sadachi, R.; Tateishi, K.; et al. Phase I clinical trial of 64Cu-ATSM for malignant brain tumors. J. Clin. Oncol. 2024, 42, 2052. [Google Scholar] [CrossRef]
- Almasi, T.; Jabbari, K.; Gholipour, N.; Kheirabadi, A.M.; Beiki, D.; Shahrokhi, P.; Akhlaghi, M. Synthesis, characterization, and in vitro and in vivo 68Ga radiolabeling of thiosemicarbazone Schiff base derived from dialdehyde dextran as a promising pool imaging agente. Int. J. Biol. Macromol. 2019, 125, 915–921. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Yousefnia, H.; Shafaii, K.; Novinrouz, A.; Rajamand, A.A. Preparation and Biodistribution Studies of a Radiogallium-Acetylacetonate Bis(Thiosemicarbazone) Complex in Tumor-Bearing Rodents. Iran. J. Pharm. Res. 2012, 11, 523–531. [Google Scholar]
- Prado, V.S.; Leitao, R.C.F.; Silva, F.; Gano, L.; Santos, I.C.; Marques, F.L.N.; Paulo, A.; Deflon, V.M. Gallium and indium complexes with new hexadentate bis(semicarbazone) and bis(thiosemicarbazone) chelators. Dalton Trans. 2021, 50, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Fuks, L.; Gniazdowska, E.; Kozminski, P.; Mieczkowski, J. Technetium(I) tricarbonyl complexed with the N-heterocyclic aldehyde thiosemicarbazones: Potential precursors of the radiopharmaceuticals. J. Radioanal. Nucl. Chem. 2012, 292, 255–259. [Google Scholar] [CrossRef]
- Halder, K.K.; Nayak, D.K.; Baishya, R.; Sarkar, B.R.; Sinha, S.; Ganguly, S.; Debnath, M.C. 99mTc-labeling of ciprofloxacin and nitrofuryl thiosemicarbazone using fac-[99mTc(CO)3(H2O)3] core: Evaluation of their efficacy as infection imaging agents. Metallomics 2011, 3, 1041–1048. [Google Scholar] [CrossRef]
- Pino-Cuevas, A.; Raposinho, P.D.; Fernandes, C.; Paulo, A.; Abram, U.; Carballo, R.; Vazquez-L’opez, E.M. Thiosemicarbazonate complexes with affinity for amyloid-β fibers: Synthesis, characterization and biological studies. Future Med. Chem. 2019, 11, 2527–2544. [Google Scholar] [CrossRef]
- Argibay-Otero, S.; Núñez, A.; Muñoz, L.; Carballo, R.; Fernandes, C.; Paulo, A.; Vázquez-López, E.M. Study of Mixed Re(I)/99mTc(I) Thiosemicarbazonate Complexes: Estrogen Receptor Affinity and Stability in Biological Media. Eur. J. Inorg. Chem. 2024, 27, e202400131. [Google Scholar] [CrossRef]
- Argibay-Otero, S.; Gano, L.; Fernandes, C.; Paulo, A.; Carballo, R.; Vázquez-López, E.M. Chemical and biological studies of Re(I)/Tc(I) thiosemicarbazonate complexes relevant for the design of radiopharmaceuticals. J. Inorg. Biochem. 2020, 203, 110917. [Google Scholar] [CrossRef]
- Kelderman, C.A.; Davey, P.R.; Ma, M.T.; de Veer, M.; Salimova, E.; Donnelly, P.S.; Paterson, B.M. Hexadentate technetium-99m bis(thiosemicarbazonato) complexes: Synthesis, characterisation and biodistribution. Dalton Trans. 2022, 51, 14064. [Google Scholar] [CrossRef]
