Potentiation of Antibiotic Activity of Aztreonam against Metallo-β-Lactamase-Producing Multidrug-Resistant Pseudomonas aeruginosa by 3-O-Substituted Difluoroquercetin Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking Study
2.2. Chemicals and Reagents
2.3. Synthesis
2.4. Microorganisms
2.5. Assessment of Antibacterial Activity
2.6. Inhibition of Efflux Pumps: EtBr Accumulation
2.7. Inhibition of β-Lactamase
2.8. Antibiotics-Potentiation Activity
(in combination with ATM)/MIC (test compound alone)
2.9. Time–Kill Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Clinical MBL-Producing P. aeruginosa Strains Showed High-Level Antibiotics Resistance
3.2. Efflux Pump and β-Lactamase Inhibitors Potentiated ATM against Highly Resistant MBL-Producing P. aeruginosa
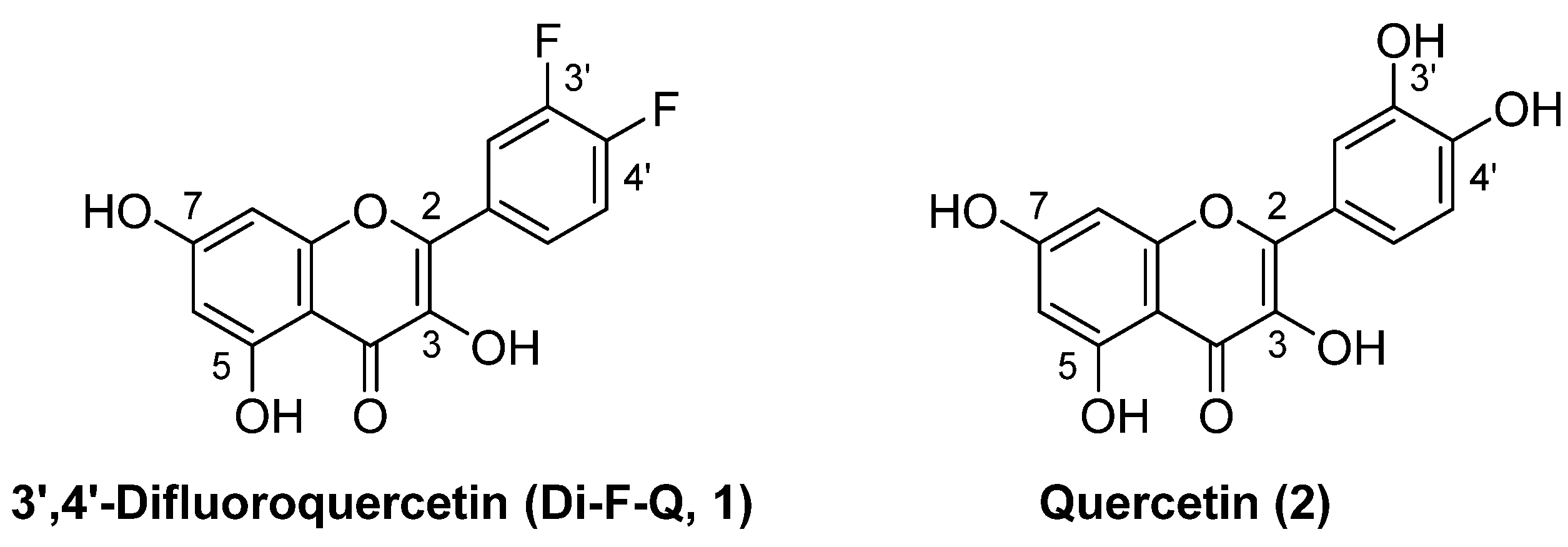
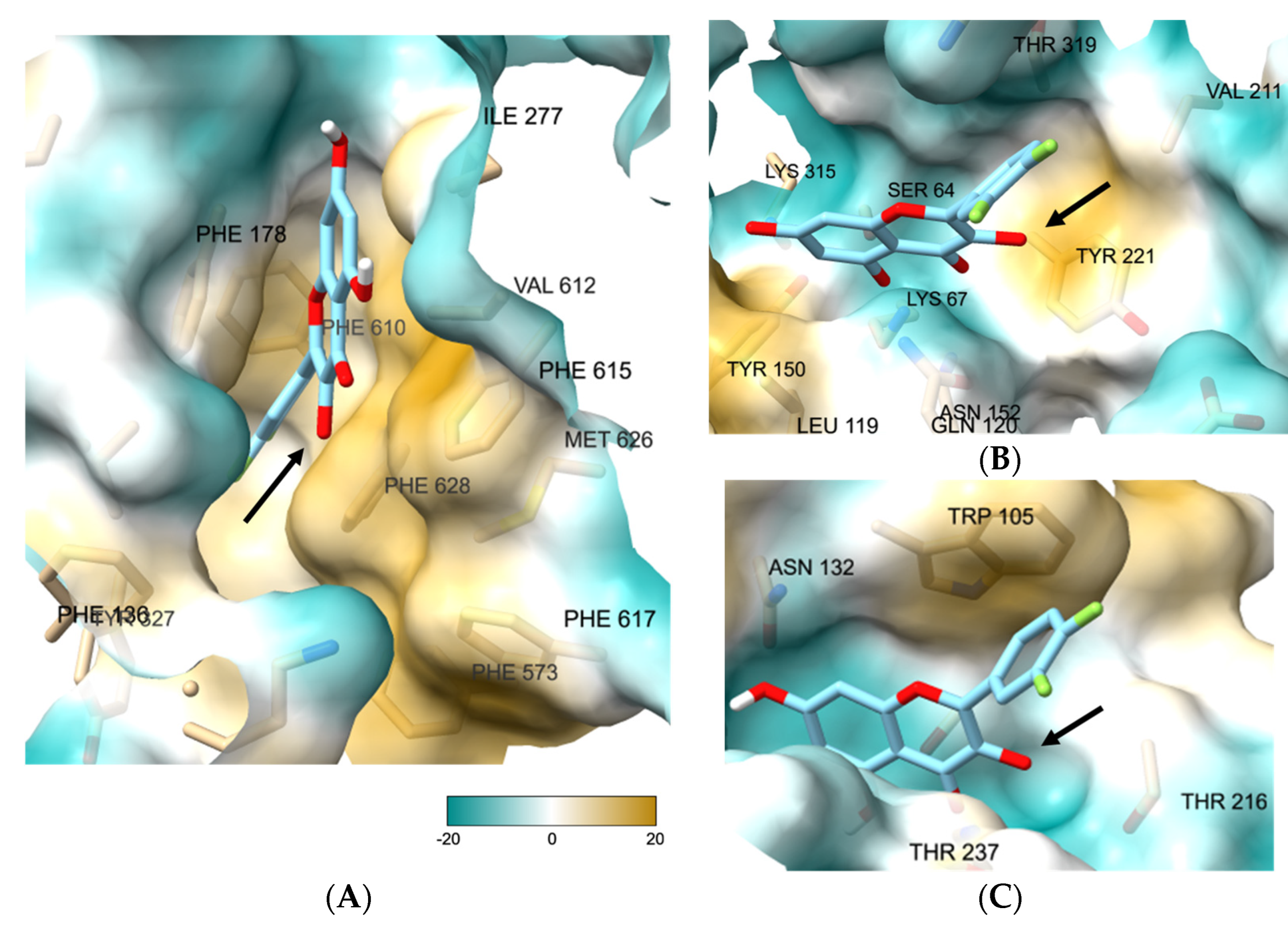
3.3. 3-O-Substituted di-F-Q (1) Showed Multitarget Inhibitory Activity against Efflux Pumps and β-Lactamases
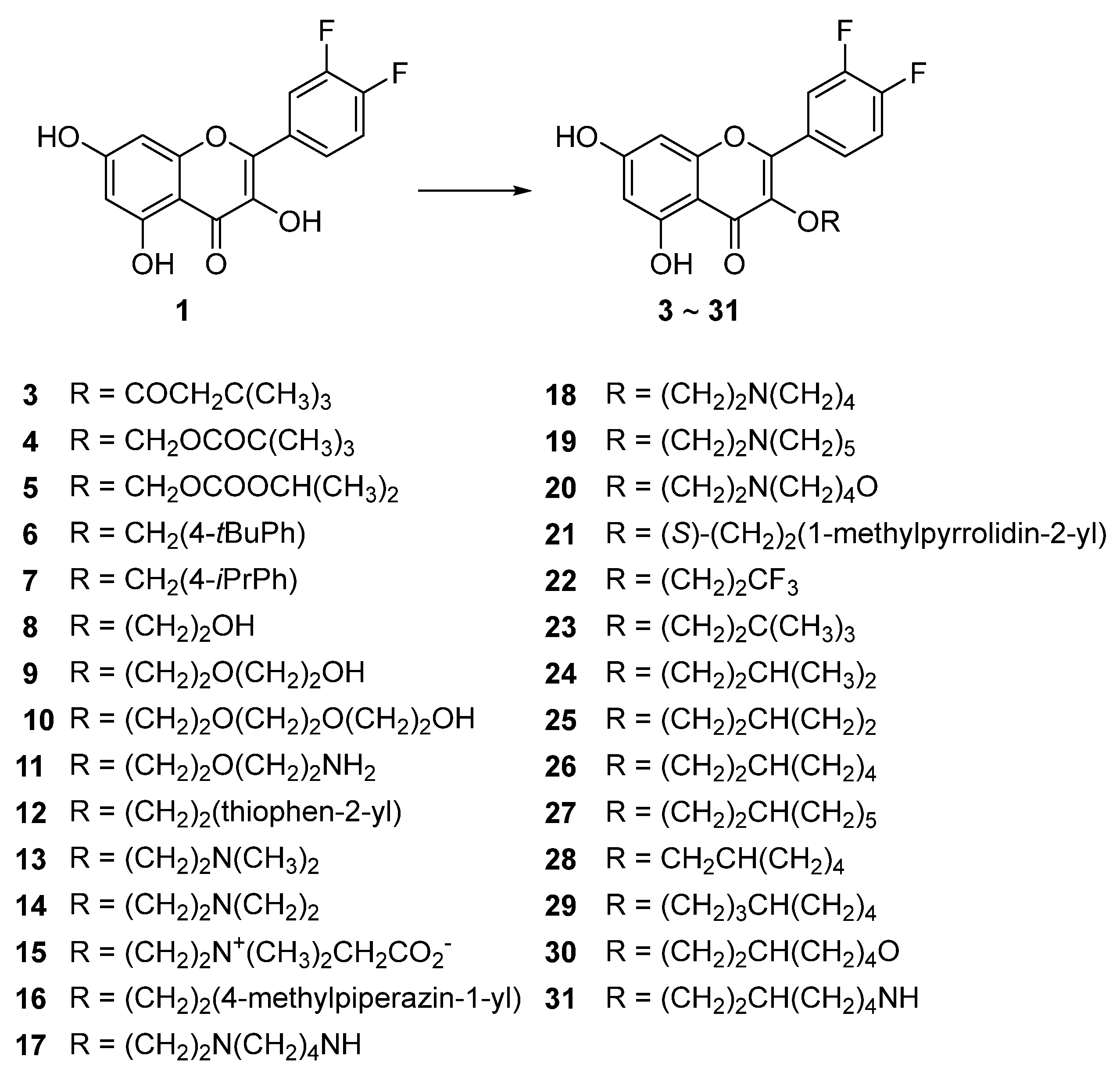
3.4. Syntheses of the Title Compounds, 3-O-Alkyl-di-F-Q Derivatives (3–31)
3.5. Compounds 23, 26, and 27 Showed Concentration-Dependent Efflux Pump Inhibitory Activity in MBL-Producing P. aeruginosa
3.6. Compounds 23, 26, and 27 Exhibited Broad-Spectrum β-Lactamase Inhibitory Activity
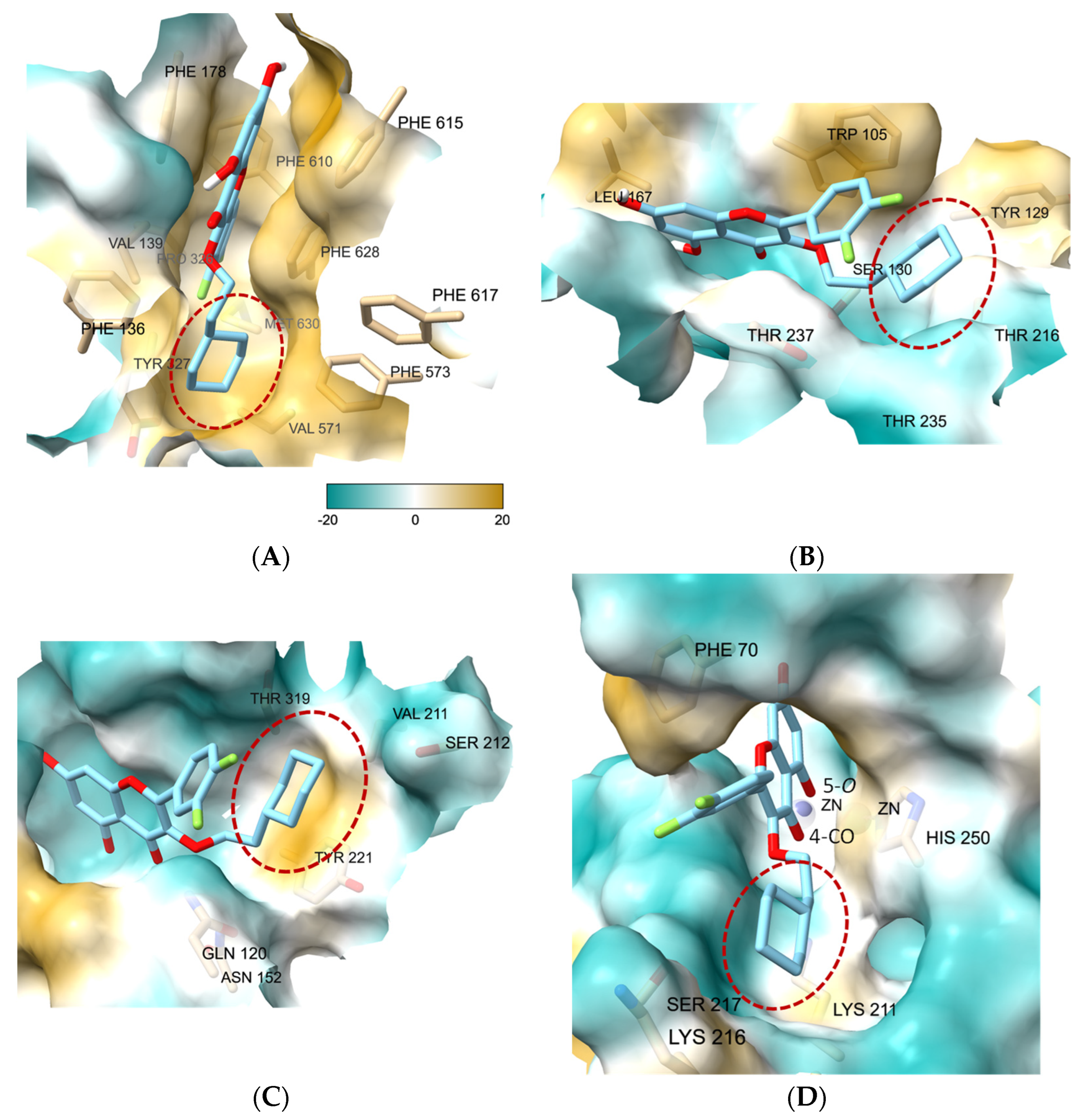
3.7. Molecular Docking Study of 27 Showed the Binding Role of Its 3-O-Substituent to the Efflux Pump and the Broad-Spectrum β-Lactamases
3.8. Antimicrobial Activity of ATM Was Significantly Increased by Combination with 27
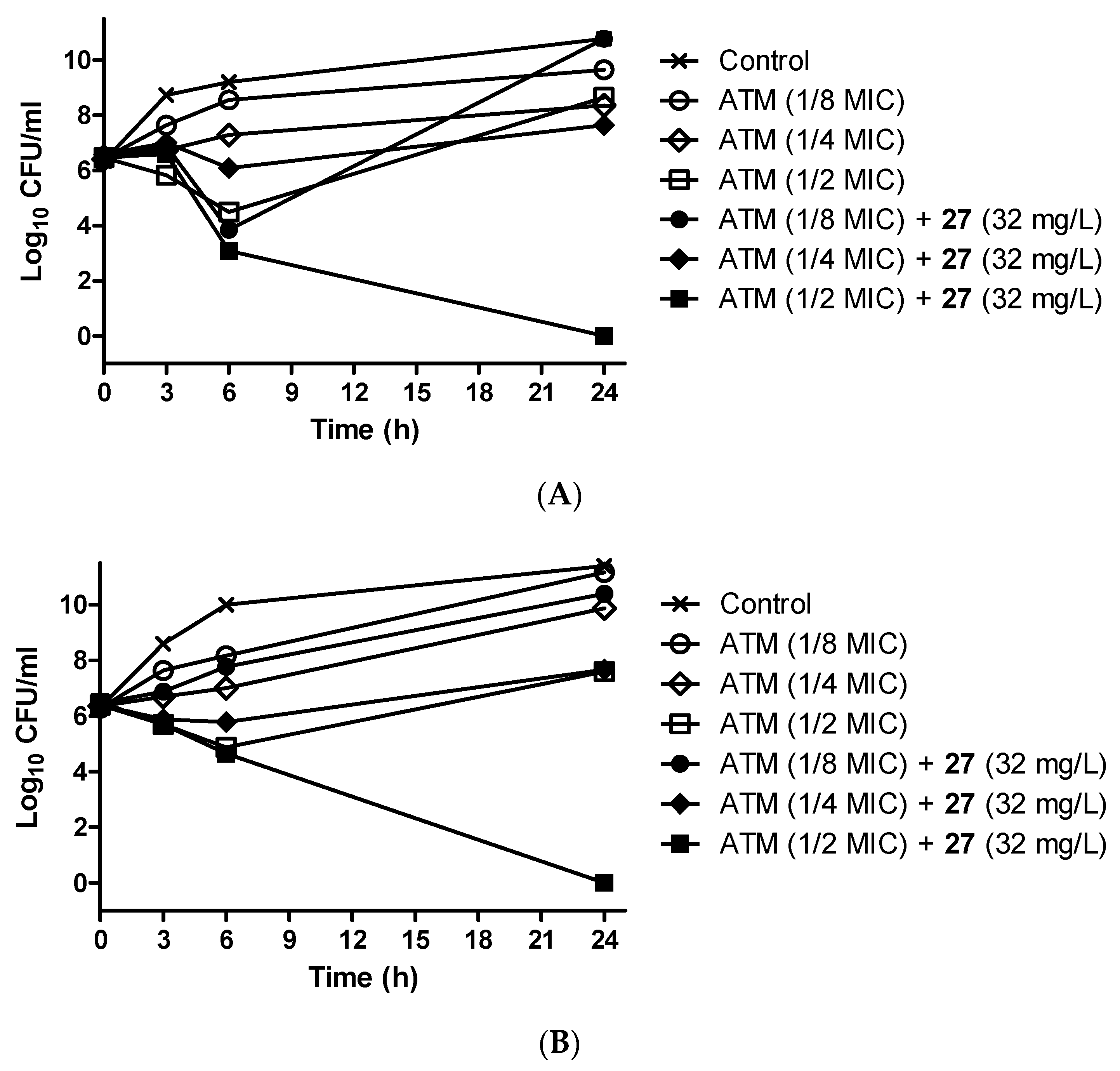
3.9. Antibiotic-Potentiating Activity of 27 Was Confirmed by Time–Kill Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, T.; Fenn, S.J.; Hardie, K.R. JMM Profile: Carbapenems: A broad-spectrum antibiotic. J. Med. Microbiol. 2021, 70, 001462. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M. Antibiotics resistance: The last resort. Nature 2013, 499, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.S.; Donkor, E.S. Carbapenem resistance: A review. Med. Sci. 2018, 6, 1. [Google Scholar] [CrossRef]
- Meletis, G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T. Emerging carbapenemases: A global perspective. Int. J. Antimicrob. Agents 2010, 36, S8–S14. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Caliskan-Aydogan, O.; Alocilja, E.C. A review of carbapenem resistance in Enterobacterales and its detection techniques. Microorganisms 2023, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Arer, V.; Kar, D. Biochemical exploration of β-lactamase inhibitors. Front. Genet. 2023, 13, 1060736. [Google Scholar] [CrossRef]
