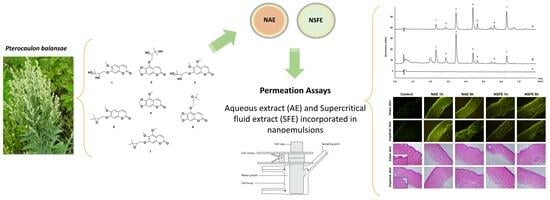
Topical Nanoemulsions as Delivery Systems for Green Extracts of Pterocaulon balansae Aiming at the Treatment of Sporotrichosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Materials
2.3. Strains
2.4. Pterocaulon Balansae Extraction
2.5. Preparation of Nanoemulsions
2.6. Physicochemical Properties of Nanoemulsions
2.6.1. Droplet Size, Polydispersity Index (PDI), and ζ-Potential
2.6.2. pH and Viscosity Measurements
2.6.3. NBD-PE-Labelled Nanoemulsions
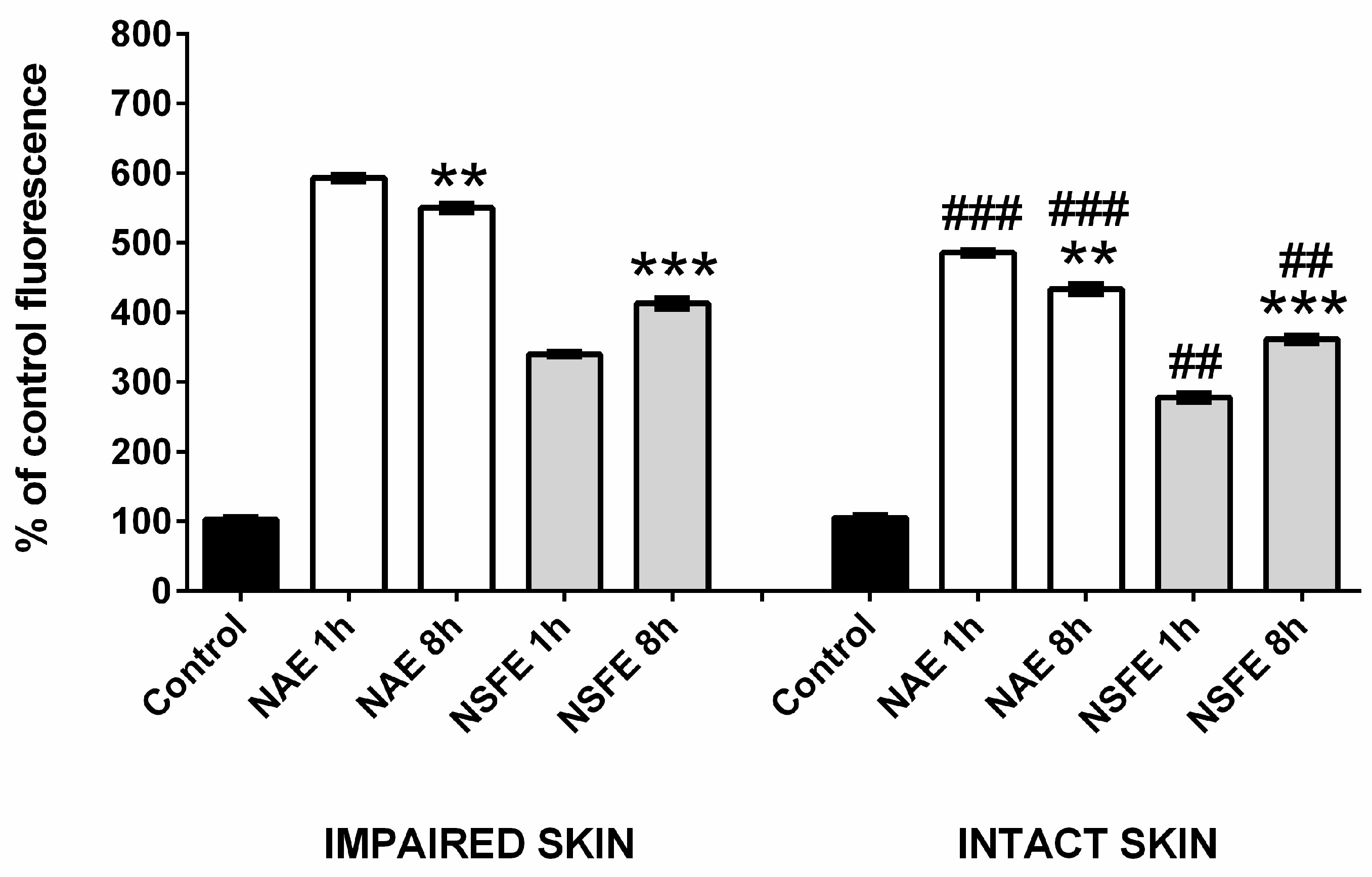
2.7. Permeation/Retention Assay in Intact and Impaired Porcine Skin
2.8. Histological and Confocal Microscopy
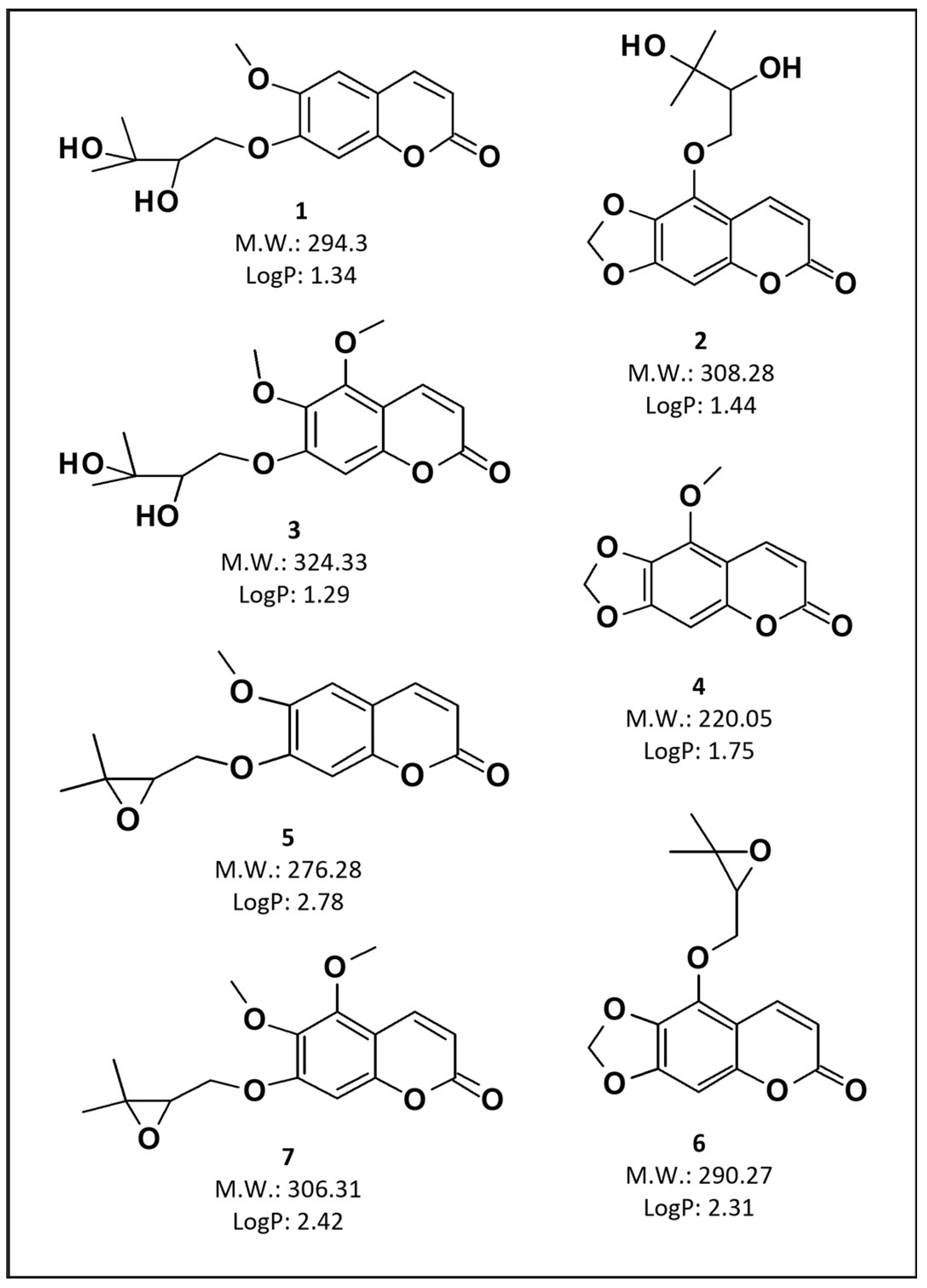
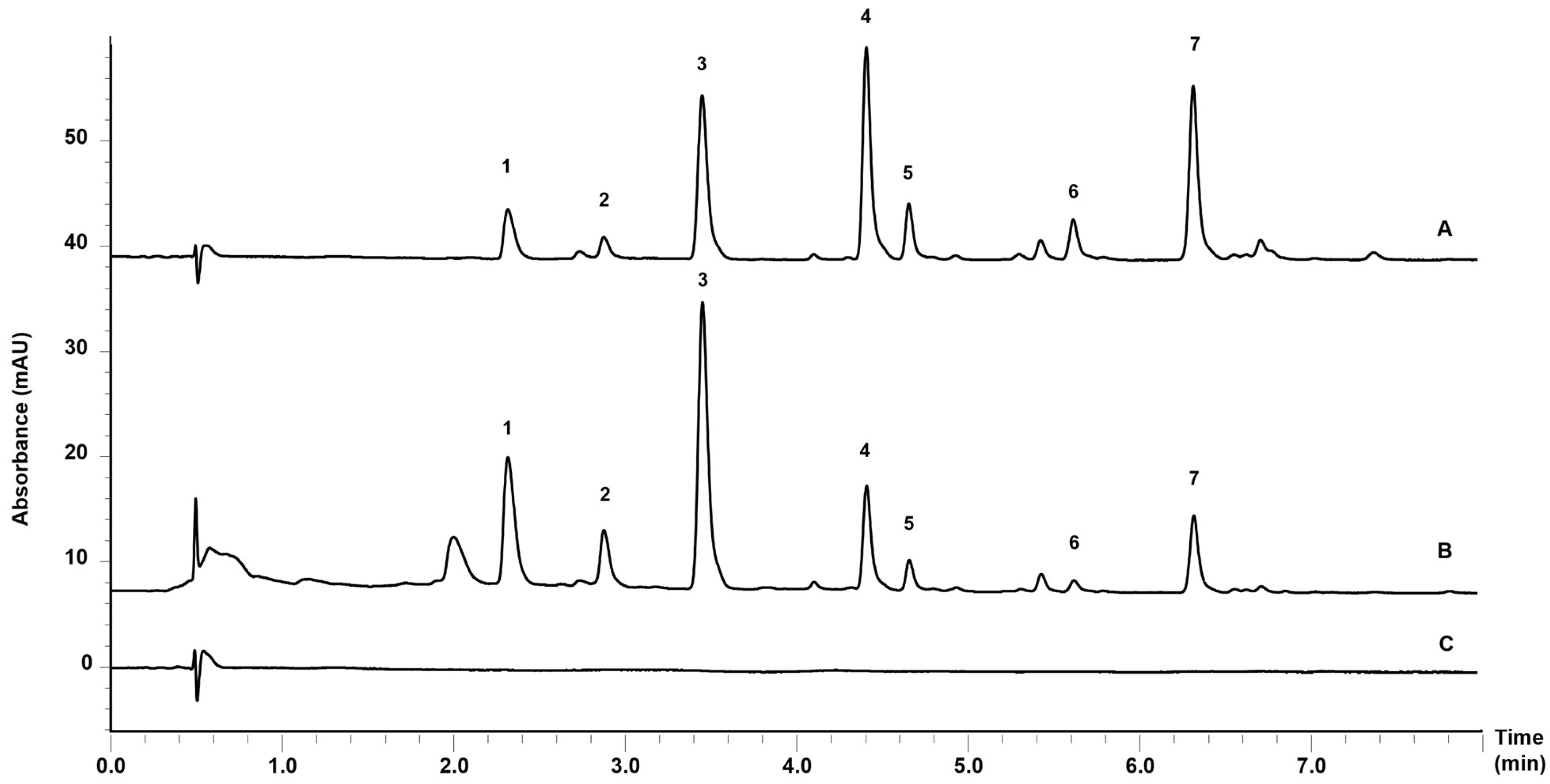
2.9. Ultra Fast Liquid Chromatography Analyses
2.10. Antifungal Activity
2.10.1. Minimum Inhibitory Concentration (MIC)
2.10.2. Hyphal Damage Assay with MTT
2.11. Statistical Analyses
3. Results and Discussion
3.1. Preparation and Characterization of Nanoemulsions
3.2. Permeation/Retention Assay in Intact and Impaired Skin
3.3. Microscopy Analyses
3.4. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khwaza, V.; Aderibigbe, B.A. Antifungal activities of natural products and their hybrid molecules. Pharmaceutics 2023, 15, 2673. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Prusty, J.S.; Kumar, A. Coumarins: Antifungal effectiveness and future therapeutic scope. Mol. Divers. 2020, 24, 1367–1383. [Google Scholar] [CrossRef]
