Mannose-Decorated Solid-Lipid Nanoparticles for Alveolar Macrophage Targeted Delivery of Rifampicin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
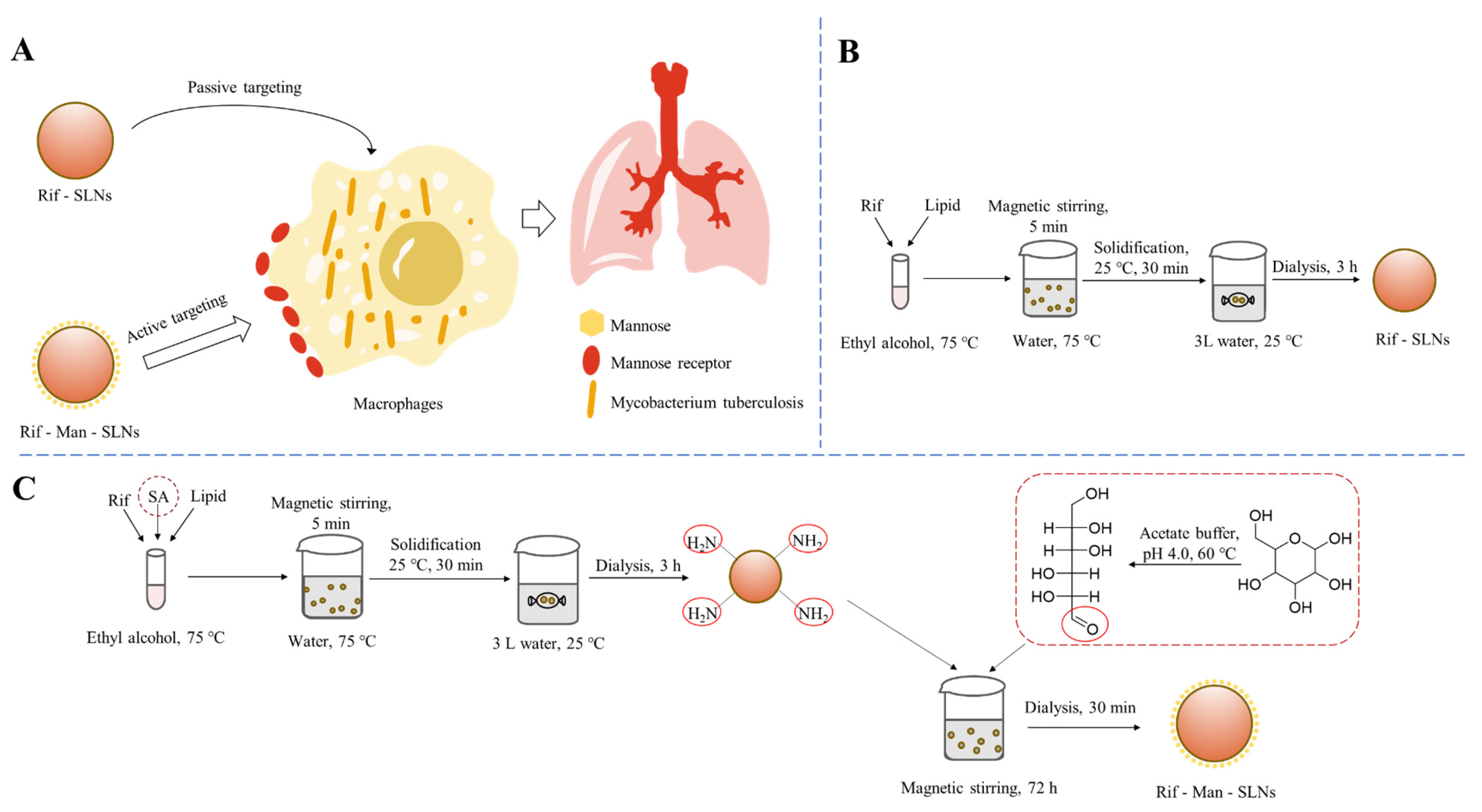
2.2. Formulation of Rif-Loaded SLNs (Rif-SLNs)
2.3. Mannose Functionalization
2.4. Particle Size, PDI and Zeta Potential
2.5. Drug Loading
2.6. Appearance and Morphology
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Powder X-ray Diffractometer (P-XRD)
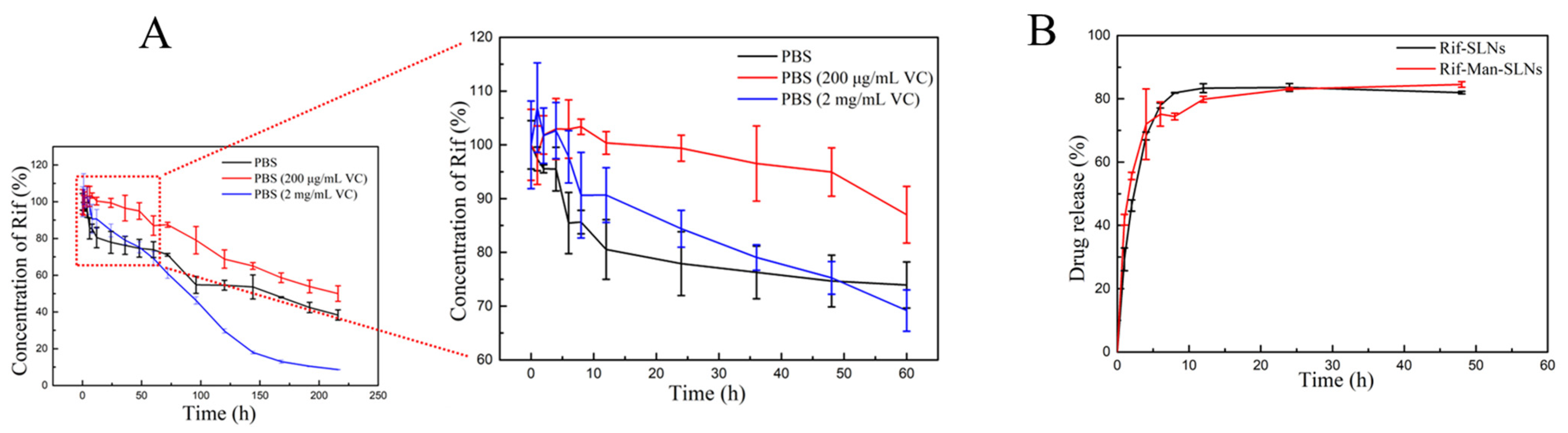
2.9. In Vitro Drug Release
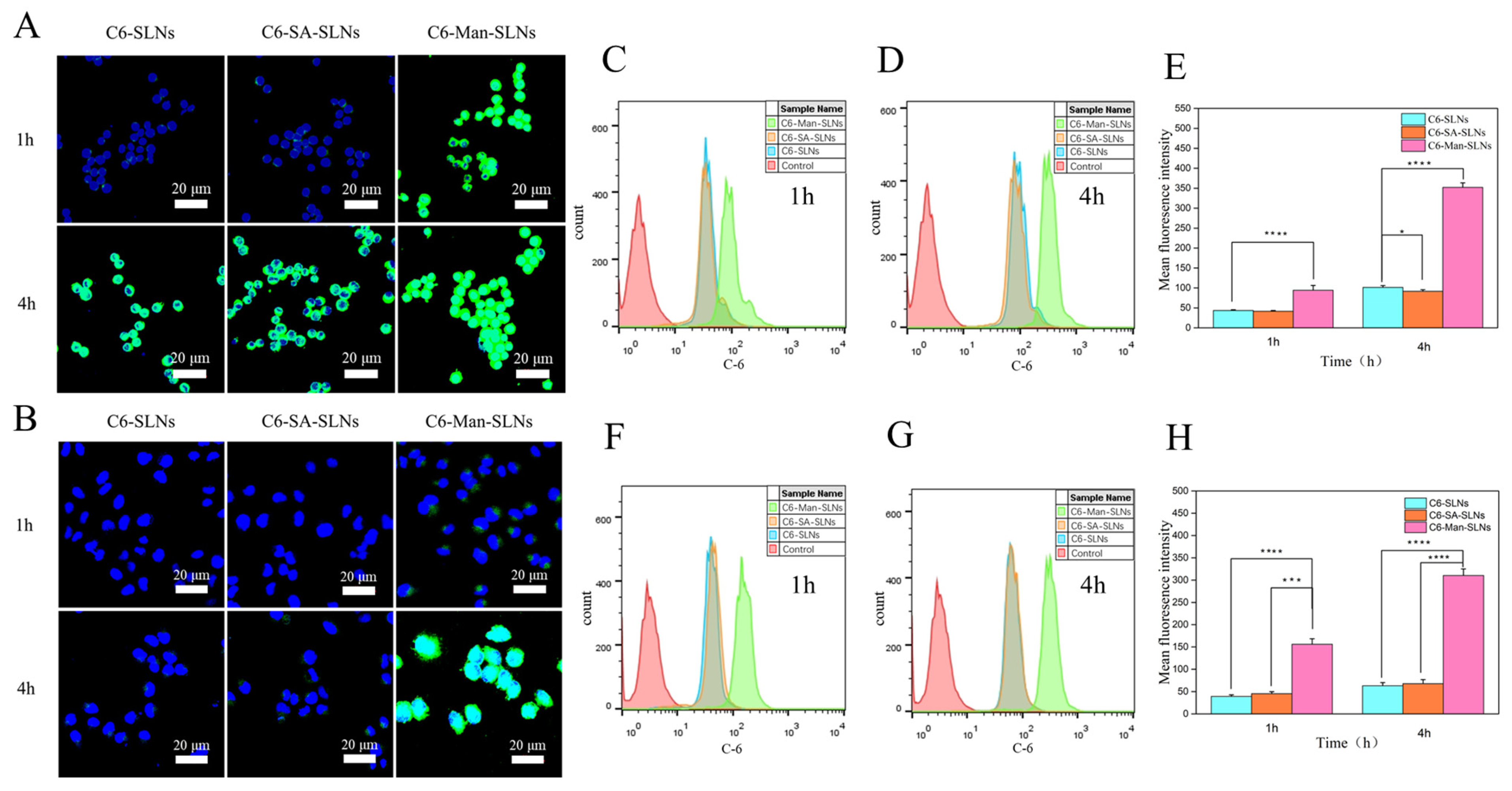
2.10. Cellular Uptake
2.10.1. Confocal Laser Scanning Microscopy (CLSM)
2.10.2. Flow Cytometer
2.11. Cytotoxicity
2.12. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Physico-Chemical Characterization of SLNs
3.2. FT-IR and XRD Analyses
3.3. Drug Release
3.4. Cellular Uptake
3.5. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denney, L.; An, H.; Ho, L.P.; Zheng, Y. Alveolar and lung interstitial macrophages: Definitions, functions, and roles in lung fibrosis. J. Leukoc. Biol. 2021, 110, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.R.; Schmid, M.C. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediat. Inflamm. 2017, 2017, 9624760. [Google Scholar] [CrossRef] [PubMed]
- Saradna, A.; Do, D.C.; Kumar, S.; Fu, Q.L.; Gao, P. Macrophage polarization and allergic asthma. Transl. Res. J. Lab. Clin. Med. 2018, 191, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Guirado, E.; Schlesinger, L.S.; Kaplan, G. Macrophages in tuberculosis: Friend or foe. Semin. Immunopathol. 2013, 35, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, A.O.; Glaudemans, A.; Maes, A.; Van de Wiele, C.; Dierckx, R.; Vorster, M.; Sathekge, M.M. Tuberculosis. Semin. Nucl. Med. 2018, 48, 108–130. [Google Scholar] [CrossRef]