- Liu, G. Direct interaction between S-methyl dithiocarbazate and pertechnetate-99m. Nucl. Med. Biol. 1997, 24, 119–122. [Google Scholar] [CrossRef]
- Carta, D.; Jentschel, C.; Thieme, S.; Salvarese, N.; Morellato, N.; Refosco, F.; Ruzza, P.; Bergmann, R.; Pietzsch, H.-J.; Bolzati, C. Assessment of the best N3− donors in preparation of [M(N)(PNP)]-based (M = 99mTc-; 188Re) target-specific radiopharmaceuticals: Comparison among succinic dihydrazide (SDH), N-methyl-S-methyl dithiocarbazate (HDTCZ) and PEGylated N-methyl-S-methyl dithiocarbazate (HO2C-PEG600-DTCZ). Nucl. Med. Biol. 2014, 41, 570–581. [Google Scholar] [CrossRef]
- de Fernandes, A.G.A.; Viana, R.B.; Moreno-Fuquen, R.; Gatto, C.C.; Lang, E.S.; Modolo, M.Z.; Silva, A.K.; Lemos, S.S.; Hagenbach, A.; Abram, U.; et al. Oxorhenium(V) complexes with a benzyldithiocarbazate ligand: Synthesis, crystal structure, spectroscopic and DFT analyses. J. Mol. Struct. 2022, 1250, 131875. [Google Scholar] [CrossRef]
- Oba-Shinjo, S.M.; Correay, M.; Riccay, T.I.; Molognoni, F.; Pinhal, M.A.; Neves, I.A.; Marie, S.K.; Sampaio, L.O.; Nader, H.B.; Chammas, R.; et al. Melanocyte Transformation Associated with Substrate Adhesion Impediment. Neoplasia 2006, 8, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, P.A.; Hermann, W.A.; Artus, G.; Abram, U.; Kaden, T.A. Metal carbonyl syntheses XXII. Low pressure carbonylation of [MOCl4]− and [MO4]−: The technetium(I) and rhenium(I) complexes [NEt4]2[MCl3(CO)3]. J. Organomet. Chem. 1995, 493, 119–127. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.M.; Pitombeira, M.S.; Souza, L.E.; Navarro Marques, F.L.; Buchpiguel, C.A.; Real, C.C.; Faria, D.P. 11C-PK11195 plasma metabolization has the same rate in multiple sclerosis patients and healthy controls: A cross-sectional study. Neural Regen. Res. 2021, 16, 2494–2498. [Google Scholar]
- Maurya, M.R.; Kumar, A.; Bhat, A.R.; Azam, A.; Bader, C.; Rehder, D. Dioxo- and Oxovanadium(V) Complexes of Thiohydrazone ONS Donor Ligands: Synthesis, Characterization, Reactivity, and Antiamoebic Activity. Inorg. Chem. 2006, 45, 1260–1269. [Google Scholar] [CrossRef]
- Salem, N.M.; El-Sayed, L.; Iskander, M.F. Metal complexes derived from hydrazoneoxime ligands: IV. Molecular and supramolecular structures of some nickel(II) complexes derived from diacetylmonoxime S-benzyldithiocarbazonate. Polyhedron 2008, 27, 3215–3226. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Heravi, M.M.; Takjoo, R.; Frank, W. Synthesis and Spectroscopic Characterization of the Benzaldehyde Schiff Baseof S-Allyldithiocarbazate and its Copper(II) Complex and the X-ray CrystalStructure of Bis[S-allyl-β-N-benzylidene)dithiocarbazato]Copper(II). Z. Anorg. Allg. Chem. 2008, 634, 972–976. [Google Scholar] [CrossRef]