- Koley, M.; Han, J.; Soloshonok, V.A.; Mojumder, S.; Javahershenas, R.; Makarem, A. Latest developments in coumarin-based anticancer agents: Mechanism of action and structure-activity relationship studies. RSC Med. Chem. 2024, 15, 10–54. [Google Scholar] [CrossRef]
- Srikrishna, D.; Godugu, C.; Kumar Dubey, P. A review on pharmacological properties of coumarins. Mini-Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Medeiros-Neves, B.; Teixeira, H.F.; von Poser, G.L. The genus Pterocaulon (Asteraceae)—A review on traditional medicinal uses, chemical constituents and biological properties. J. Ethnopharmacol. 2018, 224, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Stopiglia, C.D.O.; da Rocha Vianna, D.; de Carvalho Meirelles, G.; Teixeira, H.; von Poser, G.L.; Scroferneker, M.L. Antifungal activity of Pterocaulon species (Asteraceae) against Sporothrix schenckii. J. Mycol. Med. 2011, 21, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.K. Sporotrichosis: An Overview and Therapeutic Options. Dermatol. Res. Pract. 2014, 2014, 272376. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Hagen, F.; de Camargo, Z.P. A spotlight on Sporothrix and Sporotrichosis. Mycopathologia 2022, 187, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.M.; Oliveira, M.M.E.; Pires, R.H. Sporotrichosis: A review of a neglected disease in the last 50 years in Brazil. Microorganisms 2022, 10, 2152. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, J.; Dai, X.; Li, X. Extraction and analysis of chemical compositions of natural products and plants. Separations 2023, 10, 598. [Google Scholar] [CrossRef]
