Aerosol of Enoximone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex, Biopharmaceutical Evidence for ARDS Applicability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Quantification of ENXM
2.3. Determination of the Stoichiometry of the Inclusion Complex (Job’s Plot)
2.4. Phase Solubility Study
2.5. Assessment of a Solution for Nebulization Based on ENXM and HPβCD
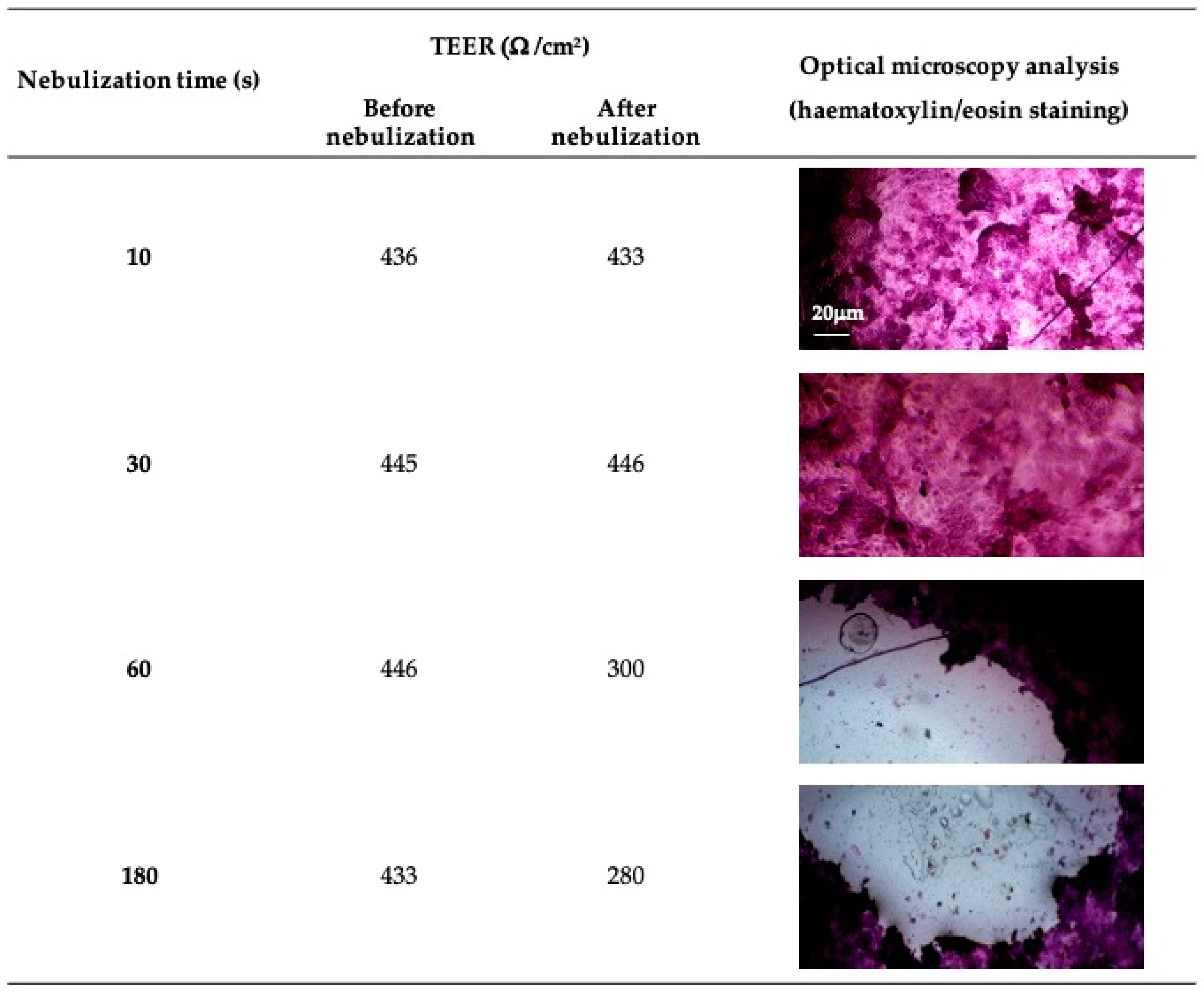
2.6. Aerodynamic Evaluation of the Nebulized Solution
2.7. Biological Investigations
