Novel Dry Hyaluronic Acid–Vancomycin Complex Powder for Inhalation, Useful in Pulmonary Infections Associated with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hyaluronic Acid-Vancomycin Complex
2.3. Physicochemical Characterization of HA-VAN25 in Solid State
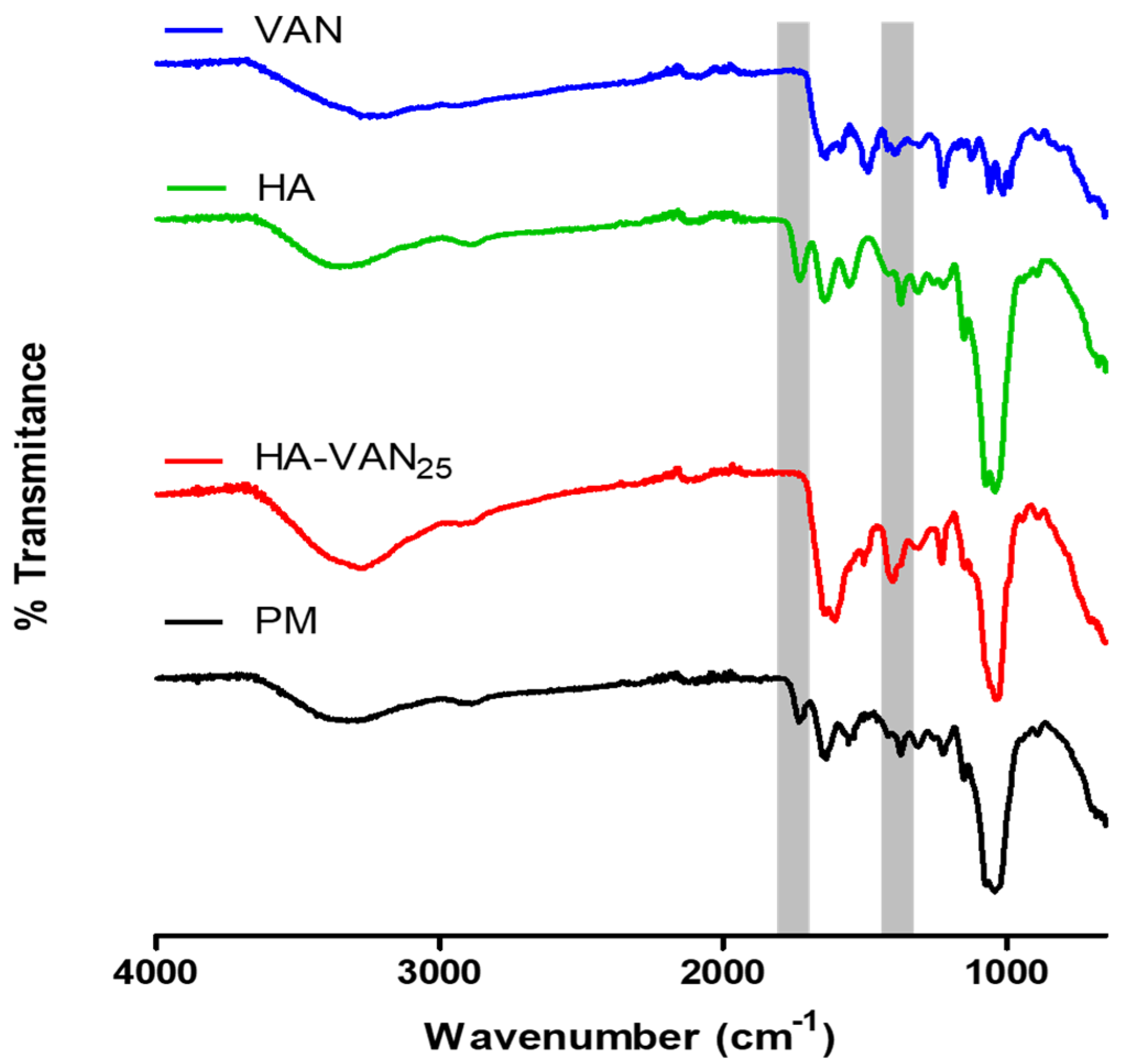
2.3.1. Infrared Spectroscopy
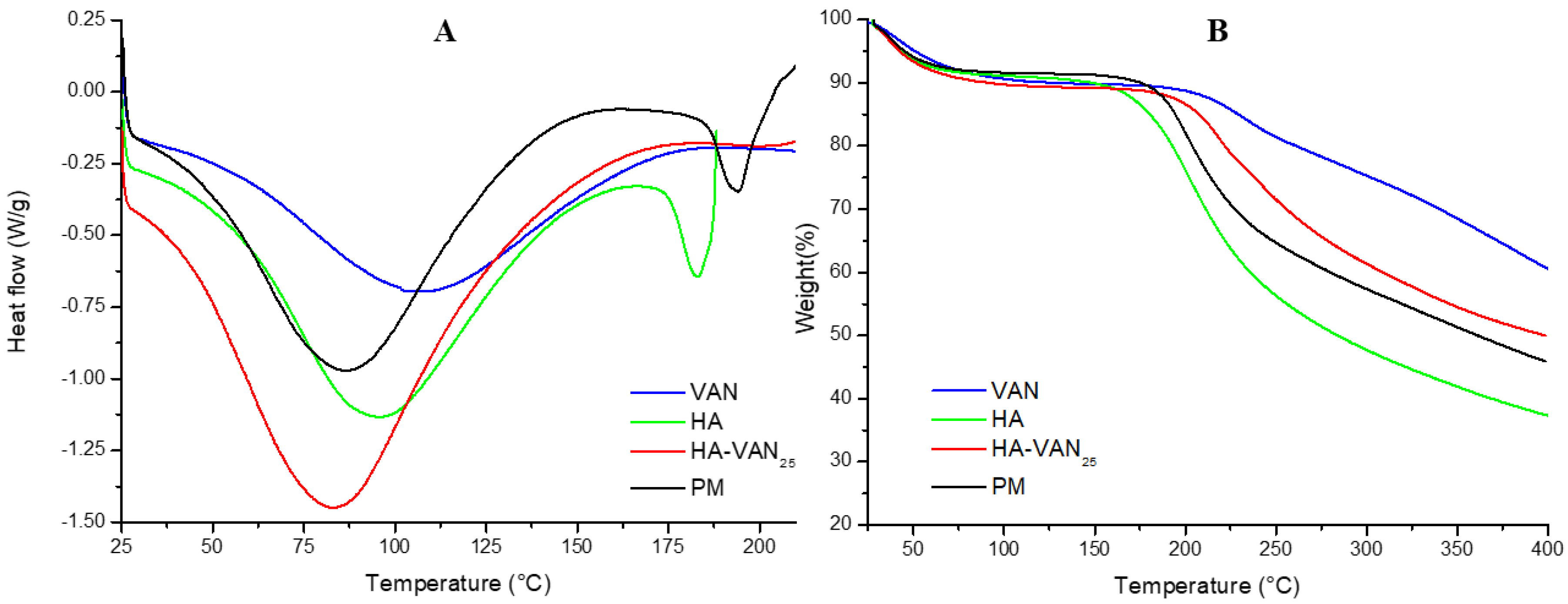
2.3.2. Thermal Analysis
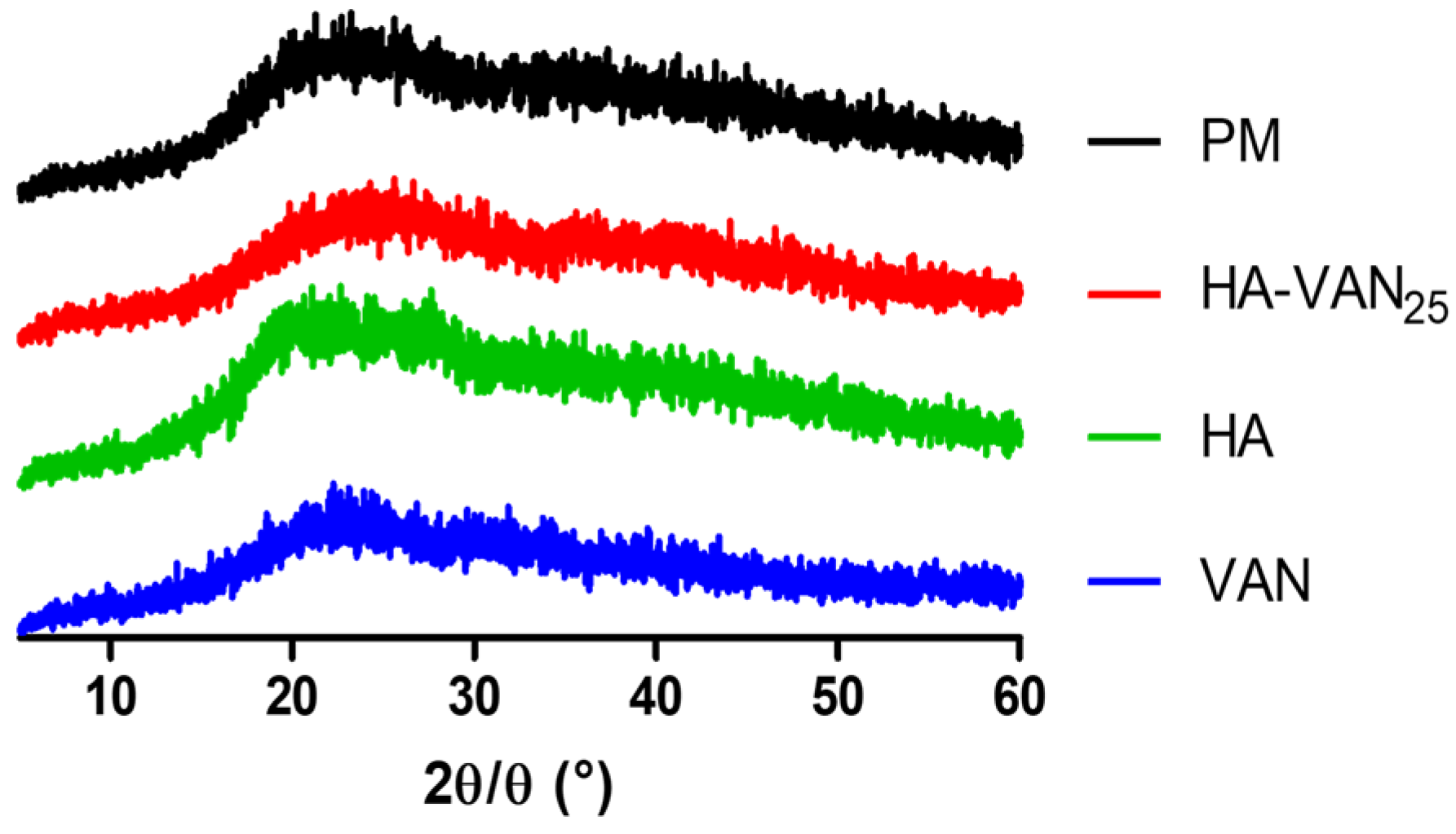
2.3.3. X-ray Diffraction
2.4. Powder Characterization
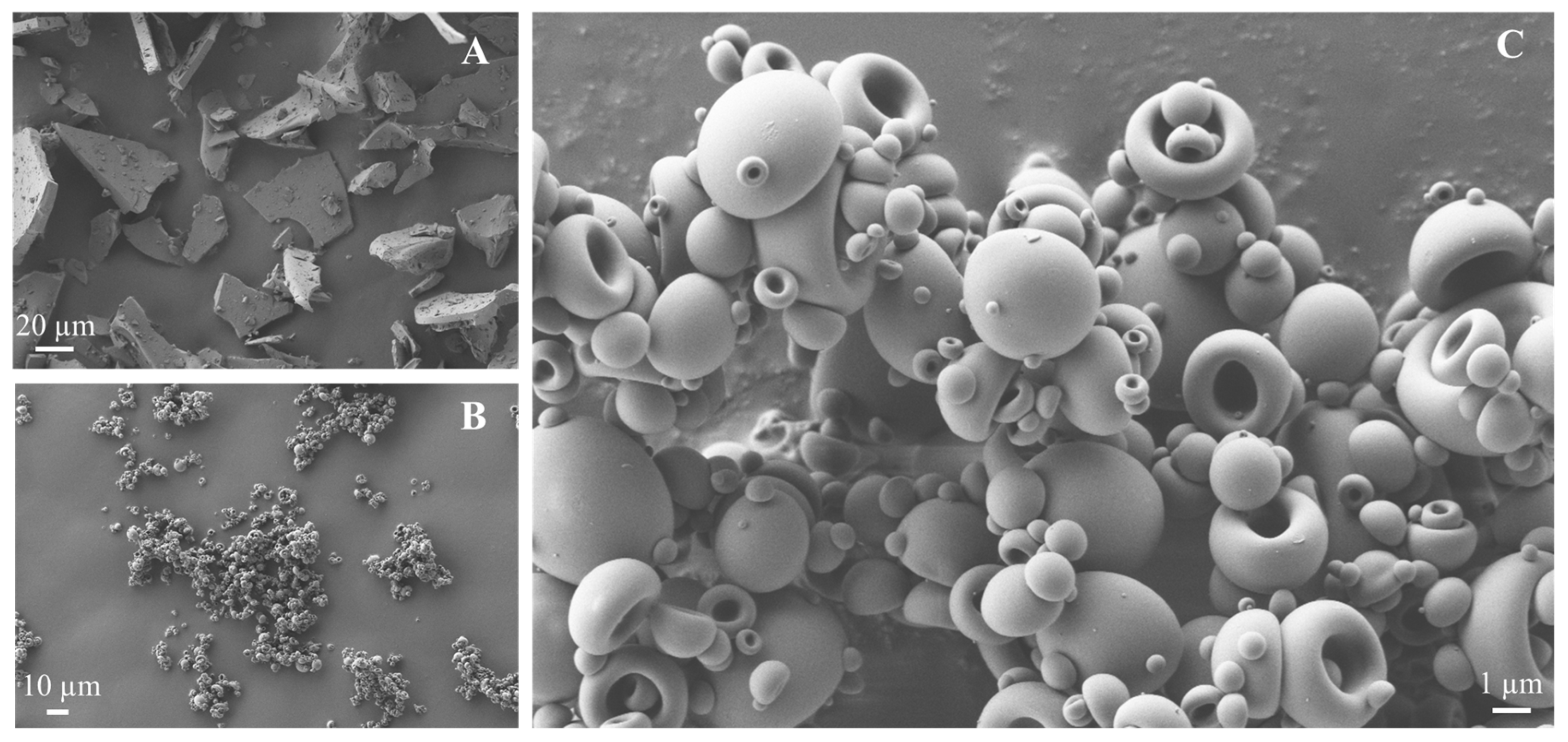
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Density Determination and Flow Properties
2.4.3. Particle Size Distribution by Laser Diffraction
2.5. In Vitro Biopharmaceutical Performance of HA-VAN25 Complex
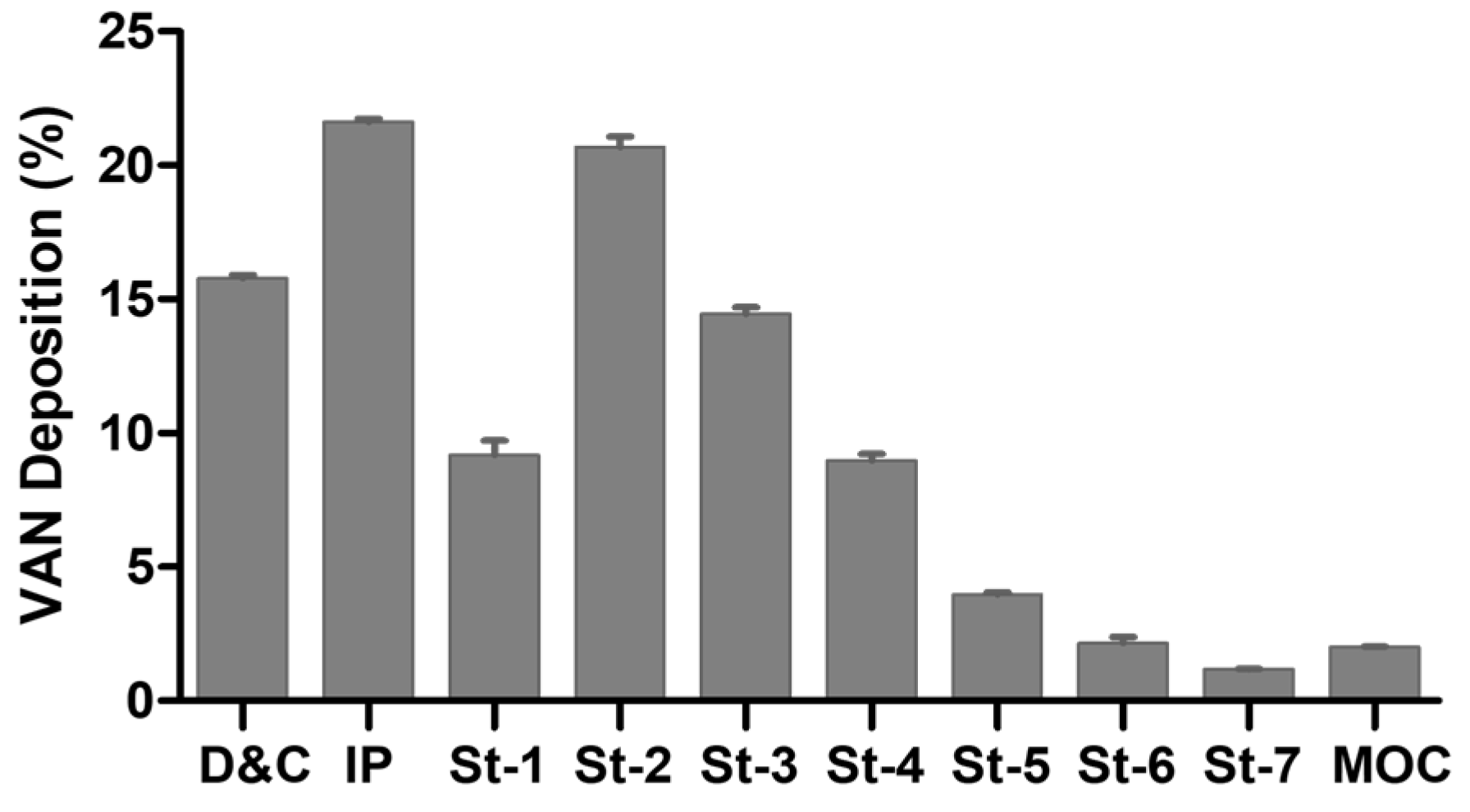
2.5.1. Aerodynamic Performance Assessment
2.5.2. Analytical Quantification of Vancomycin
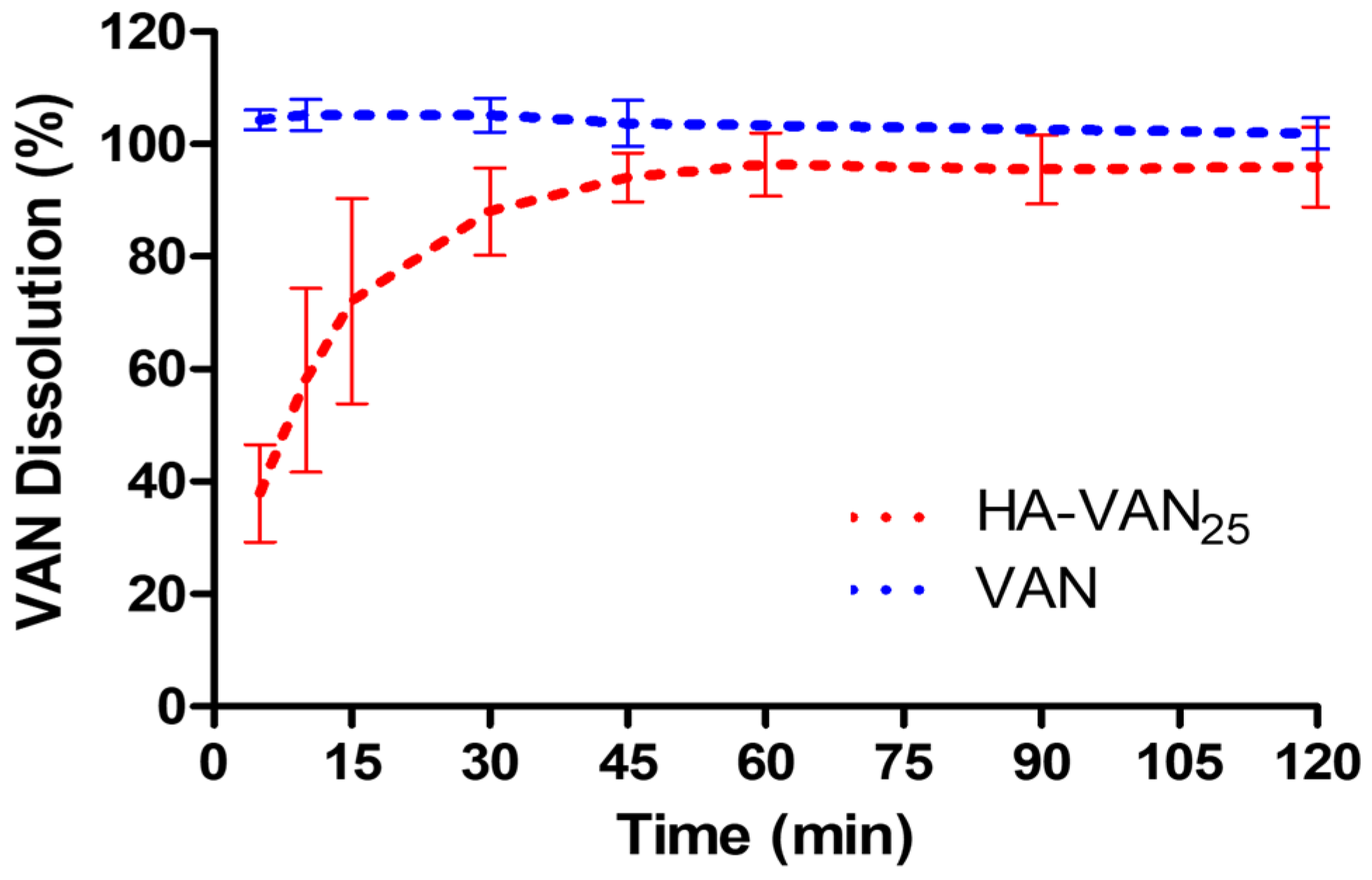
2.5.3. Dissolution Study
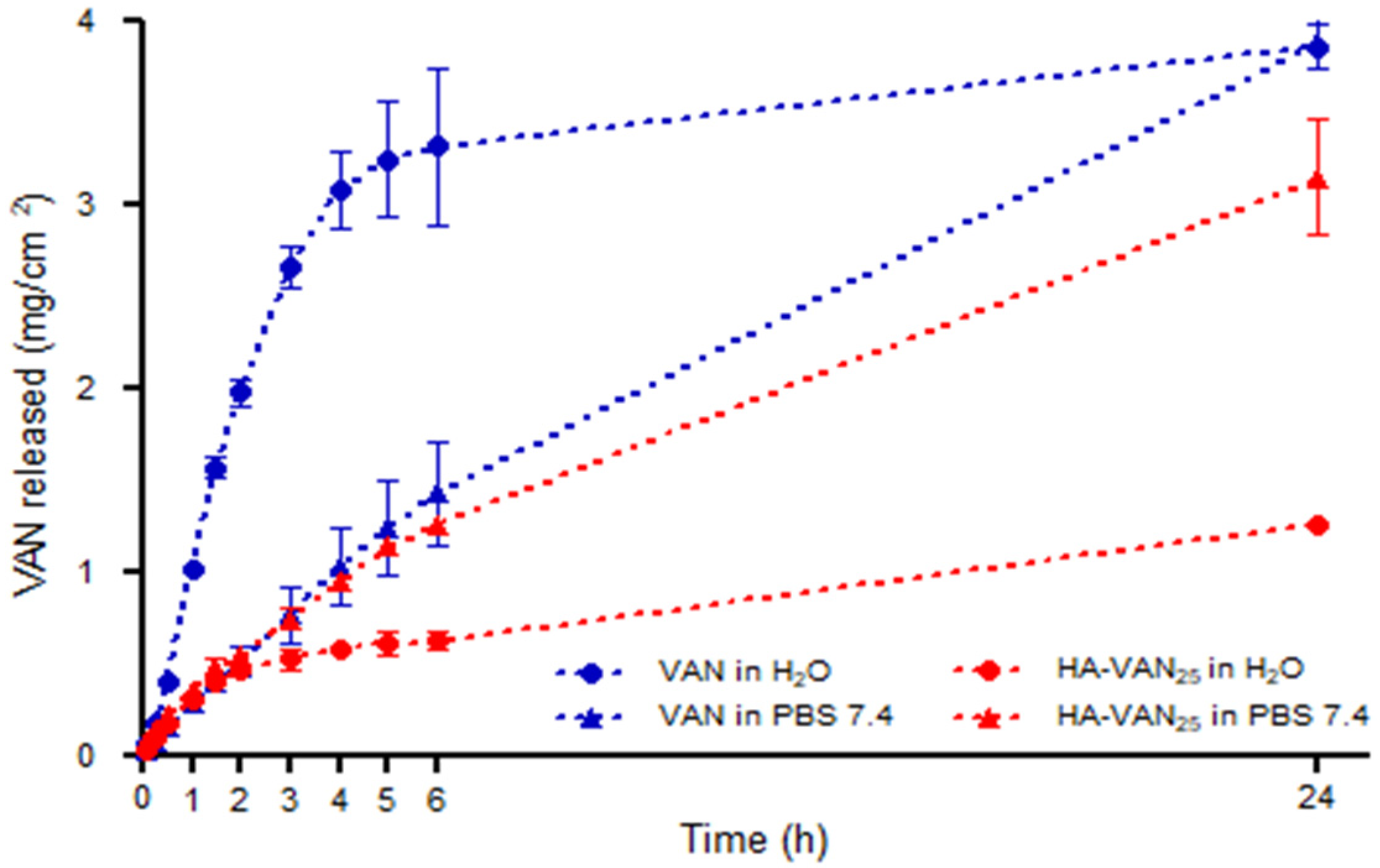
2.5.4. Franz’ Cell Diffusion Study
2.6. Antibacterial Activity Assays
2.7. Statistical Analysis
3. Results and Discussion
3.1. HA-VAN25 Complex Preparation
3.2. Physicochemical Characterization of HA-VAN25 in the Solid State
3.3. Powder Characterization
3.4. In Vitro Biopharmaceutical Performance of HA-VAN25 Complex
3.5. Antibacterial Activity Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, T.; Ramsey, B.W. Cystic Fibrosis: A Review. JAMA 2023, 329, 1859–1871. [Google Scholar] [CrossRef]
- Collaco, J.M.; Dickinson, K.M. Cystic fibrosis. Pediatr. Rev. 2021, 42, 55–67. [Google Scholar]
- Blanchard, A.C.; Waters, V.J. Opportunistic Pathogens in Cystic Fibrosis: Epidemiology and Pathogenesis of Lung Infection. J. Pediatr. Infect. Dis. Soc. 2022, 11 (Suppl. 2), S3–S12. [Google Scholar] [CrossRef]
