Amorphous Polymer–Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymer–Phospholipid/Polymer–Xylitol Mixtures
2.2.2. Preparation of Hot-Melt Extruded Systems
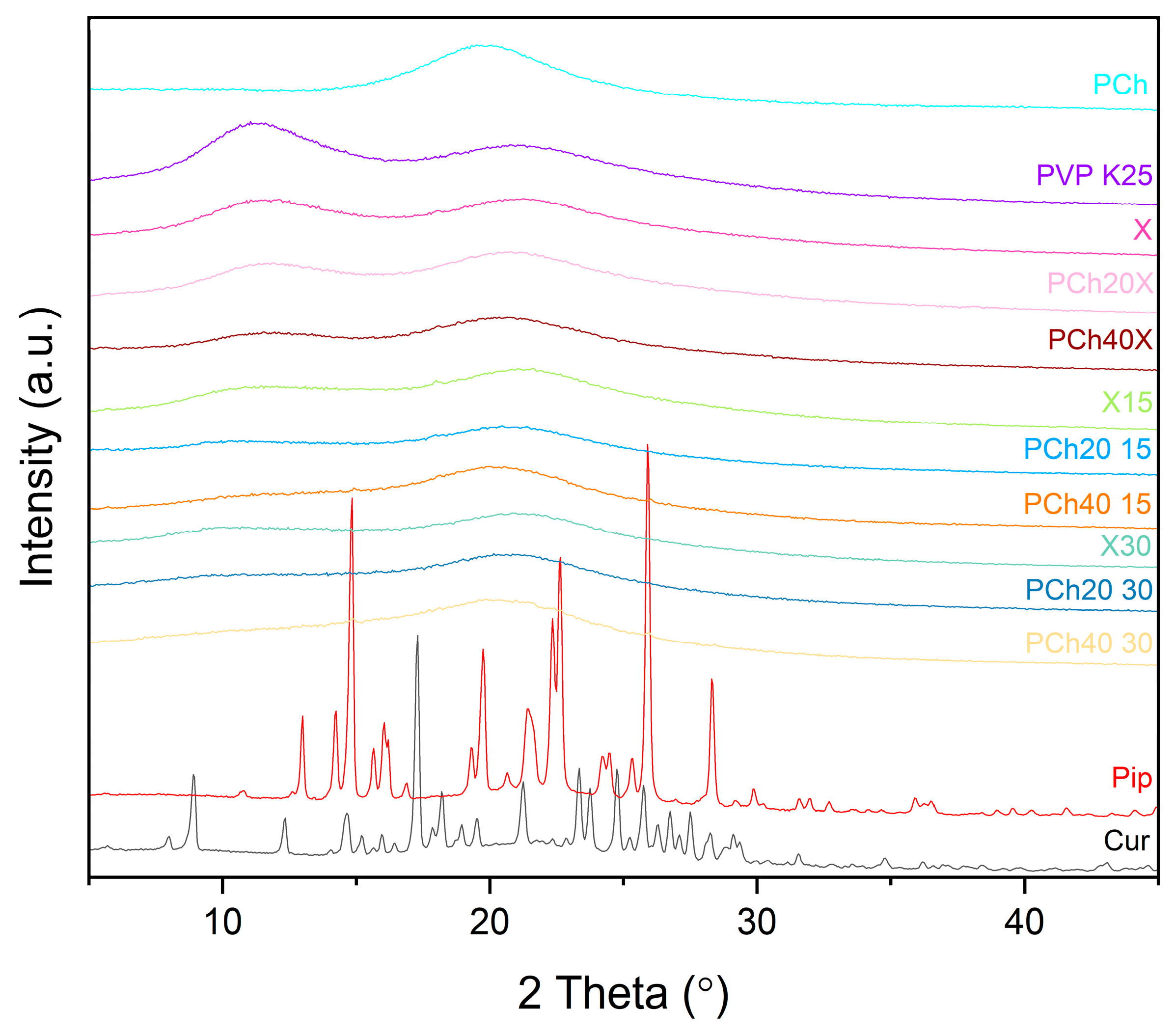
2.2.3. X-ray Powder Diffraction (XRPD)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Fourier-Transform Infrared Spectroscopy (FTIR-ATR)
2.2.6. Physical Stability
2.2.7. High-Performance Liquid Chromatography (HPLC) Analysis
2.2.8. Preparation of Media for Solubility Studies and Dissolution
2.2.9. Solubility Studies
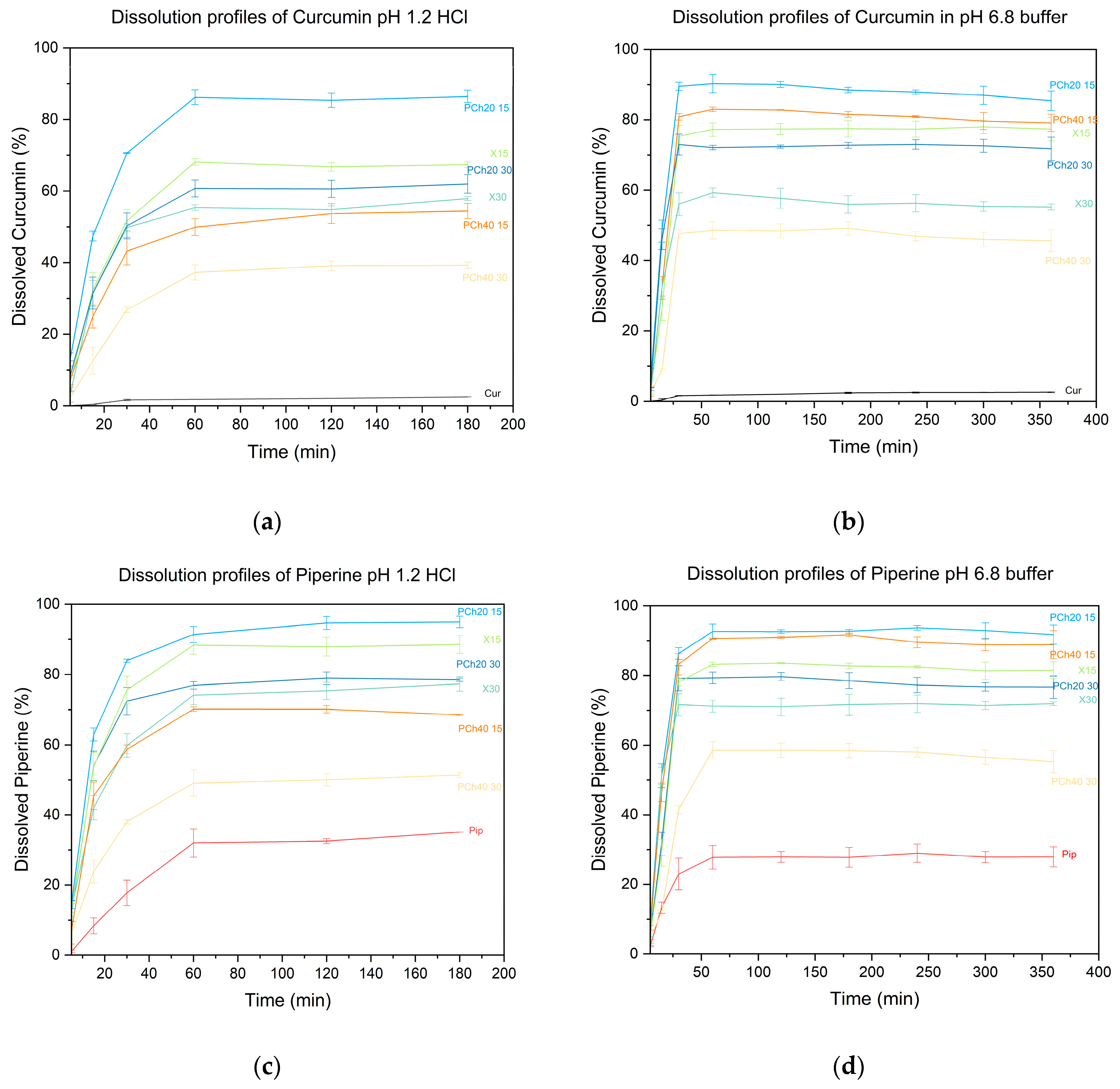
2.2.10. Dissolution Studies
2.2.11. Permeability Studies
2.2.12. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Singh, A.; Worku, Z.A.; Van den Mooter, G. Oral formulation strategies to improve solubility of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1361–1378. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Da Silva Júnior, W.F.; de Oliveira Pinheiro, J.G.; Moreira, C.D.; de Souza, F.J.J.; de Lima, Á.A.N. Alternative technologies to improve solubility and stability of poorly water-soluble drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–305. [Google Scholar]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Javeri, I.; Chand, N. Curcumin. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 435–445. [Google Scholar]
- Haq, I.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phyther. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Piperine and its role in chronic diseases. Anti-Inflamm. Nutraceuticals Chronic Dis. 2016, 928, 173–184. [Google Scholar]
- Shalaev, E.; Wu, K.; Shamblin, S.; Krzyzaniak, J.F.; Descamps, M. Crystalline mesophases: Structure, mobility, and pharmaceutical properties. Adv. Drug Deliv. Rev. 2016, 100, 194–211. [Google Scholar] [CrossRef]
- Lehmkemper, K.; Kyeremateng, S.O.; Heinzerling, O.; Degenhardt, M.; Sadowski, G. Impact of polymer type and relative humidity on the long-term physical stability of amorphous solid dispersions. Mol. Pharm. 2017, 14, 4374–4386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Fricker, G.; Kromp, T.; Wendel, A.; Blume, A.; Zirkel, J.; Rebmann, H.; Setzer, C.; Quinkert, R.-O.; Martin, F.; Müller-Goymann, C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010, 27, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Drescher, S.; van Hoogevest, P. The phospholipid research center: Current research in phospholipids and their use in drug delivery. Pharmaceutics 2020, 12, 1235. [Google Scholar] [CrossRef]
