Development of 5-Fluorouracil/pH-Responsive Adjuvant-Embedded Extracellular Vesicles for Targeting αvβ3 Integrin Receptors in Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
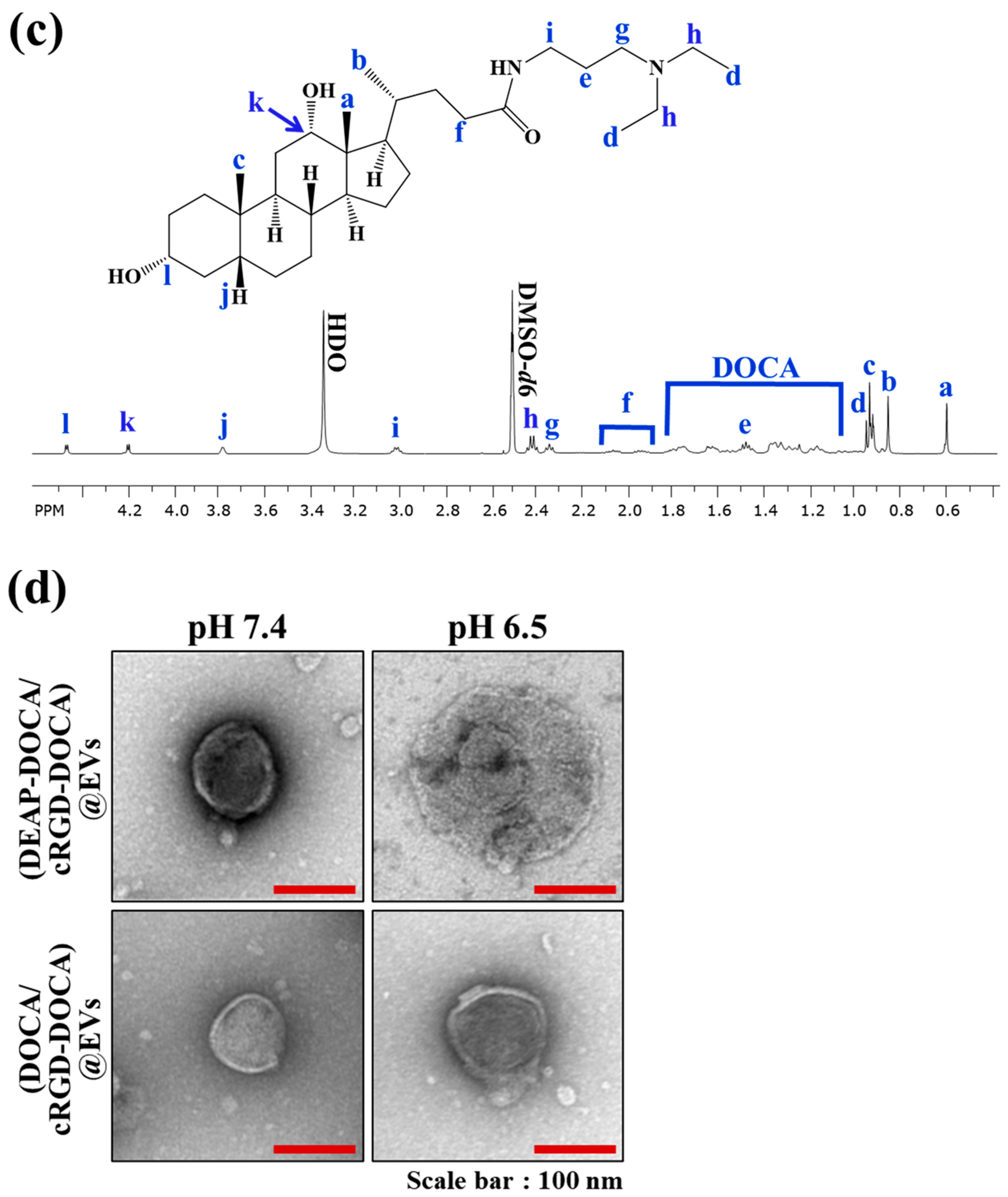
2.2. DEAP-DOCA and cRGD-DOCA Synthesis
2.3. Isolation of EVs
2.4. Preparation of EV Samples
2.5. Characterization of the EV Samples
2.6. In Vitro 5-FU Release Test
2.7. Cell Culture
2.8. In Vitro Cytotoxicity Test
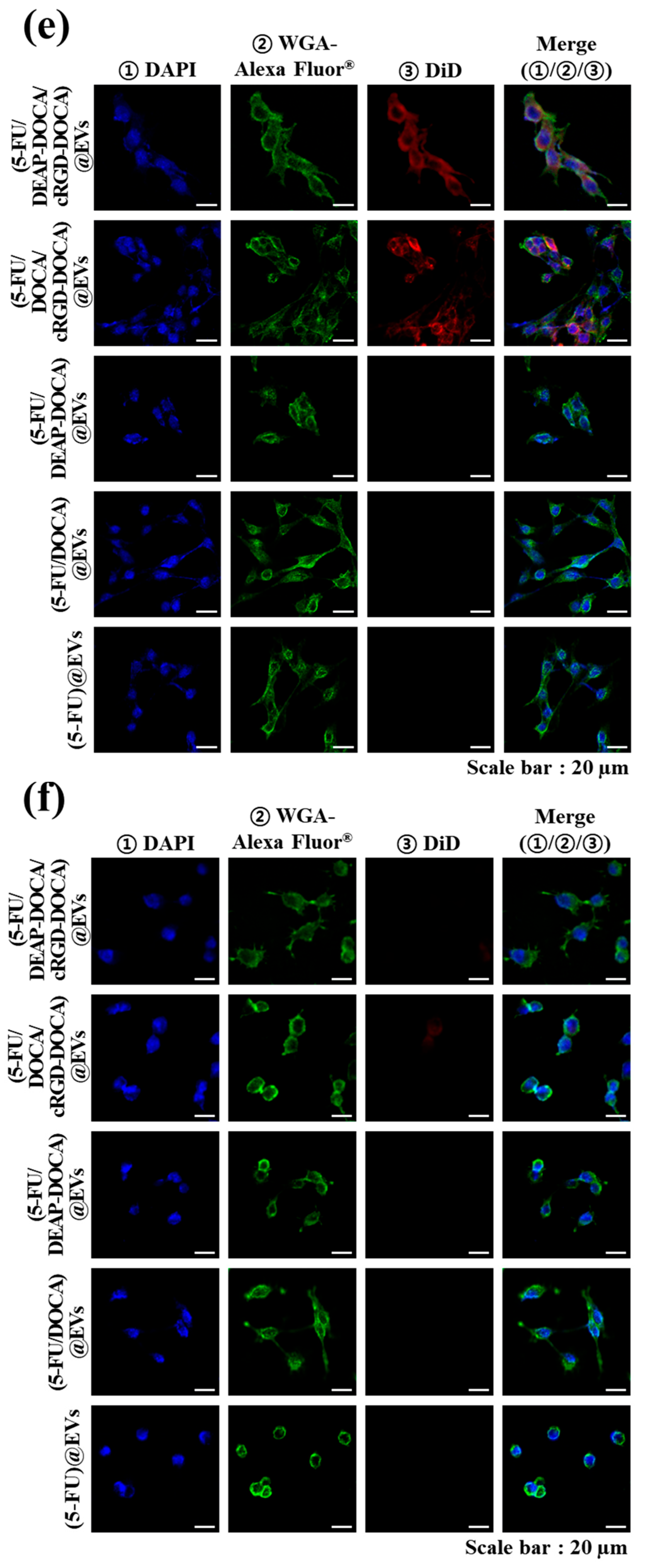
2.9. In Vitro Cellular Uptake Test
2.10. Animal Care
2.11. In Vivo Tumor Inhibition Test
2.12. In Vivo Biodistribution
2.13. Statistics
3. Results and Discussion
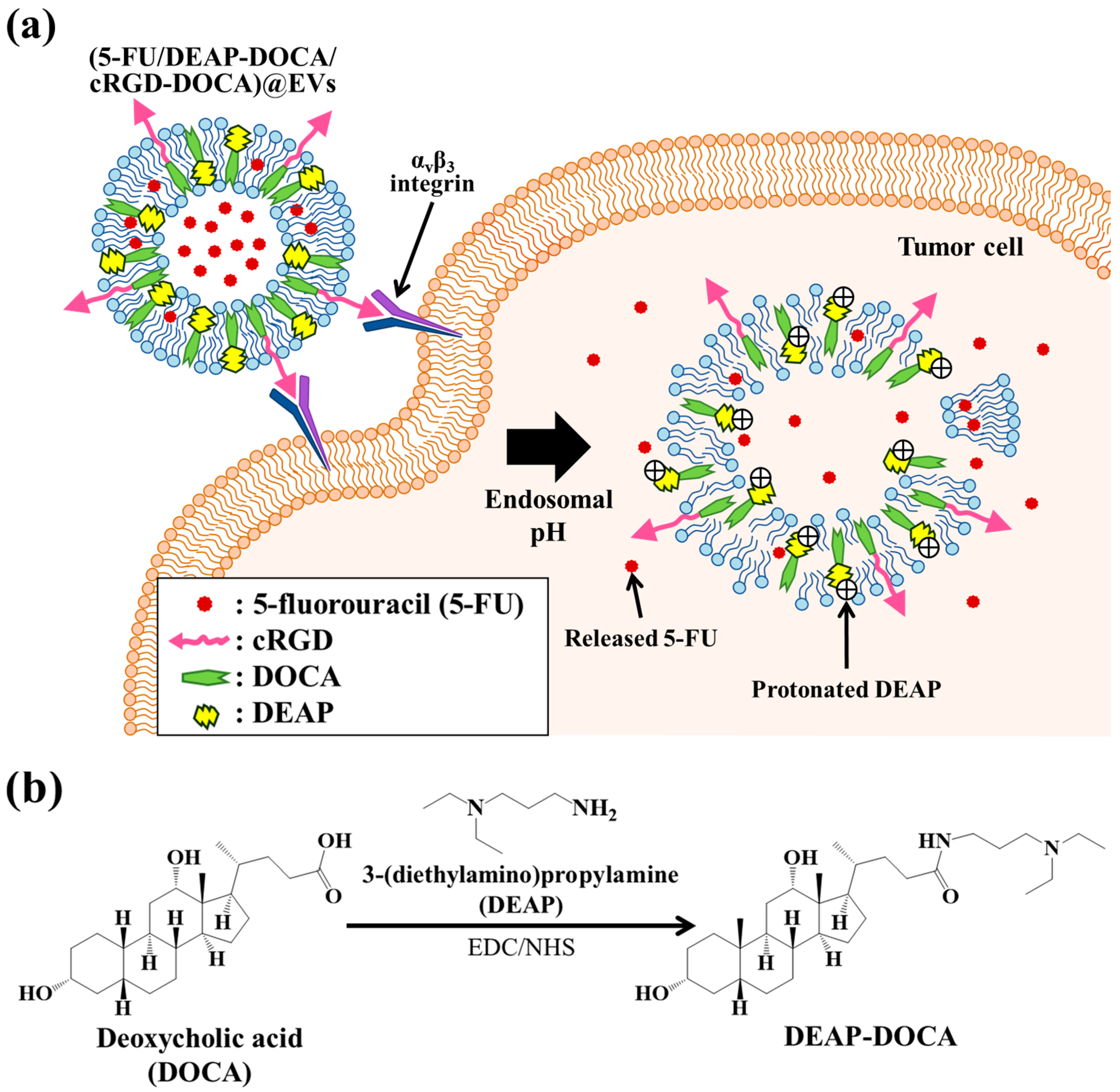
3.1. Fabrication of the EV Samples
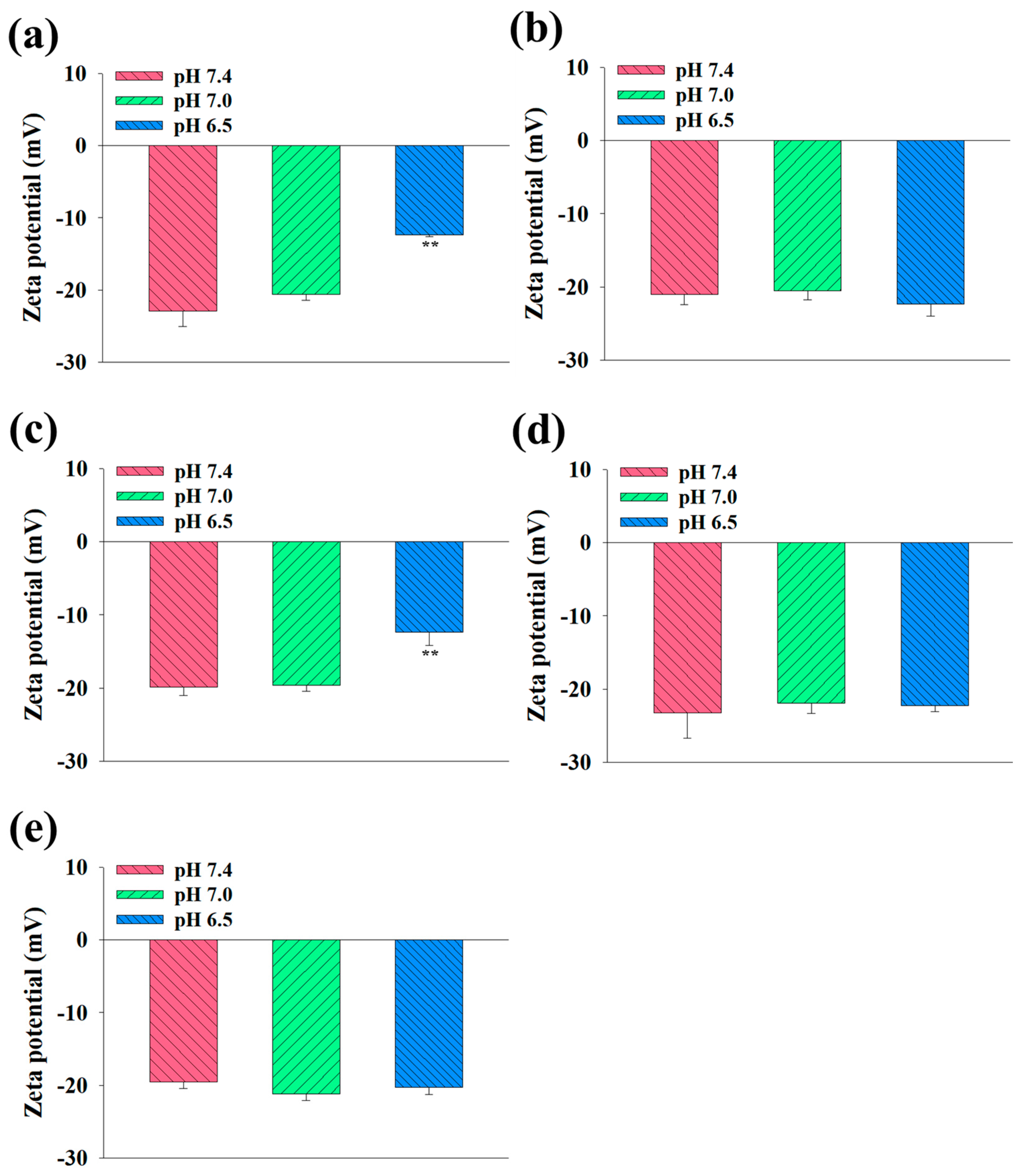
3.2. Characterization of the EV Samples
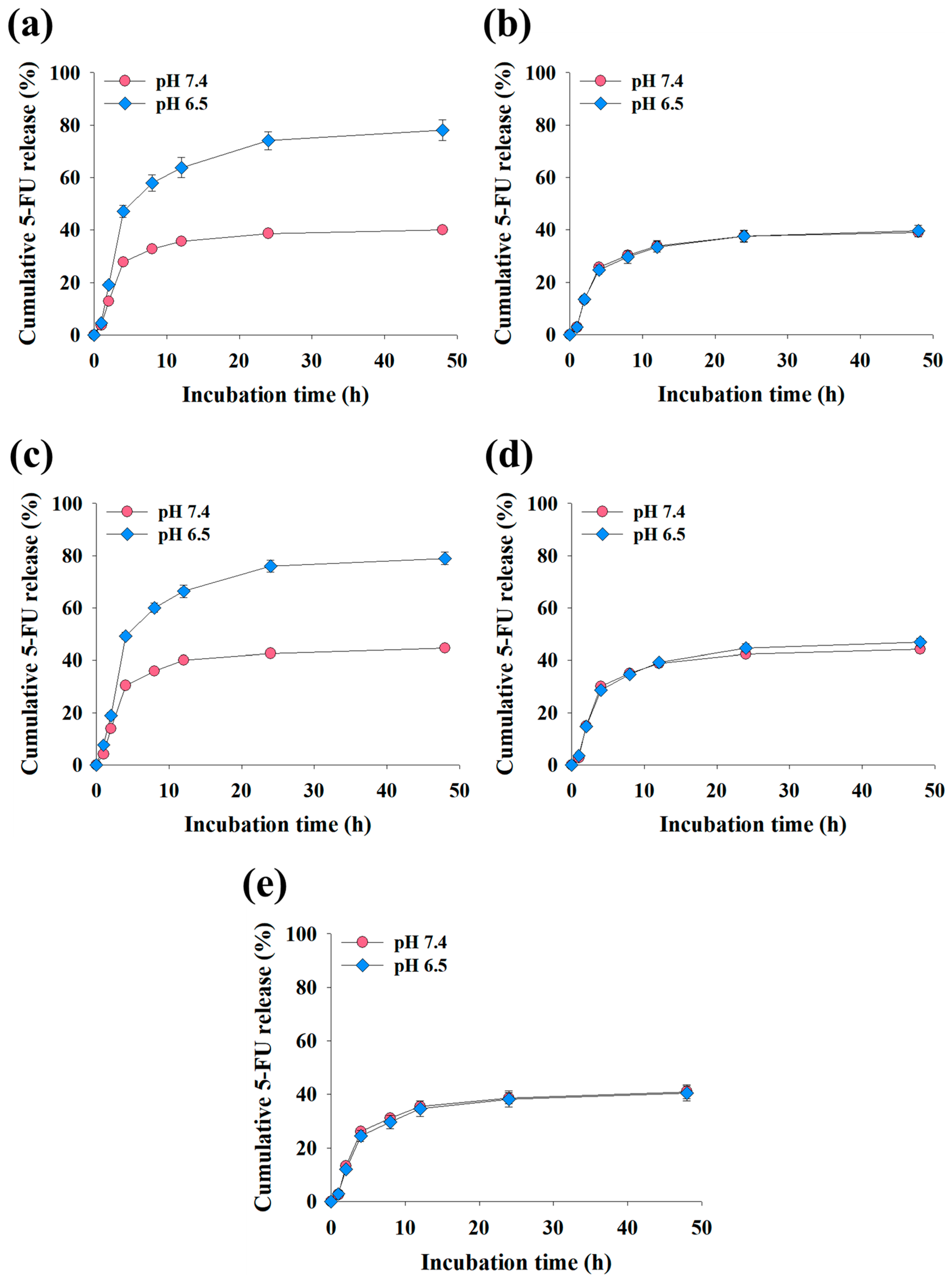
3.3. In Vitro 5-FU Release of the EV Samples
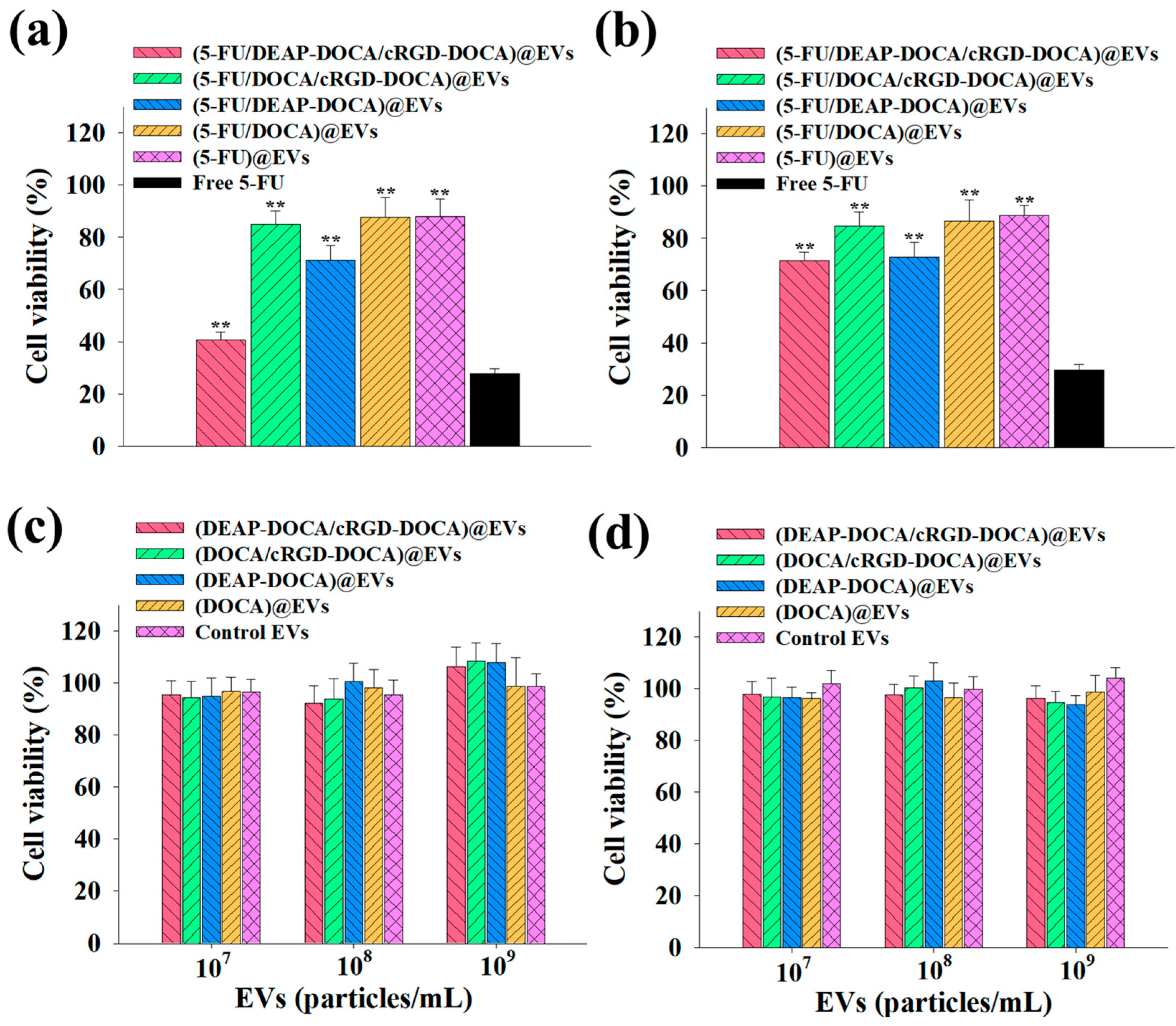
3.4. In Vitro Cell Cytotoxicity of the EV Samples
3.5. In Vivo Tumor Inhibition of the EV Samples
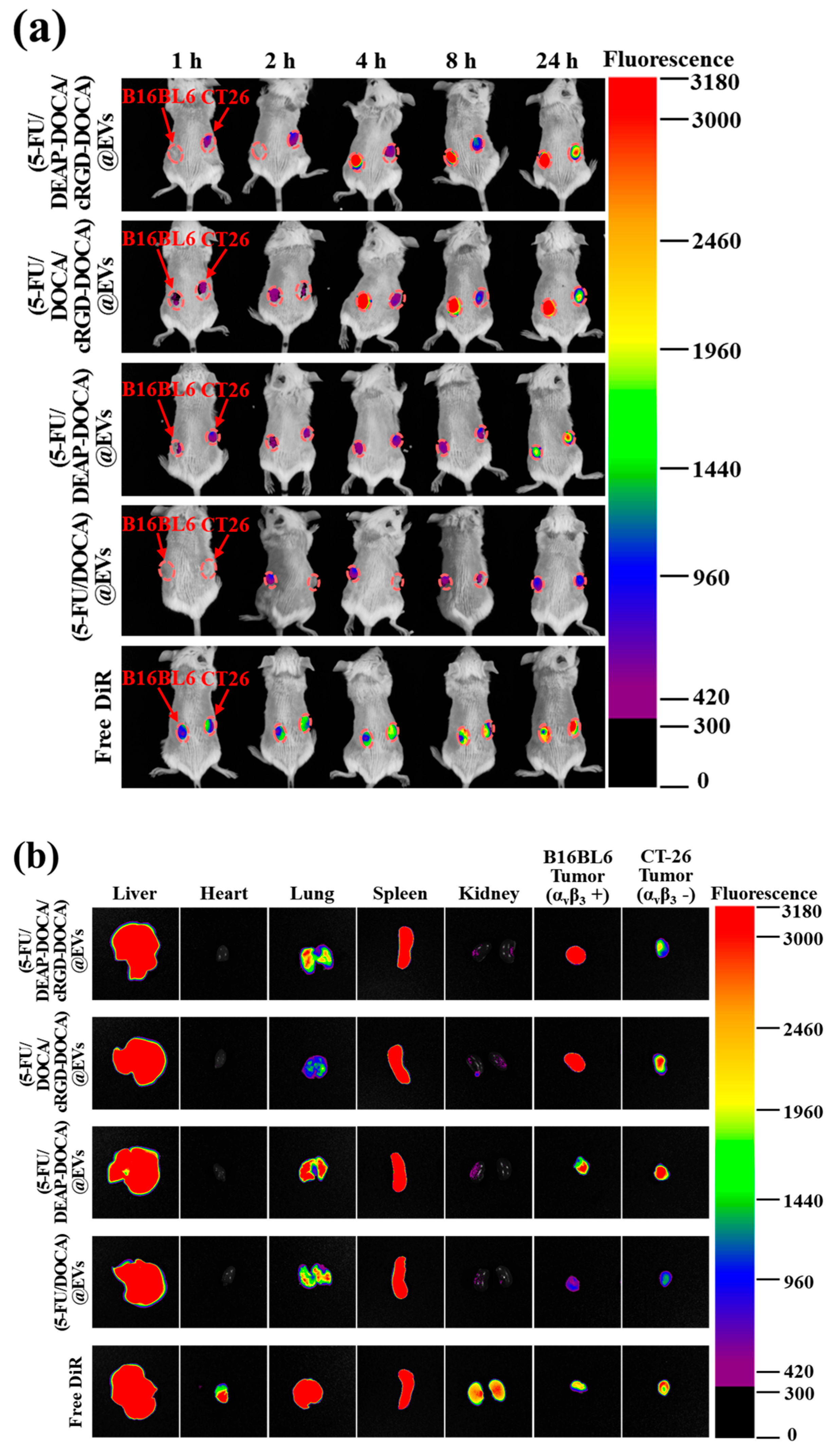
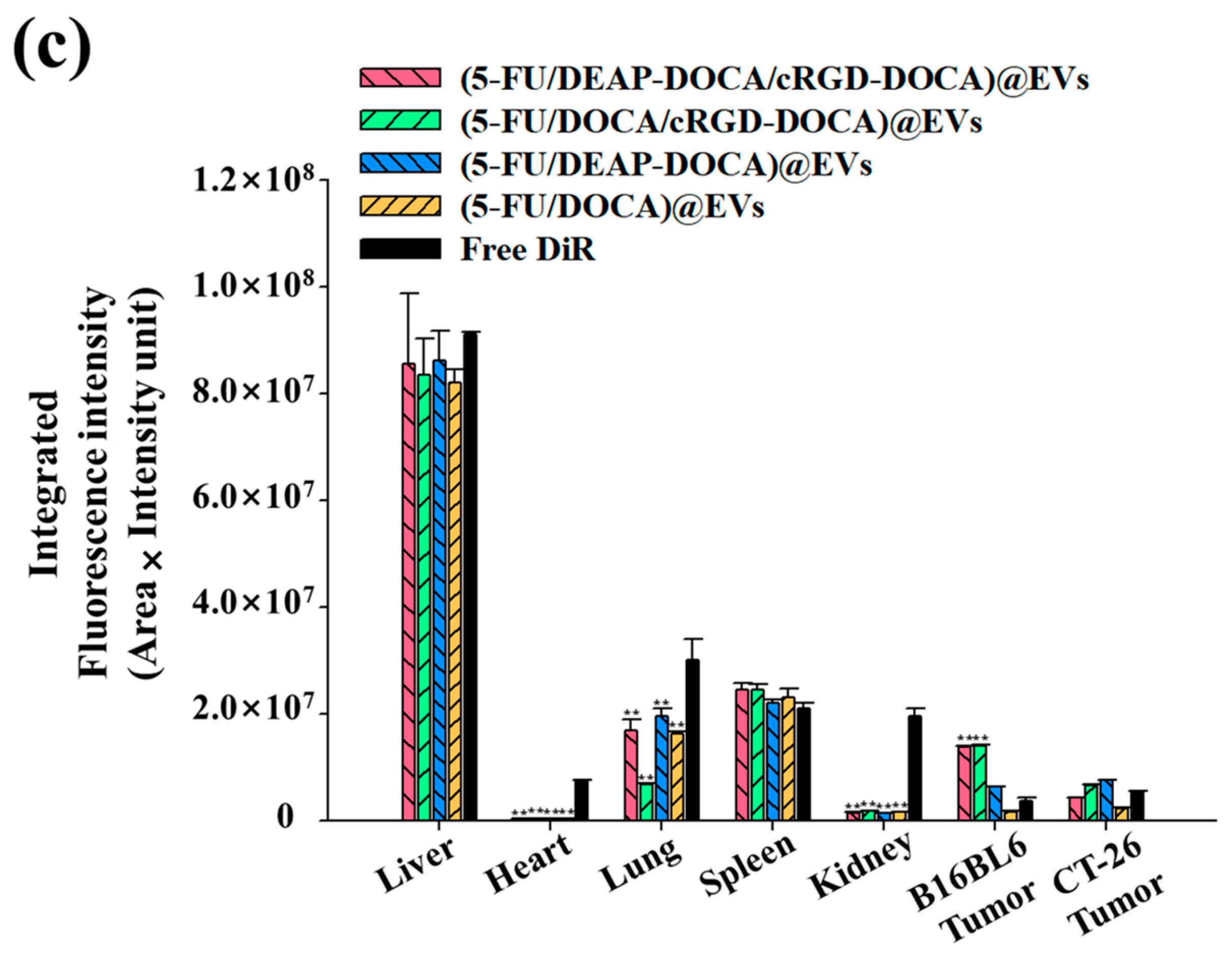
3.6. In Vivo Biodistribution of the EV Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Debele, T.A.; Peng, S.; Tsai, H.C. Drug carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, C.Q.; Post, M.; Simard, B.; Deslandes, Y.; Hsieh, T.H. Design of nanoparticles as drug carriers for cancer therapy. Cancer Genom. Proteom. 2006, 3, 147–157. [Google Scholar]