- Ufimtseva, E.; Eremeeva, N.; Bayborodin, S.; Umpeleva, T.; Vakhrusheva, D.; Skornyakov, S. Mycobacterium tuberculosis with different virulence reside within intact phagosomes and inhibit phagolysosomal biogenesis in alveolar macrophages of patients with pulmonary tuberculosis. Tuberculosis 2019, 114, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Maphasa, R.E.; Meyer, M.; Dube, A. The Macrophage Response to Mycobacterium tuberculosis and Opportunities for Autophagy Inducing Nanomedicines for Tuberculosis Therapy. Front. Cell. Infect. Microbiol. 2020, 10, 618414. [Google Scholar] [CrossRef]
- Trousil, J.; Syrova, Z.; Dal, N.K.; Rak, D.; Konefal, R.; Pavlova, E.; Matejkova, J.; Cmarko, D.; Kubickova, P.; Pavlis, O.; et al. Rifampicin Nanoformulation Enhances Treatment of Tuberculosis in Zebrafish. Biomacromolecules 2019, 20, 1798–1815. [Google Scholar] [CrossRef]
- Queval, C.J.; Brosch, R.; Simeone, R. The Macrophage: A Disputed Fortress in the Battle against Mycobacterium tuberculosis. Front. Microbiol. 2017, 8, 2284. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, C.R., Jr.; Barry, C.E., III; Lange, C. Treatment of tuberculosis. N. Engl. J. Med. 2015, 373, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.D.; Fattal, E.; Tsapis, N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015, 478, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Tornheim, J.A.; Dooley, K.E. The Global Landscape of Tuberculosis Therapeutics. Annu. Rev. Med. 2019, 70, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Khadka, P.; Dummer, J.; Hill, P.C.; Das, S.C. Considerations in preparing for clinical studies of inhaled rifampicin to enhance tuberculosis treatment. Int. J. Pharm. 2018, 548, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Loo, C.Y.; Traini, D.; Young, P.M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Gautam, L.; Sharma, R.; Dube, D.; Vyas, S.; Vyas, S.P. Dual antitubercular drug loaded liposomes for macrophage targeting: Development, characterisation, ex vivo and in vivo assessment. J. Microencapsul. 2021, 38, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Pawde, D.M.; Viswanadh, M.K.; Mehata, A.K.; Sonkar, R.; Narendra; Poddar, S.; Burande, A.S.; Jha, A.; Vajanthri, K.Y.; Mahto, S.K.; et al. Mannose receptor targeted bioadhesive chitosan nanoparticles of clofazimine for effective therapy of tuberculosis. Saudi Pharm. J. 2020, 28, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.; Choi, Y.; Tanaka, M.; Choi, J. Inhalable nanoparticles delivery targeting alveolar macrophages for the treatment of pulmonary tuberculosis. J. Biosci. Bioeng. 