- Roy, S.; Mandal, T.N.; Barik, A.K.; Gupta, S.; Butcher, R.J.; Nethaji, M.; Kar, S.K. Syntheses, characterization and X-ray crystal structures of Co(III) and Mn(II) complexes of pyrimidine derived Schiff base ligands. Polyhedron 2008, 27, 593–601. [Google Scholar] [CrossRef]
- Pavan, F.R.; Maia, P.I.S.; Leite, S.R.A.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q.F. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti—Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Kirsch, S.; Jankowsky, R.; Leibnitz, P.; Spies, H.; Johannsen, B. Crystal and solution structure of oxo rhenium(V) complexes with cysteine and cysteine methyl ester. JBIC J. Biol. Inorg. Chem. 1999, 4, 48–55. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Chen, Y.-C.; Li, D.-K.; Chuang, M.-H. Technetium-99m-Labeled Autologous Serum Albumin: A Personal-Exclusive Source of Serum Component. J. Biomed. Biotechnol. 2011, 413802. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, N.A.; Park, J.Y.; Kim, K.; Kim, Y.J.; Shin, J.H.; Lee, Y.-S.; Im, H.-J.; Jeong, J.M.; Khalid, M.; Cheon, G.J.; et al. Development of 99m Tc-Labeled Human Serum Albumin with Prolonged Circulation by Chelate-then-Click Approach: A Potential Blood Pool Imaging Agent. Mol. Pharm. 2019, 16, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.J.; Lewis, J.S.; Mullen, G.E.D.; Welch, M.J.; Blower, P.J. Copper bis(thiosemicarbazone) complex as hypoxia imaging agents: Structure-activity relationships. J. Biol. Inorg. Chem. 2002, 7, 249–259. [Google Scholar] [CrossRef]
- Mirković, M.; Janković, D.; Vranješ-Durić, S.; Radović, M.; Stanković, D.; Mijin, D.; Nikolić, N. Novel tetradentate diamine dioxime ligands: Synthesis, characterization and in vivo behavior of their 99mTc-complexes. Appl. Organometal. Chem. 2012, 26, 347–355. [Google Scholar] [CrossRef]
- Faheem, A.R.; Bokhari, T.H.; Roohi, S.; Mushtaq, A.; Sohaib, M. 99mTc-Daunorubicin a potential brain imaging and theranostic agent: Synthesis, quality control, characterization, biodistribution and scintigraphy. Nucl. Med. Biol. 2013, 40, 148–152. [Google Scholar] [CrossRef]
- Belhaj-Tayeb, H.; Briane, D.; Vergote, J.; Kothan, S.; Léger, G.; Bendada, S.-E.; Tofighi, M.; Tamgac, F.; Cao, A.; Moretti, J.-L. In vitro and in vivo study of 99mTc-MIBI encapsulated in PEG-liposomes: A promising radiotracer for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 502–509. [Google Scholar] [CrossRef]
- Noronha, O.P.D.; Sewatkar, A.B.; Ganatra, R.D. Factors Affecting the Preparation of 99mTc-Sulfur Colloid. Eur. J. Nucl. Med. 1978, 3, 47–54. [Google Scholar] [CrossRef]
- Massey, M.D.; Steven, J.S. Residual Spleen Found on Denatured Red Blood Cell Scan Following Negative Colloid Scans. J. Nucl. Med. 1991, 32, 2286–2287. [Google Scholar]