- Jin, K.T.; Lu, Z.B.; Chen, J.Y.; Liu, Y.Y.; Lan, H.R.; Dong, H.Y.; Yang, F.; Zhao, Y.Y.; Chen, X.Y. Recent trends in nanocarrier-based targeted chemotherapy: Selective delivery of anticancer drugs for effective lung, colon, cervical, and breast cancer treatment. J. Nanomater. 2020, 2020, 9184284. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Hou, F.; Wibowo, D.; Zhang, F.; Zhao, D.; Gao, H.; Zhao, C.X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano 2019, 13, 7410–7424. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Tsou, Y.H.; Wang, B.; Ho, W.; Hu, B.; Tang, P.; Sweet, S.; Zhang, X.Q.; Xu, X. Nanotechnology-mediated drug delivery for the treatment of obesity and its related comorbidities. Adv. Healthc. Mater. 2019, 8, 1801184. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered extracellular vesicles for cancer therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.L.; Kralj-Iglič, V. Pathological and therapeutic significance of tumor-derived extracellular vesicles in cancer cell migration and metastasis. Cancers 2023, 15, 4425. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fei, X.; Wang, J.; Cai, Z. Tumor-derived extracellular vesicles: Regulators of tumor microenvironment and the enlightenment in tumor therapy. Pharmacol. Res. 2020, 159, 105041. [Google Scholar] [CrossRef]