- Epps, Q.J.; Epps, K.L.; Young, D.C.; Zobell, J.T. State of the art in cystic fibrosis pharmacology optimization of antimicrobials in the treatment of cystic fibrosis pulmonay exacerbations: III. Executive summary. Pediatr. Pulmonol. 2021, 56, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- McDermott, G.; Reece, E.; Renwick, J. Microbiology of the Cystic Fibrosis Airway. In Encyclopedia of Microbiology; Academic Press: Cambridge, MA, USA, 2019; pp. 186–198. [Google Scholar]
- Fielbaum, Ó. Updated Treatment of Cystic Fibrosis. Rev. Medica Clin. Las Condes 2017, 28, 60–71. [Google Scholar]
- Abdelaziz, M.M.; Hefnawy, A.; Anter, A.; Abdellatif, M.M.; Khalil, M.A.F.; Khalil, I.A. Respirable spray dried vancomycin coated magnetic nanoparticles for localized lung delivery. Int. J. Pharm. 2022, 611, 121318. [Google Scholar] [CrossRef] [PubMed]
- Lindley, B.; Bhakta, Z.; Gray, K.; Watanabe, A.; Leclair, L.; Young, D.C. Pharmacokinetics of intermittent dosed intravenous vancomycin in adult persons with cystic fibrosis. Pediatr. Pulmonol. 2022, 57, 2646–2651. [Google Scholar] [CrossRef]
- Waterer, G.; Lord, J.; Hofmann, T.; Jouhikainen, T. Phase I, dose-escalating study of the safety and pharmacokinetics of inhaled dry-powder vancomycin (AeroVAnc) in volunteers and patients with cystic fibrosis: A New Approach to Therapy for Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2020, 64, 1–7. [Google Scholar] [CrossRef]
- McKinzie, C.J.; Chen, L.; Ehlert, K.; Grisso, A.G.; Linafelter, A.; Lubsch, L.; O’Brien, C.E.; Pan, A.C.; Wright, B.A.; Elson, E.C. Off-label use of intravenous antimicrobials for inhalation in patients with cystic fibrosis. Pediatr. Pulmonol. 2019, 54, S27–S45. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Quan, G.; Peng, T.; Huang, Z.; Singh, V.; Lu, M.; Wu, C. Development of fine solid-crystal suspension with enhanced solubility, stability, and aerosolization performance for dry powder inhalation. Int. J. Pharm. 2017, 533, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Battistini, F.D.; Olivera, M.E.; Manzo, R.H. Equilibrium and release properties of hyaluronic acid-drug complexes. Eur. J. Pharm. Sci. 2013, 49, 588–594. [Google Scholar] [CrossRef]
