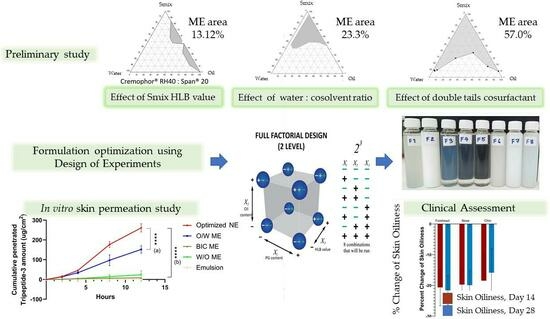
Microemulsions and Nanoemulsions for Topical Delivery of Tripeptide-3: From Design of Experiment to Anti-Sebum Efficacy on Facial Skin
Abstract
1. Introduction
2. Materials and Methods
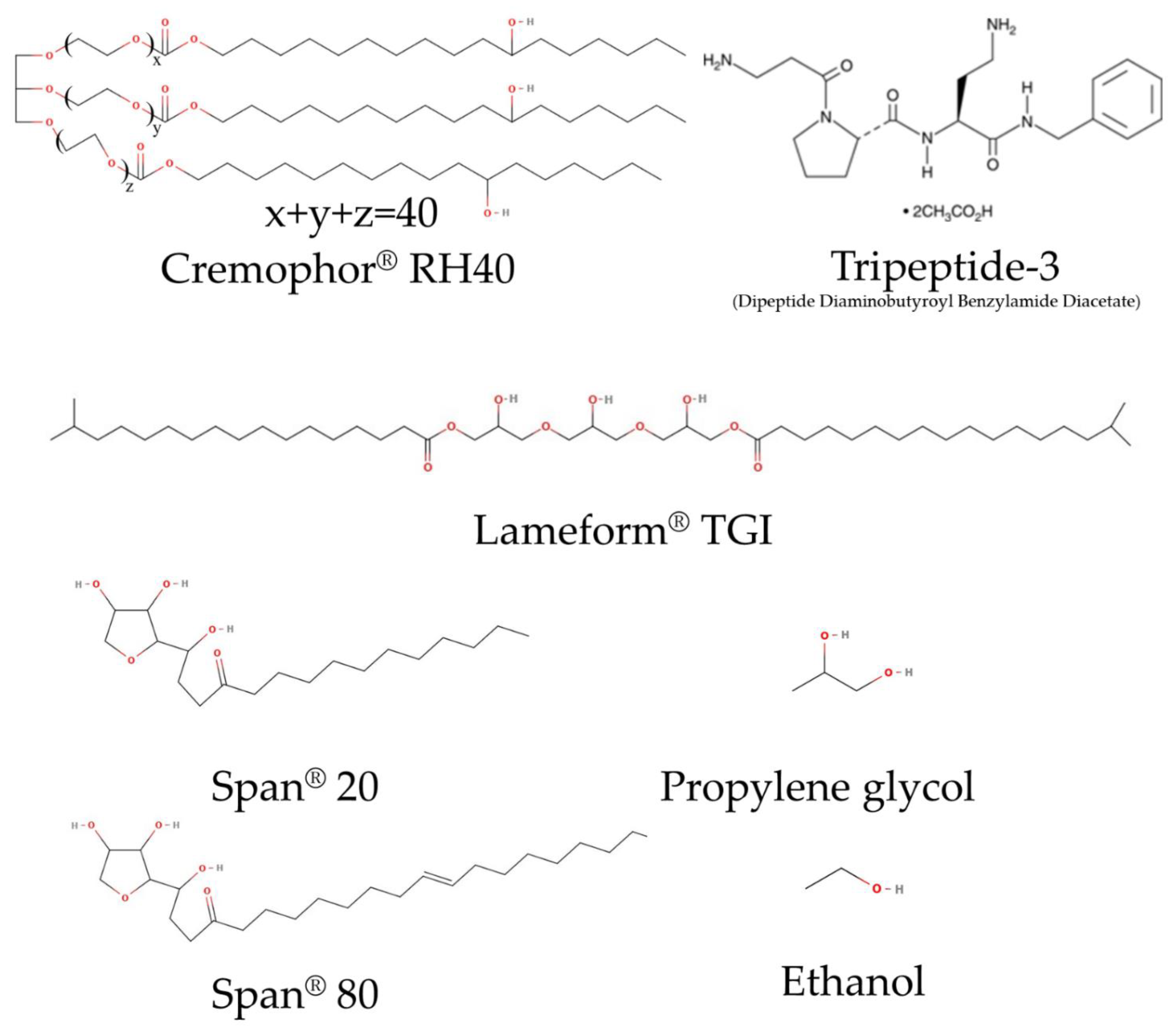
2.1. Materials
2.2. Construction of Phase Diagram to Identify Factors Affecting Micro- or Nanoemulsion Formulation
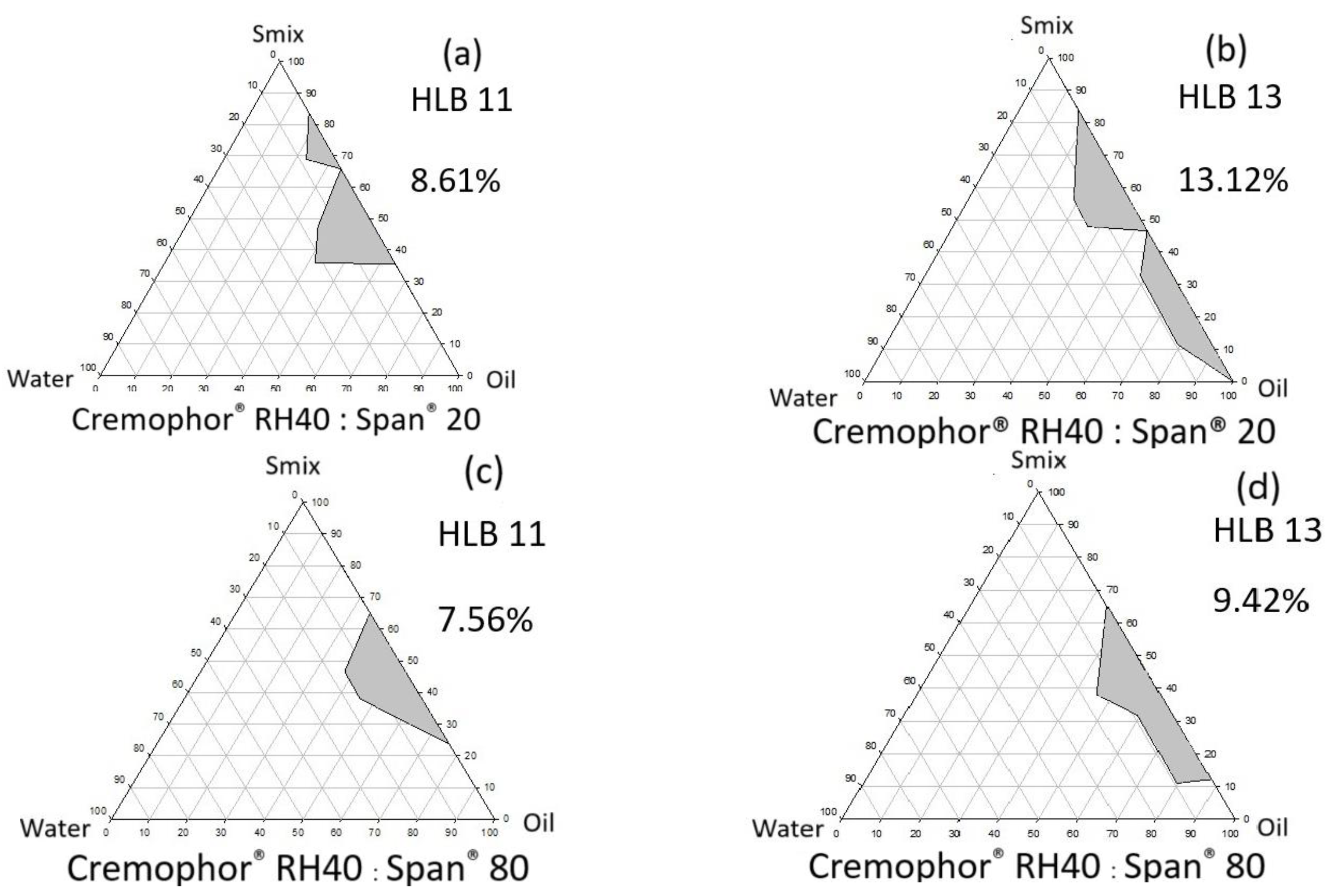
2.2.1. Effects of HLB Value of Surfactant and Single-Tail Co-Surfactant System
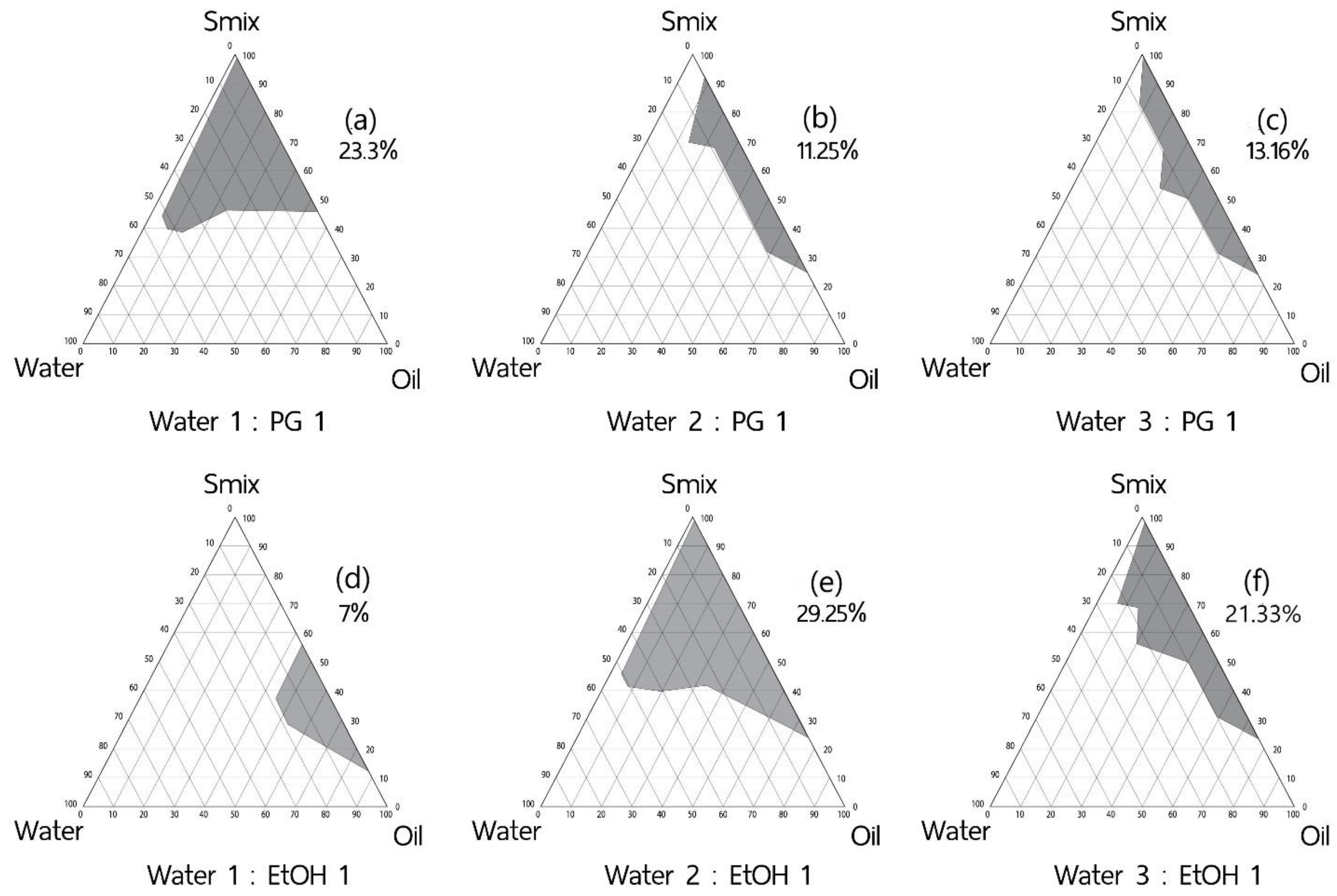
2.2.2. Effect of Co-Solvent
2.2.3. Effect of Co-Surfactant Hydrocarbon Tails
2.2.4. Design of Experiment Program (DoE) with Design Expert® for Low Surfactant Micro- or Nanoemulsions
2.3. Characterization of Studied Colloidal Dispersion
2.3.1. Polarized Light Microscopy
2.3.2. Droplet Size and Distribution Determination
2.3.3. Turbidity Measurement
2.3.4. Electrical Conductivity
2.3.5. Viscosity Measurement
2.4. Determination of Tripeptide-3 with High-Performance Liquid Chromatography (HPLC)
2.5. Stability Tests of Colloidal Dispersion
2.5.1. Stress Testing by Heating–Cooling Cycles
2.5.2. Stress Testing by Freeze–Thaw Cycles
2.5.3. Stress Testing by Centrifugation
2.5.4. pH Challenge Test
2.6. Skin Permeation Study
2.7. Skin Irritation Test and Efficacy Test of Tripeptide-3 Optimized Formulation in Human Volunteers
2.7.1. Ethics Consideration
2.7.2. Human Volunteers
2.7.3. Skin Irritation Test
2.7.4. Efficacy Test
2.8. Statistical Evaluation
3. Results & Discussion
3.1. Factors Affecting Micro- or Nanoemulsion Formulation
3.1.1. Effects of Surfactant HLB Value and Single-Tail Co-Surfactant System
3.1.2. Effect of Co-Solvents
3.1.3. Effect of Co-Surfactant Hydrocarbon Tails
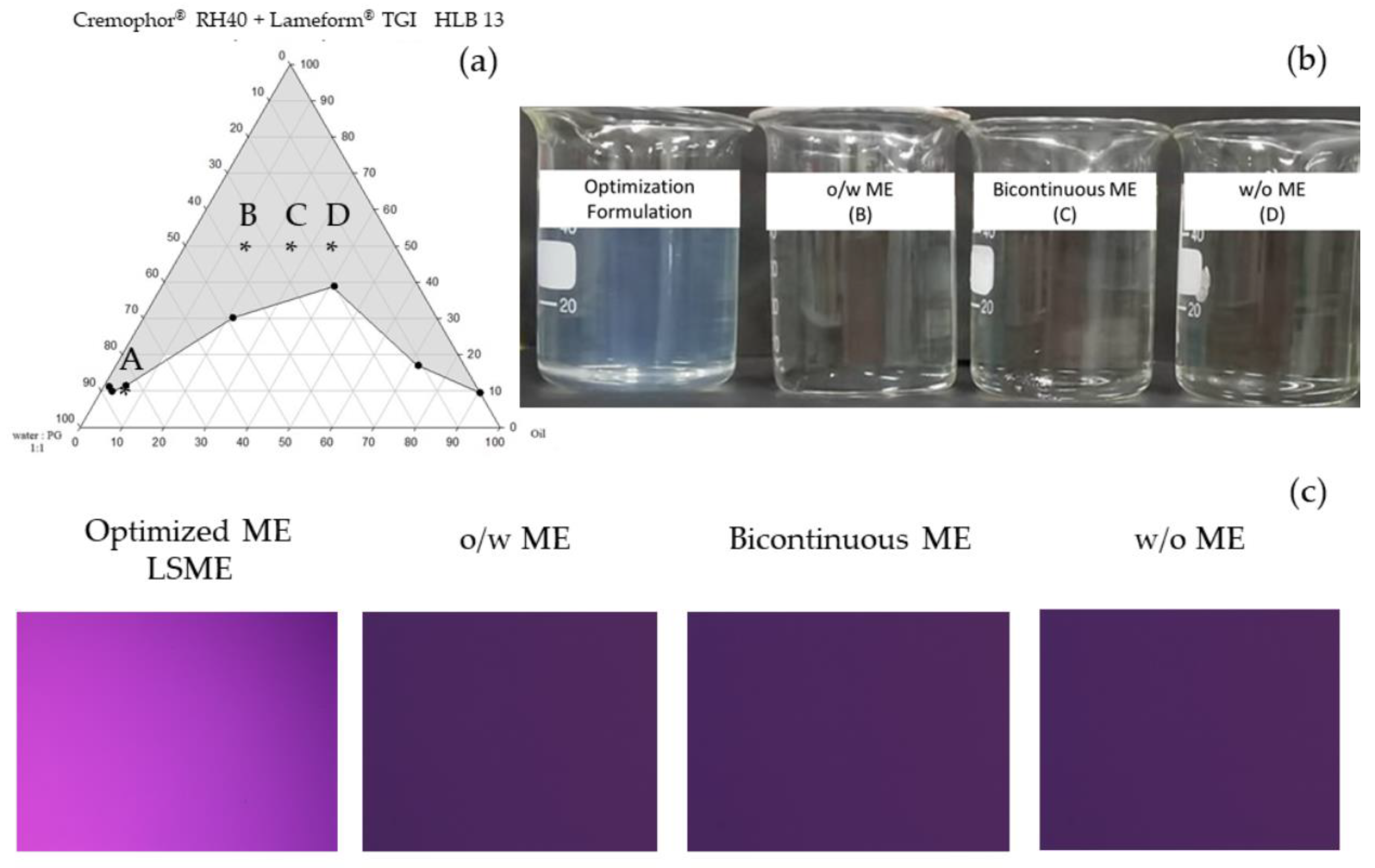
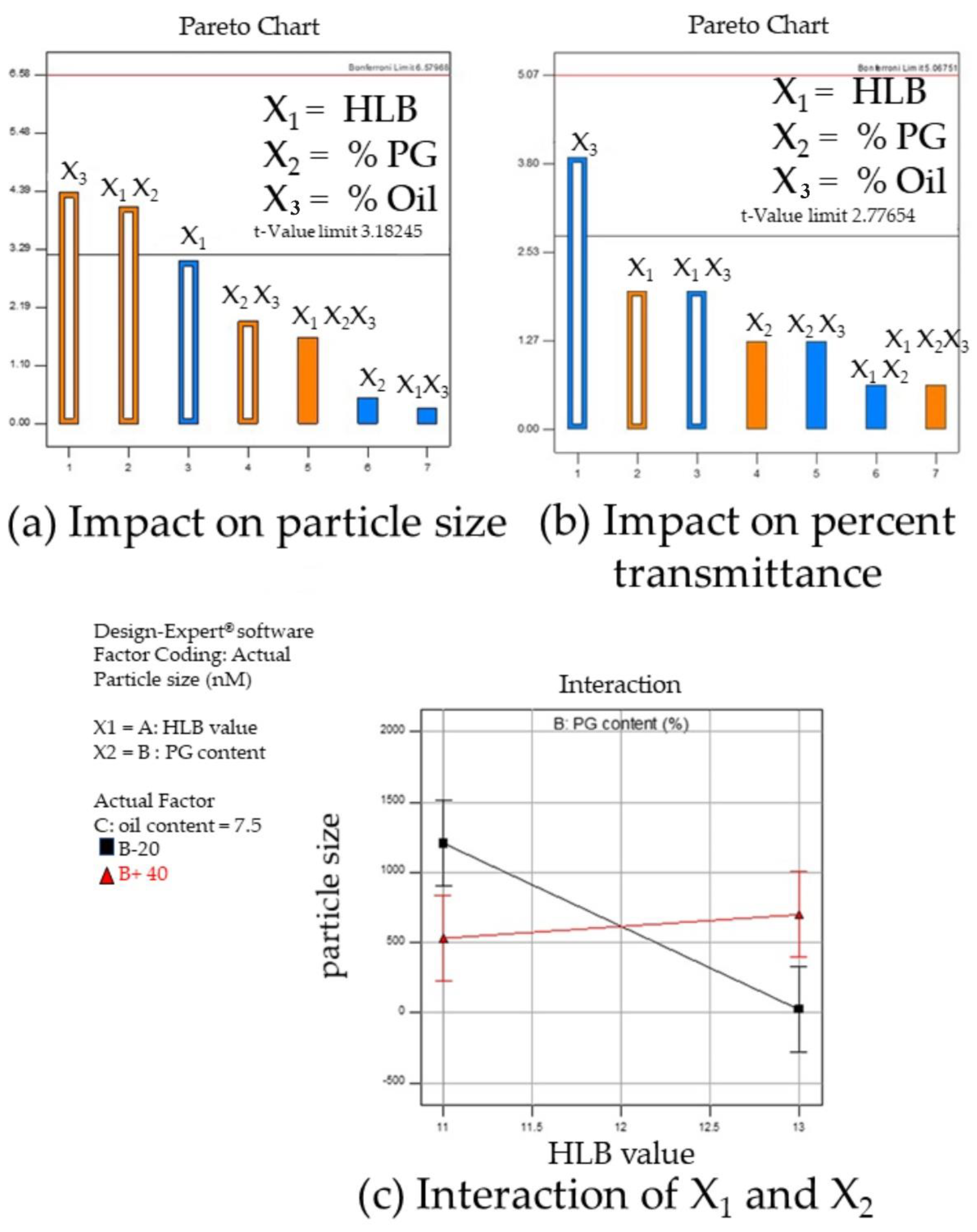
3.1.4. Design of Experiment Program (DoE) with Design Expert® for Low Surfactant Micro- or Nanoemulsions
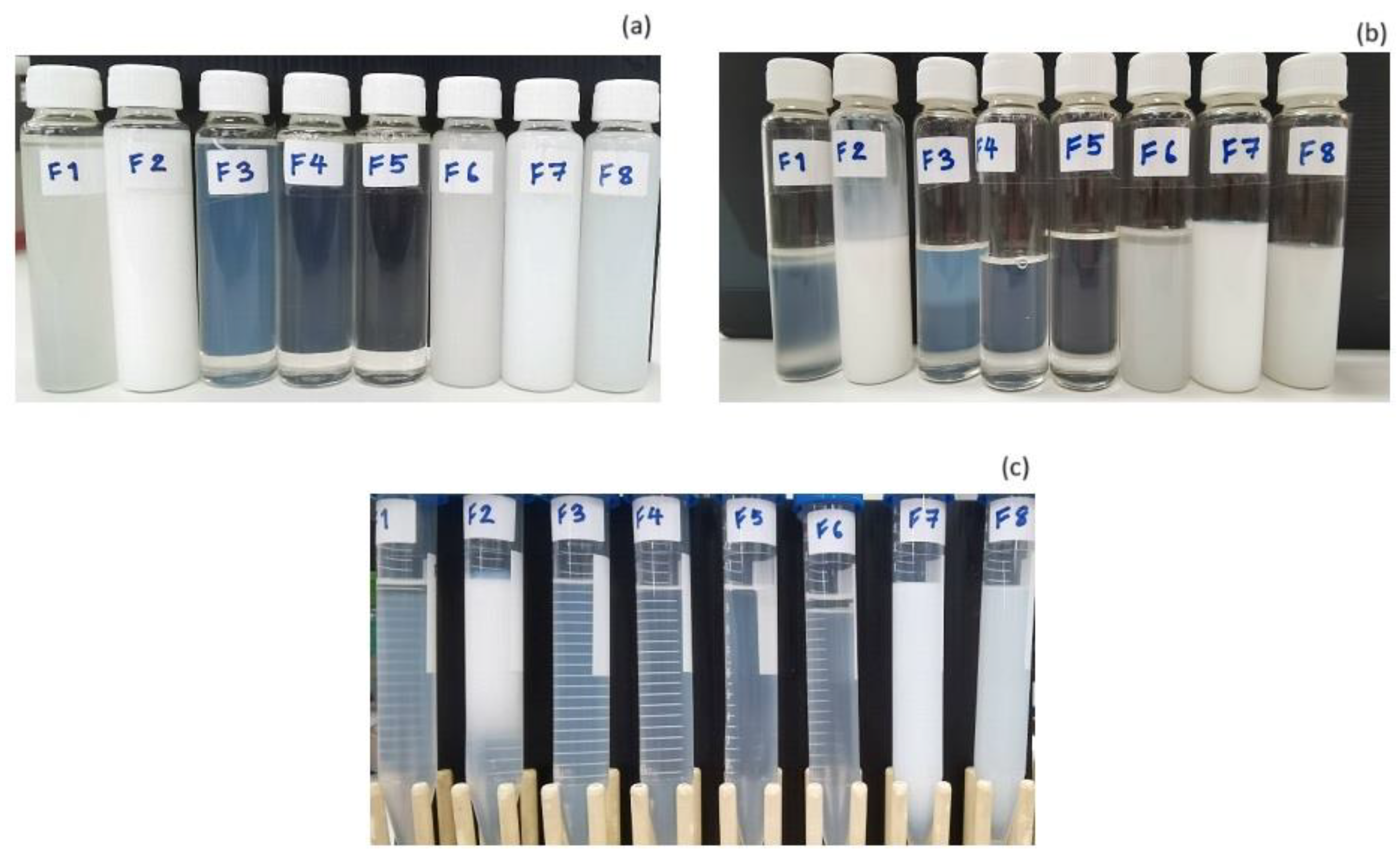