- Dahan, A.; Beig, A.; Lindley, D.; Miller, J.M. The solubility–permeability interplay and oral drug formulation design: Two heads are better than one. Adv. Drug Deliv. Rev. 2016, 101, 99–107. [Google Scholar] [CrossRef]
- Beig, A.; Miller, J.M.; Lindley, D.; Carr, R.A.; Zocharski, P.; Agbaria, R.; Dahan, A. Head-to-head comparison of different solubility-enabling formulations of etoposide and their consequent solubility–permeability interplay. J. Pharm. Sci. 2015, 104, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Kim, J.Y.; Bai, Y.; Pabla, N. Role of Passive diffusion, Transporters, and membrane trafficking-mediated processes in cellular drug transport. Clin. Pharmacol. Ther. 2017, 101, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, H.; Sun, L.; Tong, L.; Zhang, T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 2014, 93, 54–61. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin–phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Jo, K.; Cho, J.M.; Lee, H.; Kim, E.K.; Kim, H.C.; Kim, H.; Lee, J. Enhancement of aqueous solubility and dissolution of celecoxib through phosphatidylcholine-based dispersion systems solidified with adsorbent carriers. Pharmaceutics 2018, 11, 1. [Google Scholar] [CrossRef]
- Brinkmann-Trettenes, U.; Barnert, S.; Bauer-Brandl, A. Single step bottom-up process to generate solid phospholipid nano-particles. Pharm. Dev. Technol. 2014, 19, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, W.; Ye, J.; Hao, H.; Zhou, J.; Wang, R.; Liu, Y. A novel matrix dispersion based on phospholipid complex for improving oral bioavailability of baicalein: Preparation, in vitro and in vivo evaluations. Drug Deliv. 2017, 24, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Han, J.; Jiang, A.; Huang, R.; Fu, T.; Wang, L.; Zheng, Q.; Li, W.; Li, J. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int. J. Pharm. 2019, 561, 9–18. [Google Scholar] [CrossRef]
- Pacult, J.; Rams-Baron, M.; Chmiel, K.; Jurkiewicz, K.; Antosik, A.; Szafraniec, J.; Kurek, M.; Jachowicz, R.; Paluch, M. How can we improve the physical stability of co-amorphous system containing flutamide and bicalutamide? The case of ternary amorphous solid dispersions. Eur. J. Pharm. Sci. 2019, 136, 104947. [Google Scholar] [CrossRef] [PubMed]