- Reddy, N.; Shungube, M.; Arvidsson, P.I.; Baijnath, S.; Kruger, H.G.; Govender, T.; Naicker, T. A 2018-2019 patent review of metallo beta-lactamase inhibitors. Expert Opin. Ther. Pat. 2020, 30, 541–555. [Google Scholar] [CrossRef]
- Mojica, M.F.; Bonomo, R.A.; Fast, W. B1-metallo-β-lactamases: Where do we stand? Curr. Drug Targets 2016, 17, 1029–1050. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Rubio-Aparicio, D.; Nelson, K.; Sun, D.; Tsivkovski, R.; Castanheira, M.; Lindley, J.; Loutit, J.; Dudley, M. In vitro activity of the ultrabroad-spectrum beta-lactamase inhibitor QPX7728 in combination with multiple beta-lactam antibiotics against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2021, 65, e00210-21. [Google Scholar] [CrossRef]
- Yasmin, M.; Fouts, D.E.; Jacobs, M.R.; Haydar, H.; Marshall, S.H.; White, R.; D’Souza, R.; Lodise, T.P.; Rhoads, D.D.; Hujer, A.M.; et al. Monitoring ceftazidime-avibactam and aztreonam concentrations in the treatment of a bloodstream infection caused by a multidrug-resistant Enterobacter sp. carrying both Klebsiella pneumoniae carbapenemase-4 and New Delhi metallo-β-lactamase-1. Clin. Infect. Dis. 2020, 71, 1095–1098. [Google Scholar] [CrossRef]
- Marshall, S.; Hujer, A.M.; Rojas, L.J.; Papp-Wallace, K.M.; Humphries, R.M.; Spellberg, B.; Hujer, K.M.; Marshall, E.K.; Rudin, S.D.; Perez, F.; et al. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017, 61, e02243-16. [Google Scholar] [CrossRef]
- Shaw, E.; Rombauts, A.; Tubau, F.; Padullés, A.; Càmara, J.; Lozano, T.; Cobo-Sacristán, S.; Sabe, N.; Grau, I.; Rigo-Bonnin, R.; et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J. Antimicrob. Chemother. 2018, 73, 1104–1106. [Google Scholar] [CrossRef]
- Alm, R.A.; Johnstone, M.R.; Lahiri, S.D. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: Role of a novel insertion in PBP3. J. Antimicrob. Chemother. 2015, 70, 1420–1428. [Google Scholar] [CrossRef]
- Kazmierczak, K.M.; Rabine, S.; Hackel, M.; McLaughlin, R.E.; Biedenbach, D.J.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 1067–1078. [Google Scholar] [CrossRef]
- Periasamy, H.; Joshi, P.; Palwe, S.; Shrivastava, R.; Bhagwat, S.; Patel, M. High prevalence of Escherichia coli clinical isolates in India harbouring four amino acid inserts in PBP3 adversely impacting activity of aztreonam/avibactam. J. Antimicrob. Chemother. 2020, 75, 1650–1651. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.-D.; Nordmann, P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-β-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Iyobe, S.; Inoue, M.; Mitsuhashi, S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1991, 35, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Biedenbach, D.J.; Kazmierczak, K.