- Makdud, I.; Malakar, S.; Rao, M.V.; Kumar, N.; Sahu, J.K. Recent advancement in ultrasound-assisted novel technologies for the extraction of bioactive compounds from herbal plants: A review. Food Sci. Biotechnol. 2023, 32, 1763–1782. [Google Scholar]
- Nene, S.; Shah, S.; Rangaraj, N.; Mehra, N.K.; Singh, P.K.; Srivastava, S. Lipid based nanocarriers: A novel paradigm for topical antifungal therapy. J. Drug Deliv. Sci. Technol. 2021, 62, 102397. [Google Scholar] [CrossRef]
- Harwansha, R.K.; Deshmukha, R.; Rahmanb, M.A. Nanoemulsion promising nanocarrier system for delivery of herbal bioactives.pdf. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Medeiros-Neves, B.; Nemitz, M.; Brazil, N.; Schuh, R.; Steppe, M.; von Poser, G.; Teixeira, H. Determination of coumarins from Pterocaulon balansae by an Ultra-Fast Liquid Chromatography method in topical applications. Rev. Bras. Farmacogn. 2020, 30, 765–773. [Google Scholar] [CrossRef]
- Torres, F.C.; Medeiros-Neves, B.; Ferreira Teixeira, H.; Kawanoa, D.; Eifler-Lima, V.L.; Cassel, E.; Vargas, R.M.F.; von Poser, G.L. Supercritical CO2 extraction as a selective method for the obtainment of coumarins from Pterocaulon balansae (Asteraceae). J. CO2 Util. 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Argenta, D.F.; de Mattos, C.B.; Misturini, F.D.; Koester, L.S.; Bassani, V.L.; Maria Oliveira Simões, C.; Teixeira, H.F. Factorial design applied to the optimization of lipid composition of topical antiherpetic nanoemulsions containing isoflavone genistein. Int. J. Nanomed. 2014, 9, 4737–4747. [Google Scholar] [CrossRef][Green Version]
