pH-Triggered Controlled Release of Chlorhexidine Using Chitosan-Coated Titanium Silica Composite for Dental Infection Prevention
Abstract
1. Introduction
2. Materials and Methods
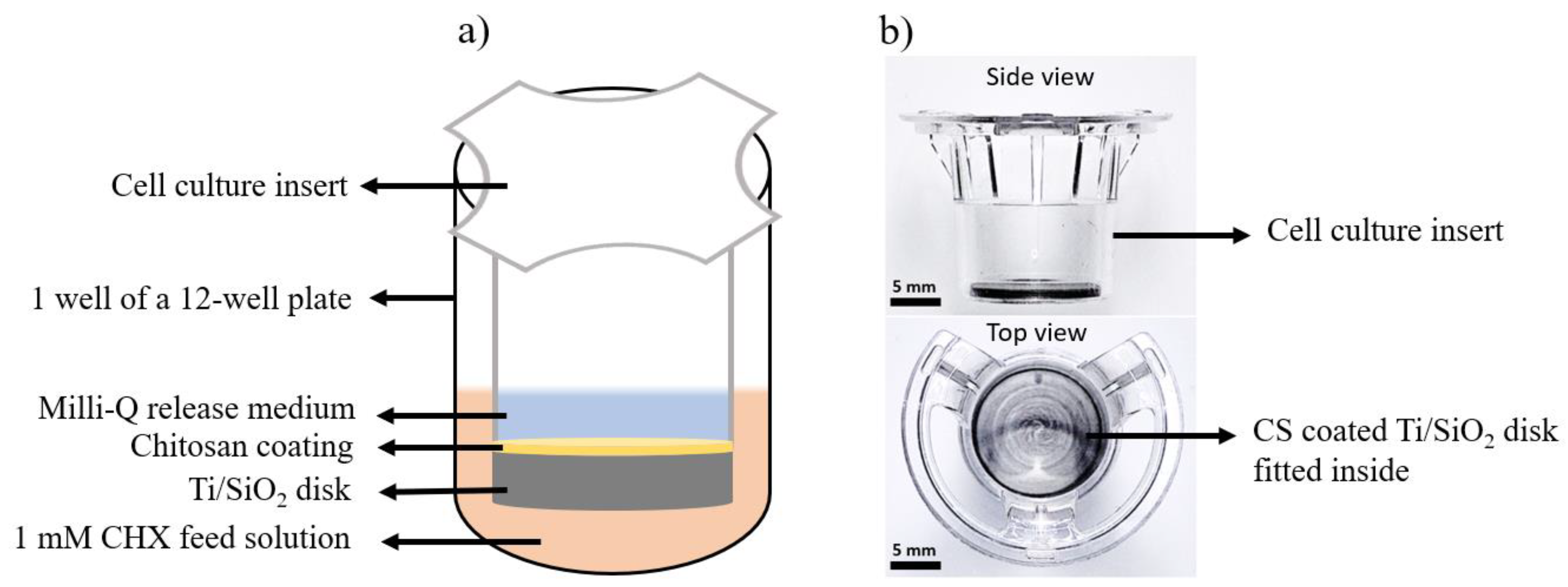
2.1. Fabrication of Ti/SiO2/CS Disk and CS Films
2.2. Characterization of Ti/SiO2/CS Disk and CS Films
2.3. Drug-Release Studies
2.4. Antimicrobial Study
3. Results
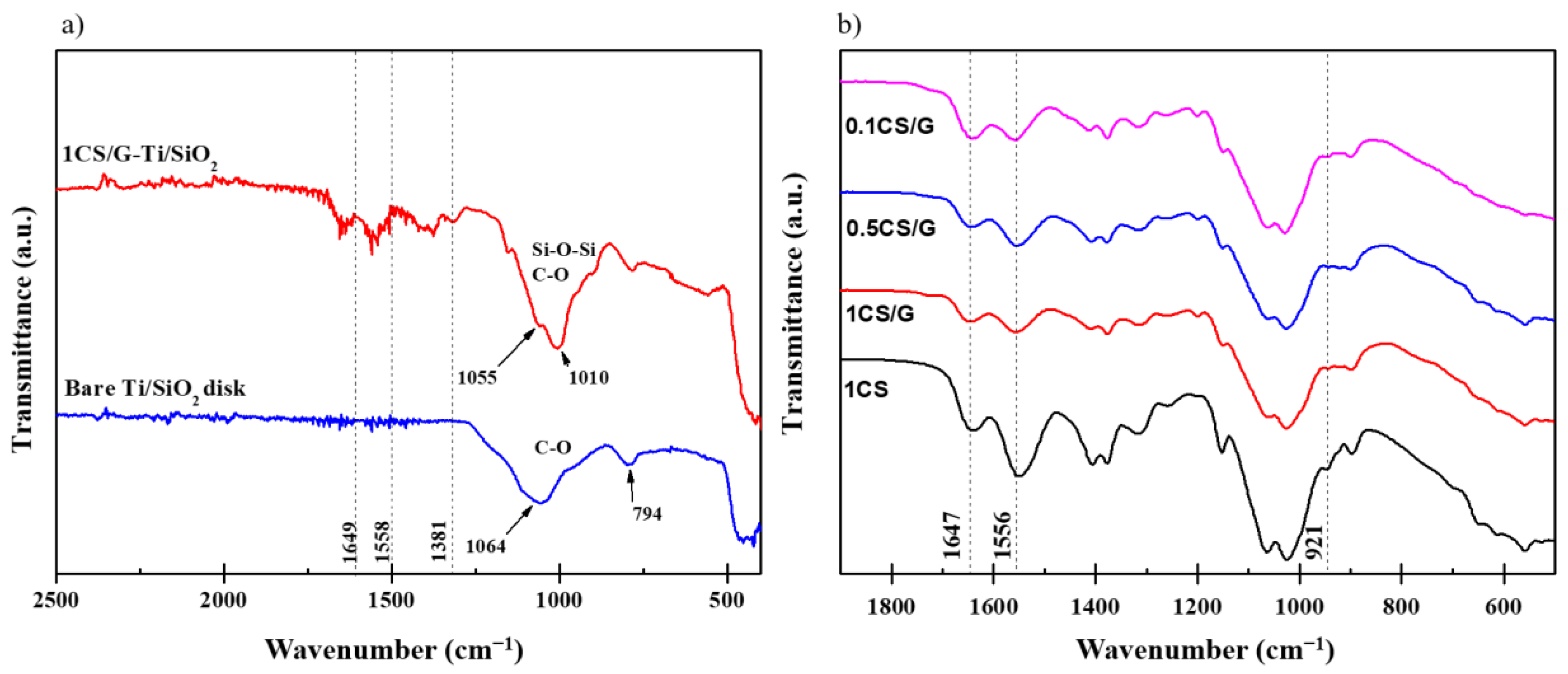
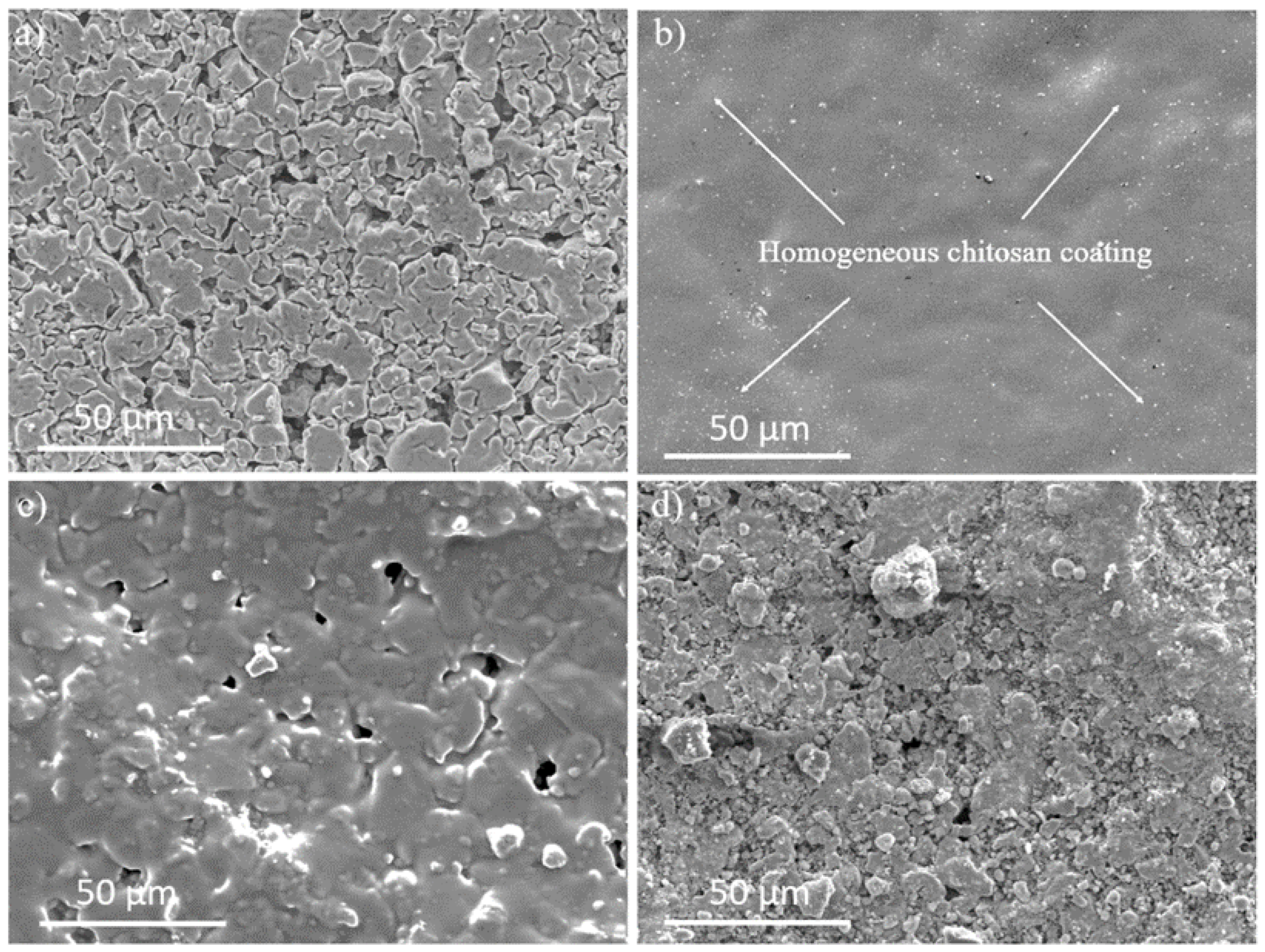
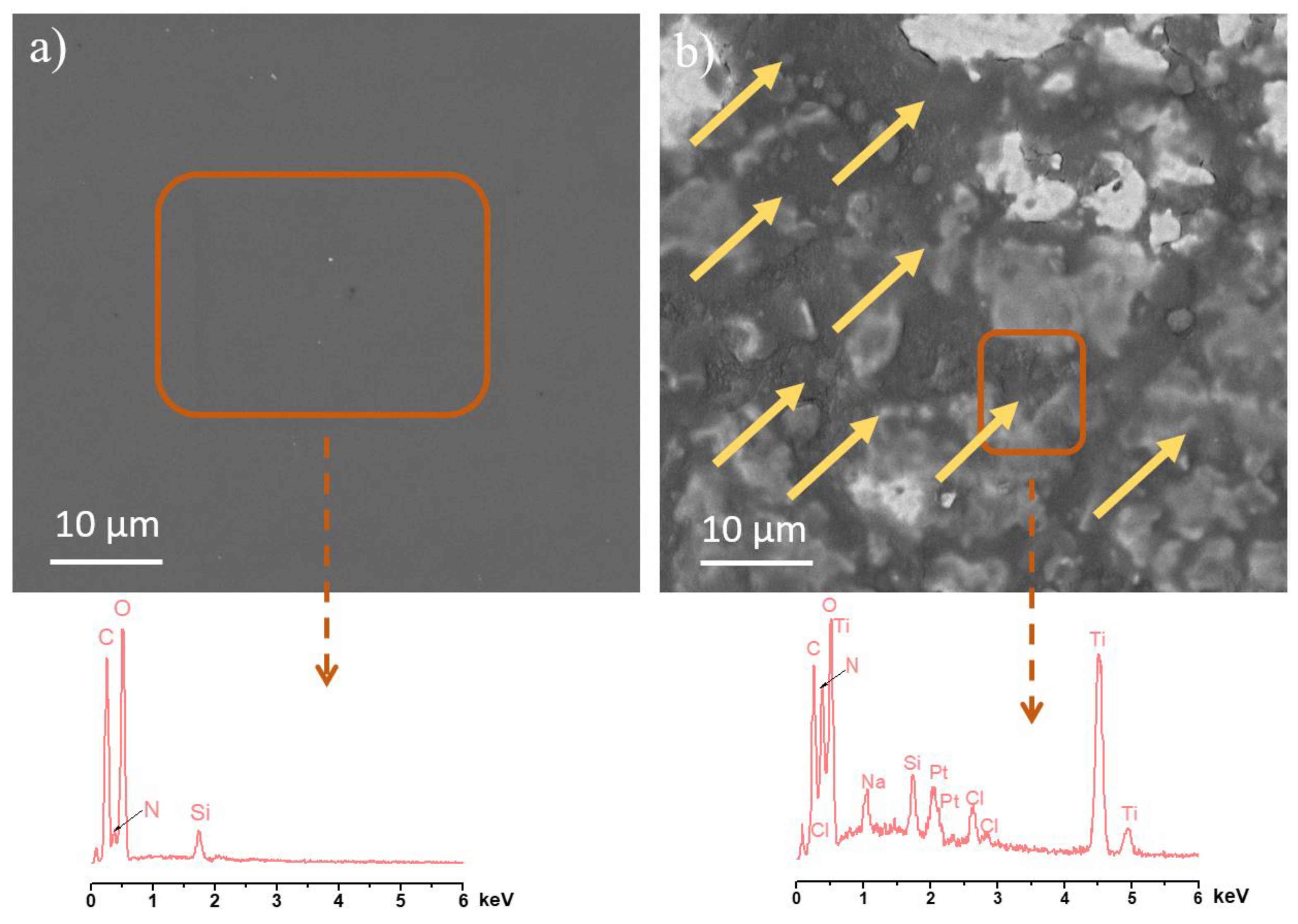
3.1. Characterization of Ti/SiO2/CS Disks and CS Films
3.2. Chlorhexidine-Release Study
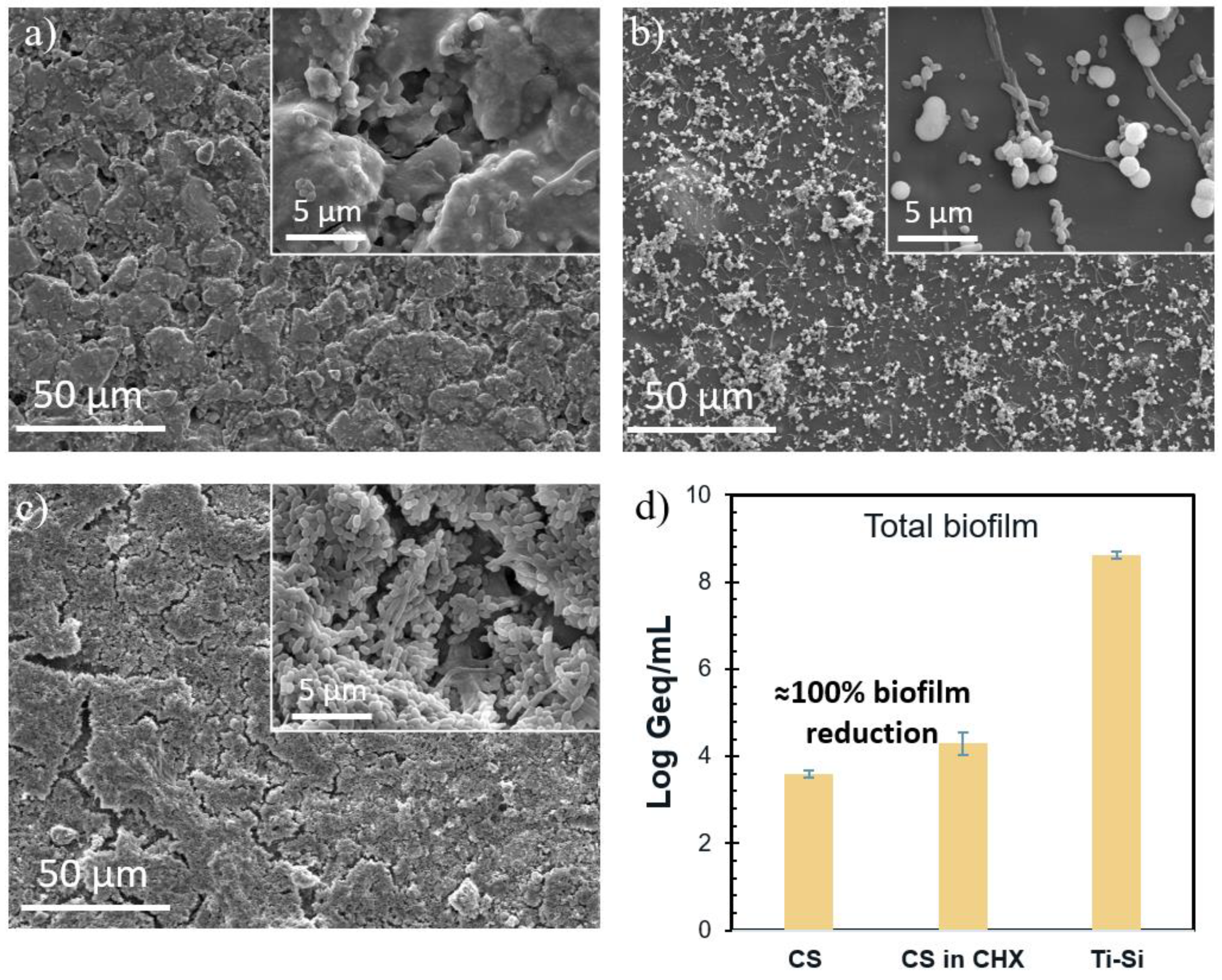
3.3. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999–2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Mundathaje, M.; Prabhu, A.G. Knowledge, attitude, and awareness of patients regarding dental implants: A cross-sectional study. J. Int. Oral Health 2018, 10, 278. [Google Scholar] [CrossRef]
- Widmer, A.F. New Developments in Diagnosis and Treatment of Infection in Orthopedic Implants. Clin. Infect. Dis. 2001, 33, S94–S106. [Google Scholar] [CrossRef] [PubMed]