2021, 132, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Sarmento, B.; Seabra, V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur. J. Pharm. Sci. 2018, 114, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Maity, S.; Ghosh, B.; Chakraborty, T.; Mondal, A.; Bishayee, A. Assessment of the antidiabetic potentiality of glyburide loaded glyceryl monostearate solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 55, 101451. [Google Scholar] [CrossRef]
- Nimje, N.; Agarwal, A.; Saraogi, G.K.; Lariya, N.; Rai, G.; Agrawal, H.; Agrawal, G.P. Mannosylated nanoparticulate carriers of rifabutin for alveolar targeting. J. Drug Target. 2009, 17, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Agarwal, A.; Majumder, S.; Lariya, N.; Khaya, A.; Agrawal, H.; Majumdar, S.; Agrawal, G.P. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J. Control. Release 2010, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.H.; Xu, Y.; Chen, S.Q.; Cheng, B.; Hu, F.Q.; You, J.; Du, Y.Z.; Yuan, H. Transport Mechanisms of Solid Lipid Nanoparticles across Caco-2 Cell Monolayers and their Related Cytotoxicology. ACS Appl. Mater. Interfaces 2016, 8, 5929–5940. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Roy, S.; Bhaumik, K.N.; Kshetrapal, P.; Pillai, J. Comparative study of oral lipid nanoparticle formulations (LNFs) for chemical stabilization of antitubercular drugs: Physicochemical and cellular evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 540–558. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Faria, V.; Goncalves, L.M.; Taboada, P.; Remunan-Lopez, C.; Almeida, A.J. Rifabutin-loaded solid lipid nanoparticles for inhaled antitubercular therapy: Physicochemical and in vitro studies. Int. J. Pharm. 