- Palena, M.C.; García, M.C.; Manzo, R.H.; Jimenez-Kairuz, A.F. Self-organized drug-interpolyelectrolyte nanocomplexes loaded with anionic drugs. Characterization and in vitro release evaluation. J. Drug Deliv. Sci. Technol. 2015, 30, 45–53. [Google Scholar] [CrossRef]
- Ardusso, M.S.; Manzo, R.H.; Jimenez-Kairuz, A.F. Comparative study of three structurally related acid polyelectrolytes as carriers of basic drugs: Carbomer, Eudragit L-100 and S-100. Supramol. Chem. 2010, 22, 289–296. [Google Scholar] [CrossRef]
- Romero, V.L.; Manzo, R.H.; Alovero, F.L. Enhanced bacterial uptake and bactericidal properties of ofloxacin loaded on bioadhesive hydrogels against Pseudomonas aeruginosa. J. Chemother. 2010, 22, 328–334. [Google Scholar] [CrossRef]
- Battistini, D.; Flores-Martin, J.; Olivera, M.E.; Genti-Raimondi, S.; Manzo, R.H. Hyaluronan as drug carrier. the in vitro efficacy and selectivity of Hyaluronan-Doxorubicin complexes to affect the viability of overexpressing CD44 receptor cells. Eur. J. Pharm. Sci. 2014, 65, 122–129. [Google Scholar] [CrossRef]
- Lamas, A.; Marshburn, J.; Stober, V.P.; Donaldson, S.H.; Garantziotis, S. Effects of inhaled high-molecular weight hyaluronan in inflammatory airway disease. Respir. Res. 2016, 17, 123. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Máiz Carro, L.; Martínez-García, M.A. Use of Hyaluronic Acid (HA) in Chronic Airway Diseases. Cells 2020, 9, 2210. [Google Scholar] [CrossRef]
- Di Cicco, M.; Peroni, D.; Sepich, M.; Tozzi, M.G.; Comberiati, P.; Cutrera, R. Hyaluronic acid for the treatment of airway diseases in children: Little evidence for few indications. Pediatr. Pulmonol. 2020, 55, 2156–2169. [Google Scholar] [CrossRef]
- United State Pharmacopeia and National Formulary (USP-NF30). <616>, Bulk Density and Tapped Density of Powders; The United States Pharmacopeial Convention: Rockville, MD, USA, 2012; pp. 5637–5640. [Google Scholar]