- OECD. OECD Guideline for the Testing of Chemicals 428: Skin Absorption: In Vitro Method; OECD: Paris, Francs, 2004; pp. 1–8. [Google Scholar]
- Kaushik, V.; Keck, C.M. Influence of mechanical skin treatment (massage, ultrasound, microdermabrasion, tape stripping and microneedling) on dermal penetration efficacy of chemical compounds. Eur. J. Pharm. Biopharm. 2021, 169, 29–36. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. In Approved Standard—Second Edition; Libk. 28; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; ISBN 1562386689. [Google Scholar]
- Chiou, C.C.; Mavrogiorgos, N.; Tillem, E.; Hector, R.; Walsh, T.J. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2001, 45, 3310–3321. [Google Scholar] [CrossRef]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and microemulsions in biomedicine: From theory to practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route. A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef]
- Patel, M.; Patel, A.; Desai, J.; Patel, S. Cutaneous pharmacokinetics of topically applied novel dermatological formulations. AAPS PharmSciTech 2024, 25, 46. [Google Scholar] [CrossRef]
- Nagasa, G.D.; Belete, A. Review on nanomaterials and nano-scaled systems for topical and systemic delivery of antifungal drugs. J. Multidiscip. Healthc. 2022, 15, 1819–1840. [Google Scholar] [CrossRef]

| Composition | NB | NAE | NSFE |
|---|---|---|---|