2016, 497, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Diab, R.; Brillault, J.; Bardy, A.; Gontijo, A.V.; Olivier, J.C. Formulation and in vitro characterization of inhalable polyvinyl alcohol-free rifampicin-loaded PLGA microspheres prepared with sucrose palmitate as stabilizer: Efficiency for ex vivo alveolar macrophage targeting. Int. J. Pharm. 2012, 436, 833–839. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, W.; Zhou, X.; Xu, F.; Zou, J.; Zhang, Q.; Zhao, Y.; He, H.; Yang, H.; Liu, J. Reactive oxygen species (ROS)-responsive size-reducible nanoassemblies for deeper atherosclerotic plaque penetration and enhanced macrophage-targeted drug delivery. Bioact. Mater. 2023, 19, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.C.; Magalhaes, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomededicine 2017, 12, 2721–2736. [Google Scholar] [CrossRef]
- Pei, Y.; Yeo, Y. Drug delivery to macrophages: Challenges and opportunities. J. Control. Release 2016, 240, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Hu, X.; Zhou, J.; Liu, S.; Xie, Z. Glutathione-responsive paclitaxel dimer nanovesicles with high drug content. Biomater. Sci. 2017, 5, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Agnihotri, V.V.; Patil, K.Y.; Pardeshi, S.R.; Surana, S.J. Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Int. J. Biol. Macromol. 2020, 165 Pt A, 445–459. [Google Scholar] [CrossRef]
- Kraisit, P.; Hirun, N.; Mahadlek, J.; Limmatvapirat, S. Fluconazole-loaded solid lipid nanoparticles (SLNs) as a potential carrier for buccal drug delivery of oral candidiasis treatment using the Box-Behnken design. J. Drug Deliv. Sci. Technol. 2021, 63, 102437. [Google Scholar] [CrossRef]
- Sawant, K.K.; Dodiya, S.S. Recent advances and patents on solid lipid nanoparticles. Recent Pat. Drug Deliv. Formul. 2008, 2, 120–135. [Google Scholar] [CrossRef]
- Venkateswarlu, V.; Manjunath, K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Release 2004, 95, 627–638. [Google Scholar] [CrossRef]