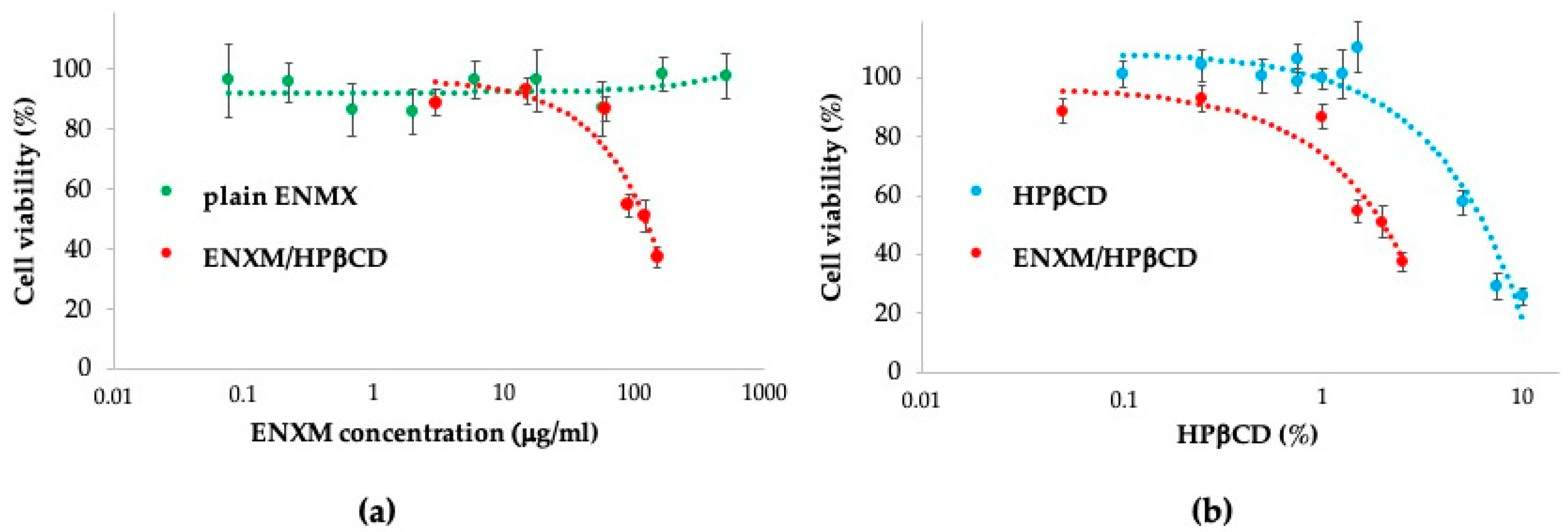
2.8. Cell Viability Studies by WST-1 Assay
- Plain ENXM: a stock solution of ENXM in DMSO (1 mg/mL) was prepared, from which dilutions were made in the RPMI-1640 medium to achieve ENXM concentrations in the range of 0.072–1000 μg/mL. All solutions contained 1% DMSO;
- HPβCD: HPβCD solutions in the RPMI-1640 medium, ranging from 0.01% to 10%;
- ENXM/HPβCD solution, with dilutions made in the RPMI-1640 medium, corresponding to 3–150 μg/mL of ENXM and 0.05–2.5% of cyclodextrin, respectively.