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. In vitro activity of aztreonam–avibactam against a global collection of Gram-negative pathogens from 2012 and 2013. Antimicrob. Agents Chemother. 2015, 59, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Mesaros, N.; Nordmann, P.; Plésiat, P.; Roussel-Delvallez, M.; van Eldere, J.; Glupczynski, Y.; van Laethem, Y.; Jacobs, F.; Lebecque, P.; Malfroot, A.; et al. Pseudomonas aeruginosa: Resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infect. 2007, 13, 560–578. [Google Scholar] [CrossRef]
- Giamarellou, H. Prescribing guidelines for severe Pseudomonas infections. J. Antimicrob. Chemother. 2002, 49, 229–233. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.E. Resistance in nonfermenting gram-negative bacteria: Multidrug resistance to the maximum. Am. J. Med. 2006, 119, S29–S36. [Google Scholar] [CrossRef]
- Thomson, J.M.; Bonomo, R.A. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: Beta-lactams in peril! Curr. Opin. Microbiol. 2005, 8, 518–524. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Hocquet, D.; Nordmann, P.; El Garch, F.; Cabanne, L.; Plesiat, P. Involvement of the MexXY–OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1347–1351. [Google Scholar] [CrossRef]
- Quale, J.; Bratu, S.; Gupta, J.; Landman, D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2006, 50, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Le Thomas, I.; Couetdic, G.; Clermont, O.; Brahimi, N.; Plesiat, P.; Bingen, E. In vivo selection of a target⁄efflux double mutant of Pseudomonas aeruginosa by ciprofloxacin therapy. J. Antimicrob. Chemother. 2001, 48, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Sugimoto, Y.; Yoneyama, H.; Nakae, T. High-level fluoroquinolone resistance in Pseudomonas aeruginosa due to interplay of the MexAB–OprM efflux pump and the DNA gyrase mutation. Microbiol. Immunol. 2002, 46, 391–395. [Google Scholar] [CrossRef]
- Niga, T.; Ito, H.; Oyamada, Y.; Yamagishi, J.-I.; Kadono, M.; Nishino, T.; Gotoh, N.; Inoue, M. Cooperation between alteration of DNA gyrase genes and over-expression of MexB and MexX confers high-level fluoroquinolone resistance in Pseudomonas aeruginosa strains isolated from a patient who received a liver transplant followed by treatment with fluoroquinolones. Microbiol. Immunol. 2005, 49, 443–446. [Google Scholar]