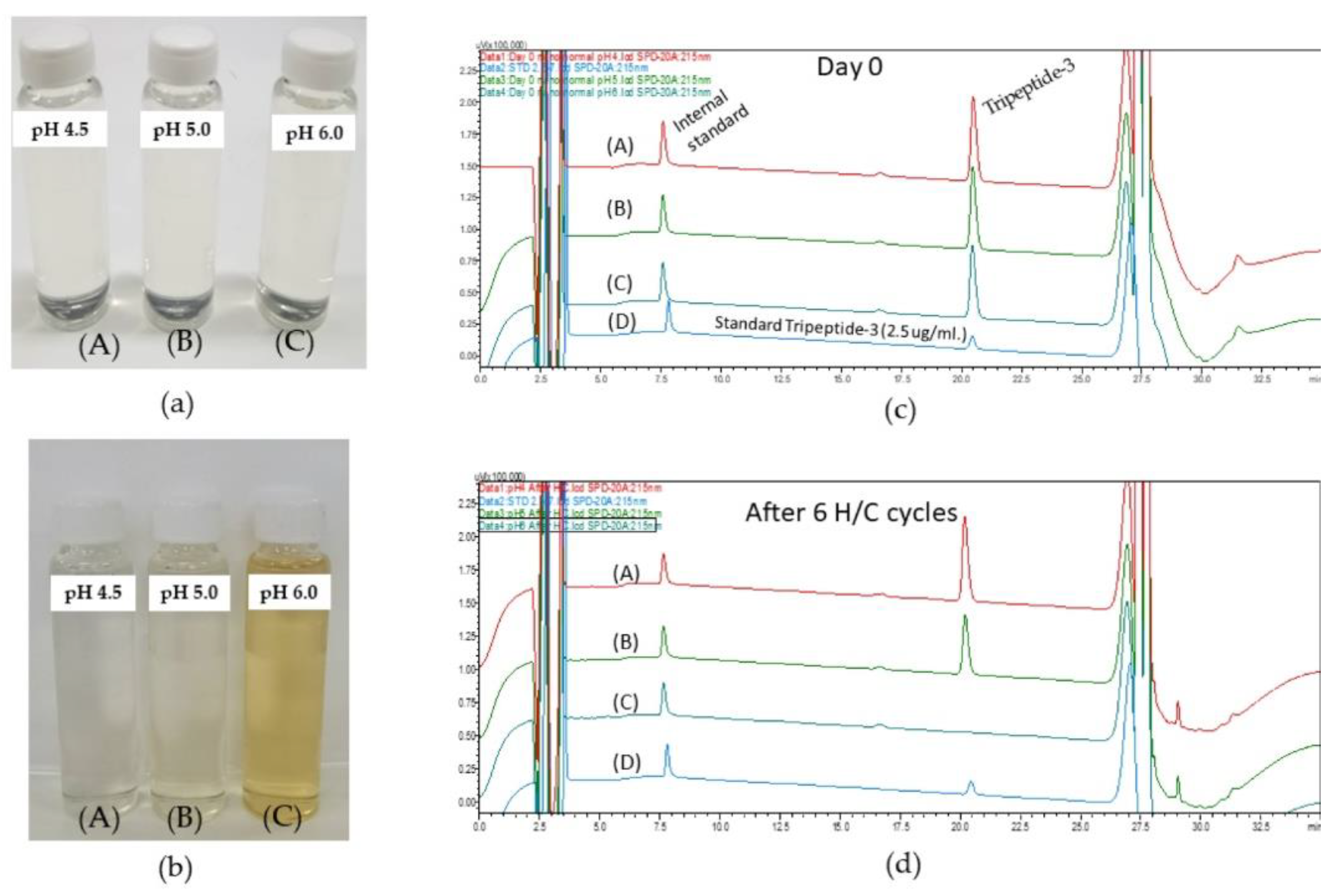
3.2. Stability Tests of Colloidal Dispersion from DoE
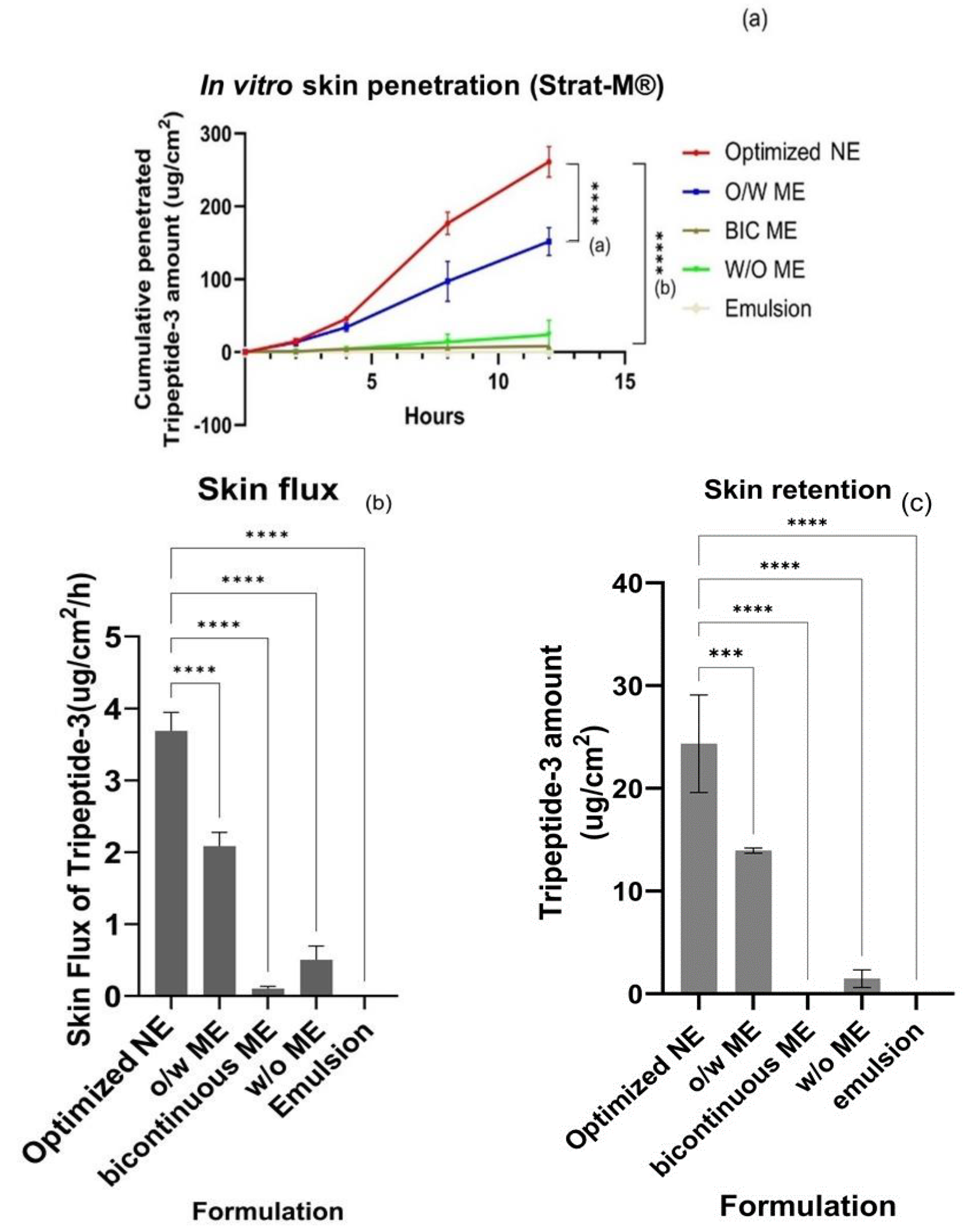
3.3. Skin Permeation of Tripeptide-3 Micro- and Nano-Formulations
3.4. Clinical Evaluation
3.4.1. Skin Irritation Test in Volunteers
3.4.2. Efficacy Evaluation of the Tripeptide-3 NE Product in Volunteers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadra, P.; Deb, A. A Literature review on acne due to hormonal changes and lifestyle. IJONS 2020, 10, 18507–18521. [Google Scholar]
- Bukvić Mokos, Z.; Kralj, M.; Basta-Juzbašić, A.; Lakoš Jukić, I. Seborrheic dermatitis: An update. ADC 2012, 20, 98–104. [Google Scholar]
- Wu, Y.; Niu, Y.; Zhong, S.; Liu, H.; Zhen, Y.; Saint-Leger, D.; Verschoore, M. A preliminary investigation of the impact of oily skin on quality of life and concordance of self-perceived skin oiliness and skin surface lipids (sebum). Int. J. Cosmet. Sci. 2013, 35, 442–447. [Google Scholar] [CrossRef]
- Rose, A.E.; David, J.G. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol. Surg. 2013, 39, 443–448. [Google Scholar] [CrossRef]
- Kraeling, M.; Zhou, W.; Wang, P.; Ogunsola, O. In vitro skin penetration of acetyl hexapeptide-8 from a cosmetic formulation. Cutan. Ocul. Toxicol. 2015, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Remo, C.; Dominik, I.; Eileen, J.; Eliane, U.W. Method for the Treatment of Skin Disorders Using Dipeptide Diaminobutylroyl Benzylamide Diacetate. U.S. Patent 10561702B2, 18 February 2020. [Google Scholar]
- Talianu, M.-T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuţa, V.; Jinga, V.; Popa, L. Foray into concepts of design and evaluation of microemulsions as a modern approach for topical applications in acne pathology. Nanomaterials 2020, 10, 2292. [Google Scholar] [CrossRef]
- Mortazavi, S.; Moghimi, H. Skin permeability, a dismissed necessity for anti-wrinkle peptide performance. Int. J. Cosmet. Sci. 2022, 44, 232–248. [Google Scholar] [CrossRef] [PubMed]