- Vullendula, S.K.A.; Nair, A.R.; Yarlagadda, D.L.; Sree, K.S.N.; Bhat, K.; Dengale, S.J. Polymeric solid dispersion Vs co-amorphous technology: A critical comparison. J. Drug Deliv. Sci. Technol. 2022, 78, 103980. [Google Scholar] [CrossRef]
- Gautam, C.S.; Saha, L. Fixed dose drug combinations (FDCs): Rational or irrational: A view point. Br. J. Clin. Pharmacol. 2008, 65, 795. [Google Scholar] [CrossRef]
- Mitra, A.; Wu, Y. Challenges and opportunities in achieving bioequivalence for fixed-dose combination products. AAPS J. 2012, 14, 646–655. [Google Scholar] [CrossRef]
- Desai, D.; Wang, J.; Wen, H.; Li, X.; Timmins, P. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharm. Dev. Technol. 2013, 18, 1265–1276. [Google Scholar] [CrossRef]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Yu, D.; Kan, Z.; Shan, F.; Zang, J.; Zhou, J. Triple strategies to improve oral bioavailability by fabricating coamorphous forms of ursolic acid with piperine: Enhancing water-solubility, permeability, and inhibiting cytochrome p450 isozymes. Mol. Pharm. 2020, 17, 4443–4462. [Google Scholar] [CrossRef]
- Singh, S.; Jamwal, S.; Kumar, P. Piperine enhances the protective effect of curcumin against 3-NP induced neurotoxicity: Possible neurotransmitters modulation mechanism. Neurochem. Res. 2015, 40, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Wei Tan, A.C.; Leong, W.H.; Yin Chia, A.Y.; Vijayabalan, S.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Synergistic effects of curcumin and piperine as potent acetylcholine and amyloidogenic inhibitors with significant neuroprotective activity in SH-SY5Y cells via computational molecular modeling and in vitro assay. Front. Aging Neurosci. 2019, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Chopra, K.; Rongzhu, L.; Kulkarni, S.K. Protective effect of curcumin and its combination with piperine (bioavailability enhancer) against haloperidol-associated neurotoxicity: Cellular and neurochemical evidence. Neurotox. Res. 2011, 20, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.M.; Nguyen, J.H.; Becker, C.; Francke, N.M.; Jørgensen, E.B.; Holm, P.; Holm, R.; Mu, H.; Rades, T.; Langguth, P. Influence of polymer molecular weight on in vitro dissolution behavior and in vivo performance of celecoxib: PVP amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2016, 101, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Asgreen, C.; Knopp, M.M.; Skytte, J.; Löbmann, K. Influence of the polymer glass transition temperature and molecular weight on drug amorphization kinetics using ball milling. Pharmaceutics 2020, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, X.; Zhu, W.; Zhang, X.; Di, L. Preparation of Curcumin-Eudragit® E PO Solid Dispersions with Gradient Temperature through Hot-Melt Extrusion. Molecules 2021, 26, 4964. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.; Kelly, A.L.; Gough, T.; Jadhav, V.; Singh, K.K.; Paradkar, A. Application of hot melt extrusion for improving bioavailability of artemisinin a thermolabile drug. Drug Dev. Ind. Pharm. 2018, 44, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Tajber, L.; Miklaszewski, A.; Cielecka-Piontek, J. Sweeteners Show a Plasticizing Effect on PVP K30—A Solution for the Hot-Melt Extrusion of Fixed-Dose Amorphous Curcumin-Hesperetin Solid Dispersions. Pharmaceutics 2024, 16, 659. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Löbmann, K.; Rades, T.; Grohganz, H. On the role of salt formation and structural similarity of co-formers in co-amorphous drug delivery systems. Int. J. Pharm. 2018, 535, 86–94. [Google Scholar] [CrossRef]
- Wdowiak, K.; Pietrzak, R.; Tykarska, E.; Cielecka-Piontek, J. Hot-Melt Extrusion as an Effective Technique for Obtaining an Amorphous System of Curcumin and Piperine with Improved Properties Essential for Their Better Biological Activities. Molecules 2023, 28, 3848. [Google Scholar] [CrossRef]
- Löbmann, K.; Strachan, C.; Grohganz, H.; Rades, T.; Korhonen, O.; Laitinen, R. Co-amorphous simvastatin and glipizide combinations show improved physical stability without evidence of intermolecular interactions. Eur. J. Pharm. Biopharm. 2012, 81, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Lindenberg, E.; Rades, T.; Grohganz, H.; Löbmann, K. Influence of preparation pathway on the glass forming ability. Int. J. Pharm. 2017, 521, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, N.; Kuentz, M. Glass-forming ability of compounds in marketed amorphous drug products. Eur. J. Pharm. Biopharm. 2017, 112, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rades, T.; Grohganz, H. The influence of moisture on the storage stability of co-amorphous systems. Int. J. Pharm. 2021, 605, 120802. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Taylor, L.S. Effect of polymer hygroscopicity on the phase behavior of amorphous solid dispersions in the presence of moisture. Mol. Pharm. 2010, 7, 477–490. [Google Scholar] [CrossRef]