- AlJasser, R.N.; A AlSarhan, M.; Alotaibi, D.H.; AlOraini, S.; Ansari, A.S.; Habib, S.R.; Zafar, M.S. Analysis of prosthetic factors affecting peri-implant health: An in vivo retrospective study. J. Multidiscip. Health 2021, 14, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Nakayama, Y.; Tatsumi, J.; Kubota, T.; Sato, S.; Nishida, T.; Takeuchi, Y.; Onitsuka, T.; Sakagami, R.; Nozaki, T.; et al. Prevalence and risk factors for peri-implant diseases in Japanese adult dental patients. J. Oral Sci. 2017, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- VanEpps, J.S.; Younger, J.G. Implantable device-related infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, Y.-W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Braem, A.; Van Mellaert, L.; Mattheys, T.; Hofmans, D.; De Waelheyns, E.; Geris, L.; Anné, J.; Schrooten, J.; Vleugels, J. Staphylococcal biofilm growth on smooth and porous titanium coatings for biomedical applications. J. Biomed. Mater. Res. Part A 2014, 102, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.; Lamont-Friedrich, S.J.; Michl, T.D.; Griesser, H.J.; Coad, B.R. The importance of fungal pathogens and antifungal coatings in medical device infections. Biotechnol. Adv. 2018, 36, 264–280. [Google Scholar] [CrossRef]