- Poole, K.; Srikumar, R. Multidrug efflux in Pseudomonas aeruginosa: Components, mechanisms and clinical significance. Curr. Top. Med. Chem. 2001, 1, 59–71. [Google Scholar] [CrossRef]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of efflux pumps on antimicrobial resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, C.; Zhang, X.; Wang, D.; Pan, X.; Liu, H.; Zhu, G.; Bai, F.; Cheng, Z.; Wu, W.; et al. A MexR mutation which confers aztreonam resistance to Pseudomonas aeruginosa. Front. Microbiol. 2021, 12, 659808. [Google Scholar] [CrossRef]
- Jorth, P.; McLean, K.; Ratjen, A.; Secor, P.R.; Bautista, G.E.; Ravishankar, S.; Rezayat, A.; Garudathri, J.; Harrison, J.J.; Harwood, R.A.; et al. Evolved aztreonam resistance is multifactorial and can produce hypervirulence in Pseudomonas aeruginosa. mBio 2017, 8, e00517. [Google Scholar] [CrossRef]
- Waditzer, M.; Bucar, F. Flavonoids as inhibitors of bacterial efflux pumps. Molecules 2021, 26, 6904. [Google Scholar] [CrossRef]
- Dos Santos, J.F.S.; Tintino, S.R.; da Silva, A.R.P.; Dos S Barbosa, C.R.; Scherf, J.R.; de S Silveira, Z.; de Freitas, T.S.; de Lacerda Neto, L.J.; Barros, L.M.; de A Menezes, I.R.; et al. Enhancement of antibiotic activity by quercetin against Staphylococcus aureus efflux pumps. J. Bioenerg. Biomembr. 2021, 53, 157–167. [Google Scholar] [CrossRef]
- Kho, W.; Kim, M.K.; Jung, M.; Chong, Y.P.; Kim, Y.S.; Park, K.-H.; Chong, Y. Strain-specific anti-biofilm and antibiotic-potentiating activity of 3′,4′-difluoroquercetin. Sci. Rep. 2020, 10, 14162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Cheng, B.; Gao, L.; Qin, C.; Zhang, L.; Zhang, X.; Wang, J.; Wan, Y. Discovery of quercetin and its analogs as potent OXA-48 beta-lactamase inhibitors. Front. Pharmacol. 2022, 13, 926104. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Tripathi, A. Quercetin inhibits carbapenemase and efflux pump activities among carbapenem-resistant Gram-negative bacteria. APMIS 2020, 128, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Benin, B.M.; Hillyer, T.; Shin, W.S. Quercetin, a potential metallo-β-lactamase inhibitor for use in combination therapy against β-lactam antibiotic-resistant bacteria. Open J. Pathol. Toxicol. Res. 2021, 1, OJPTR.MS.ID.000503. [Google Scholar]
- Nakashima, R.; Sakurai, K.; Yamasaki, S.; Hayashi, K.; Nagata, C.; Hoshino, K.; Onodera, Y.; Nishino, K.; Yamaguchi, A. Structural basis for the inhibition of bacterial multidrug exporters. Nature 2013, 500, 102–107. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, S.; Balius, T.E.; Singh, I.; Levit, A.; Moroz, Y.S.; O’Meara, M.J.; Che, T.; Algaa, E.; Tolmachova, K.; et al. Ultra-large library docking for discovering new chemotypes. Nature 2019, 566, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of cyclic boronic acid QPX7728, an ultrabroad-spectrum inhibitor of serine and metallo-β-lactamases. J. Med. Chem. 2020, 63, 7491–7507. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The Vega suite of programs: A versatile platform for cheminformatics and drug design projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci. 2018, 27, 14–25. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, M.K.; Mok, H.; Choo, H.; Chong, Y. Separation of quercetin’s biological activity from its oxidative property through bioisosteric replacement of the catecholic hydroxyl groups with fluorine atoms. J. Agric. Food Chem. 2012, 60, 6499–6506. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI M100-ED33:2023; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2023. [Google Scholar]
- Paixão, L.; Rodrigues, L.; Couto, I.; Martins, M.; Fernandes, P.; de Carvalho, C.C.; Monteiro, G.A.; Sansonetty, F.; Amaral, L.; Viveiros, M. Fluorometric determination of ethidium bromide efflux kinetics in Escherichia coli. J. Biol. Eng. 2009, 3, 18. [Google Scholar] [CrossRef]
- Pemberton, O.A.; Jaishankar, P.; Akhtar, A.; Adams, J.L.; Shaw, L.N.; Renslo, A.R.; Chen, Y. Heteroaryl phosphonates as noncovalent inhibitors of both serine and metallocarbapenemases. J. Med. Chem. 2019, 62, 8480–8496. [Google Scholar] [CrossRef]
- Liu, B.; Trout, R.E.L.; Chu, G.-H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Discovery of taniborbactam (VNRX-5133): A broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from Gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, Y.A.; Kim, J.; Jeong, S.H.; Shin, J.H.; Shin, K.S.; Shin, J.H.; Kim, Y.R.; Kim, H.S.; Uh, Y.; et al. Emergence of NDM-1-producing Pseudomonas aeruginosa sequence type 773 clone: Shift of carbapenemases molecular epidemiology and spread of 16S rRNA methylase genes in Korea. Ann. Lab. Med. 2023, 43, 196–199. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: A retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Tripathi, A. Demonstration of bactericidal and synergistic activity of quercetin with meropenem among pathogenic carbapenem-resistant Escherichia coli and Klebsiella pneumoniae. Microb. Pathog. 2020, 143, 104120. [Google Scholar] [CrossRef]
- Sariyer, E.; Saral, A. Evaluation of quercetin as a potential β-lactamase CTX-M-15 inhibitor via molecular docking, dynamic simulations, and MMGBSA. Turk. J. Chem. 2021, 45, 1045–1056. [Google Scholar] [CrossRef]