| Medium chain triglycerides (MCT) (%) | 16.0 | 16.0 | 16.0 |
| Egg lecithin (%) | 4.0 | 4.0 | 4.0 |
| Polysorbate 80 (%) | 1.0 | 1.0 | 1.0 |
| AE (mg/mL) | - | 1.60 | - |
| SFE (mg/mL) | - | - | 1.60 |
| Water to | 100.0 | 100.0 | 100.0 |
| Parameter | NB | NAE | NSFE |
|---|---|---|---|
| Size (nm) | 133.02 (3.87) a | 162.02 (6.59) ac | 127.49 (4.18) c |
| PDI | 0.145 (0.02) | 0.111 (0.02) | 0.096 (0.03) |
| ζ-potential (mV) | −38.92 (1.77) b | −32.62 (6.03) | −21.20 (3.42) b |
| pH | 4.69 (0.03) ab | 5.33 (0.01) ac | 4.46 (0.08) bc |
| Viscosity (cP) | 0.96 (0.007) ab | 1.08 (0.011) a | 1.06 (0.005) b |
| Total coumarin content (mg/mL) | - | 1.448 (0.04) | 1.461 (0.06) |
| NAE Mean (SD) | NSFE Mean (SD) | ||
|---|---|---|---|
| µg/cm2 | µg/cm2 | ||
| Intact skin | Stratum corneum | 0.86 (0.23) | 0.52 (0.06) |
| Viable epidermis | 1.16 (0.40) | 1.03 (0.13) | |
| Dermis | 1.98 (0.39) a | 2.15 (0.14) c | |
| Receptor fluid | 0.70 (0.11) b | 0.59 (0.05) d | |
| Impaired skin | Epidermis | 1.02 (0.74) | 0.91 (0.16) |
| Dermis | 2.85 (0.81) a | 2.79 (0.42) c | |
| Receptor fluid | 1.76 (0.51) b | 2.24 (0.23) d |
| Samples | Sporothrix schenkii | ||||
|---|---|---|---|---|---|
| Ss 31 UCS | Ss MLS | Ss Sta Casa 1 | Ss ATCC 201679 | Ss STT | |
| AE | >1000 | >1000 | >1000 | >1000 | >1000 |
| SFE | >1000 | >1000 | >1000 | >1000 | >1000 |
| NAE | 250 | 250 | 250 | 250 | 250 |