- Sabale, V.; Vora, S. Formulation and evaluation of microemulsion-based hydrogel for topical delivery. Int. J. Pharm. Investig. 2012, 2, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Eqbal, A.; Ansari, V.; Hafeez, A.; Ahsan, F.; Imran, M.; Tanweer, S. Recent applications of nanoemulsion based drug delivery system: A review. Res. J. Pharm. Technol. 2021, 14, 2852–2858. [Google Scholar] [CrossRef]
- Kawakami, K.; Yoshikawa, T.; Moroto, Y.; Kanaoka, E.; Takahashi, K.; Nishihara, T.; Masuda, K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. J. Control. Release 2002, 81, 65–74. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Bhatt, P.; Madhav, S. A detailed review on nanoemulsion drug delivery system. Int. J. Pharm. Sci. Res. 2011, 2, 2482–2489. [Google Scholar]
- Reza, K.H. Nanoemulsion as a novel transdermal drug delivery system. Int. J. Pharm. Sci. Res. 2011, 8, 1938–1946. [Google Scholar]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielinska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Delmas, T.; Piraux, H.; Couffin, A.C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How to prepare and stabilize very small nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar] [CrossRef]
- Sole, I.; Solans, C.; Maestro, A.; Gonzalez, C.; Gutierrez, J.M. Study of nano-emulsion formation by dilution of microemulsions. J. Colloid. Interface Sci. 2012, 376, 133–139. [Google Scholar] [CrossRef]
- Sommer, E.; Neubert, R.H.H.; Mentel, M.; Tuchscherer, B.; Mrestani, Y.; Wohlrab, J. Dermal peptide delivery using enhancer molecules and colloidal carrier systems. Part III: Tetrapeptide GEKG. Eur. J. Pharm. Sci. 2018, 124, 137–144. [Google Scholar] [CrossRef]
- Alireza, S.; Sharma, A.C.; Patel, D.; Sayani, A. Low-surfactant microemulsions for enhanced topical delivery of poorly soluble drug. J. Pharm. Pharm. Sci. 2011, 14, 315–324. [Google Scholar] [CrossRef][Green Version]