- Barber, T.W.; Dixon, A.; Smith, M.; Yap, K.S.K.; Kalff, V. Ga-68 octreotate PET/CT and Tc-99m heat-denatured red blood cell SPECT/CT imaging of an intrapancreatic accessory spleen. J. Med. Imaging Radiat. Oncol. 2016, 60, 227–229. [Google Scholar] [CrossRef]
- Tanaka, T.; Furukawa, T.; Fujieda, S.; Kasamatsu, S.; Yonekura, Y.; Fujibayashi, Y. Double-tracer autoradiography with Cu-ATSM/FDG and immunohistochemical interpretation in four different mouse implanted tumor models. Nucl. Med. Biol. 2006, 33, 743–750. [Google Scholar] [CrossRef]
- Tzanopoulou, S.; Sagnou, M.; Paravatou-Petsotas, M.; Gourni, E.; Loudos, G.; Xanthopoulos, S.; Lafkas, D.; Kiaris, H.; Varvarigou, A.; Pirmettis, I.C.; et al. Evaluation of Re and 99mTc Complexes of 2-(4′-Aminophenyl)benzothiazole as Potential Breast Cancer Radiopharmaceuticals. J. Med. Chem. 2010, 53, 4633–4641. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Hosseinimehr, S.J.; Khalaj, A.; Noaparast, Z.; Abedi, S.M.; Sabzevari, O. 99mTc-radiolabeled GE11-modified peptide for ovarian tumor targeting. DARU J. Pharm. Sci. 2017, 25, 13. [Google Scholar] [CrossRef]


| Parameter Evaluation | Values |
|---|---|
| Empirical formula | C36H33N4O3ReS4 |
| Formula weight | 884.10 |
| Temperature (K) | 200 |
| Wavelength (Å) | 0.71073 |
| Crystal system | Triclinic |
| Space group | P ī |
| Unit cell dimensions | |
| a (Å) | 8.172(2) |
| b (Å) | 15.097(4) |
| c (Å) | 16.168(4) |
| α (°) | 110.804(2) |
| β (°) | 98.755(2) |
| γ (°) | 95.717(2) |
| Volume | 1.81737(8) |
| Z | 2 |
| D calc (g/cm3) | 1.616 |
| Absorption coefficient (mm−1) | 3.614 |
| F(000) | 880 |
| 2θ range for data collection (o) | 2.65 to 24.77 |
| Index ranges | −9 ≤ h ≤ 9, −17 ≤ k ≤ 16, 0 ≤ l ≤ 19 |
| Reflections collected | 6120 |
| Independent reflections | 6120 [R(int) = 0.0000] |
| Completeness to 2θ = 50.00 | 98.3% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 6120/0/433 |
| Goodness-of-fit on F2 | 1.116 |
| R indices (all data) | R1 = 0.0573, wR2 = 0.0798 |
| Final R indices [I > 2σ(I)] | R1 = 0.0387, wR2 = 0.0748 |
| Largest difference peak and hole (e Å−3) | 0.688 and −0.635 |
| Distances (pm) | Angles (°) | ||
|---|---|---|---|
| Re-O(1) | 168.04(4) | O(1)-Re-O(2) | 156.9(2) |
| Re-O(2) | 206.1(4) | O(1)-Re-N(1) | 98.6(2) |
| Re-N(1) | 207.1(5) | O(1)-Re-N(12) | 82.9(2) |
| Re-N(12) | 225.5(5) | O(1)-Re-S(1) | 98.1(2) |
| Re-S(1) | 230.8(2) | O(1)-Re-S(12) | 102.2(2) |
| Re-S(12) | 233.5(2) | O(2)-Re-N(1) | 78.5(2) |
| C(4)-O(2) | 130.4(7) | O(2)-Re-N(12) | 75.7(2) |
| C(42)-O(22) | 123.3(8) | O(2)-Re-S(1) | 103.7(1) |
| C(2)-N(1) | 133.0(7) | O(2)-Re-S(12) | 82.9(1) |
| C(6)-N(2) | 128.2(8) | N(1)-Re-N(12) | 103.5(2) |
| C(6)-S(1) | 175.9(7) | N(1)-Re-S(1) | 79.2(1) |
| N(1)-Re-S(12) | 159.1(1) | ||
| N(12)-Re-S(1) | 177.0(1) | ||