| NSFE | 250 | 250 | 250 | 250 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros-Neves, B.; Heidrich, D.; Schuh, R.S.; Brazil, N.T.; Fachel, F.N.S.; Cassel, E.; Vargas, R.M.F.; Scroferneker, M.L.; von Poser, G.L.; Koester, L.S.; et al. Topical Nanoemulsions as Delivery Systems for Green Extracts of Pterocaulon balansae Aiming at the Treatment of Sporotrichosis. Pharmaceutics 2024, 16, 492. https://doi.org/10.3390/pharmaceutics16040492
Medeiros-Neves B, Heidrich D, Schuh RS, Brazil NT, Fachel FNS, Cassel E, Vargas RMF, Scroferneker ML, von Poser GL, Koester LS, et al. Topical Nanoemulsions as Delivery Systems for Green Extracts of Pterocaulon balansae Aiming at the Treatment of Sporotrichosis. Pharmaceutics. 2024; 16(4):492. https://doi.org/10.3390/pharmaceutics16040492
Chicago/Turabian StyleMedeiros-Neves, Bruna, Daiane Heidrich, Roselena Silvestri Schuh, Nathalya Tesch Brazil, Flávia Nathiely Silveira Fachel, Eduardo Cassel, Rubem Mário Figueiró Vargas, Maria Lúcia Scroferneker, Gilsane Lino von Poser, Letícia Scherer Koester, and et al. 2024. "Topical Nanoemulsions as Delivery Systems for Green Extracts of Pterocaulon balansae Aiming at the Treatment of Sporotrichosis" Pharmaceutics 16, no. 4: 492. https://doi.org/10.3390/pharmaceutics16040492
APA StyleMedeiros-Neves, B., Heidrich, D., Schuh, R. S., Brazil, N. T., Fachel, F. N. S., Cassel, E., Vargas, R. M. F., Scroferneker, M. L., von Poser, G. L., Koester, L. S., & Teixeira, H. F. (2024). Topical Nanoemulsions as Delivery Systems for Green Extracts of Pterocaulon balansae Aiming at the Treatment of Sporotrichosis. Pharmaceutics, 16(4), 492. https://doi.org/10.3390/pharmaceutics16040492