- Mucha, I.; Karolewicz, B.; Górniak, A. Stability Studies of Amorphous Ibrutinib Prepared Using the Quench-Cooling Method and Its Dispersions with Soluplus®. Polymers 2024, 16, 1961. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017, 39, 166–179. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Elvang, P.A.; Bauer-Brandl, A.; Brandl, M. A dynamic in vitro permeation study on solid mono-and diacyl-phospholipid dispersions of celecoxib. Eur. J. Pharm. Sci. 2019, 127, 199–207. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, H.; Wang, Y.; Wang, R.; Xu, J.; Zhang, C. Amorphous Solid Dispersions: Role of the Polymer and Its Importance in Physical Stability and In Vitro Performance. Pharmaceutics 2022, 14, 1747. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Understanding the generation and maintenance of supersaturation during the dissolution of amorphous solid dispersions using modulated DSC and 1H NMR. Int. J. Pharm. 2018, 536, 414–425. [Google Scholar] [CrossRef]
- Fong, S.Y.K.; Martins, S.M.; Brandl, M.; Bauer-Brandl, A. Solid phospholipid dispersions for oral delivery of poorly soluble drugs: Investigation into celecoxib incorporation and solubility-in vitro permeability enhancement. J. Pharm. Sci. 2016, 105, 1113–1123. [Google Scholar] [CrossRef]
- Edueng, K.; Mahlin, D.; Larsson, P.; Bergström, C.A.S. Mechanism-based selection of stabilization strategy for amorphous formulations: Insights into crystallization pathways. J. Control. Release 2017, 256, 193–202. [Google Scholar] [CrossRef]
- Knopp, M.M.; Wendelboe, J.; Holm, R.; Rades, T. Effect of amorphous phase separation and crystallization on the in vitro and in vivo performance of an amorphous solid dispersion. Eur. J. Pharm. Biopharm. 2018, 130, 290–295. [Google Scholar] [CrossRef]
- Dahan, A.; Beig, A.; Ioffe-Dahan, V.; Agbaria, R.; Miller, J.M. The twofold advantage of the amorphous form as an oral drug delivery practice for lipophilic compounds: Increased apparent solubility and drug flux through the intestinal membrane. AAPS J. 2013, 15, 347–353. [Google Scholar] [CrossRef]
- Nogami, S.; Minoura, K.; Kiminami, N.; Kitaura, Y.; Uchiyama, H.; Kadota, K.; Tozuka, Y. Stabilizing effect of the cyclodextrins additive to spray-dried particles of curcumin/polyvinylpyrrolidone on the supersaturated state of curcumin. Adv. Powder Technol. 2021, 32, 1750–1756. [Google Scholar] [CrossRef]