- United State Pharmacopeia and National Formulary (USP-NF). <1174> Powder Flow; The United States Pharmacopeial Convention: Rockville, MD, USA, 2012; pp. 801–804. [Google Scholar]
- United State Pharmacopeia and National Formulary (USP-NF). <601>. Aerosols, Metered-Dose Inhalers and Dry Powder Inhalers; The United States Pharmacopeial Convention: Rockville, MD, USA, 2012; pp. 232–252. [Google Scholar]
- Peppas, N.A.; Gurny, R.; Doelker, E.; Buri, P. Modelling of drug diffusion through swellable polymeric systems. J. Memb. Sci. 1980, 7, 241–253. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Lewis, J.S. The clinical and laboratory standards institute subcommittee on Antimicrobial susceptibility testing: Background, organization, functions, and processes. J. Clim. Microbiol. 2020, 58, e01864-19. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement Production of Hyaluronic Acid by Streptococcus zooepidemicus in Sugarcane Molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef]
- Hao, C.; Zhou, D.; Xu, J.; Hong, S.; Wei, W.; Zhao, T.; Huang, H.; Fang, W. One-pot synthesis of vancomycin-encapsulated ZIF-8 nanoparticles as multivalent and photocatalytic antibacterial agents for selective-killing of pathogenic gram-positive bacteria. J. Mater. Sci. 2021, 56, 9434–9444. [Google Scholar] [CrossRef]
- Sullivan, B.P.; El-Gendy, N.; Kuehl, C.; Berkland, C. Pulmonary Delivery of Vancomycin Dry Powder Aerosol to Intubated Rabbits. Mol. Pharm. 2015, 12, 2665–2674. [Google Scholar] [CrossRef]
- Vasi, A.M.; Popa, M.I.; Butnaru, M.; Dodi, G.; Verestiuc, L. Chemical functionalization of hyaluronic acid for drug delivery applications. Mater. Sci. Eng. C 2014, 38, 177–185. [Google Scholar] [CrossRef]