- Sjuts, H.; Vargiu, A.V.; Kwasny, S.M.; Nguyen, S.T.; Kim, H.-S.; Ding, X.; Ornik, A.R.; Ruggerone, P.; Bowlin, T.L.; Nikaido, H.; et al. Molecular basis for inhibition of AcrB multidrug efflux pump by novel and powerful pyranopyridine derivatives. Proc. Natl. Acad. Sci. USA 2016, 113, 3509–3514. [Google Scholar] [CrossRef]
- Viveiros, M.; Martins, A.; Paixão, L.; Rodrigues, L.; Martins, M.; Couto, I.; Fähnrich, E.; Kern, W.V. Amaral, Demonstration of intrinsic efflux activity of Escherichia coli K-12 AG100 by automated ethidium bromide method. Int. J. Antimicrob. Agents 2008, 31, 458–462. [Google Scholar] [CrossRef]
- Barnes, M.D.; Winkler, M.L.; Taracila, M.A.; Page, M.G.; Desarbre, E.; Kreiswirth, B.N.; Shields, R.H.; Nguyen, M.-H.; Clancy, C.; Spellberg, B.; et al. Klebsiella pneumoniae Carbapenemase-2 (KPC-2), substitutions at Ambler position Asp179, and resistance to ceftazidime-avibactam: Unique antibiotic-resistant phenotypes emerge from β-lactamase protein engineering. mBio 2017, 8, e00528-17. [Google Scholar] [CrossRef] [PubMed]
- Lohans, C.T.; Brem, J.; Schofield, C.J. New Delhi metallo-β-lactamase 1 catalyzes avibactam and aztreonam hydrolysis. Antimicrob. Agents Chemother. 2017, 61, e01224-17. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]

| Isolates, MBLs Produced | MIC Value (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PIP-TZB | CAZ-AVI | CAZ | FEP | ATM | IPM | MEM | AMK | GEN | CST | CIP | |
| PA-001, blaNDM | >64/4 | >8/4 | >16 | >16 | 32 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-007, blaNDM | >64/4 | >8/4 | >16 | 16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-008, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-011, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-012, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | ≤4 | 8 | ≤1 | >2 |
| PA-017, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-019, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-020, blaNDM | >64/4 | >8/4 | >16 | 16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-023, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-025, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-028, blaNDM | >64/4 | >8/4 | >16 | >16 | 8 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-034, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-036, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-038, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-039, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | ≤4 | ≤2 | ≤1 | >2 |