- Shi, C.; Tong, Q.; Fang, J.; Wang, C.; Wu, J.; Wang, W. Preparation, characterization and in vivo studies of amorphous solid dispersion of berberine with hydrogenated phosphatidylcholine. Eur. J. Pharm. Sci. 2015, 74, 11–17. [Google Scholar] [CrossRef]
- Kuche, K.; Bhargavi, N.; Dora, C.P.; Jain, S. Drug-phospholipid complex—A go through strategy for enhanced oral bioavailability. AAPS PharmSciTech 2019, 20, 43. [Google Scholar] [CrossRef]
- Fong, S.Y.K.; Brandl, M.; Bauer-Brandl, A. Phospholipid-based solid drug formulations for oral bioavailability enhancement: A meta-analysis. Eur. J. Pharm. Sci. 2015, 80, 89–110. [Google Scholar] [CrossRef]
- Jacobsen, A.-C.; Ejskjær, L.; Brandl, M.; Holm, R.; Bauer-Brandl, A. Do phospholipids boost or attenuate drug absorption? In vitro and in vivo evaluation of mono-and diacyl phospholipid-based solid dispersions of celecoxib. J. Pharm. Sci. 2021, 110, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Pawar, Y.B.; Munjal, B.; Arora, S.; Karwa, M.; Kohli, G.; Paliwal, J.K.; Bansal, A.K. Bioavailability of a lipidic formulation of curcumin in healthy human volunteers. Pharmaceutics 2012, 4, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.B.; Arya, H.; Fu, I.-H.; Yeh, T.-K.; Periyasamy, L.; Hsieh, H.-P.; Coumar, M.S. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef] [PubMed]





| Name | Percentage of Ingredient | |||
|---|---|---|---|---|
| Actives | Polymer | Xylitol | Phospholipid | |
| X30 | 30 | 63.83 | 6.17 | 0 |
| PCh20 30 | 30 | 48.27 | 1.73 | 20 |
| PCh40 30 | 30 | 29.14 | 0.86 | 40 |
| X15 | 15 | 77.51 | 7.49 | 0 |
| PCh20 15 | 15 | 62.75 | 2.25 | 20 |
| PCh40 15 | 15 | 43.71 | 1.29 | 40 |
| Compound | ||||||
|---|---|---|---|---|---|---|
| Curcumin | Piperine | |||||
| Conc. [mg/mL] | Improv. [-fold] | Conc. [mg/mL] | Improv. [-fold] | |||
| System | pH 1.2 HCl | Raw | 0.00011 ± 0.00001 e | N/A | 0.009 ± 0.0006 e | N/A |
| X30 | 1.112 ± 0.091 c | 10109 | 1.082 ± 0.084 c | 120 | ||
| PCh20 30 | 1.243 ± 0.102 c | 11300 | 1.193 ± 0.107 c | 133 | ||
| PCh40 30 | 0.468 ± 0.076 d | 4255 | 0.456 ± 0.061 d | 51 | ||
| X15 | 1.637 ± 0.113 b | 14881 | 1.592 ± 0.119 b | 177 | ||
| PCh20 15 | 1.871 ± 0.126 a | 17009 | 1.881 ± 0.123 a | 209 | ||
| PCh40 15 | 0.536 ± 0.082 d | 4873 | 0.524 ± 0.077 d | 58 | ||
| pH 6.8 Phosphate buffer | Raw | 0.00014 ± 0.00002 e | N/A | 0.008 ± 0.0004 f | N/A | |