- Olivera, M.E.; Manzo, R.H.; Alovero, F.; Jimenez-Kairuz, A.F.; Ramírez-Rigo, M.V. Chapter 13—Polyelectrolyte-drug ionic complexes as nanostructured drug carriers to design solid and liquid oral delivery systems. In Micro and Nanotechnologies; Andronescu, E., Grumezescu, G.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 365–408. [Google Scholar]
- Chen, L.; Okuda, T.; Lu, X.Y.; Chan, H.K. Amorphous powders for inhalation drug delivery. Adv. Drug Deliv. Rev. 2016, 100, 102–115. [Google Scholar] [CrossRef]
- Parumasivam, T.; Ashhurst, A.S.; Nagalingam, G.; Britton, W.J.; Chan, H.K. Inhalation of Respirable Crystalline Rifapentine Particles Induces Pulmonary Inflammation. Mol. Pharm. 2017, 14, 328–335. [Google Scholar] [CrossRef]
- Carro, L.M.; Blanco-Aparicio, M. New Inhaled Antibiotics and Forms of Administration. Open Respir. Arch. 2020, 2, 251–264. [Google Scholar]
- Goyal, A.K.; Garg, T.; Bhandari, S.; Rath, G. Chapter 22—Advancement in pulmonary drug delivery systems for treatment of tuberculosis. In Micro and Nanotechnologies; Andronescu, E., Grumezescu, G.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Chaurasiya, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2021, 13, 31. [Google Scholar] [CrossRef]
- Peng, T.; Lin, S.; Niu, B.; Wang, X.; Huang, Y.; Zhang, X.; Li, G.; Pan, X.; Wu, C. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm. Sin. B 2016, 6, 308–318. [Google Scholar] [CrossRef]
- Martinelli, F.; Balducci, A.G.; Kumar, A.; Sonvico, F.; Forbes, B.; Bettini, R.; Buttini, F. Engineered sodium hyaluronate respirable dry powders for pulmonary drug delivery. Int. J. Pharm. 2017, 517, 286–295. [Google Scholar] [CrossRef]
- Ceschan, N.E.; Rosas, M.D.; Olivera, M.E.; Dugour, A.V.; Figueroa, J.M.; Bucalá, V.; Ramírez-Rigo, M.V. Development of a Carrier-Free Dry Powder Ofloxacin Formulation With Enhanced Aerosolization Properties. J. Pharm. Sci. 2020, 103, 2787–2797. [Google Scholar] [CrossRef]
