Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies
Abstract
1. Introduction
2. Self-Assembled Peptides for Drug Delivery Systems
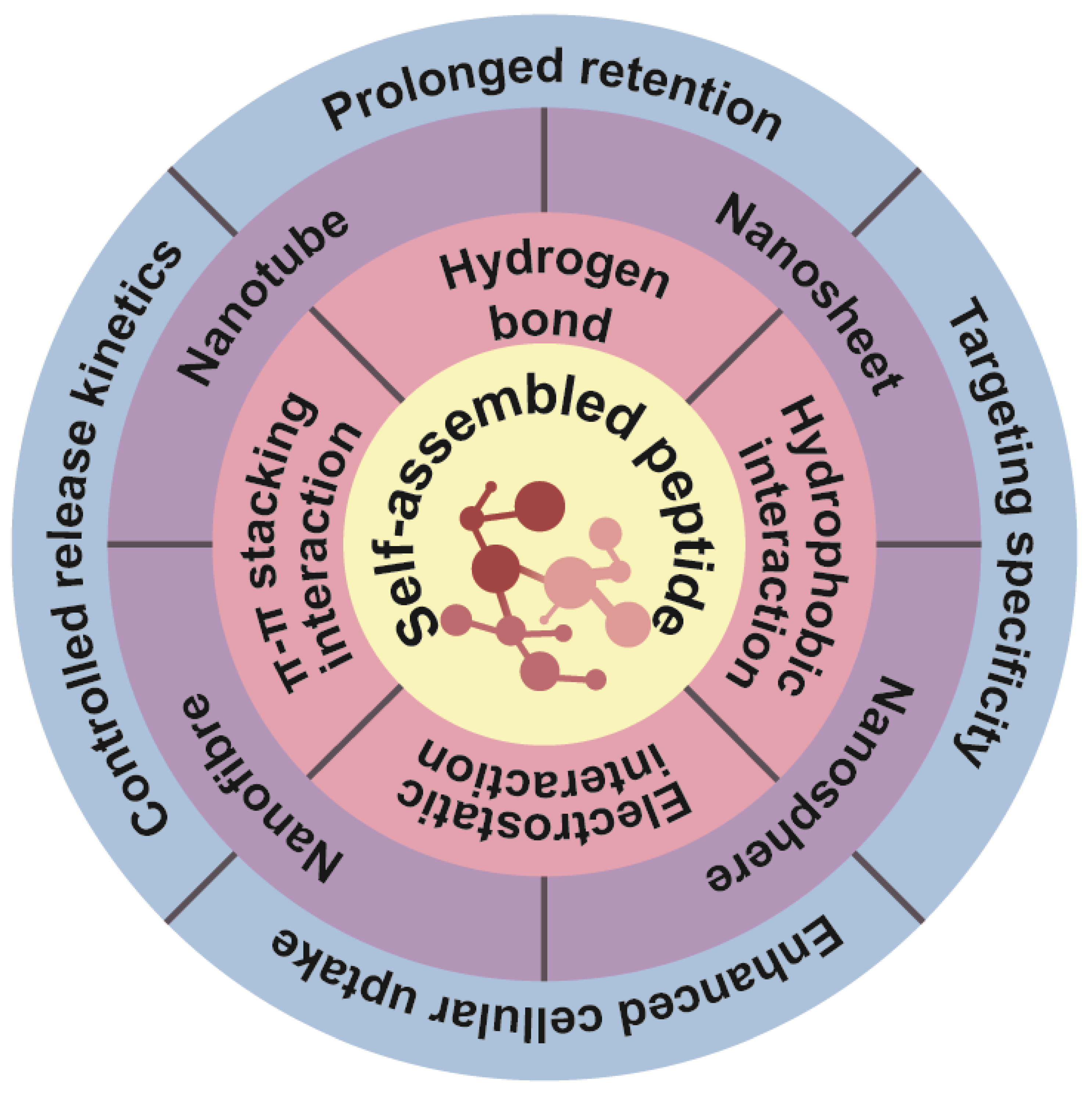
2.1. Drivers of Peptide Self-Assembly
2.1.1. Hydrogen Bonds
2.1.2. Hydrophobic Interactions
2.1.3. Electrostatic Interactions
2.1.4. π-π Stacking Interactions
2.2. Secondary Structure in Peptide Self-Assembly
2.2.1. α-Helix Structure
2.2.2. β-Sheet Structure
2.2.3. β-Turn Structure
2.2.4. Random Coil
2.3. Classification of Peptide Self-Assembly
2.3.1. Spontaneous Self-Assembly
2.3.2. Triggered Self-Assembly
pH-Triggered Self-Assembly
Temperature-Triggered Self-Assembly
Light-Triggered Self-Assembly
Receptor–Ligand Binding-Triggered Self-Assembly
2.4. Peptide Amphiphilic Molecules Self-Assemble into Structures
2.4.1. Nanofiber Assemblies
2.4.2. Nanotube Assemblies
2.4.3. Nanosheet Assemblies
2.4.4. Nanosphere Assemblies
2.5. Advantages of Self-Assembled Peptides
2.5.1. Environmental Responsiveness
2.5.2. Adjustability of Assembly Structure
2.5.3. Reversibility
2.5.4. Suitable Histocompatibility
2.6. Application of Self-Assembled Peptides for Drug Delivery
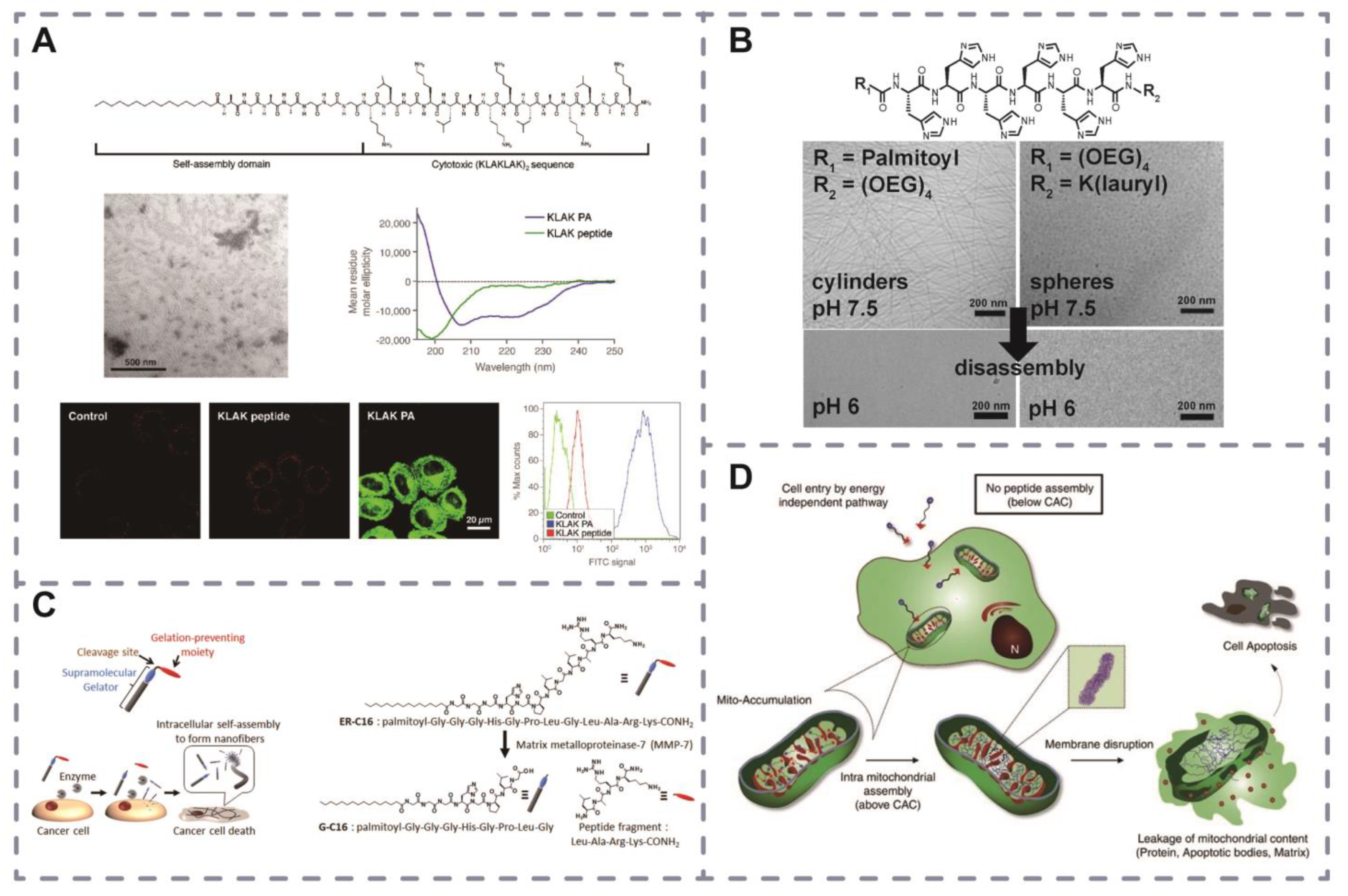
3. Functional Peptide-Modified Drug Delivery Systems
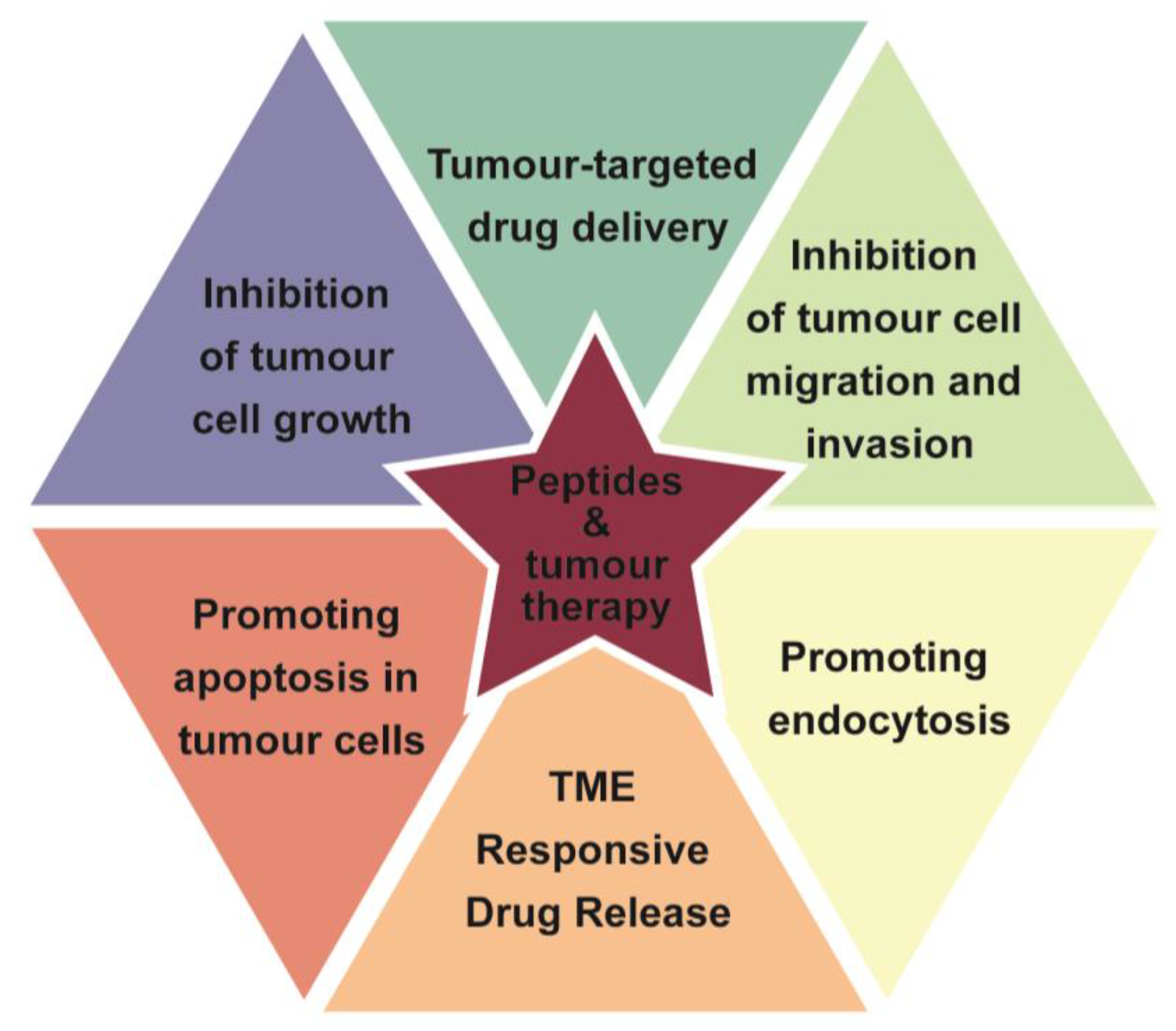
3.1. Classification of Functional Peptides
3.1.1. Cell-Penetrating Peptide (CPP)
3.1.2. Cell-Targeting Peptides (CTPs)
3.1.3. Stimulus-Responsive Peptide
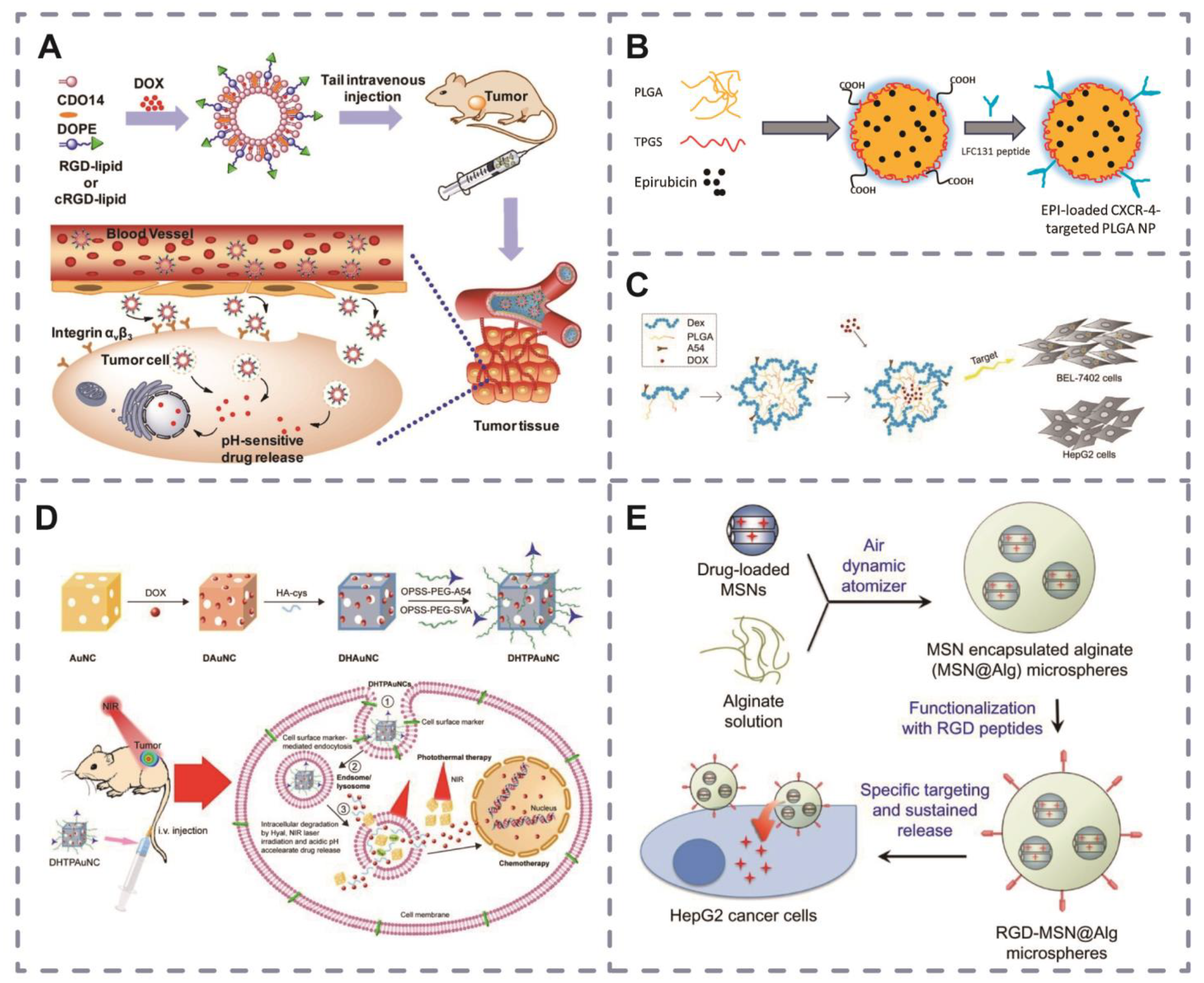
3.2. Peptide-Modified Nanomaterials
3.2.1. Peptide-Modified Liposomes
3.2.2. Peptide-Modified Polymers
3.2.3. Peptide-Modified Inorganic Nanoparticles
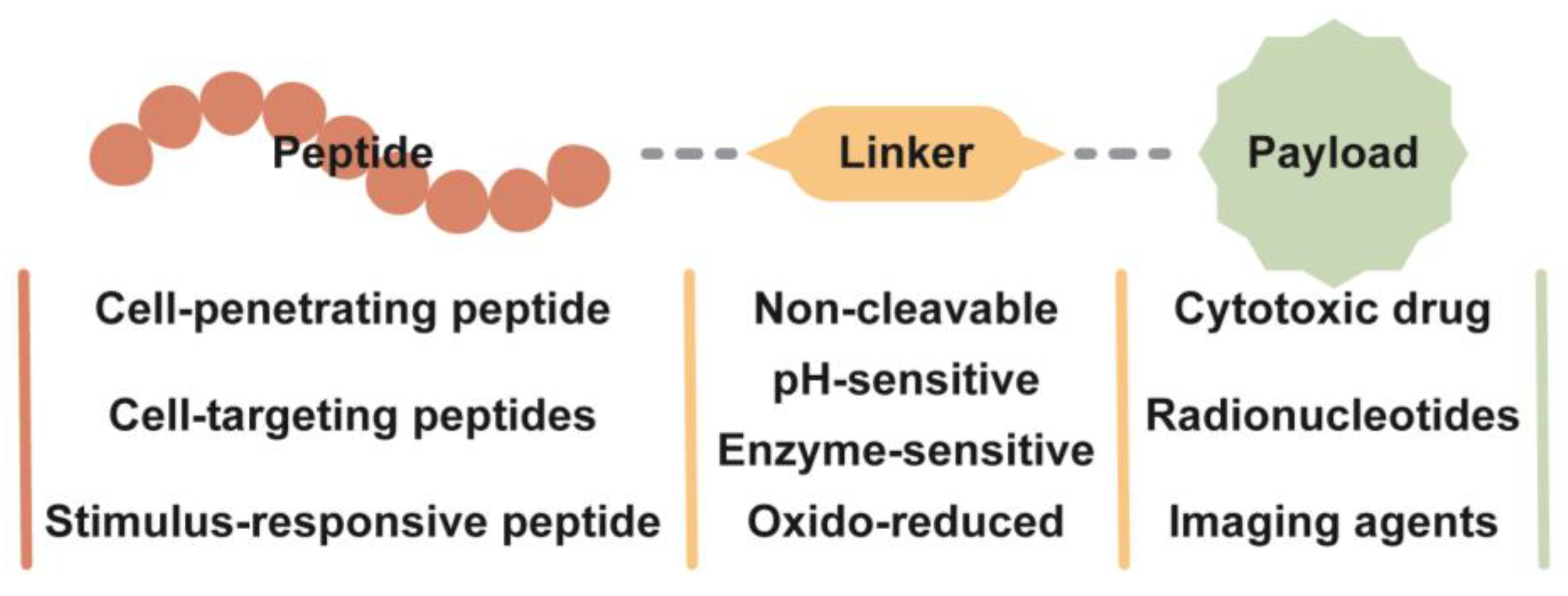
3.3. Peptide–Drug Conjugate
- (1)
- Their small molecular size (2–20 kDa) facilitates easier penetration into the tumor stroma and cells;
- (2)
- PDCs are not subject to FcR, RES, or ADA pathway-mediated non-pharmacological elimination, which effectively improves drug utilization;
- (3)
- PDCs can be produced in prokaryotic nuclei or through chemical synthesis, simplifying production and scalability;
- (4)
- PDCs can be conjugated with clinically proven cytotoxic agents like Adriamycin and Paclitaxel, which are proven cytotoxic molecules, to prepare target agents, significantly reducing off-target toxicity and enhancing PDC formulation platform technology’s feasibility;
- (5)
- Certain targeted peptides in PDCs can alter cell entry mechanisms to effectively kill drug-resistant tumors, addressing the challenge of traditional chemotherapy’s ineffectiveness against drug-resistant tumors.
3.3.1. Linker in the Peptide–Drug Conjugate
- (1)
- Suitable stability during the somatic circulation before reaching the target site to avoid systemic toxicity caused by drug release at non-pathological sites;
- (2)
- After being phagocytosed by target cells, PDC can be triggered by the special microenvironment within target cells to rapidly break off and release drug molecules;
- (3)
- The hydrophobicity should not be too strong; otherwise, PDCs are prone to poor in vivo stability and decreased drug efficacy due to hydrophobic aggregation and, at the same time, produce strong systemic toxicity and immune side effects.
Non-Cleavable Linkers
Cleavable Linker
3.3.2. Applications of Peptide–Drug Conjugate
3.3.3. Advances in Clinical Studies of Peptide–Drug Conjugate
4. Strategies to Improve Peptide Drug Delivery
4.1. Modification of Peptide Structure
4.1.1. Cyclic Peptide Formation
4.1.2. Use of D-Amino Acids
4.1.3. Altering Single or Multiple Amino Acids in a Peptide
4.2. Modification of Peptide Ends
4.2.1. Hydrophobic Modification of Peptides
4.2.2. Hydrophilic Modification of Peptides
4.3. Physical Encapsulation of Peptides
4.4. Novel Technology for Peptides: Microfluidics
4.5. AI-Enabled Peptide Design and Synthesis
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Samec, T.; Boulos, J.; Gilmore, S.; Hazelton, A.; Alexander-Bryant, A. Peptide-based delivery of therapeutics in cancer treatment. Mater. Today Bio. 2022, 14, 100248. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Pochan, D.; Scherman, O. Introduction: Molecular Self-Assembly. Chem. Rev. 2021, 121, 13699–13700. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.J.; Langenstein, M.G.; Pochan, D.J.; Kloxin, C.J.; Saven, J.G. Peptide Design and Self-assembly into Targeted Nanostructure and Functional Materials. Chem. Rev. 2021, 121, 13915–13935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Q.; Wu, Y.; Li, X.; Zhou, Y.; Wang, Z.; Liang, H.; Ding, F.; Hong, S.; Steinmetz, N.F.; et al. Molecularly Stimuli-Responsive Self-Assembled Peptide Nanoparticles for Targeted Imaging and Therapy. ACS Nano 2023, 17, 8004–8025. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.-M.; Zhang, M.-R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 3146. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Luginbuhl, K.M.; Simon, J.R.; Dzuricky, M.; Berger, R.; Varol, H.S.; Huang, F.C.; Buehne, K.L.; Mayne, N.R.; Weitzhandler, I.; et al. Genetically encoded lipid-polypeptide hybrid biomaterials that exhibit temperature-triggered hierarchical self-assembly. Nat. Chem. 2018, 10, 496–505. [Google Scholar] [CrossRef]
- Pitz, M.E.; Nukovic, A.M.; Elpers, M.A.; Alexander-Bryant, A.A. Factors Affecting Secondary and Supramolecular Structures of Self-Assembling Peptide Nanocarriers. Macromol. Biosci. 2022, 22, e2100347. [Google Scholar] [CrossRef] [PubMed]