- Lyndon, J.A.; Boyd, B.J.; Birbilis, N. Metallic implant drug/device combinations for controlled drug release in orthopaedic applications. J. Control. Release 2014, 179, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Juodkazis, S.; Ivanova, E.P. Nanofabrication of mechano-bactericidal surfaces. Nanoscale 2017, 9, 16564–16585. [Google Scholar] [CrossRef] [PubMed]
- Braem, A.; De Cremer, K.; Delattin, N.; De Brucker, K.; Neirinck, B.; Vandamme, K.; Martens, J.A.; Michiels, J.; Vleugels, J.; Cammue, B.P.; et al. Novel anti-infective implant substrates: Controlled release of antibiofilm compounds from mesoporous silica-containing macroporous titanium. Colloids Surf. B Biointerfaces 2015, 126, 481–488. [Google Scholar] [CrossRef] [PubMed]
- De Cremer, K.; Braem, A.; Gerits, E.; De Brucker, K.; Vandamme, K.; Martens, J.; Vleugels, J.; Cammue, B.; Thevissen, K. Controlled release of chlorhexidine from a mesoporous silica-containing macroporous titanium dental implant prevents microbial biofilm formation. Eur. Cells Mater. 2017, 33, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.A.; Wells, C.M.; McGraw, G.S.; Pulgarin, D.A.V.; Whitaker, M.D.; Pruitt, R.L.; Bumgardner, J.D. Chitosan coatings to control release and target tissues for therapeutic delivery. Ther. Deliv. 