New Chalcone-Derived Molecule for the Topical Regulation of Hyperpigmentation and Skin Aging
Abstract
1. Introduction
2. Materials and Methods
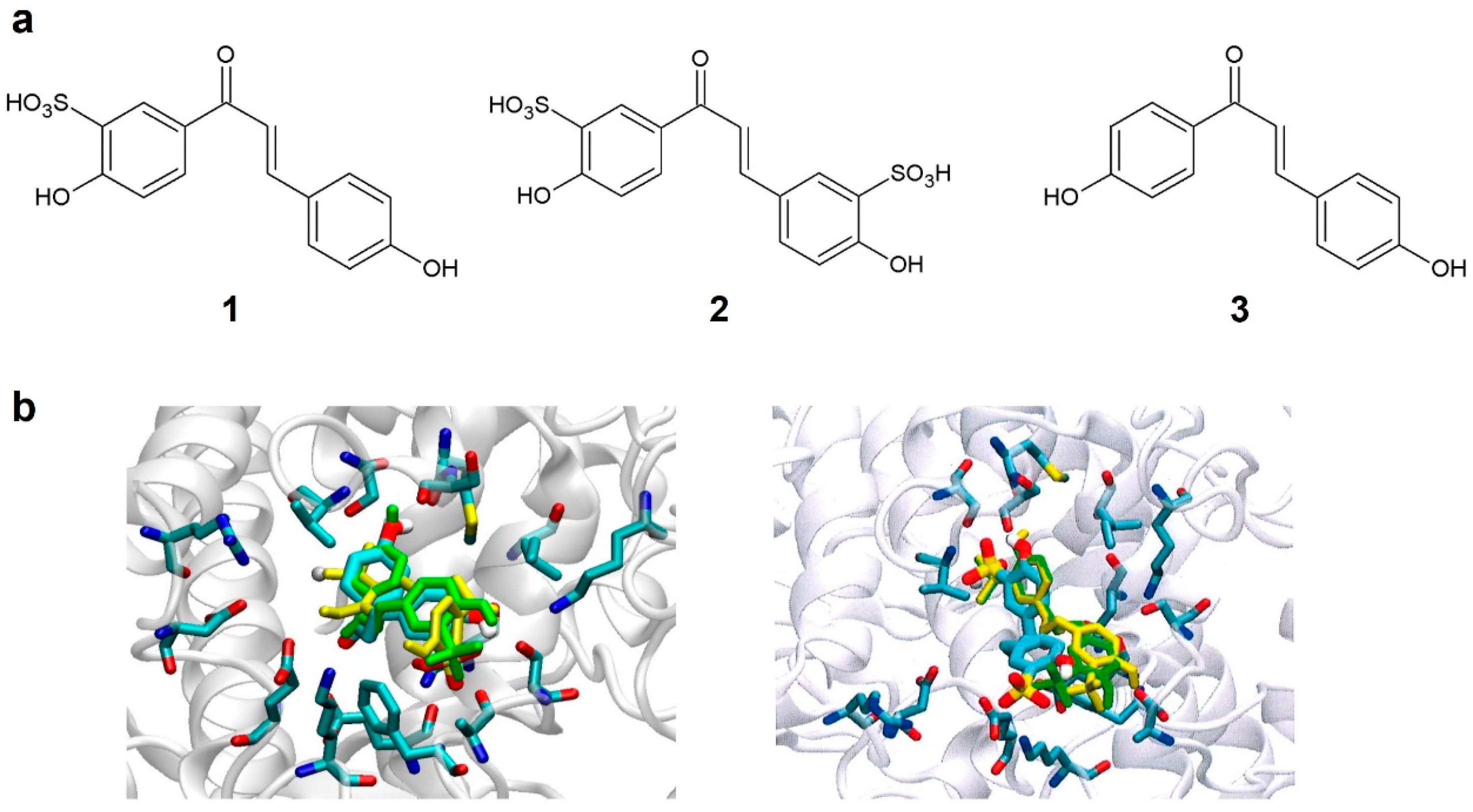
2.1. Computational Design
2.2. Synthesis of Compounds
2.2.1. Synthesis of 5-Acetyl-2-hydroxybenzenesulfonic Acid (Intermediate)
2.2.2. Synthesis of 5-[(E)-3-(p-Hydroxyphenyl)acryloyl]-2-hydroxybenzenesulfonic Acid (Compound 1)
2.2.3. Synthesis of 5-[(E)-2-(4-Hydroxy-3-sulfobenzoyl)-1-ethenyl]-2-hydroxybenzenesulfonic Acid (Compound 2)