- Schafer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- de Souza Neto, A.V.; Balla, D.Q.; Candido, T.M.; Rosado, C.; Baby, A.R.; Pessoa, F.V.L.S. Effect of an emollient emulsion containing 15.0% of caprylic/capric triglyceride on the urocanic acid of the stratum corneum. Life 2023, 13, 876. [Google Scholar] [CrossRef]
- Lee, S.; Chow, P.; Yagnik, C. Developing eco-friendly skin care formulations with microemulsions of essential oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Fleancu, M.C.; Olteanu, N.L.; Rogozea, A.E.; Crisciu, A.V.; Pincovschi, I.; Mihaly, M. Physical–chemical parameters promoting phase changes in non-ionic environmental-friendly microemulsions. Fluid. Phase Equilibria 2013, 337, 18–25. [Google Scholar] [CrossRef]
- Racoviţa, R.C.; Crişciu, A.V.; Sima, S.; Secuianu, C. A convenient hybrid method for obtaining liquid–liquid equilibrium data in ternary systems. J. Chem. Eng. Data 2020, 65, 3384–3392. [Google Scholar] [CrossRef]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P. Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic-lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech 2014, 15, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dunham, A. Pharmacy Calculations; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Ragheb, R.; Nobbmann, U. Multiple scattering effects on intercept, size, polydispersity index, and intensity for parallel (VV) and perpendicular (VH) polarization detection in photon correlation spectroscopy. Sci. Rep. 2020, 10, 21768. [Google Scholar] [CrossRef] [PubMed]
- Gogate, S.U.; Schwartz, P.A.; Agharkar, S.N. Effect of unpurified Cremophor EL on the solution stability of paclitaxel. Pharm. Dev. Technol. 2009, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W.; Kim, S.J.; Hwang, I.A.; Park, K.C. Evaluation of facial skin type by sebum secretion: Discrepancies between subjective descriptions and sebum secretion. Ski. Res. Technol. 2002, 8, 168–172. [Google Scholar] [CrossRef]