- Tominaga, N.; Yoshioka, Y.; Ochiya, T. A novel platform for cancer therapy using extracellular vesicles. Adv. Drug Deliv. Rev. 2015, 95, 50–55. [Google Scholar] [CrossRef]
- Bie, N.; Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular vesicles for improved tumor accumulation and penetration. Adv. Drug Deliv. Rev. 2022, 188, 114450. [Google Scholar] [CrossRef]
- Ghoshal, K.; Jacob, S.T. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem. Pharmacol. 1997, 53, 1569–1575. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol. Pharm. 2006, 3, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.; Sachpekidis, C.; Kolocouris, A.; Dimitrakopoulou-Strauss, A.; Bouziotis, P. PET diagnostic molecules utilizing multimeric cyclic RGD peptide analogs for imaging integrin αvβ3 receptors. Molecules 2021, 26, 1792. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Le Breton, A.; Préat, V. RGD-based strategies to target alpha (v) beta (3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Asati, S.; Pandey, V.; Soni, V. RGD peptide as a targeting moiety for theranostic purpose: An update study. Int. J. Pept. Res. Ther. 2019, 25, 49–65. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.M.; Oh, K.T.; Lee, E.S. Extremely small-sized globular poly (ethylene glycol)-cyclic RGD conjugates targeting integrin αvβ3 in tumor cells. Int. J. Pharm. 