2015, 6, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Beenken, K.E.; Smith, J.K.; Skinner, R.A.; McLaren, S.G.; Bellamy, W.; Gruenwald, M.J.; Spencer, H.J.; Jennings, J.A.; Haggard, W.O.; Smeltzer, M.S. Chitosan coating to enhance the therapeutic efficacy of calcium sulfate-based antibiotic therapy in the treatment of chronic osteomyelitis. J. Biomater. Appl. 2014, 29, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Albright, V.; Zhuk, I.; Wang, Y.; Selin, V.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C.; Sukhishvili, S.A. Self-defensive antibiotic-loaded layer-by-layer coatings: Imaging of localized bacterial acidification and pH-triggering of antibiotic release. Acta Biomater. 2017, 61, 66–74. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, Z.; Perinpanayagam, H.; Zhu, J. Development of Robust Chitosan–Silica Class II Hybrid Coatings with Antimicrobial Properties for Titanium Implants. Coatings 2020, 10, 534. [Google Scholar] [CrossRef]
- Buşilă, M.; Muşat, V.; Textor, T.; Mahltig, B. Synthesis and characterization of antimicrobial textile finishing based on Ag:ZnO nanoparticles/chitosan biocomposites. RSC Adv. 2015, 5, 21562–21571. [Google Scholar] [CrossRef]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef]