- Meetham, P.; Kanlayavattanakul, M.; Lourith, N. Development and clinical efficacy evaluation of anti-greasy green tea tonner on facial skin. Rev. Bras. Farmacogn. 2018, 28, 214–217. [Google Scholar] [CrossRef]
- Basketter, D.A.; York, M.; McFadden, J.P.; Robinson, M.K. Determination of skin irritation potential in the human 4-h patch test. Contact Dermat. 2004, 50, 1–4. [Google Scholar] [CrossRef]
- Jırova, D.J.; Basetter, D.; Liebsch, M.; Bendova, H.; Kejlova, K.; Marriott, M.; Kandárová, H. Comparison of human skin irritation patch test data with in vitro skin irritation assays and animal data. Contact Dermat. 2010, 62, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hallas, J.; Pottegard, A. Use of self-controlled designs in pharmacoepidemiology. J. Intern. Med. 2014, 275, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Singhan, A.; Indranupakorn, I. Microemulsion development for enhancing the stability of green tea. Isan J. Pharm. Sci. 2015, 11, 40–56. [Google Scholar]
- Millard, J.; Núñez, F.; Yalkowsky, S. Solubilization by cosolvents. Establishing useful constants for the log-linear model. Int. J. Pharm. 2002, 245, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef] [PubMed]
- Poomanee, W.; Kongin, K.; Sriputorn, K.; Leelapornpisid, P. Application of factorial experimental design for optimization and development of color lipstick containing antioxidant-rich sacha inchi oil. Pak. J. Pharm. Sci. 2021, 34, 1437–1444. [Google Scholar]
- Hu, X.; Wang, H.; Liu, Y. Statistical analysis of main and interaction effects on Cu(II) and Cr(VI) decontamination by nitrogen-doped magnetic graphene oxide. Sci. Rep. 2016, 6, 34378. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, G.; Zhou, Y.; Banga, A.K. Adapalene microemulsion for transfollicular drug delivery. J. Pharm. Sci. 2013, 102, 2622–2631. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Safaya, M.; Rotliwala, Y.C. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Fernando, R.; Laerte, D.; Rosa, S.; Thalita, H.; Daniele, S.; Cleônia, A.; Talita, G.; Larissa, R.; Patrícia, G.; Antonio, T.; et al. Preparation and characterization of nanoemulsion containing a natural naphthoquinone. Química Nova 2018, 41, 756–761. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Kichou, H.; Bonnier, F.; Dancik, Y.; Bakar, J.; Michael-Jubeli, R.; Caritá, A.C.; Perse, X.; Soucé, M.; Rapetti, L.; Tfayli, A.; et al. Strat-M® positioning for skin permeation studies: A comparative study including EpiSkin® RHE, and human skin. Int. J. Pharm. 2023, 647, 123488. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Fan, W.; Yu, Q.; Dong, X.; Qi, J.; Zhu, Q.; Zhao, W.; Wu, W.; Chen, Z.; Li, Y.; et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: Implications for transdermal delivery and immunization. Oncotarget 2017, 8, 38214–38226. [Google Scholar] [CrossRef] [PubMed]
- Endly, D.; Miller, R. OILY SKIN: A review of treatment options. J. Clin. Aesthet. Dermatol. 2017, 10, 49–55. [Google Scholar] [PubMed]
- Pai, V.V.; Bhandari, P.; Shukla, P. Topical peptides as cosmeceuticals. Indian. J. Dermatol. Venereol. Leprol. 2017, 83, 9–18. [Google Scholar] [CrossRef]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Guerle-Cavero, R.; Balfagón-Costa, A. Study of elastin, hydrolyzed collagen and collagen-like products in a tri-layered chitosan membrane to test anti-aging skin properties. Int. J. Mol. Sci. 2023, 24, 11016. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Ski. Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.; Campos, P. Correlations between sebaceous glands activity and porphyrins in the oily skin and hair and immediate effects of dermocosmetic formulations. J. Cosmet. Dermatol. 2020, 19, 3100–3106. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Kuo, S.; Wang, Y.; Jiang, Y.; Liu, Y.-T.; Gallo, R.L.; Huang, C.-M. Porphyrin metabolisms in human skin commensal propionibacterium acnes bacteria potential application to monitor human radiation risk. Curr. Med. Chem. 2013, 20, 562–568. [Google Scholar] [PubMed]