- Pinheiro, M.; Ribeiro, R.; Vieira, A.; Andrade, F.; Reis, S. Design of a nanostructured lipid carrier intended to improve the treatment of tuberculosis. Drug Des. Dev. Ther. 2016, 10, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Yuan, H.; Zhang, H.H.; Fang, M. Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization. Int. J. Pharm. 2002, 239, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Mishra, D.K.; Jain, N.; Rajoriya, V.; Jain, A.K. Mannosylated solid lipid nanoparticles for lung-targeted delivery of Paclitaxel. Drug Dev. Ind. Pharm. 2015, 41, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Srivastava, R.; Naidu, V. Niclosamide loaded cationic Solid Lipid Nanoparticles for treatment of Cancer. In Proceedings of the 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), Sendai, Japan, 22–25 August 2016; pp. 245–248. [Google Scholar]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection—Preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Jindal, S.; Singh, M.; Sharma, G.; Kaur, I.P. Nano-formulation of rifampicin with enhanced bioavailability: Development, characterization and in-vivo safety. Int. J. Pharm. 2015, 485, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Ashokraj, Y.; Bharatam, P.V.; Pillai, O.; Panchagnula, R. Solid-state characterization of rifampicin samples and its biopharmaceutic relevance. Eur. J. Pharm. Sci. 2004, 22, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Shishoo, C.J.; Shah, S.A.; Rathod, I.S.; Savale, S.S.; Kotecha, J.S.; Shah, P.B. Stability of rifampicin in dissolution medium in presence of isoniazid. Int. J. Pharm. 1999, 190, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.; Padilla, D.J.; Garcia-Contreras, L.; Verberkmoes, J.L.; Durbin, D.; Peloquin, C.A.; Elbert, K.J.; Hickey, A.J.; Edwards, D.A. Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm. Res. 2009, 26, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Bachhav, S.S.; Dighe, V.D.; Kotak, D.; Devarajan, P.V. Rifampicin Lipid-Polymer hybrid nanoparticles (LIPOMER) for enhanced Peyer’s patch uptake. Int. J. Pharm. 2017, 532, 612–622. [Google Scholar] [CrossRef]