- Sis, M.J.; Webber, M.J. Drug Delivery with Designed Peptide Assemblies. Trends Pharmacol. Sci. 2019, 40, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yang, H.; Gao, S.; Li, L.; Fu, X.; Wei, Q. Research progress on self-assembled nanodrug delivery systems. J. Mater. Chem. B 2022, 10, 1908–1922. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Zhao, X. Designed peptide amphiphiles as scaffolds for tissue engineering. Adv. Colloid Interface Sci. 2023, 314, 102866. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Liu, J.; Zhang, N.; Ji, X.; Han, Y.; Wang, Y. Assembly and Evolution of Gemini-Type Peptide Amphiphile with a Di-Lysine Spacer. Langmuir 2019, 35, 6154–6160. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Paramonov, S.E.; Jun, H.W.; Hartgerink, J.D. Self-assembly of peptide-amphiphile nanofibers: The roles of hydrogen bonding and amphiphilic packing. J. Am. Chem. Soc. 2006, 128, 7291–7298. [Google Scholar] [CrossRef]
- Wang, C.; Fu, L.; Hu, Z.; Zhong, Y. A mini-review on peptide-based self-assemblies and their biological applications. Nanotechnology 2021, 33, 062004. [Google Scholar] [CrossRef]
- Löwik, D.W.P.M.; Garcia-Hartjes, J.; Meijer, J.T.; van Hest, J.C.M. Tuning secondary structure and self-assembly of amphiphilic peptides. Langmuir 2005, 21, 524–526. [Google Scholar] [CrossRef]
- Nambiar, M.; Schneider, J.P. Peptide hydrogels for affinity-controlled release of therapeutic cargo: Current and potential strategies. J. Pept. Sci. 2022, 28, e3377. [Google Scholar] [CrossRef]
- Papapostolou, D.; Smith, A.M.; Atkins, E.D.T.; Oliver, S.J.; Ryadnov, M.G.; Serpell, L.C.; Woolfson, D.N. Engineering nanoscale order into a designed protein fiber. Proc. Natl. Acad. Sci. USA 2007, 104, 10853–10858. [Google Scholar] [CrossRef]
- Sun, X.; Quan, S.; Wang, B.; Wang, Q.; Li, W.; Xiao, J. Peptide-triggered self-assembly of collagen mimetic peptides into nanospheres by electrostatic interaction and π-π stacking. J. Mater. Chem. B 2023, 11, 4677–4683. [Google Scholar] [CrossRef]
- Carny, O.; Shalev, D.E.; Gazit, E. Fabrication of coaxial metal nanocables using a self-assembled peptide nanotube scaffold. Nano Lett. 2006, 6, 1594–1597. [Google Scholar] [CrossRef]
- Song, Z.; Chen, X.; You, X.; Huang, K.; Dhinakar, A.; Gu, Z.; Wu, J. Self-assembly of peptide amphiphiles for drug delivery: The role of peptide primary and secondary structures. Biomater. Sci. 2017, 5, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W.; Dehsorkhi, A.; Castelletto, V.; Furzeland, S.; Atkins, D.; Seitsonen, J.; Ruokolainen, J. Reversible helical unwinding transition of a self-assembling peptide amphiphile. Soft Matter 2013, 9, 9290–9293. [Google Scholar] [CrossRef]
- Beesley, J.L.; Woolfson, D.N. The de novo design of α-helical peptides for supramolecular self-assembly. Curr. Opin. Biotechnol. 2019, 58, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, Y.Y.; Zhang, W.; Li, L.; Zhang, Y.; Zhang, L.; Liu, H.; Ni, J.M.; Wang, R. Design of new acid-activated cell-penetrating peptides for tumor drug delivery. PeerJ 2017, 5, e3429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Park, Y.; Park, I.S.; Park, Y.S.; Kim, Y.; Hahm, K.S.; Shin, S.Y. Improvement of bacterial cell selectivity of melittin by a single Trp mutation with a peptoid residue. Protein. Peptide Lett. 2006, 13, 719–725. [Google Scholar] [CrossRef]
- Laxio Arenas, J.; Kaffy, J.; Ongeri, S. Peptides and peptidomimetics as inhibitors of protein-protein interactions involving β-sheet secondary structures. Curr. Opin. Chem. Biol. 2019, 52, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, Y.; Zuo, P.; Miao, S.; Hu, B.; Kang, Y.; Liu, W.; Yang, Q.; Ren, H.; et al. α-Helix-Mediated Protein Adhesion. J. Am. Chem. Soc. 2023, 145, 17125–17135. [Google Scholar] [CrossRef]
- Singh, A.; Joseph, J.P.; Gupta, D.; Sarkar, I.; Pal, A. Pathway driven self-assembly and living supramolecular polymerization in an amyloid-inspired peptide amphiphile. Chem. Commun. 2018, 54, 10730–10733. [Google Scholar] [CrossRef]
- Li, X.; Sabol, A.L.; Wierzbicki, M.; Salveson, P.J.; Nowick, J.S. An Improved Turn Structure for Inducing β-Hairpin Formation in Peptides. Angew. Chem. 2021, 60, 22776–22782. [Google Scholar] [CrossRef]
- De Brevern, A.G. A Perspective on the (Rise and Fall of) Protein β-Turns. Int. J. Mol. Sci. 2022, 23, 2314. [Google Scholar] [CrossRef]
- Robinson, J.A. Max Bergmann lecture Protein epitope mimetics in the age of structural vaccinology. J. Pept. Sci. 2013, 19, 127–140. [Google Scholar] [CrossRef]
- Duti, I.J.; Florian, J.R.; Kittel, A.R.; Amelung, C.D.; Gray, V.P.; Lampe, K.J.; Letteri, R.A. Peptide Stereocomplexation Orchestrates Supramolecular Assembly of Hydrogel Biomaterials. J. Am. Chem. Soc. 2023, 145, 18468–18476. [Google Scholar] [CrossRef]
- Guler, M.O.; Stupp, S.I. A self-assembled nanofiber catalyst for ester hydrolysis. J. Am. Chem. Soc. 2007, 129, 12082–12083. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, S.; Santoso, S.; Gong, H.Y.; Watson, N.; Zhang, S.G. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc. Natl. Acad. Sci. USA 2002, 99, 5355–5360. [Google Scholar] [CrossRef]
- Xing, R.; Yuan, C.; Fan, W.; Ren, X.; Yan, X. Biomolecular glass with amino acid and peptide nanoarchitectonics. Sci. Adv. 2023, 9, eadd8105. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, S.; Handgraaf, J.W.; Tellers, E.E.; Popescu, D.C.; Overhand, M.; Kjaer, K.; Vaiser, V.; Sommerdijk, N.A.J.M.; Rapaport, H.; Kros, A. Two-dimensional ordered β-sheet lipopeptide monolayers. J. Am. Chem. Soc. 2006, 128, 13959–13966. [Google Scholar] [CrossRef]
- Altman, M.; Lee, P.; Rich, A.; Zhang, S.G. Conformational behavior of ionic self-complementary peptides. Protein. Sci. 2000, 9, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Gan, H.X.; Tong, Y.W. pH-Controlled Hierarchical Self-Assembly of Peptide Amphiphile. Macromolecules 2015, 48, 2647–2653. [Google Scholar] [CrossRef]