| PA-044, blaNDM | >64/4 | >8/4 | >16 | >16 | 16 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-002, blaIMP | >64/4 | >8/4 | >16 | >16 | 64 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-016, blaIMP | >64/4 | >8/4 | >16 | >16 | 64 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-027, blaIMP | 64/4 | 8/4 | >16 | >16 | 32 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-029, blaIMP | >64/4 | >8/4 | >16 | >16 | 64 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| PA-030, blaIMP | >64/4 | >8/4 | >16 | >16 | 64 | >8 | >32 | >32 | >8 | ≤1 | >2 |
| Isolates, MBLs Produced | MIC Value (mg/L) 2 | ||||||
|---|---|---|---|---|---|---|---|
| ATM-ARMA | |||||||
| ATM Alone | ATM-AVI | ATM-CZA | ATM-CCCP | ATM-PAβN | ATM-1 | ATM-CCCP-AVI | |
| PA-025, blaNDM | 16 | 16 | 16 | – | – | 8 (8) | – |
| PA-038, blaNDM | 16 | 16 | 16 | 4 (256) | 4 (128) | 8 (8) | – |
| PA-002, blaIMP | 64 | 64 | 16 (128) | 16 (256) | 32 (128) | 16 (16) | 4 (256/256) |
| PA-003, blaVIM | 32 | 16 (128) | 16 (128) | 8 (256) | 8 (128) | 16 (16) | 4 (256/256) |
| Compound | β-Lactamases, IC50 (μM) | Compound | β-Lactamases, IC50 (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AmpC | KPC-2 | OXA-48 | VIM-2 | NDM-1 | AmpC | KPC-2 | OXA-48 | VIM-2 | NDM-1 | ||
| 1 | >50 | >50 | >100 | >100 | >50 | 17 | >50 | >50 | >100 | >100 | >50 |
| 3 | 19.2 | 22.2 | 21.2 | 20.3 | 24.6 | 18 | >50 | >50 | >100 | >100 | >50 |
| 4 | 25.4 | 33.2 | 28.7 | 19.3 | 15.7 | 19 | >50 | >50 | >100 | >100 | >50 |
| 5 | >50 | >50 | 42.7 | >100 | >50 | 20 | >50 | >50 | >100 | >100 | >50 |
| 6 | >50 | >50 | 37.8 | >100 | 14.1 | 21 | >50 | >50 | >100 | >100 | >50 |
| 7 | >50 | 21.5 | 6.0 | 13.1 | 16.7 | 22 | >50 | >50 | 39.8 | >100 | >50 |
| 8 | >50 | >50 | >100 | >100 | >50 | 23 | 18.9 | 17.3 | 14.8 | 11.9 | 11.4 |
| 9 | >50 | >50 | >100 | >100 | >50 | 24 | >50 | 39.4 | 19.1 | 42.1 | >50 |
| 10 | >50 | >50 | >100 | >100 | >50 | 25 | >50 | 22.8 | 11.4 | 13.9 | 22.7 |
| 11 | >50 | >50 | >100 | >100 | >50 | 26 | 21.0 | 11.5 | 8.0 | 9.0 | 15.9 |
| 12 | >50 | >50 | >100 | >100 | >50 | 27 | 15.3 | 11.3 | 6.3 | 4.4 | 10.1 |
| 13 | >50 | >50 | >100 | >100 | >50 | 28 | >50 | 40.8 | 9.2 | 7.8 | 21.8 |
| 14 | >50 | >50 | >100 | >100 | >50 | 29 | >50 | 28.3 | 5.5 | 5.1 | 26.7 |
| 15 | >50 | >50 | >100 | >100 | >50 | 30 | >50 | >50 | 35.9 | >100 | >50 |
| 16 | >50 | >50 | >100 | >100 | >50 | 31 | >50 | >50 | >100 | >100 | >50 |
| Isolates, MBLs Produced | Effects of Combination on the MIC of ATM | |||||
|---|---|---|---|---|---|---|
| ATM (Alone) | ATM-27 | ATM-AVI | ATM-CCCP | ATM-CCCP-AVI | ||
| MIC 1 | FD 2 (FICI) 3 | MIC 1 | MIC 1 | MIC 1 | ||
| PA-001, blaNDM | 32 | 16 (8) | 2 (A) | 16 (128) | 16 (32) | 16 (16/64) |
| PA-007, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 8 (64) | 8 (64/– 4) |
| PA-008, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 8 (64) | 8 (64/– 4) |