- Pavinatto, A.V.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Ligler, F.S.; Lingerfelt, B.M.; Price, R.P.; Schoen, P.E. Development of uniform chitosan thin-film layers on silicon chips. Langmuir 2001, 17, 5082–5084. [Google Scholar] [CrossRef]
- Gan, Q.; Zhu, J.; Yuan, Y.; Liu, H.; Qian, J.; Li, Y.; Liu, C. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J. Mater. Chem. B 2015, 3, 2056–2066. [Google Scholar] [CrossRef]
- Zayed, N.; Figueiredo, J.; Van Holm, W.; Boon, N.; Bernaerts, K.; Teughels, W. Mode of killing determines the necrotrophic response of oral bacteria. J. Oral Microbiol. 2023, 15, 2184930. [Google Scholar] [CrossRef] [PubMed]
- Yanming, D.; Congyi, X.U.; Jianwei, W. Determination of degree of substitution for N-acylated chitosan using IR spectra. Sci. China 2001, 44, 216–224. [Google Scholar]
- Ostrowska-Czubenko, J.; Gierszewska, M.; Pieróg, M. pH-responsive hydrogel membranes based on modified chitosan: Water transport and kinetics of swelling. J. Polym. Res. 2015, 22, 153. [Google Scholar] [CrossRef]
- Edmiston, C.E.; McBain, A.J.; Roberts, C.; Leaper, D. Clinical and Microbiological Aspects of Biofilm-Associated Surgical Site Infections. Adv. Exp. Med. Biol. 2015, 830, 47–67. [Google Scholar] [CrossRef]