- Rughani, R.V.; Branco, M.C.; Pochan, D.; Schneider, J.P. Design of a Shear-Thin Recoverable Peptide-Based Hydrogel Capable of Intrafibrillar Photopolymerization. Macromolecules 2010, 43, 7924–7930. [Google Scholar] [CrossRef]
- Haines, L.A.; Rajagopal, K.; Ozbas, B.; Salick, D.A.; Pochan, D.J.; Schneider, J.P. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 2005, 127, 17025–17029. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Xu, X.D.; Li, S.Y.; Zhuo, R.X.; Zhang, X.Z. Photo-switched self-assembly of a gemini α-helical peptide into supramolecular architectures. Nanoscale 2013, 5, 6270–6274. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Lu, S.; Wang, N.; Xu, H.; Cox, H.; Li, R.; Waigh, T.; Han, Y.; Wang, Y.; Lu, J.R. Enzyme-Triggered Morphological Transition of Peptide Nanostructures for Tumor-Targeted Drug Delivery and Enhanced Cancer Therapy. Acs. Appl. Mater Inter. 2019, 11, 16357–16366. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, M.; Deng, S.; Zhu, X.; Song, Y.; Song, E. Enzyme-Triggered Transforming of Assembly Peptide-Modified Magnetic Resonance-Tuned Probe for Highly Sensitive Imaging of Bacterial Infection In Vivo. Small 2023, 19, e2208249. [Google Scholar] [CrossRef] [PubMed]
- Straley, K.S.; Heilshorn, S.C. Dynamic, 3D-Pattern Formation Within Enzyme-Responsive Hydrogels. Adv. Mater. 2009, 21, 4148–4152. [Google Scholar] [CrossRef]
- Todd, S.J.; Scurr, D.J.; Gough, J.E.; Alexander, M.R.; Ulijn, R.V.J.L. Enzyme-activated RGD ligands on functionalized poly (ethylene glycol) monolayers: Surface analysis and cellular response. Langmuir 2009, 25, 7533–7539. [Google Scholar] [CrossRef]
- Yang, Z.M.; Liang, G.L.; Wang, L.; Xu, B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramoleclar hydrogel in vivo. J. Am. Chem. Soc. 2006, 128, 3038–3043. [Google Scholar] [CrossRef]
- Sedighi, M.; Shrestha, N.; Mahmoudi, Z.; Khademi, Z.; Ghasempour, A.; Dehghan, H.; Talebi, S.F.; Toolabi, M.; Préat, V.; Chen, B.; et al. Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications. Polymers 2023, 15, 1160. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef]
- Ma, J.; Cai, B.; Zhang, S.; Jian, T.; De Yoreo, J.J.; Chen, C.L.; Baneyx, F. Nanoparticle-Mediated Assembly of Peptoid Nanosheets Functionalized with Solid-Binding Proteins: Designing Heterostructures for Hierarchy. Nano Lett. 2021, 21, 1636–1642. [Google Scholar] [CrossRef]
- Zelzer, M.; Ulijn, R.V. Next-generation peptide nanomaterials: Molecular networks, interfaces and supramolecular functionality. Chem. Soc. Rev. 2010, 39, 3351–3357. [Google Scholar] [CrossRef]
- Castelletto, V.; Cheng, G.; Stain, C.; Connon, C.J.; Hamley, I.W. Self-Assembly of a Peptide Amphiphile Containing L-Carnosine and Its Mixtures with a Multilamellar Vesicle Forming Lipid. Langmuir 2012, 28, 11599–11608. [Google Scholar] [CrossRef]
- Fowler, M.; Siddique, B.; Duhamel, J. Effect of Sequence on the Ionization Behavior of a Series of Amphiphilic Polypeptides. Langmuir 2013, 29, 4451–4459. [Google Scholar] [CrossRef]
- Garcia, A.M.; Kurbasic, M.; Kralj, S.; Melchionna, M.; Marchesan, S. A biocatalytic and thermoreversible hydrogel from a histidine-containing tripeptide. Chem. Commun. 2017, 53, 8110–8113. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J. Chemistry-Seamless proteins tie up their loose ends. Science 2006, 311, 1563–1564. [Google Scholar] [CrossRef] [PubMed]
- El Khalifi, M.; Bentin, J.; Duverger, E.; Gharbi, T.; Boulahdour, H.; Picaud, F. Encapsulation capacity and natural payload delivery of an anticancer drug from boron nitride nanotube. Phys. Chem. Chem. Phys. 2016, 18, 24994–25001. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.L.; Coulter, S.M.; Pentlavalli, S.; Laverty, G. Pharmaceutical Formulation and Characterization of Dipeptide Nanotubes for Drug Delivery Applications. Macromol. Biosci. 2020, 20, 2000115. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Cox, H.; Mansfield, E.D.H.; Ellacott, S.H.; Peltier, R.; Brendel, J.C.; Hartlieb, M.; Waigh, T.A.; Perrier, S. Dual self-assembly of supramolecular peptide nanotubes to provide stabilisation in water. Nat. Commun. 2019, 10, 4708. [Google Scholar] [CrossRef]
- Insua, I.; Montenegro, J. 1D to 2D Self Assembly of Cyclic Peptides. J. Am. Chem. Soc. 2020, 142, 300–307. [Google Scholar] [CrossRef]
- Merg, A.D.; Touponse, G.; van Genderen, E.; Zuo, X.B.; Bazrafshan, A.; Blum, T.; Hughes, S.; Salaita, K.; Abrahams, J.P.; Conticello, V.P. 2D Crystal Engineering of Nanosheets Assembled from Helical Peptide Building Blocks. Angew. Chem. Int. Edit 2019, 58, 13507–13512. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Liu, H.; Wang, R.; Wei, J.; Cheng, C.; Zheng, Y.; Pan, Y.; He, X.; Ding, M.; Tan, H.; Fu, Q. Conformation-Directed Micelle-to-Vesicle Transition of Cholesterol-Decorated Polypeptide Triggered by Oxidation. J. Am. Chem. Soc. 2018, 140, 6604–6610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, S.; Huo, M.; van Hest, J.C.M.; Che, H. Recent advances in permeable polymersomes: Fabrication, responsiveness, and applications. Chem. Sci. 2023, 14, 7411–7437. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliver Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Booth, R.; Insua, I.; Ahmed, S.; Rioboo, A.; Montenegro, J. Supramolecular fibrillation of peptide amphiphiles induces environmental responses in aqueous droplets. Nat. Commun. 2021, 12, 6421. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Choi, W.T.; Heller, W.T.; Ke, Z.L.; Wright, E.R.; Champion, J.A. Engineering Globular Protein Vesicles through Tunable Self-Assembly of Recombinant Fusion Proteins. Small 2017, 13, 1700399. [Google Scholar] [CrossRef]