2017, 528, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Noh, G.J.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Cyclic RGD-conjugated hyaluronate dot bearing cleavable doxorubicin for multivalent tumor targeting. Biomacromolecules 2020, 21, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Gao, Y.J.; Qiao, Z.Y.; Fan, G.; Liu, Y.; Zhang, D.; Wang, H. A photoacoustic approach for monitoring the drug release of pH-sensitive poly(β-amino ester)s. J. Mater. Chem. B 2014, 2, 6271–6282. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, E.; Lee, E.S. Macrophage membrane-derived pH-responsive nanovesicles to target tumor cells with integrin α4β1 receptor. Macromol. Res. 2023, 32, 261–271. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, H.; Lee, J.M.; Na, K.; Lee, E.S. pH-responsive starch microparticles for a tumor-targeting implant. Polym. Adv. Technol. 2018, 29, 1372–1376. [Google Scholar] [CrossRef]
- Qiao, Z.Y.; Qiao, S.L.; Fan, G.; Fan, Y.S.; Chen, Y.; Wang, H. One-pot synthesis of pH-sensitive poly (RGD-co-β-amino ester)s for targeted intracellular drug delivery. Polym. Chem. 2014, 5, 844–853. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Noh, G.J.; Lee, E.S. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018, 202, 323–333. [Google Scholar] [CrossRef]