- Pavithra, D.; Doble, M. Biofilm formation, bacterial adhesion and host response on polymeric implants—Issues and prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.H.N.; D’Haeyer, C.; Thevissen, K.; Braem, A. Development of mesoporous bioactive glass-containing macroporous titanium for controlled release of antimicrobial drugs. J. Am. Ceram. Soc. 2022, 105, 1882–1895. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Sankar, V.; Hearnden, V.; Hull, K.; Juras, D.V.; Greenberg, M.S.; Kerr, A.; Lockhart, P.; Patton, L.; Porter, S.; Thornhill, M. Local drug delivery for oral mucosal diseases: Challenges and opportunities. Oral Dis. 2011, 17, 73–84. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Wu, D.-Q.; Chu, C.-C. Effect of the Crosslinking Level on the Properties of Temperature-Sensitive Poly(N-Isopropylacrylamide) Hydrogels. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 582–593. [Google Scholar] [CrossRef]
- Yongabi, D.; Khorshid, M.; Gennaro, A.; Jooken, S.; Duwé, S.; Deschaume, O.; Losada-Pérez, P.; Dedecker, P.; Bartic, C.; Wübbenhorst, M.; et al. QCM-D Study of Time-Resolved Cell Adhesion and Detachment: Effect of Surface Free Energy on Eukaryotes and Prokaryotes. ACS Appl. Mater. Interfaces 2020, 12, 18258–18272. [Google Scholar] [CrossRef]