- Yin, X.Y.; Chen, Z.X.; Chen, Y.; Xie, Y.; Xiong, B.J.; Jiang, H.; Zhu, J.T. Lipidated gemini peptide amphiphiles with enhanced loading capacity and cell membrane affinity for drug delivery. Colloid Surf. B 2020, 195, 111271. [Google Scholar] [CrossRef]
- Delfi, M.; Sartorius, R.; Ashrafizadeh, M.; Sharifi, E.; Zhang, Y.; De Berardinis, P.; Zarrabi, A.; Varma, R.S.; Tay, F.R.; Smith, B.R.; et al. Self-assembled peptide and protein nanostructures for anti-cancer therapy: Targeted delivery, stimuli-responsive devices and immunotherapy. Nano Today 2021, 38, 101119. [Google Scholar] [CrossRef] [PubMed]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Del Rio, G.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef]
- Standley, S.M.; Toft, D.J.; Cheng, H.; Soukasene, S.; Chen, J.; Raja, S.M.; Band, V.; Band, H.; Cryns, V.L.; Stupp, S.I. Induction of Cancer Cell Death by Self-assembling Nanostructures Incorporating a Cytotoxic Peptide. Cancer Res. 2010, 70, 3020–3026. [Google Scholar] [CrossRef]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Law, B.; Weissleder, R.; Tung, C.H. Protease-sensitive fluorescent nanofibers. Bioconjugate Chem. 2007, 18, 1701–1704. [Google Scholar] [CrossRef][Green Version]
- Wu, H.M.; Zhou, T.; Tian, L.; Xia, Z.C.; Xu, F. Self-Assembling RADA16-I Peptide Hydrogel Scaffold Loaded with Tamoxifen for Breast Reconstruction. BioMed Res. Int. 2017, 2017, 3656193. [Google Scholar] [CrossRef]
- Callmann, C.E.; Barback, C.V.; Thompson, M.P.; Hall, D.J.; Mattrey, R.F.; Gianneschi, N.C. Therapeutic Enzyme-Responsive Nanoparticles for Targeted Delivery and Accumulation in Tumors. Adv. Mater. 2015, 27, 4611–4615. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Finbloom, J.A.; Chen, F.; Toft, D.J.; Cryns, V.L.; Stupp, S.I. pH and Amphiphilic Structure Direct Supramolecular Behavior in Biofunctional Assemblies. J. Am. Chem. Soc. 2014, 136, 14746–14752. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Seifert, J.; Romano, N.C.; Gao, M.; Helmus, J.J.; Jaroniec, C.P.; Modarelli, D.A.; Parquette, J.R. Amphiphilic Self-Assembly of an n-Type Nanotube. Angew. Chem. Int. Edit 2010, 49, 7688–7691. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, Y.; Shi, J.F.; Zhou, J.; Medina, J.E.; Zhou, R.; Yuan, D.; Yang, C.H.; Wang, H.M.; Yang, Z.M.; et al. Enzyme-Instructed Intracellular Molecular Self-Assembly to Boost Activity of Cisplatin against Drug-Resistant Ovarian Cancer Cells. Angew. Chem. Int. Edit 2015, 54, 13307–13311. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Shi, J.F.; Li, J.; Yuan, D.; Alberti, K.A.; Xu, Q.B.; Xu, B. Pericellular Hydrogel/Nanonets Inhibit Cancer Cells. Angew. Chem. Int. Edit 2014, 53, 8104–8107. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Xu, B. Nanoscale assemblies of small molecules control the fate of cells. Nano Today 2015, 10, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Fukuoka, Y.; Morimoto, Y.; Honjo, T.; Koda, D.; Goto, M.; Maruyama, T. Cancer Cell Death Induced by the Intracellular Self-Assembly of an Enzyme-Responsive Supramolecular Gelator. J. Am. Chem. Soc. 2015, 137, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Jeena, M.T.; Palanikumar, L.; Go, E.M.; Kim, I.; Kang, M.G.; Lee, S.; Park, S.; Choi, H.; Kim, C.; Jin, S.M.; et al. Mitochondria localization induced self-assembly of peptide amphiphiles for cellular dysfunction. Nat. Commun. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, G.L.; Zhai, S.D.; Zhang, W.J.; Xing, D. Specific photoacoustic cavitation through nucleus targeted nanoparticles for high-efficiency tumor therapy. Nano Res. 2020, 13, 719–728. [Google Scholar] [CrossRef]
- Tripathi, P.P.; Arami, H.; Banga, I.; Gupta, J.; Gandhi, S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 2018, 9, 37252. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.; Zabit, S.; Hauser, N.; Farouz, S.; Melloul, O.; Hirbawi, J.; Lorberboum-Galski, H. TAT for Enzyme/Protein Delivery to Restore or Destroy Cell Activity in Human Diseases. Life 2021, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Ablan, F.D.O.; Spaller, B.L.; Abdo, K.I.; Almeida, P.F. Charge Distribution Fine-Tunes the Translocation of α-Helical Amphipathic Peptides across Membranes. Biophys. J. 2016, 111, 1738–1749. [Google Scholar] [CrossRef]
- Vasconcelos, L.; Madani, F.; Arukuusk, P.; Pärnaste, L.; Gräslund, A.; Langel, U. Effects of cargo molecules on membrane perturbation caused by transportan10 based cell-penetrating peptides. Biochim. Et Biophys. Acta 2014, 1838, 3118–3129. [Google Scholar] [CrossRef]
- Bolhassani, A.; Jafarzade, B.S.; Mardani, G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides 2017, 87, 50–63. [Google Scholar] [CrossRef]
- Xue, G.; Liu, Z.; Wang, L.; Zu, L. The role of basic residues in the fragmentation process of the lysine rich cell-penetrating peptide TP10. J. Mass Spectrom. 2015, 50, 220–227. [Google Scholar] [CrossRef]
- Jones, S.; Howl, J. Applications of cell-penetrating peptides as signal transduction modulators for the selective induction of apoptosis. Cell-Penetrating Pept. Methods Protoc. 2011, 291–303. [Google Scholar] [CrossRef]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal. Transduct. Tar. 2023, 8, 293. [Google Scholar] [CrossRef]
- Argyros, O.; Karampelas, T.; Asvos, X.; Varela, A.; Sayyad, N.; Papakyriakou, A.; Davos, C.H.; Tzakos, A.G.; Fokas, D.; Tamvakopoulos, C. Peptide-Drug Conjugate GnRH-Sunitinib Targets Angiogenesis Selectively at the Site of Action to Inhibit Tumor Growth. Cancer Res. 2016, 76, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Ning, Y.S.; Chen, J.L.; Duan, X.C.; Song, N.; Ding, D.; Su, X.C.; Yu, Z.L. Proline Isomerization-Regulated Tumor Microenvironment-Adaptable Self-Assembly of Peptides for Enhanced Therapeutic Efficacy. Nano Lett. 2019, 19, 7965–7976. [Google Scholar] [CrossRef] [PubMed]