| N(12)-Re-S(12) | 81.0(1) | ||
| S(1)-Re-S(12) | 96.05(6) | ||
| Experimental Parameter | Rf Values | Radioactive Concentration (%) at Time | |||
|---|---|---|---|---|---|
| 0 | 1 h | 3 h | 6 h | ||
| PBS 7.4 | 0–0.3 0.7–1 | 2.8 4.4 | 3.1 6 | 3 6.3 | 3.2 6.3 |
| L-His | 0–0.3 0.7–1 | 2.8 4.4 | 2.2 3.8 | 0.6 5.9 | 2.4 7.2 |
| L-Cys | 0–0.3 0.7–1 | 2.8 4.4 | 6.5 5.5 | 9 9.7 | 14.9 15.4 |
| Plasma | 0–0.3 0.7–1 | 2.8 4.4 | 17.7 2.4 | 38.6 3.0 | 74.5 5.2 |
| Samples | 15 min | 60 min | 120 min |
|---|---|---|---|
| Blood | 0.41 ± 0.16 | 0.17 ± 0.04 | 0.17 ± 0.04 |
| Brain | 0.02 ± 0.01 | 0.01 ± 0 | 0.02 ± 0.01 |
| Thyorid | 0.51 ± 0.14 | 1.08 ± 0.46 | 0.45 ± 0.24 |
| Heart | 0.21 ± 0.06 | 0.2 ± 0.09 | 0.06 ± 0.04 |
| Lungs | 0.54 ± 0.19 | 0.82 ± 0.52 | 0.48 ± 0.24 |
| Kidneys | 0.6 ± 0.04 | 1.3 ± 1.04 | 2.15 ± 0.25 |
| Stomach | 0.7 ± 0.73 | 1.42 ± 0.94 | 1.42 ± 0.01 |
| Spleen | 63.11 ± 2.93 | 60.11 ± 6.74 | 55.46 ± 2.9 |
| Liver | 26.47 ± 4.67 | 29.07 ± 4.95 | 31.91 ± 2.73 |
| Large intestine | 0.07 ± 0 | 0.1 ± 0.3 | 0.24 ± 0.02 |
| Small intestine | 0.14 ± 0.04 | 0.32 ± 0.08 | 0.74 ± 0.1 |
| Bone | 2.58 ± 3.9 | 0.71 ± 0.36 | 3.26 ± 0.16 |
| Muscle | 0.05 ± 0.01 | 0.12 ± 0.15 | 0.04 ± 0.01 |
| B16-F10 | 0.34 ± 0.07 | 0.31 ± 0.09 | 0.25 ± 0.05 |
| TM1M | 0.26 ± 0.08 | 0.22 ± 0.05 | 0.20 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araujo Fernandes, A.G.; Lafratta, A.E.; Luz, C.P.; Levy, D.; de Paula Faria, D.; Buchpiguel, C.A.; Abram, U.; Deflon, V.M.; Navarro Marques, F.L. [99mTc]Technetium and Rhenium Dithiocarbazate Complexes: Chemical Synthesis and Biological Assessment. Pharmaceutics 2025, 17, 100. https://doi.org/10.3390/pharmaceutics17010100
de Araujo Fernandes AG, Lafratta AE, Luz CP, Levy D, de Paula Faria D, Buchpiguel CA, Abram U, Deflon VM, Navarro Marques FL. [99mTc]Technetium and Rhenium Dithiocarbazate Complexes: Chemical Synthesis and Biological Assessment. Pharmaceutics. 2025; 17(1):100. https://doi.org/10.3390/pharmaceutics17010100
Chicago/Turabian Stylede Araujo Fernandes, André Gustavo, Alyne Eloise Lafratta, Carolina Portela Luz, Debora Levy, Daniele de Paula Faria, Carlos Alberto Buchpiguel, Ulrich Abram, Victor Marcelo Deflon, and Fabio Luiz Navarro Marques. 2025. "[99mTc]Technetium and Rhenium Dithiocarbazate Complexes: Chemical Synthesis and Biological Assessment" Pharmaceutics 17, no. 1: 100. https://doi.org/10.3390/pharmaceutics17010100
APA Stylede Araujo Fernandes, A. G., Lafratta, A. E., Luz, C. P., Levy, D., de Paula Faria, D., Buchpiguel, C. A., Abram, U., Deflon, V. M., & Navarro Marques, F. L. (2025). [99mTc]Technetium and Rhenium Dithiocarbazate Complexes: Chemical Synthesis and Biological Assessment. Pharmaceutics, 17(1), 100. https://doi.org/10.3390/pharmaceutics17010100