- Geng, T.; Leung, E.; Chamley, L.W.; Wu, Z. Functionalisation of extracellular vesicles with cyclic-RGDyC potentially for glioblastoma targeted intracellular drug delivery. Biomater. Adv. 2023, 149, 213388. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, P.; Yan, F.; Yuan, M.; Yuan, H.; Du, Y.; Yan, J.; Song, Q.; Zhang, T.; Hu, D. Engineered Extracellular Vesicles for Delivery of an IL-1 Receptor Antagonist Promote Targeted Repair of Retinal Degeneration. Small 2023, 19, 2302962. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xing, H.; Wu, M.C.; Shen, F.; Chen, Y.; Yang, T. Extracellular-vesicles delivered tumor-specific sequential nanocatalysts can be used for MRI-informed nanocatalytic Therapy of hepatocellular carcinoma. Theranostics 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Deng, J.; Zhao, Y.; Tao, T. Cyclic RGD peptide-modified liposomal drug delivery system: Enhanced cellular uptake in vitro and improved pharmacokinetics in rats. Int. J. Nanomed. 2012, 7, 3803–3811. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, X. Exosomes-derived miR-125-5p from cartilage endplate stem cells regulates autophagy and ECM metabolism in nucleus pulposus by targeting SUV38H1. Exp. Cell Res. 2022, 414, 113066. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.L.; Mahung, C.; Wallet, S.M.; Barnett, A.; Cairns, B.A.; Coleman Jr, L.G.; Maile, R. Plasma extracellular vesicles released after severe burn injury modulate macrophage phenotype and function. J. Leukoc. Biol. 2022, 111, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, E.S. Tumor extracellular vesicles carrying antitumor (KLAKLAK)2 peptide and tumor-specific antigens for improved tumor therapy. J. Pharm. Investig. 2023, 53, 505–516. [Google Scholar] [CrossRef]
- Arıca, B.; Çalış, S.; Kaş, H.S.; Sargon, M.F.; Hıncal, A.A. 5-Fluorouracil encapsulated alginate beads for the treatment of breast cancer. Int. J. Pharm. 2002, 242, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Tolentino, M.Q.; Hartmann, A.K.; Loe, D.T.; Rouge, J.L. Controlled release of small molecules and proteins from DNA-surfactant stabilized metal organic frameworks. J. Mater. Chem. B 2020, 8, 5627–5635. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.; Yu, H.S.; Na, K.; Oh, K.T.; Lee, E.S. Dendritic cell-targeted pH-responsive extracellular vesicles for anticancer vaccination. Pharmaceutics 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.Y.; Choi, S.B.; Gan, C.Y. The potential roles of Pinto bean (Phaseolus vulgaris cv. Pinto) bioactive peptides in regulating physiological functions: Protease activating, lipase inhibiting and bile acid binding activities. J. Funct. Food 2017, 33, 67–75. [Google Scholar] [CrossRef]