| Factors | Level | ||
|---|---|---|---|
| Low Level | High Level | ||
| X 1 | HLB value | 11 | 13 |
| X 2 | Propylene glycol content | 20% | 40% |
| X 3 | Oil content | 5% | 10% |
| HLB | Surfactant Mixing Ratio (% w/w) | ME Area (%) | |
|---|---|---|---|
| Cremophor® RH40 | Span® 80 | ||
| 7 | 25.2 | 74.8 | 0 |
| 9 | 43.9 | 56.1 | 0 |
| 11 | 62.6 | 37.4 | 7.56 |
| 13 | 81.3 | 18.7 | 9.42 |
| HLB | Cremophor® RH40 | Span® 20 | |
| 7 | N/A | N/A | N/A 1 |
| 9 | 6.3 | 93.8 | 0 |
| 11 | 37.5 | 62.5 | 8.61 |
| 13 | 68.8 | 31.3 | 13.12 |
| Electrical Conductivity (μS/cm) | |||
|---|---|---|---|
| Optimized NE | O/W ME | Bicontinuous ME | W/O ME |
| 3663.58 ± 5.8 | 208.53 ± 0.7 | 81.92 ± 0.1 | 28.40 ± 0.1 |
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Residuals of Response 1 | Response 2 | Residuals of Response 2 | ||
|---|---|---|---|---|---|---|---|---|
| Std | Run (F) | A:HLB Value | B:PG Content (%) | C:Oil Content (%) | Particle Size (nm) | Turbidity (% Transmittance) | ||
| 7 | 1 | 11 | 40 | 10 | 901.9 ± 911.20 | −353.35 | 0.0 ± 0.01 | 0.000 |
| 5 | 2 | 11 | 20 | 10 | 1608.6 ± 1040.90 | 353.35 | 0.0 ± 0.01 | 0.000 |
| 3 | 3 | 11 | 40 | 5 | 81.2 ± 11.50 | −12.55 | 51.2 ± 0.01 | 5.310 |
| 2 | 4 | 13 | 20 | 5 | 25.7 ± 1.20 | −1.3 | 70.6 ± 0.58 | −5.565 |
| 4 | 5 | 13 | 40 | 5 | 28.3 ± 1.80 | 1.3 | 87.3 ± 0.01 | 0.565 |
| 8 | 6 | 13 | 40 | 10 | 1290.6 ± 1098.20 | 201.1 | 0.0 ± 0.00 | −0.003 |
| 6 | 7 | 13 | 40 | 10 | 106.3 ± 3.90 | 12.55 | 0.0 ± 0.00 | −5.310 |
| 1 | 8 | 11 | 20 | 5 | 888.4 ± 809.10 | −201.1 | 0.1 ± 0.01 | 0.003 |
| Formulation | Particle Size (nm) Day 0 | Percent Transmittance (%) | Centrifuge at 10,000 rpm 15 min | Heating/Cooling 6 Cycles | Particle Size (nm) After H/C |
|---|---|---|---|---|---|
| 3 | 81.2 ± 11.50 | 51.2 ± 0.01 | Stable | x | N/A |
| 4 | 25.7 ± 1.20 | 70.6 ± 0.58 | Stable | Stable | 22.5 ± 1.80 |