- Charati, M.B.; Lee, I.; Hribar, K.C.; Burdick, J.A. Light-Sensitive Polypeptide Hydrogel and Nanorod Composites. Small 2010, 6, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.Y.; Liu, X.Y.; Wu, J.L.; Li, X.C.; Tang, Z.Q.; Deng, Y.T.; Sun, X.M.; Chen, K.; Gao, Z.Q.; Bai, J.K. pH-triggered morphological change in a self-assembling amphiphilic peptide used as an antitumor drug carrier. Nanotechnology 2020, 31, 165601. [Google Scholar] [CrossRef]
- Matt, A.; Kuttich, B.; Grillo, I.; Weissheit, S.; Thiele, C.M.; Stühn, B. Temperature induced conformational changes in the elastin-like peptide GVG(VPGVG). Soft Matter 2019, 15, 4192–4199. [Google Scholar] [CrossRef]
- De Haas, R.J.; Ganar, K.A.; Deshpande, S.; de Vries, R. pH-Responsive Elastin-Like Polypeptide Designer Condensates. ACS Appl. Mater Inter. 2023, 15, 45336–45344. [Google Scholar] [CrossRef]
- Liu, M.; Fang, X.; Yang, Y.; Wang, C. Peptide-Enabled Targeted Delivery Systems for Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 701504. [Google Scholar] [CrossRef]
- Sharma, R.; Borah, S.J.; Bhawna; Kumar, S.; Gupta, A.; Singh, P.; Goel, V.K.; Kumar, R.; Kumar, V. Functionalized Peptide-Based Nanoparticles for Targeted Cancer Nanotherapeutics: A State-of-the-Art Review. ACS Omega 2022, 7, 36092–36107. [Google Scholar] [CrossRef]
- Fu, S.; Zhao, Y.; Sun, J.; Yang, T.; Zhi, D.; Zhang, E.; Zhong, F.; Zhen, Y.; Zhang, S.; Zhang, S. Integrin αvβ3-targeted liposomal drug delivery system for enhanced lung cancer therapy. Colloids Surf. B Biointerfaces 2021, 201, 111623. [Google Scholar] [CrossRef]
- Di-Wen, S.; Pan, G.Z.; Hao, L.; Zhang, J.; Xue, Q.Z.; Wang, P.; Yuan, Q.Z. Improved antitumor activity of epirubicin-loaded CXCR4-targeted polymeric nanoparticles in liver cancers. Int. J. Pharmaceut. 2016, 500, 54–61. [Google Scholar] [CrossRef]
- Situ, J.Q.; Ye, Y.Q.; Zhu, X.L.; Yu, R.S.; You, J.; Yuan, H.; Hu, F.Q.; Du, Y.Z. Specific targeting of A54 homing peptide-functionalized dextran-g-poly(lactic--glycolic acid) micelles to tumor cells. Int. J. Nanomed. 2015, 10, 665–675. [Google Scholar] [CrossRef]
- Huang, S.; Li, C.; Wang, W.; Li, H.; Sun, Z.; Song, C.; Li, B.; Duan, S.; Hu, Y. A54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int. J. Nanomed. 2017, 12, 5163–5176. [Google Scholar] [CrossRef]
- Liao, Y.T.; Liu, C.H.; Yu, J.S.; Wu, K.C.W. Liver cancer cells: Targeting and prolonged-release drug carriers consisting of mesoporous silica nanoparticles and alginate microspheres. Int. J. Nanomed 2014, 9, 2767–2778. [Google Scholar] [CrossRef]
- Trencsényi, G.; Halmos, G.; Képes, Z. Radiolabeled NGR-Based Heterodimers for Angiogenesis Imaging: A Review of Preclinical Studies. Cancers 2023, 15, 4459. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.L.; Chang, M.L.; Fan, Y.; Shi, Y.B.; Lin, G.M. NGR-modified pH-sensitive liposomes for controlled release and tumor target delivery of docetaxel. Colloid Surf. B 2017, 160, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Yuan, W.M.; Qin, Y.; Chen, H.L.; Tang, J.; Yuan, M.Q.; Zhang, Z.R.; He, Q. Efficient Delivery of Payload into Tumor Cells in a Controlled Manner by TAT and Thiolytic Cleavable PEG Co-Modified Liposomes. Mol. Pharm. 2010, 7, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Li, J.; Cao, Z.; Yang, P.; Qiu, Y.; Yang, B.; Wang, Y.; Long, Y.; Liu, Y.; Zhang, Q.; et al. A pH-responsive cell-penetrating peptide-modified liposomes with active recognizing of integrin αvβ3 for the treatment of melanoma. J. Control. Release 2015, 217, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.T.; Xiao, Y.; Pan, M.; Li, F.; Duan, W.L.; Meng, L.; Liu, X.; Yan, F.; Zheng, H.R. Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound. J. Control. Release 2016, 243, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.F.; Zhang, W.Q.; Luo, L.M.; Song, P.; Li, D.; Du, R.; Ren, W.; Huang, D.; Lu, W.L.; Zhang, X.; et al. The antitumor activity of a doxorubicin loaded, iRGD-modified sterically-stabilized liposome on B16-F10 melanoma cells: In vitro and in vivo evaluation. Int. J. Nanomed. 2013, 8, 2473–2485. [Google Scholar] [CrossRef]
- Klutz, K.; Schaffert, D.; Willhauck, M.J.; Grünwald, G.K.; Haase, R.; Wunderlich, N.; Zach, C.; Gildehaus, F.J.; Senekowitsch-Schmidtke, R.; Göke, B.; et al. Epidermal growth factor receptor-targeted 131I-therapy of liver cancer following systemic delivery of the sodium iodide symporter gene. Mol. Ther. 2011, 19, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Han, H.H.; Wang, Z.Q.; Kuang, L.S.; Wang, L.; Yu, L.P.; Wu, M.A.; Zhou, Z.L.; Qian, M. Targeted Drug Delivery to Hepatocarcinoma by Phage-Displayed Specific Binding Peptide. Mol. Cancer Res. 2010, 8, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Su, L.; Wu, C.S.; Wu, J.L.; Zhu, C.B.; Yuan, G.Y. RGD peptide targeted lipid-coated nanoparticles for combinatorial delivery of sorafenib and quercetin against hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2016, 42, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Gong, F.; Pang, P.; Shen, M.; Zhu, K.; Cheng, D.; Liu, Z.; Shan, H. An RGD-modified MRI-visible polymeric vector for targeted siRNA delivery to hepatocellular carcinoma in nude mice. PLoS ONE 2013, 8, e66416. [Google Scholar] [CrossRef]
- Zhang, X.R.; Li, J.X.; Yan, M.X. Targeted hepatocellular carcinoma therapy: Transferrin modified, self-assembled polymeric nanomedicine for co-delivery of cisplatin and doxorubicin. Drug Dev. Ind. Pharm. 2016, 42, 1590–1599. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liang, Z.S.; Gao, F.; Luo, S.F.; Lu, G.Q. In vitro photothermal study of gold nanoshells functionalized with small targeting peptides to liver cancer cells. J. Mater. Sci.-Mater. M 2010, 21, 665–674. [Google Scholar] [CrossRef]