- Choi, M.A.; Kim, S.H.; Chung, W.Y.; Hwang, J.K.; Park, K.K. Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem. Biophys. Res. Commun. 2004, 326, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Sugihara, E.; Ohta, S.; Izuhara, K.; Funakoshi, T.; Amagai, M.; Saya, H. Periostin is a key niche component for wound metastasis of melanoma. PLoS ONE 2015, 10, e0129704. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Zhang, X.; Giangrande, P.H.; McNamara II, J.O.; Nimjee, S.M.; Sarraf-Yazdi, S.; Sullenger, B.A.; Clary, B.M. Targeted inhibition of αvβ3 integrin with an RNA aptamer impairs endothelial cell growth and survival. Biochem. Biophys. Res. Commun. 2005, 338, 956–963. [Google Scholar] [CrossRef]
- Lerner, N.; Avissar, S.; Beit-Yannai, E. Extracellular vesicles mediate signaling between the aqueous humor producing and draining cells in the ocular system. PLoS ONE 2017, 12, e0171153. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.K.; Priya, V.; Mehata, A.K.; Muthu, M.S. Abciximab coated albumin nanoparticles of rutin for improved and targeted antithrombotic effect. J. Drug Deliv. Sci. Technol. 2022, 76, 103785. [Google Scholar] [CrossRef]