| X30 | 1.155 ± 0.109 c | 8250 | 1.129 ± 0.098 d | 141 | ||
| PCh20 30 | 2.325 ± 0.114 b | 16607 | 2.347 ± 0.121 c | 293 | ||
| PCh40 30 | 0.618 ± 0.082 d | 4414 | 0.594 ± 0.074 e | 74 | ||
| X15 | 2.482 ± 0.137 b | 17729 | 2.471 ± 0.108 b,c | 309 | ||
| PCh20 15 | 3.583 ± 0.116 a | 25593 | 3.576 ± 0.123 a | 447 | ||
| PCh40 15 | 2.528 ± 0.099 b | 18057 | 2.533 ± 0.086 b | 317 | ||
| Compound | ||||||
|---|---|---|---|---|---|---|
| Curcumin | Piperine | |||||
| Conc. [mg/mL] | Improv. [-fold] | Conc. [mg/mL] | Improv. [-fold] | |||
| Model | GIT | Raw | 0.00061 ± 0.000038 g | N/A | 0.0044 ± 0.0008 g | N/A |
| X30 | 0.310 ± 0.012 e | 508 | 0.367 ± 0.016 e | 83 | ||
| PCh20 30 | 0.421 ± 0.013 d | 690 | 0.455 ± 0.025 d | 103 | ||
| PCh40 30 | 0.134 ± 0.012 f | 220 | 0.282 ± 0.018 f | 64 | ||
| X15 | 0.547 ± 0.023 c | 897 | 0.594 ± 0.022 c | 135 | ||
| PCh20 15 | 0.728 ± 0.051 a | 1193 | 0.757 ± 0.038 a | 172 | ||
| PCh40 15 | 0.651 ± 0.028 b | 1067 | 0.673 ± 0.021 b | 153 | ||
| BBB | Raw | 0.00016 ± 0.000057 f | N/A | 0.0051 ± 0.0003 f | N/A | |
| X30 | 0.178 ± 0.025 d | 1113 | 0.358 ± 0.034 d | 70 | ||
| PCh20 30 | 0.269 ± 0.023 c | 1681 | 0.409 ± 0.014 c | 80 | ||
| PCh40 30 | 0.098 ± 0.018 e | 613 | 0.263 ± 0.021 e | 52 | ||
| X15 | 0.274 ± 0.011 c | 1713 | 0.427 ± 0.013 c | 84 | ||
| PCh20 15 | 0.423 ± 0.028 a | 2644 | 0.583 ± 0.029 a | 114 | ||
| PCh40 15 | 0.346 ± 0.022 b | 2163 | 0.471 ± 0.024 b | 92 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wdowiak, K.; Miklaszewski, A.; Cielecka-Piontek, J. Amorphous Polymer–Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion. Pharmaceutics 2024, 16, 999. https://doi.org/10.3390/pharmaceutics16080999
Wdowiak K, Miklaszewski A, Cielecka-Piontek J. Amorphous Polymer–Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion. Pharmaceutics. 2024; 16(8):999. https://doi.org/10.3390/pharmaceutics16080999
Chicago/Turabian StyleWdowiak, Kamil, Andrzej Miklaszewski, and Judyta Cielecka-Piontek. 2024. "Amorphous Polymer–Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion" Pharmaceutics 16, no. 8: 999. https://doi.org/10.3390/pharmaceutics16080999
APA StyleWdowiak, K., Miklaszewski, A., & Cielecka-Piontek, J. (2024). Amorphous Polymer–Phospholipid Solid Dispersions for the Co-Delivery of Curcumin and Piperine Prepared via Hot-Melt Extrusion. Pharmaceutics, 16(8), 999. https://doi.org/10.3390/pharmaceutics16080999