2.9. Direct Nebulization on Differentiated Air–Liquid Interface (ALI) Cell Model
2.10. Drug Effect: Augmentation of Intracellular cAMP Level and Protection from Induced Oxidative Stress
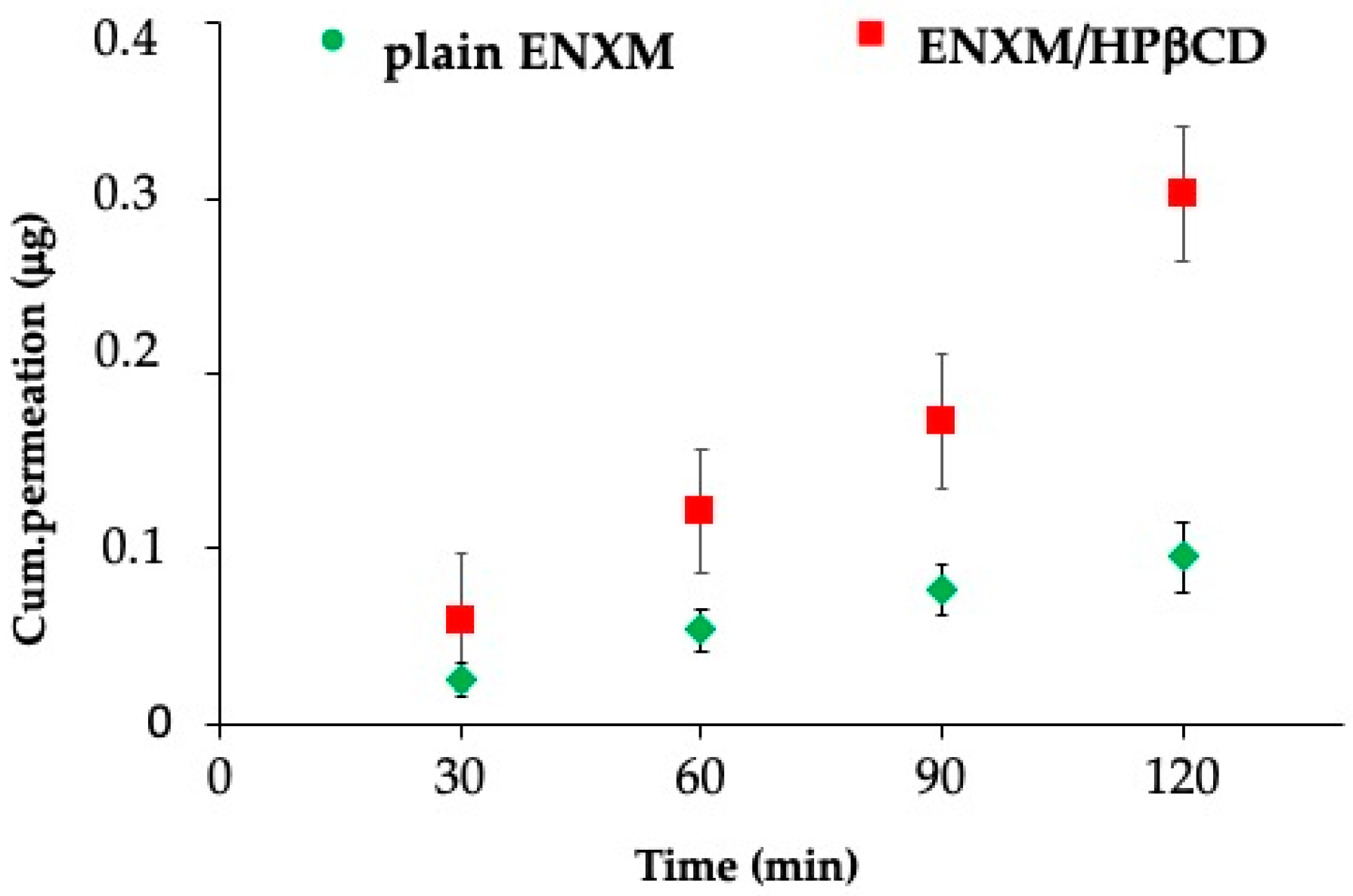
2.11. ENXM Permeation through the ALI Monolayer
2.12. Statistical Analysis
3. Results
3.1. Phase Solubility Study
3.2. Aerodynamic Evaluation of the Nebulized Solution
3.3. Cell Viability Evaluation
3.4. PDE-3 Activity Evaluation on ALI In Vitro Model and Permeation Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzo, A.N.; Aggarwal, N.R.; Thompson, B.T.; Schmidt, E.P. Advancing Precision Medicine for the Diagnosis and Treatment of Acute Respiratory Distress Syndrome. J. Clin. Med. 2023, 12, 1563. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care. Med. 2024, 209, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D. Challenges in ARDS Definition, Management, and Identification of Effective Personalized Therapies. J. Clin. Med. 2023, 12, 1381. [Google Scholar] [CrossRef]
- Vizzoni, L.; Migone, C.; Grassiri, B.; Zambito, Y.; Ferro, B.; Roncucci, P.; Mori, F.; Salvatore, A.; Ascione, E.; Crea, R.; et al. Biopharmaceutical Assessment of Mesh Aerosolised Plasminogen, a Step towards ARDS Treatment. Pharmaceutics 2023, 15, 1618. [Google Scholar] [CrossRef]
- Wang, C.; Taskinen, J.H.; Segersvärd, H.; Immonen, K.; Kosonen, R.; Tolva, J.M.; Mäyränpää, M.I.; Kovanen, P.T.; Olkkonen, V.M.; Sinisalo, J.; et al. Alterations of Cardiac Protein Kinases in Cyclic Nucleotide-Dependent Signaling Pathways in Human Ischemic Heart Failure. Front. Cardiovasc. Med. 2022, 9, 919355. [Google Scholar] [CrossRef]
- Calamera, G.; Moltzau, L.R.; Levy, F.O.; Andressen, K.W. Phosphodiesterases and Compartmentation of cAMP and cGMP Signaling in Regulation of Cardiac Contractility in Normal and Failing Hearts. Int. J. Mol. Sci. 2022, 23, 2145. [Google Scholar] [CrossRef]
- Movsesian, M.; Ahmad, F.; Hirsch, E. Functions of PDE3 Isoforms in Cardiac Muscle. J. Cardiovasc. Dev. Dis. 2018, 5, 10. [Google Scholar] [CrossRef]
- Zuo, H.; Cattani-Cavalieri, I.; Musheshe, N.; Nikolaev, V.O.; Schmidt, M. Phosphodiesterases as Therapeutic Targets for Respiratory Diseases. Pharmacol. Ther. 2019, 197, 225–242. [Google Scholar] [CrossRef]
- Shah, A.J.; Vorla, M.; Kalra, D.K. Molecular Pathways in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2022, 23, 10001. [Google Scholar] [CrossRef]
- Halpin, D. ABCD of the Phosphodiesterase Family: Interaction and Differential Activity in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2008, 3, 543–561. [Google Scholar] [CrossRef]
- Crilley, T.K.; Wanstall, J.C.; Bonnet, P. Vasorelaxant Effects of SCA40 (A Phosphodiesterase III Inhibitor) in Pulmonary Vascular Preparations in Rats. Clin. Exp. Pharmacol. Physiol. 1998, 25, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Joskova, M.; Mokry, J.; Franova, S. Respiratory Cilia as a Therapeutic Target of Phosphodiesterase Inhibitors. Front. Pharmacol. 2020, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Beute, J.; Ganesh, K.; Nastiti, H.; Hoogenboom, R.; Bos, V.; Folkerts, J.; Schreurs, M.W.J.; Hockman, S.; Hendriks, R.W.; KleinJan, A. PDE3 Inhibition Reduces Epithelial Mast Cell Numbers in Allergic Airway Inflammation and Attenuates Degranulation of Basophils and Mast Cells. Front. Pharmacol. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Kosutova, P.; Mikolka, P.; Mokra, D.; Calkovska, A. Anti-inflammatory Activity of Non-selective PDE Inhibitor Aminophylline on the Lung Tissue and Respiratory Parameters in Animal Model of ARDS. J. Inflamm. 2023, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Navarro, A.; Almudéver, P.; Lluch, J.; Morcillo, E.J.; Cortijo, J. Oxidative Stress-Induced Glucocorticoid Resistance is Prevented by Dual PDE3/PDE4 Inhibition in Human Alveolar Macrophages. Clin. Exp. Allergy 2011, 41, 535–546. [Google Scholar] [CrossRef]
- Beute, J. Use of Enoximone in the Treatment of Atopic Immune-Related Disorders, in Pharmaceutical Composition as Well As in Pharmaceutical Preparation; EP3131545A2; European Patent Office: Munich, Germany, 2017. [Google Scholar]
- Beute, J.; KleinJan, A. Oral Enoximone Allows the Reduction and Discontinuation of Inhaled Steroids and Beta2 Agonists in Asthmatic Children. Int. J. Pediatr. Adolesc. Med. 2022, 9, 147–151. [Google Scholar] [CrossRef]
- Beute, J.; Boermans, P.; Benraad, B.; Telman, J.; Diamant, Z.; KleinJan, A. PDE3-Inhibitor Enoximone Prevented Mechanical Ventilation in Patients with SARS-CoV-2 Pneumonia. Exp. Lung Res. 2021, 47, 1–12. [Google Scholar] [CrossRef]
- Beute, J.; Boermans, P.; KleinJan, A. Evaluation of Real-Life Investigational Use of Enoximone in Asthma, the Third Step in Drug Repurposing: A Preliminary Report. Can. Respir. J. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Ferro, B.; Cinelli, R.; Vegnuti, L.; Piras, A.M.; Roncucci, P. The Potential Role of Aerosolized Phosphodiesterase 3 Inhibitor Enoximone in the Management of Coronavirus Disease 2019 Hypoxemia: A Case Report. J Aerosol Med Pulm Drug Deliv. 2021, 34, 262–264. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and Derivatives in Drug Delivery: New Developments, Relevant Clinical Trials, and Advanced Products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Pilcer, G.; Amighi, K. Formulation Strategy and Use of Excipients in Pulmonary Drug Delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Pan, P.; Huang, Y.; Chen, T.; Yuan, T.; Ma, Y.; Han, G.; Li, J.; Jin, Y.; et al. Pulmonary Delivery of Resveratrol-β-Cyclodextrin Inclusion Complexes for the Prevention of Zinc Chloride Smoke-Induced Acute Lung Injury. Drug Deliv. 2022, 29, 1122–1131. [Google Scholar] [CrossRef]
- Alboni, S.; Secco, V.; Papotti, B.; Vilella, A.; Adorni, M.P.; Zimetti, F.; Schaeffer, L.; Tascedda, F.; Zoli, M.; Leblanc, P.; et al. Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells. Pathogens 2023, 12, 647. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Sun, Y.; Lu, G.; Wang, K.; Wang, Z.; Xing, J.; Gao, Y. Improvement of Pulmonary Absorption of Poorly Absorbable Macromolecules by Hydroxypropyl-β-Cyclodextrin Grafted Polyethylenimine (HP-β-CD-PEI) in Rats. Int. J. Pharm. 2015, 489, 294–303. [Google Scholar] [CrossRef]
- Tewes, F.; Brillault, J.; Couet, W.; Olivier, J.-C. Formulation of Rifampicin–Cyclodextrin Complexes for Lung Nebulization. J. Control. Release 2008, 129, 93–99. [Google Scholar] [CrossRef]