- Yuan, H.; Miao, J.; Du, Y.Z.; You, J.; Hu, F.Q.; Zeng, S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int. J. Pharm. 2008, 348, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chen, Y.; Zhang, Z.; Yin, Q.; Zheng, N.; Cheng, J. Biodegradable micelles capable of mannose-mediated targeted drug delivery to cancer cells. Macromol. Rapid Commun. 2015, 36, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Pandita, D.; Ahuja, A.; Lather, V.; Dutta, T.; Velpandian, T.; Khar, R.K. Development, characterization and in vitro assessement of stearylamine-based lipid nanoparticles of paclitaxel. Die Pharm.-Int. J. Pharm. Sci. 2011, 66, 171–177. [Google Scholar]
- Zhang, Z.; Feng, S.S. The drug encapsulation efficiency, in vitro drug release, cellular uptake and cytotoxicity of paclitaxel-loaded poly(lactide)-tocopheryl polyethylene glycol succinate nanoparticles. Biomaterials 2006, 27, 4025–4033. [Google Scholar] [CrossRef]




| Scaffolds | Particle Size (nm) | PDI | Zeta Potential (mV) | DL (%) | DEE (%) |
|---|---|---|---|---|---|
| Rif-SLNs | 167.7 ± 12.2 | 0.191 ± 0.048 | −24.4 ± 0.8 | 20.4 ± 0.7 | 88.3 ± 3.2 |
| Rif-Man-SLNs | 196.5 ± 15.5 | 0.320 ± 0.075 | 42.3 ± 1.1 | 1.0 ± 0.1 | 5.6 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bera, H.; Zhao, C.; Tian, X.; Cun, D.; Yang, M. Mannose-Decorated Solid-Lipid Nanoparticles for Alveolar Macrophage Targeted Delivery of Rifampicin. Pharmaceutics 2024, 16, 429. https://doi.org/10.3390/pharmaceutics16030429
Bera H, Zhao C, Tian X, Cun D, Yang M. Mannose-Decorated Solid-Lipid Nanoparticles for Alveolar Macrophage Targeted Delivery of Rifampicin. Pharmaceutics. 2024; 16(3):429. https://doi.org/10.3390/pharmaceutics16030429
Chicago/Turabian StyleBera, Hriday, Caizhu Zhao, Xidong Tian, Dongmei Cun, and Mingshi Yang. 2024. "Mannose-Decorated Solid-Lipid Nanoparticles for Alveolar Macrophage Targeted Delivery of Rifampicin" Pharmaceutics 16, no. 3: 429. https://doi.org/10.3390/pharmaceutics16030429
APA StyleBera, H., Zhao, C., Tian, X., Cun, D., & Yang, M. (2024). Mannose-Decorated Solid-Lipid Nanoparticles for Alveolar Macrophage Targeted Delivery of Rifampicin. Pharmaceutics, 16(3), 429. https://doi.org/10.3390/pharmaceutics16030429