- Kozáková, J.; Altay, A.; Ždímal, V.; Mašková, L.; Sonvico, F.; Quarta, E.; Rossi, A.; Buttini, F.; Colombo, G. Dry powder inhaler of colistimethate sodium for lung infections in cystic fibrosis: Optimization of powder construction. Drug Dev. Ind. Pharm. 2019, 45, 1664–1673. [Google Scholar] [CrossRef]
- Crowder, T.M.; Rosati, J.A.; Schroeter, J.D.; Hickey, A.J.; Martonen, T.B. Fundamental effects of particle morphology on lung delivery: Predictions of Stokes’ law and the particular relevance to dry powder inhaler formulation and development. Pharm. Res. 2002, 19, 239–245. [Google Scholar] [CrossRef]
- Gradon, L.; Sosnowski, T.R. Formation of particles for dry powder inhalers. Adv. Powder Technol. 2014, 25, 43–55. [Google Scholar] [CrossRef]
- Papadopoulos, D.; Hickey, A.J.; Mansour, H.M.; Telko, M.J.; Xu, Z.; Smyth, H.D.; Mulder, T.; McLean, R.; Langridge, J. Physical characterization of component particles included in dry powder inhalers. II. Dynamic characteristics. J. Pharm. Sci. 2007, 96, 1302–1319. [Google Scholar]
- Hassan, M.S.; Lau, R.W.M. Effect of particle shape on dry particle inhalation: Study of flowability, aerosolization, and deposition properties. AAPS PharmSciTech 2009, 10, 1252–1262. [Google Scholar] [CrossRef]
- Chougule, M.; Padhi, B.; Jinturkar, K.; Misra, A. Development of Dry Powder Inhalers. Recent Pat. Drug Deliv. Formul. 2008, 1, 11–21. [Google Scholar] [CrossRef]
- Basavaraj, B.V.; Saritha, N.; Bharath, S.; Deveswaran, R.; Madhavan, V. Vigna mungo mucilage—A natural polymer in the design of matrix based SR tablet of aceclofenac. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 125–130. [Google Scholar]
- Hamedani, S.; Yaqoubi, S.; Safdari, R.; Hamishehkar, H.; Nokhodchi, A. A novel particle engineering method for the production of inhalable cromolyn sodium powders by a combination of spray drier and nebulizer. J. Drug Deliv. Sci. Technol. 2022, 78, 103958. [Google Scholar] [CrossRef]
- Shur, J.; Harris, H.; Jones, M.D.; Kaerger, J.S.; Price, R. The role of fines in the modification of the fluidization and dispersion mechanism within dry powder inhaler formulations. Pharm. Res. 2008, 25, 1631–1640. [Google Scholar] [CrossRef]