- Miras, J.; Liu, C.; Blomberg, E.; Thormann, E.; Vílchez, S.; Esquena, J. pH-responsive chitosan nanofilms crosslinked with genipin. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 616, 126229. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Lai, J.-Y. In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using γ-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymer 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, R.; Lima, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef] [PubMed]

 ) of higher slope indicating an increased release in the acidic regions for CS-coated disk highlighted using curly brackets.
) of higher slope indicating an increased release in the acidic regions for CS-coated disk highlighted using curly brackets.
 ) of higher slope indicating an increased release in the acidic regions for CS-coated disk highlighted using curly brackets.
) of higher slope indicating an increased release in the acidic regions for CS-coated disk highlighted using curly brackets.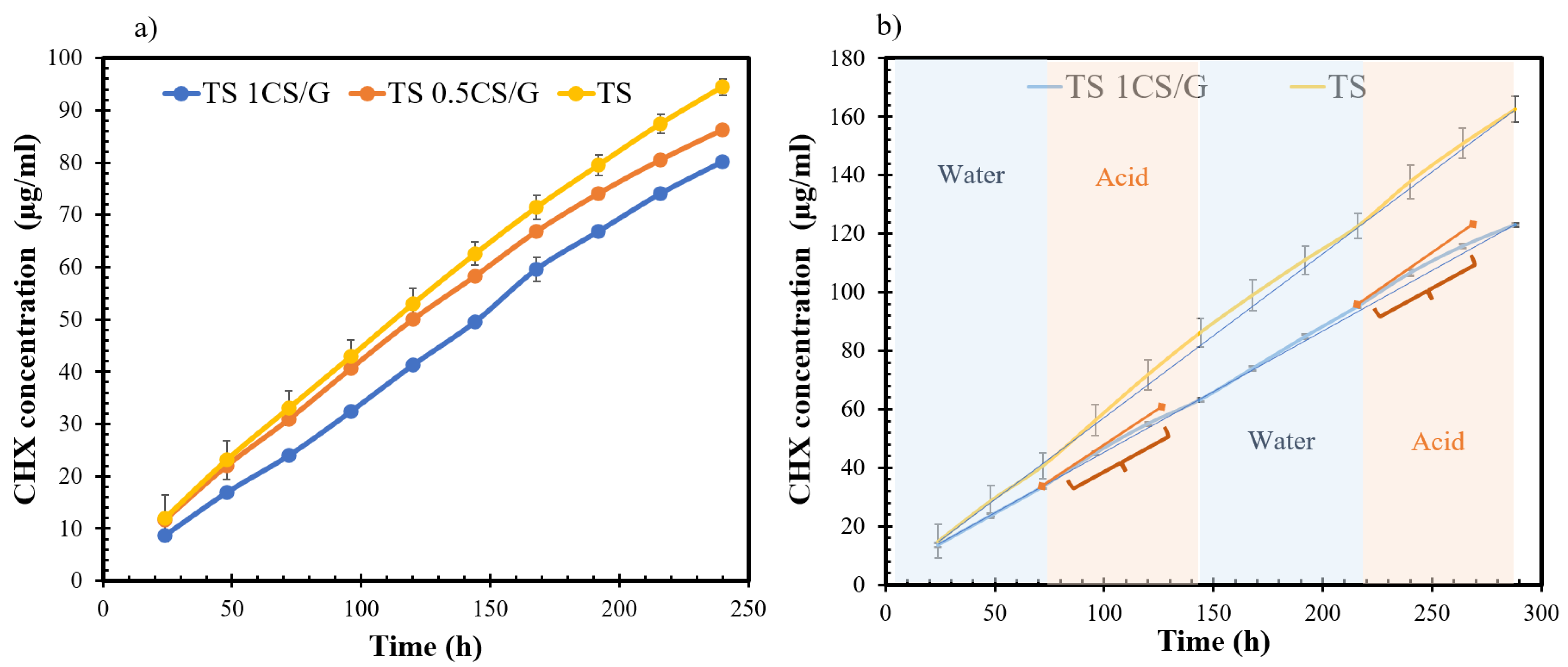
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, M.G.; Kamarudin, N.H.N.; Aktan, M.K.; Zheng, K.; Zayed, N.; Yongabi, D.; Wagner, P.; Teughels, W.; Boccaccini, A.R.; Braem, A. pH-Triggered Controlled Release of Chlorhexidine Using Chitosan-Coated Titanium Silica Composite for Dental Infection Prevention. Pharmaceutics 2024, 16, 377. https://doi.org/10.3390/pharmaceutics16030377