- Akrami, M.; Balalaie, S.; Hosseinkhani, S.; Alipour, M.; Salehi, F.; Bahador, A.; Haririan, I. Tuning the anticancer activity of a novel pro-apoptotic peptide using gold nanoparticle platforms. Sci. Rep. 2016, 6, 31030. [Google Scholar] [CrossRef]
- Bijukumar, D.; Girish, C.; Sasidharan, A.; Nair, S.; Koyakutty, M. Transferrin-conjugated biodegradable graphene for targeted radiofrequency ablation of hepatocellular carcinoma. ACS Biomater. Sci. Eng. 2015, 1, 1211–1219. [Google Scholar] [CrossRef]
- Chavda, V.P.; Solanki, H.K.; Davidson, M.; Apostolopoulos, V.; Bojarska, J. Peptide-Drug Conjugates: A New Hope for Cancer Management. Molecules 2022, 27, 7232. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S.; Gunassekaran, G.R.; Lee, S.-M.; Yoon, J.-W.; Lee, Y.-K.; Lee, B. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef]
- Pooja, D.; Gunukula, A.; Gupta, N.; Adams, D.J.; Kulhari, H. Bombesin receptors as potential targets for anticancer drug delivery and imaging. Int. J. Biochem. Cell B 2019, 114, 105567. [Google Scholar] [CrossRef]
- Tjandra, K.C.; McCarthy, N.; Yang, L.; Laos, A.J.; Sharbeen, G.; Phillips, P.A.; Forgham, H.; Sagnella, S.M.; Whan, R.M.; Kavallaris, M.; et al. Identification of Novel Medulloblastoma Cell-Targeting Peptides for Use in Selective Chemotherapy Drug Delivery. J. Med. Chem. 2020, 63, 2181–2193. [Google Scholar] [CrossRef]
- Yin, H.; Yang, J.; Zhang, Q.; Wang, H.; Xu, J.; Zheng, J. iRGD as a tumor-penetrating peptide for cancer therapy. Mol. Med. Rep. 2017, 15, 2925–2930. [Google Scholar] [CrossRef]
- He, R.J.; Finan, B.; Mayer, J.P.; DiMarchi, R.D. Peptide Conjugates with Small Molecules Designed to Enhance Efficacy and Safety. Molecules 2019, 24, 1855. [Google Scholar] [CrossRef]
- Böhme, D.; Beck-Sickinger, A.G. Drug delivery and release systems for targeted tumor therapy. J. Pept. Sci. 2015, 21, 186–200. [Google Scholar] [CrossRef]
- Dal Corso, A.; Pignataro, L.; Belvisi, L.; Gennari, C. Innovative Linker Strategies for Tumor-Targeted Drug Conjugates. Chem.-Eur. J. 2019, 25, 14740–14757. [Google Scholar] [CrossRef] [PubMed]
- Shokri, B.; Zarghi, A.; Shahhoseini, S.; Mohammadi, R.; Kobarfard, F. Design, synthesis and biological evaluation of peptide-NSAID conjugates for targeted cancer therapy. Arch. Pharm. 2019, 352, e1800379. [Google Scholar] [CrossRef] [PubMed]
- Lelle, M.; Frick, S.U.; Steinbrink, K.; Peneva, K. Novel cleavable cell-penetrating peptide–drug conjugates: Synthesis and characterization. J. Pept. Sci. 2014, 20, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Mhaka, A.M.; Rosen, D.M.; Brennen, W.N.; Dalrymple, S.; Dach, I.; Olesen, C.; Gurel, B.; DeMarzo, A.M.; Wilding, G.; et al. Engineering a Prostate-Specific Membrane Antigen-Activated Tumor Endothelial Cell Prodrug for Cancer Therapy. Sci. Transl. Med. 2012, 4, 143er4. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Mezo, G.; Tzakos, A.G. On the design principles of peptide-drug conjugates for targeted drug delivery to the malignant tumor site. Beilstein J. Org. Chem. 2018, 14, 930–954. [Google Scholar] [CrossRef]
- Bugatti, K. Brief Guide to Preparing a Peptide-Drug Conjugate. Chembiochem 2023, 24, e202300254. [Google Scholar] [CrossRef]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies. Front. Chem. 2020, 8, 571. [Google Scholar] [CrossRef]
- Burns, K.E.; Hensley, H.; Robinson, M.K.; Thévenin, D. Therapeutic Efficacy of a Family of pHLIP-MMAF Conjugates in Cancer Cells and Mouse Models. Mol. Pharmaceut. 2017, 14, 415–422. [Google Scholar] [CrossRef]
- Langer, M.; Kratz, F.; Rothen-Rutishauser, B.; Wunderli-Allenspach, H.; Beck-Sickinger, A.G. Novel peptide conjugates for tumor-specific chemotherapy. J. Med. Chem. 2001, 44, 1341–1348. [Google Scholar] [CrossRef]
- Chang, M.L.; Zhang, F.; Wei, T.; Zuo, T.T.; Guan, Y.Y.; Lin, G.M.; Shao, W. Smart linkers in polymer-drug conjugates for tumor-targeted delivery. J. Drug Target. 2016, 24, 475–491. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]
- Chen, Z.P.; Zhang, P.C.; Cheetham, A.G.; Moon, J.H.; Moxley, J.W.; Lin, Y.A.; Cui, H.G. Controlled release of free doxorubicin from peptide-drug conjugates by drug loading. J. Control. Release 2014, 191, 123–130. [Google Scholar] [CrossRef]
- Svagzdys, S.; Lesauskaite, V.; Pangonyte, D.; Saladzinskas, Z.; Tamelis, A.; Pavalkis, D. Matrix Metalloproteinase-9 Is a Prognostic Marker to Predict Survival of Patients Who Underwent Surgery Due to Rectal Carcinoma. Tohoku J. Exp. Med. 2011, 223, 67–73. [Google Scholar] [CrossRef]
- Paramasivam, A.; Raghunandhakumar, S.; Priyadharsini, J.V.; Jayaraman, G. Thymoquinone inhibits the migration of mouse neuroblastoma (Neuro-2a) cells by down-regulating MMP-2 and MMP-9. Chin. J. Nat. Med. 2016, 14, 904–912. [Google Scholar] [CrossRef]
- Min, J.Q.; Feng, Q.; Liao, W.J.; Liang, Y.M.; Gong, C.W.; Li, E.L.; He, W.F.; Yuan, R.F.; Wu, L.Q. IFITM3 promotes hepatocellular carcinoma invasion and metastasis by regulating MMP9 through p38/MAPK signaling. Febs. Open. Bio. 2018, 8, 1299–1311. [Google Scholar] [CrossRef]
- Huang, H.; Jin, H.; Zhao, H.; Wang, J.; Li, X.; Yan, H.; Wang, S.; Guo, X.; Xue, L.; Li, J.; et al. Rho GDI β promotes Sp1/MMP-2 expression and bladder cancer invasion through perturbing miR-200c-targeted JNK 2 protein translation. Mol. Oncol. 2017, 11, 1579–1594. [Google Scholar] [CrossRef]