| 5 | 28.3 ± 1.80 | 87.3 ± 0.01 | Stable | Stable | 23.58 ± 1.04 * |
| Formulation 4 | Particle Size Measurement | |||
|---|---|---|---|---|
| Day 0 Size nm (PDI) | After 6 H/C Cycles Size nm (PDI) | After 3 F/T Cycles Size nm (PDI) | Day 180 Size nm (PDI) | |
| pH 4.5 | 28.1 ± 0.7 (0.27 ± 0.010) | 26.7± 0.3 * (0.149 ± 0.028) * p = 0.0334 | 31.5 ± 0.3 ** (0.262 ± 0.014) ** p = 0.0015 | 29.1± 0.4 (0.075 ± 0.038) p = 0.0599 ns |
| pH 5.0 | 23.9 ± 0.3 ### (0.152 ± 0.002) ### p = 0.0007 vs. pH 4.5) | 30.1 ± 0.0 **** (0.258 ± 0.016) **** p < 0.0001 vs. day 0 | 26.1 ± 0.2 *** (0.154 ± 0.01) *** p = 0.0005 vs. day 0 | N/A |
| pH 6.0 | 24.3 ± 0.1 ### (0.267 ± 0.032) ### p = 0.0007 vs. pH 4.5 | 25.4± 0.5 * (0.220 ± 0.037) * p = 0.0202 vs. day 0 | 28.0 ± 0.3 *** (0.278 ± 0.015) **** p = 0.0001 vs. day 0 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magrode, N.; Poomanee, W.; Kiattisin, K.; Ampasavate, C. Microemulsions and Nanoemulsions for Topical Delivery of Tripeptide-3: From Design of Experiment to Anti-Sebum Efficacy on Facial Skin. Pharmaceutics 2024, 16, 554. https://doi.org/10.3390/pharmaceutics16040554
Magrode N, Poomanee W, Kiattisin K, Ampasavate C. Microemulsions and Nanoemulsions for Topical Delivery of Tripeptide-3: From Design of Experiment to Anti-Sebum Efficacy on Facial Skin. Pharmaceutics. 2024; 16(4):554. https://doi.org/10.3390/pharmaceutics16040554
Chicago/Turabian StyleMagrode, Nontachai, Worrapan Poomanee, Kanokwan Kiattisin, and Chadarat Ampasavate. 2024. "Microemulsions and Nanoemulsions for Topical Delivery of Tripeptide-3: From Design of Experiment to Anti-Sebum Efficacy on Facial Skin" Pharmaceutics 16, no. 4: 554. https://doi.org/10.3390/pharmaceutics16040554
APA StyleMagrode, N., Poomanee, W., Kiattisin, K., & Ampasavate, C. (2024). Microemulsions and Nanoemulsions for Topical Delivery of Tripeptide-3: From Design of Experiment to Anti-Sebum Efficacy on Facial Skin. Pharmaceutics, 16(4), 554. https://doi.org/10.3390/pharmaceutics16040554