- Malamatari, M.; Somavarapu, S.; Kachrimanis, K.; Bloxham, M.; Taylor, K.M.G.; Buckton, G. Preparation of theophylline inhalable microcomposite particles by wet milling and spray drying: The influence of mannitol as a co-milling agent. Int. J. Pharm. 2016, 514, 200–211. [Google Scholar] [CrossRef]
- Amighi, K.; Pilcer, G.; Rosière, R.; Traina, K.; Sebti, T.; Vanderbist, F. New co-spray-dried tobramycin nanoparticles-clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients. J. Pharm. Sci. 2013, 102, 1836–1846. [Google Scholar]
- Guzman, M.L.; Soria, E.A.; Laino, C.; Manzo, R.H.; Olivera, M.E. Reduced food interaction and enhanced gastrointestinal tolerability of a new system based on risedronate complexed with Eudragit E100: Mechanistic approaches from in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2016, 107, 263–272. [Google Scholar] [CrossRef]
- Vancomycin Monograph in “DrugBank Online”. Available online: https://go.drugbank.com/drugs/DB00512 (accessed on 10 May 2022).
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar]
- Fridkin, S.K.; Hageman, J.; McDougal, L.K.; Mohammed, J.; Jarvis, W.R.; Perl, T.M.; Tenover, F.C. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin. Infect. Dis. 2003, 36, 429–439. [Google Scholar] [CrossRef]
- Canal, L.A.; San, H. ¿Impacto de la concentración mínima inhibitoria de vancomicina o incorrecto manejo terapéutico en el fracaso clínico por Staphylococcus aureus resistentes a meticilina? Ann. Peidatria 2015, 85, 373–374. [Google Scholar]
| ED (mg) | EF (%) | FPF (%) | Extra-FPF (%) | MMAD (µm) |
|---|---|---|---|---|
| 7.9 ± 0.4 | 84.2 ± 0.1 | 42.9 ± 0.2 | 25.9 ± 0.1 | 4.29 ± 0.03 |
| Complex | Receptor Medium | |||||
|---|---|---|---|---|---|---|
| H20 | PBS 7.4 | |||||
| k | n | R2 | k | n | R2 | |
| HA-VAN25 | 0.5 | 0.60 | 0.96 | 0.4 | 0.67 | 0.99 |