- Lee, J.; Oh, E.-T.; Lee, H.J.; Lee, E.; Kim, H.G.; Park, H.J.; Kim, C. Tuning of Peptide Cytotoxicity with Cell Penetrating Motif Activatable by Matrix Metalloproteinase-2. ACS Omega 2022, 7, 29684–29691. [Google Scholar] [CrossRef]
- Song, Q.; Chuan, X.X.; Chen, B.L.; He, B.; Zhang, H.; Dai, W.B.; Wang, X.Q.; Zhang, Q. A smart tumor targeting peptide-drug conjugate, pHLIP-SS-DOX: Synthesis and cellular uptake on MCF-7 and MCF-7/Adr cells. Drug Deliv. 2016, 23, 1734–1746. [Google Scholar] [CrossRef]
- Duan, Z.; Chen, C.; Qin, J.; Liu, Q.; Wang, Q.; Xu, X.; Wang, J. Cell-penetrating peptide conjugates to enhance the antitumor effect of paclitaxel on drug-resistant lung cancer. Drug Deliv. 2017, 24, 752–764. [Google Scholar] [CrossRef]
- Lindgren, M.; Rosenthal-Aizman, K.; Saar, K.; Eiríksdóttir, E.; Jiang, Y.; Sassian, M.; Östlund, P.; Hällbrink, M.; Langel, Ü. Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem. Pharmacol. 2006, 71, 416–425. [Google Scholar] [CrossRef]
- Nishimura, Y.; Takeda, K.; Ezawa, R.; Ishii, J.; Ogino, C.; Kondo, A. A display of pH-sensitive fusogenic GALA peptide facilitates endosomal escape from a Bio-nanocapsule via an endocytic uptake pathway. J. Nanobiotechnology 2014, 12, 11. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, T.; Perche, F.; Taigind, A.; Torchilin, V.P. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc. Natl. Acad. Sci. USA 2013, 110, 17047–17052. [Google Scholar] [CrossRef]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J. Control. Release 2015, 212, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Melphalan flufenamide (Melflufen): First approval. Drugs 2021, 81, 963–969. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Nhàn, N.T.T.; Yamada, T.; Yamada, K.H. Peptide-Based Agents for Cancer Treatment: Current Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 2931. [Google Scholar] [CrossRef]
- Lai, X.; Tang, J.; ElSayed, M.E.H. Recent advances in proteolytic stability for peptide, protein, and antibody drug discovery. Expert Opin. Drug Discov. 2021, 16, 1467–1482. [Google Scholar] [CrossRef]
- Barman, P.; Joshi, S.; Sharma, S.; Preet, S.; Sharma, S.; Saini, A. Strategic Approaches to Improvise Peptide Drugs as Next Generation Therapeutics. Int. J. Pept. Res. Ther. 2023, 29, 61. [Google Scholar] [CrossRef]
- Yao, J.F.; Yang, H.; Zhao, Y.Z.; Xue, M. Metabolism of peptide drugs and strategies to improve their metabolic stability. Curr. Drug Metab. 2018, 19, 892–901. [Google Scholar] [CrossRef]
- Gonzalez-Cruz, A.O.; Hernandez-Juarez, J.; Ramírez-Cabrera, M.A.; Balderas-Renteria, I.; Arredondo-Espinoza, E. Peptide-based drug-delivery systems: A new hope for improving cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 72, 103362. [Google Scholar] [CrossRef]
- Bogdanowich-Knipp, S.J.; Jois, D.S.S.; Siahaan, T.J. The effect of conformation on the solution stability of linear vs. cyclic RGD peptides. J. Pept. Res. 1999, 53, 523–529. [Google Scholar] [CrossRef]
- Chan, L.Y.; Zhang, V.M.; Huang, Y.h.; Waters, N.C.; Bansal, P.S.; Craik, D.J.; Daly, N.L. Cyclization of the antimicrobial peptide gomesin with native chemical ligation: Influences on stability and bioactivity. ChemBioChem 2013, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Wu, M.H.; Chang, Q.; Zhao, X. Stapling strategy enables improvement of antitumor activity and proteolytic stability of host-defense peptide hymenochirin-1B. Rsc. Adv. 2018, 8, 22268–22275. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, T.D.; Hong, B.S.; Kim, O.Y.; Yoon, W.H.; Chae, C.B.; Gho, Y.S. A serum-stable branched dimeric anti-VEGF peptide blocks tumor growth via anti-angiogenic activity. Exp. Mol. Med. 2010, 42, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Tugyi, R.; Uray, K.; Iván, D.; Fellinger, E.; Perkins, A.; Hudecz, F. Partial D-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 413–418. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, W. Enhanced glioma-targeting and stability of LGICP peptide coupled with stabilized peptide DA7R. Acta Pharm. Sin. B 2018, 8, 106–115. [Google Scholar] [CrossRef]
- Chen, L.; Tu, Z.G.; Voloshchuk, N.; Liang, J.F. Lytic peptides with improved stability and selectivity designed for cancer treatment. J. Pharm. Sci. 2012, 101, 1508–1517. [Google Scholar] [CrossRef]
- Seebach, D.; Lukaszuk, A.; Patora-Komisarska, K.; Podwysocka, D.; Gardiner, J.; Ebert, M.O.; Reubi, J.C.; Cescato, R.; Waser, B.; Gmeiner, P.J.C.; et al. On the Terminal Homologation of Physiologically Active Peptides as a Means of Increasing Stability in Human Serum–Neurotensin, Opiorphin, B27-KK10 Epitope, NPY. Chem. Biodivers. 2011, 8, 711–739. [Google Scholar] [CrossRef] [PubMed]
- Ayo, A.; Laakkonen, P. Peptide-Based Strategies for Targeted Tumor Treatment and Imaging. Pharmaceutics 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Björklund, M.; Valtanen, H.; Savilahti, H.; Koivunen, E. Use of intein-directed peptide biosynthesis to improve serum stability and bioactivity of a gelatinase inhibitory peptide. Comb. Chem. High T Scr. 2003, 6, 29–35. [Google Scholar] [CrossRef]
- Pechenov, S.; Revell, J.; Will, S.; Naylor, J.; Tyagi, P.; Patel, C.; Liang, L.; Tseng, L.; Huang, Y.; Rosenbaum, A.I.; et al. Development of an orally delivered GLP-1 receptor agonist through peptide engineering and drug delivery to treat chronic disease. Sci. Rep. 2021, 11, 22521. [Google Scholar] [CrossRef]
- Han, J.; Huang, X.; Sun, L.D.; Li, Z.; Qian, H.; Huang, W.L. Novel fatty chain-modified glucagon-like peptide-1 conjugates with enhanced stability and prolonged in vivo activity. Biochem. Pharmacol. 2013, 86, 297–308. [Google Scholar] [CrossRef]