- Wang, X.; Gu, X.; Wang, H.; Sun, Y.; Wu, H.; Mao, S. Synthesis, characterization and liver targeting evaluation of self-assembled hyaluronic acid nanoparticles functionalized with glycyrrhetinic acid. Eur. J. Pharm. Sci. 2017, 96, 255–262. [Google Scholar] [CrossRef]
- Aimaletdinov, A.M.; Gomzikova, M.O. Tracking of extracellular vesicles’ biodistribution: New methods and approaches. Int. J. Mol. Sci. 2022, 23, 11312. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, E.; Lee, E.S. Development of 5-Fluorouracil/pH-Responsive Adjuvant-Embedded Extracellular Vesicles for Targeting αvβ3 Integrin Receptors in Tumors. Pharmaceutics 2024, 16, 599. https://doi.org/10.3390/pharmaceutics16050599
Kim J, Lee E, Lee ES. Development of 5-Fluorouracil/pH-Responsive Adjuvant-Embedded Extracellular Vesicles for Targeting αvβ3 Integrin Receptors in Tumors. Pharmaceutics. 2024; 16(5):599. https://doi.org/10.3390/pharmaceutics16050599
Chicago/Turabian StyleKim, Jiseung, Eunsol Lee, and Eun Seong Lee. 2024. "Development of 5-Fluorouracil/pH-Responsive Adjuvant-Embedded Extracellular Vesicles for Targeting αvβ3 Integrin Receptors in Tumors" Pharmaceutics 16, no. 5: 599. https://doi.org/10.3390/pharmaceutics16050599
APA StyleKim, J., Lee, E., & Lee, E. S. (2024). Development of 5-Fluorouracil/pH-Responsive Adjuvant-Embedded Extracellular Vesicles for Targeting αvβ3 Integrin Receptors in Tumors. Pharmaceutics, 16(5), 599. https://doi.org/10.3390/pharmaceutics16050599