| PA-011, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 8 (64) | 8 (64/– 4) |
| PA-012, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 8 (64) | 8 (64/– 4) |
| PA-017, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-019, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-020, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-023, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-025, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-028, blaNDM | 8 | 1 (32) | 8 (S) | 8 4 | 8 4 | 8 4 |
| PA-034, blaNDM | 16 | 4 (16) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-036, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-038, blaNDM | 16 | 4 (8) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-039, blaNDM | 16 | 4 (8) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-044, blaNDM | 16 | 4 (32) | 4 (S) | 16 4 | 16 4 | 16 4 |
| PA-002, blaIMP | 64 | 8 (32) | 8 (S) | 64 4 | 64 4 | 64 4 |
| PA-016, blaIMP | 64 | 8 (16) | 8 (S) | 64 4 | 64 4 | 64 4 |
| PA-027, blaIMP | 32 | 8 (8) | 4 (S) | 32 4 | 32 4 | 32 4 |
| PA-029, blaIMP | 64 | 16 (16) | 4 (S) | 64 4 | 64 4 | 64 4 |
| PA-030, blaIMP | 64 | 32 (8) | 2 (A) | 64 4 | 64 4 | 64 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, T.; Kim, M.K.; Ahn, J.H.; Jeong, S.; Park, K.-H.; Chong, Y. Potentiation of Antibiotic Activity of Aztreonam against Metallo-β-Lactamase-Producing Multidrug-Resistant Pseudomonas aeruginosa by 3-O-Substituted Difluoroquercetin Derivatives. Pharmaceutics 2024, 16, 185. https://doi.org/10.3390/pharmaceutics16020185
Lee S, Lee T, Kim MK, Ahn JH, Jeong S, Park K-H, Chong Y. Potentiation of Antibiotic Activity of Aztreonam against Metallo-β-Lactamase-Producing Multidrug-Resistant Pseudomonas aeruginosa by 3-O-Substituted Difluoroquercetin Derivatives. Pharmaceutics. 2024; 16(2):185. https://doi.org/10.3390/pharmaceutics16020185
Chicago/Turabian StyleLee, Seongyeon, Taegum Lee, Mi Kyoung Kim, Joong Hoon Ahn, Seri Jeong, Ki-Ho Park, and Youhoon Chong. 2024. "Potentiation of Antibiotic Activity of Aztreonam against Metallo-β-Lactamase-Producing Multidrug-Resistant Pseudomonas aeruginosa by 3-O-Substituted Difluoroquercetin Derivatives" Pharmaceutics 16, no. 2: 185. https://doi.org/10.3390/pharmaceutics16020185
APA StyleLee, S., Lee, T., Kim, M. K., Ahn, J. H., Jeong, S., Park, K.-H., & Chong, Y. (2024). Potentiation of Antibiotic Activity of Aztreonam against Metallo-β-Lactamase-Producing Multidrug-Resistant Pseudomonas aeruginosa by 3-O-Substituted Difluoroquercetin Derivatives. Pharmaceutics, 16(2), 185. https://doi.org/10.3390/pharmaceutics16020185