- Ding, Y.P.; Ji, T.J.; Zhao, Y.; Zhang, Y.L.; Zhao, X.Z.; Zhao, R.F.; Lang, J.Y.; Zhao, X.; Shi, J.; Sukumar, S.; et al. Improvement of Stability and Efficacy of C16Y Therapeutic Peptide via Molecular Self-Assembly into Tumor-Responsive Nanoformulation. Mol. Cancer Ther. 2015, 14, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Ngambenjawong, C.; Pun, S.H. Multivalent Polymers Displaying M2 Macrophage-Targeting Peptides Improve Target Binding Avidity and Serum Stability. Acs Biomater. Sci. Eng. 2017, 3, 2050–2053. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Fu, H.; Kuang, Q.F.; Zhang, L.; Zhang, Q.Y.; Liu, Y.Y.; Ran, R.; Gao, H.L.; Zhang, Z.R.; He, Q. Liposomes co-modified with cholesterol anchored cleavable PEG and octaarginines for tumor targeted drug delivery. J. Drug Target. 2014, 22, 313–326. [Google Scholar] [CrossRef]
- Cheng, D.B.; Yang, P.P.; Cong, Y.; Liu, F.H.; Qiao, Z.Y.; Wang, H. One-pot synthesis of pH-responsive hyperbranched polymer–peptide conjugates with enhanced stability and loading efficiency for combined cancer therapy. Polym. Chem. 2017, 8, 2462–2471. [Google Scholar] [CrossRef]
- Wu, M.J.; Huang, T.; Wang, J.; Chen, P.; Mi, W.W.; Ying, Y.Y.; Wang, H.L.; Zhao, D.D.; Huang, S.W. Antilung cancer effect of ergosterol and cisplatin-loaded liposomes modified with cyclic arginine-glycine-aspartic acid and octa-arginine peptides. Medicine 2018, 97, e11916. [Google Scholar] [CrossRef]
- Qu, W.; Li, Y.; Hovgaard, L.; Li, S.; Dai, W.B.; Wang, J.C.; Zhang, X.; Zhang, Q. A silica-based pH-sensitive nanomatrix system improves the oral absorption and efficacy of incretin hormone glucagon-like peptide-1. Int. J. Nanomed. 2012, 7, 4983–4994. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Xu, H.J.; Li, Y.; Song, D.M.; Wang, X.X.; Qiao, M.Q.; Gong, M. Novel application of hydrophobin in medical science: A drug carrier for improving serum stability. Sci. Rep. 2016, 6, 26461. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Otake, Y.; Nakamura, H. Peptide Synthesis Utilizing Micro-flow Technology. Chem.-Asian J. 2018, 13, 3818–3832. [Google Scholar] [CrossRef]
- Wang, W.Z.; Huang, Y.Y.; Liu, J.Z.; Xie, Y.F.; Zhao, R.; Xiong, S.X.; Liu, G.Q.; Chen, Y.; Ma, H.M. Integrated SPPS on continuous-flow radial microfluidic chip. Lab A Chip 2011, 11, 929–935. [Google Scholar] [CrossRef]
- Qiang, L.; Guo, J.; Han, Y.K.; Jiang, J.F.; Su, X.W.; Liu, H.; Qi, Q.G.; Han, L. A novel anti drug screening system based on high-throughput microfluidic chips. Sci. Rep. 2019, 9, 8087. [Google Scholar] [CrossRef]
- Martin, V.; Egelund, P.H.G.; Johansson, H.; Thordal Le Quement, S.; Wojcik, F.; Sejer Pedersen, D. Greening the synthesis of peptide therapeutics: An industrial perspective. RSC Adv. 2020, 10, 42457–42492. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, M.R.; Rahman, F.; Rahaman, M.S.; Khan, M.S.; Abrar, S.; Ray, T.K.; Uddin, M.B.; Kali, M.S.K.; Dua, K.; et al. Emerging Promise of Computational Techniques in Anti-Cancer Research: At a Glance. Bioengineering 2022, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Mijalis, A.J.; Thomas, D.A.; Simon, M.D.; Adamo, A.; Beaumont, R.; Jensen, K.F.; Pentelute, B.L. A fully automated flow-based approach for accelerated peptide synthesis. Nat. Chem. Biol. 2017, 13, 464–466. [Google Scholar] [CrossRef]
- Brown, P.; Butterworth, S.; Hann, M.; Jeffrey, P.; Porter, R.; Swarbrick, M.; Ward, S. Recent Clinical Disclosures and SMR Award. Highlights from The Society for Medicines Research Symposium. London, UK-5 December 2019. Drugs Future 2020, 45, 63–69. [Google Scholar] [CrossRef]

| Role in Self-Assembly | Amino Acid Name |
|---|---|
| Aliphatic hydrophobic groups provide hydrophobic forces | A, L, I, V, M |
| Aromatic ring hydrophobic groups provide π-π stacking | F, Y, W |
| Hydrophilic groups provide hydrogen bonding forces | N, Q, S, T |
| Charged groups provide electrostatic forces | H, R, K, E, D |
| Disulfide bond | C |
| Spatial positional resistance provides flexibility | G |
| Spatial positional resistance provides rigidity | P |
| Drug Name | Company | Indications | R&D Stage |
|---|---|---|---|
| Lutathera | Novartis | Gastrointestinal pancreatic | Approved |
| neuroendocrine tumors | |||
| Pepaxto | Oncopeptides | Multiple myeloma | Approved |
| SNG 1005 | Shenogen Pharma | Brain metastatic | Phase III |
| Group&Angiochem | |||
| AN-152 | AEterna Zentaris | Ovarian cancer | Phase III |
| EP-100 | Esperance Pharmaceuticals | Breast cancer, ovarian cancer | Phase II |
| CBP-1008 | Coherent Biopharma (CBP) | Breast cancer | Phase II |
| CBP-1018 | Lung cancer | Phase I | |
| BT-1718 | Bicycle Therapeutics | Non-small cell lung cancer | Phase I/II |
| BT5528 | Solid tumor | Phase I/II | |
| BT8009 | Solid tumor | Phase I/II | |
| CBX-12 | Cybrexa Therapeutics | Tumor | Phase I |
| TH1902 | Theratechnologies | Triple-negative breast cancer | Phase I |
| BGC 0228 | BrightGene | Advanced solid tumors | Phase I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, L.; Liu, C.; Luo, Y.; Chen, D. Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies. Pharmaceutics 2024, 16, 240. https://doi.org/10.3390/pharmaceutics16020240
Wang Y, Zhang L, Liu C, Luo Y, Chen D. Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies. Pharmaceutics. 2024; 16(2):240. https://doi.org/10.3390/pharmaceutics16020240
Chicago/Turabian StyleWang, Yubo, Lu Zhang, Chen Liu, Yiming Luo, and Dengyue Chen. 2024. "Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies" Pharmaceutics 16, no. 2: 240. https://doi.org/10.3390/pharmaceutics16020240
APA StyleWang, Y., Zhang, L., Liu, C., Luo, Y., & Chen, D. (2024). Peptide-Mediated Nanocarriers for Targeted Drug Delivery: Developments and Strategies. Pharmaceutics, 16(2), 240. https://doi.org/10.3390/pharmaceutics16020240