Srivastava MG, Kamarudin NHN, Aktan MK, Zheng K, Zayed N, Yongabi D, Wagner P, Teughels W, Boccaccini AR, Braem A. pH-Triggered Controlled Release of Chlorhexidine Using Chitosan-Coated Titanium Silica Composite for Dental Infection Prevention. Pharmaceutics. 2024; 16(3):377. https://doi.org/10.3390/pharmaceutics16030377
Chicago/Turabian StyleSrivastava, Mrinal Gaurav, Nur Hidayatul Nazirah Kamarudin, Merve Kübra Aktan, Kai Zheng, Naiera Zayed, Derick Yongabi, Patrick Wagner, Wim Teughels, Aldo R. Boccaccini, and Annabel Braem. 2024. "pH-Triggered Controlled Release of Chlorhexidine Using Chitosan-Coated Titanium Silica Composite for Dental Infection Prevention" Pharmaceutics 16, no. 3: 377. https://doi.org/10.3390/pharmaceutics16030377
APA StyleSrivastava, M. G., Kamarudin, N. H. N., Aktan, M. K., Zheng, K., Zayed, N., Yongabi, D., Wagner, P., Teughels, W., Boccaccini, A. R., & Braem, A. (2024). pH-Triggered Controlled Release of Chlorhexidine Using Chitosan-Coated Titanium Silica Composite for Dental Infection Prevention. Pharmaceutics, 16(3), 377. https://doi.org/10.3390/pharmaceutics16030377