| Samples | Inhibition Halo (mm) | ||
|---|---|---|---|
| ATCC 29213 | ATCC 25923 | ATCC 43300 | |
| HA | 8 ± 0.0 | 8 ± 0.0 | 8 ± 0.0 |
| VAN | 29 ± 0.5 * | 31 ± 0.5 * | 30 ± 0.5 * |
| HA-VAN25 | 30 ± 0.5 * | 31 ± 0.5 * | 31 ± 0.5 * |
| 29213 | 25923 | 43300 | ||||
|---|---|---|---|---|---|---|
| Samples | MIC | MBC | MIC | MBC | MIC | MBC |
| HA | >2500 | -- | >2500 | -- | >2500 | -- |
| VAN | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 |
| HA-VAN25 | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magi, M.S.; de Lafuente, Y.; Quarta, E.; Palena, M.C.; Ardiles, P.d.R.; Páez, P.L.; Sonvico, F.; Buttini, F.; Jimenez-Kairuz, A.F. Novel Dry Hyaluronic Acid–Vancomycin Complex Powder for Inhalation, Useful in Pulmonary Infections Associated with Cystic Fibrosis. Pharmaceutics 2024, 16, 436. https://doi.org/10.3390/pharmaceutics16040436
Magi MS, de Lafuente Y, Quarta E, Palena MC, Ardiles PdR, Páez PL, Sonvico F, Buttini F, Jimenez-Kairuz AF. Novel Dry Hyaluronic Acid–Vancomycin Complex Powder for Inhalation, Useful in Pulmonary Infections Associated with Cystic Fibrosis. Pharmaceutics. 2024; 16(4):436. https://doi.org/10.3390/pharmaceutics16040436
Chicago/Turabian StyleMagi, María S., Yanina de Lafuente, Eride Quarta, María C. Palena, Perla del R. Ardiles, Paulina L. Páez, Fabio Sonvico, Francesca Buttini, and Alvaro F. Jimenez-Kairuz. 2024. "Novel Dry Hyaluronic Acid–Vancomycin Complex Powder for Inhalation, Useful in Pulmonary Infections Associated with Cystic Fibrosis" Pharmaceutics 16, no. 4: 436. https://doi.org/10.3390/pharmaceutics16040436
APA StyleMagi, M. S., de Lafuente, Y., Quarta, E., Palena, M. C., Ardiles, P. d. R., Páez, P. L., Sonvico, F., Buttini, F., & Jimenez-Kairuz, A. F. (2024). Novel Dry Hyaluronic Acid–Vancomycin Complex Powder for Inhalation, Useful in Pulmonary Infections Associated with Cystic Fibrosis. Pharmaceutics, 16(4), 436. https://doi.org/10.3390/pharmaceutics16040436