- Evrard, B.; Bertholet, P.; Gueders, M.; Flament, M.-P.; Piél, G.; Delattre, L.; Gayot, A.; Leterme, P.; Foidart, J.; Cataldo, D. Cyclodextrins as a Potential Carrier in Drug Nebulization. J. Control. Release 2004, 96, 403–410. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP). Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use, EMA/CHMP/495747/2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-and-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 9 October 2017).
- European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP). In Report Published in Support of the ‘Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use’ (EMA/CHMP/495747/2013). EMA/CHMP/333892/2013 Committee for Human Medicinal Products (CHMP). Available online: https://www.ema.europa.eu/en/documents/report/cyclodextrins-used-excipients-report-published-support-questions-and-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 9 October 2017).
- Albert, M.-A.; Rocks, N.; Ait Mahmoude, N.; Petit, L.; Maes, P.; Cataldo, D. Late Breaking Abstract—An innovative inhaled solution of budesonide-cyclodextrin complex is safe and prevents the methacholine-induced hyperreactivity in asthmatics better than a commercial budesonide suspension: A breezy story (BOREAS). In Proceedings of the ERS International Congress, Milan, Italy, 9–13 September 2023. [Google Scholar]
- Job, P. Formation and Stability of Inorganic Complexes in Solution. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
- Grassiri, B.; Knoll, P.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Bernkop-Schnürch, A. Thiolated Hydroxypropyl-β-cyclodextrin: A Potential Multifunctional Excipient for Ocular Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2612. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Preparations for Inhalation: Aerodynamic Assessment of Fine Particles; Council of Europe: Strasbourg, France, 2014; Volume E, pp. 276–286. [Google Scholar]
- Cidem, A.; Bradbury, P.; Traini, D.; Ong, H.X. Modifying and Integrating in vitro and ex vivo Respiratory Models for Inhalation Drug Screening. Front. Bioeng. Biotechnol. 2020, 8, 581995. [Google Scholar] [CrossRef]
- Stolfa, I.; Page, C. Phosphodiesterase inhibitors and lung diseases. Adv. Pharmacol. 2023, 98, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Hye, T.; Moinuddin, S.M.; Sarkar, T.; Nguyen, T.; Saha, D.; Ahsan, F. An evolving perspective on novel modified release drug delivery systems for inhalational therapy. Expert Opin. Drug Deliv. 2023, 20, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.M.; Grassiri, B.; Migone, C.; Zambito, Y.; Healy, A.M.; Ehrhardt, C.; Roncucci, P.; Ferro, B. Aerosolised Phosphodiesterase 3 Inhibitor Enoximone in the Treatment of COVID-19 Pneumonia: In Vitro Evaluations Supporting Clinical Evidence. In Proceedings of the Drug Delivery to the Lungs, Edinburgh International Conference Centre and Virtual, Edinburgh, UK, 7–9 December 2022. [Google Scholar]
- Grassiri, B.; Cesari, A.; Balzano, F.; Migone, C.; Kali, G.; Bernkop-Schnürch, A.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers 2022, 14, 3170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hong, S.; Tan, S.S.K.; Peng, T.; Goh, L.Y.H.; Lam, K.H.; Chow, K.T.; Gokhale, R. Polysorbates versus Hydroxypropyl Beta-Cyclodextrin (HPβCD): Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies. Molecules 2022, 27, 6497. [Google Scholar] [CrossRef]
- Váradi, J.; Hermenean, A.; Gesztelyi, R.; Jeney, V.; Balogh, E.; Majoros, L.; Malanga, M.; Fenyvesi, É.; Szente, L.; Bácskay, I.; et al. Pharmacokinetic Properties of Fluorescently Labelled Hydroxypropyl-Beta-Cyclodextrin. Biomolecules 2019, 9, 509. [Google Scholar] [CrossRef]
- Barski, P.; Surdacki, M.; Saj, A.; Wróblewska, A.; Ornat, M.; Pawelak, A.; Pompa, D.; Jurgiel, J.; Ermisch, V.; Hirnle, A.; et al. Isotonic Saline Nebulization and Lung Function in Children with Mild Respiratory Ailments. Physiol. Res. 2020, 69, 131–137. [Google Scholar] [CrossRef]
- Petpiroon, N.; Netkueakul, W.; Sukrak, K.; Takahashi, K. Development of lung tissue models and their applications. Life Sci. 2023, 334, 122208. [Google Scholar] [CrossRef]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef]
- Mokra, D.; Mokry, J. Phosphodiesterase Inhibitors in Acute Lung Injury: What Are the Perspectives? Int. J. Mol. Sci. 2021, 22, 1929. [Google Scholar] [CrossRef]
- Fernandes, I.G.; De Brito, C.A.; Dos Reis, V.M.S.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and Other Respiratory Viruses: What Does Oxidative Stress Have to Do with It? Oxid. Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef]


| Time | Sample | Crystal Size | TSI | ||||
|---|---|---|---|---|---|---|---|
| (min) | Length μm ± SD | Width μm ± SD | Delivered mg ± SD | First Stage % | Second Stage % | Filter % | |
| 1 | ENXM/HPβCD | Absence of crystals | Absence of crystals | 0.12 ± 0.03 | 53 ± 6 | 35 ± 10 | 12 ± 7 |
| 3 | ENXM/HPβCD | Absence of crystals | Absence of crystals | 0.14 ± 0.04 | 47 ± 6 | 42 ± 6 | 11 ± 3 |
| 1 | 1:17 Perfan® | 17 ± 4 | 11 ± 3 | 0.05 ± 0.01 | 71 ± 13 | 23 ± 7 | 6 ± 3 |
| 3 | 1:17 Perfan® | 18 ± 4 | 11 ± 3 | 0.07 ± 0.01 | 60 ± 7 | 29 ± 10 | 11 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migone, C.; Grassiri, B.; Vizzoni, L.; Fabiano, A.; Ferro, B.; Zambito, Y.; Piras, A.M. Aerosol of Enoximone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex, Biopharmaceutical Evidence for ARDS Applicability. Pharmaceutics 2024, 16, 1221. https://doi.org/10.3390/pharmaceutics16091221
Migone C, Grassiri B, Vizzoni L, Fabiano A, Ferro B, Zambito Y, Piras AM. Aerosol of Enoximone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex, Biopharmaceutical Evidence for ARDS Applicability. Pharmaceutics. 2024; 16(9):1221. https://doi.org/10.3390/pharmaceutics16091221
Chicago/Turabian StyleMigone, Chiara, Brunella Grassiri, Lucia Vizzoni, Angela Fabiano, Baldassare Ferro, Ylenia Zambito, and Anna Maria Piras. 2024. "Aerosol of Enoximone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex, Biopharmaceutical Evidence for ARDS Applicability" Pharmaceutics 16, no. 9: 1221. https://doi.org/10.3390/pharmaceutics16091221
APA StyleMigone, C., Grassiri, B., Vizzoni, L., Fabiano, A., Ferro, B., Zambito, Y., & Piras, A. M. (2024). Aerosol of Enoximone/Hydroxypropyl-β-Cyclodextrin Inclusion Complex, Biopharmaceutical Evidence for ARDS Applicability. Pharmaceutics, 16(9), 1221. https://doi.org/10.3390/pharmaceutics16091221