2.2.4. Synthesis of (E)-1,3-Bis(p-hydroxyphenyl)-2-propen-1-one (Compound 3)
2.3. Solubility Assay
2.4. HPLC Conditions
2.5. Cell Cultures
2.6. Cell Viability Assay
2.7. ATAC-Seq Study
2.8. Gene Expression and miRNA Level Quantification by qPCR
2.9. Tyrosinase Inhibition Assay
2.10. Melanin Inhibition Assay
2.11. Anti-Glycation Effect
2.12. Antioxidant Activity Assay
2.13. Wound Healing Assay
2.14. RHPE Depigmentation Efficacy Assay
2.15. Skin Irritation Test
2.16. Statistical Analysis
3. Results
3.1. Computational Approach for the Identification of Novel Tyrosinase Inhibitors and Chemical Synthesis
3.2. Solubility Study
3.3. Tyrosinase Activity
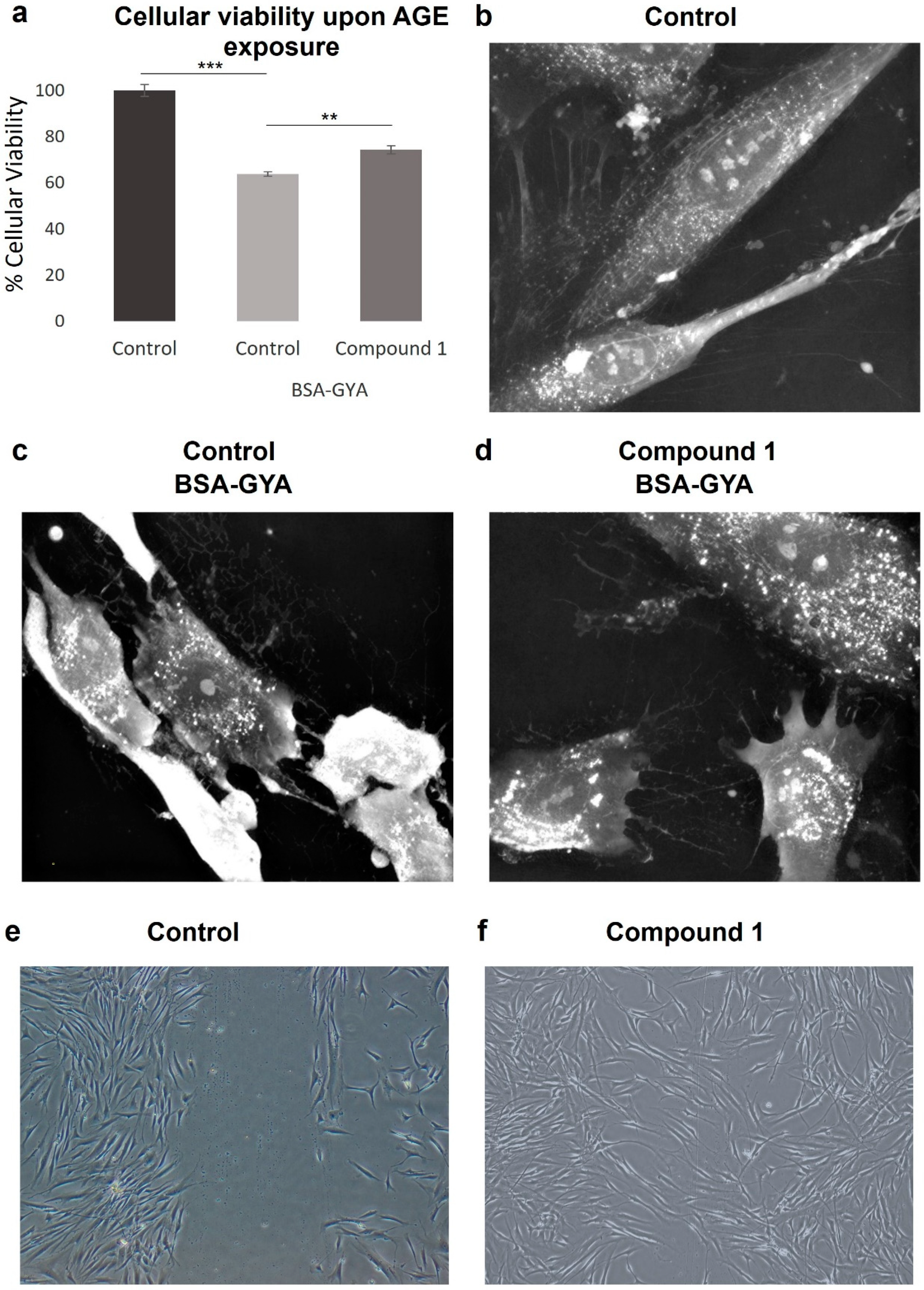
3.4. Cell Viability
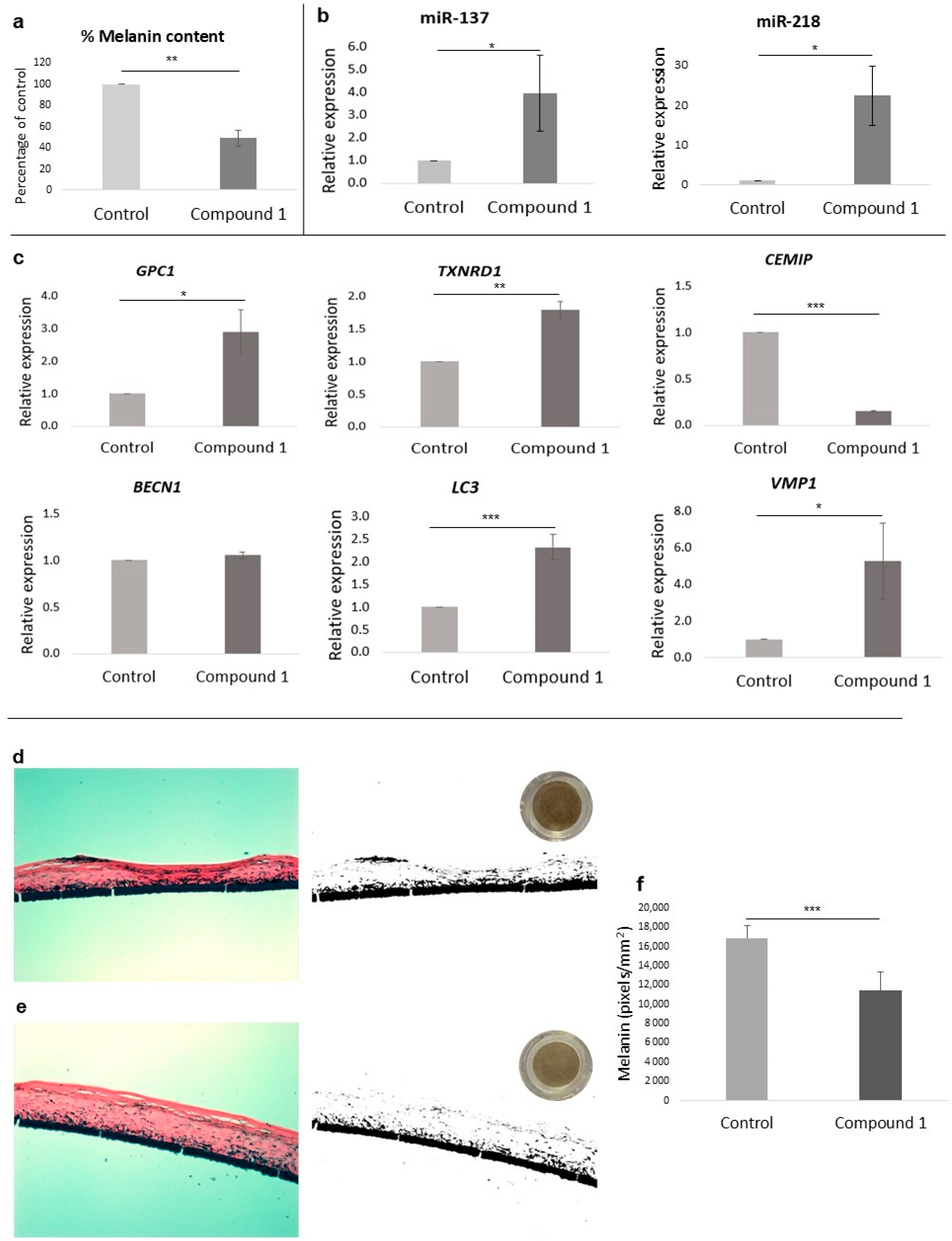
3.5. Melanin Inhibition
3.6. Epigenetic Effect on Depigmentation
3.7. Effects on Chromatin Accessibility
3.8. Effects on Anti-Aging Gene Expression
3.9. Anti-Glycation Activity
3.10. Wound Healing Effect
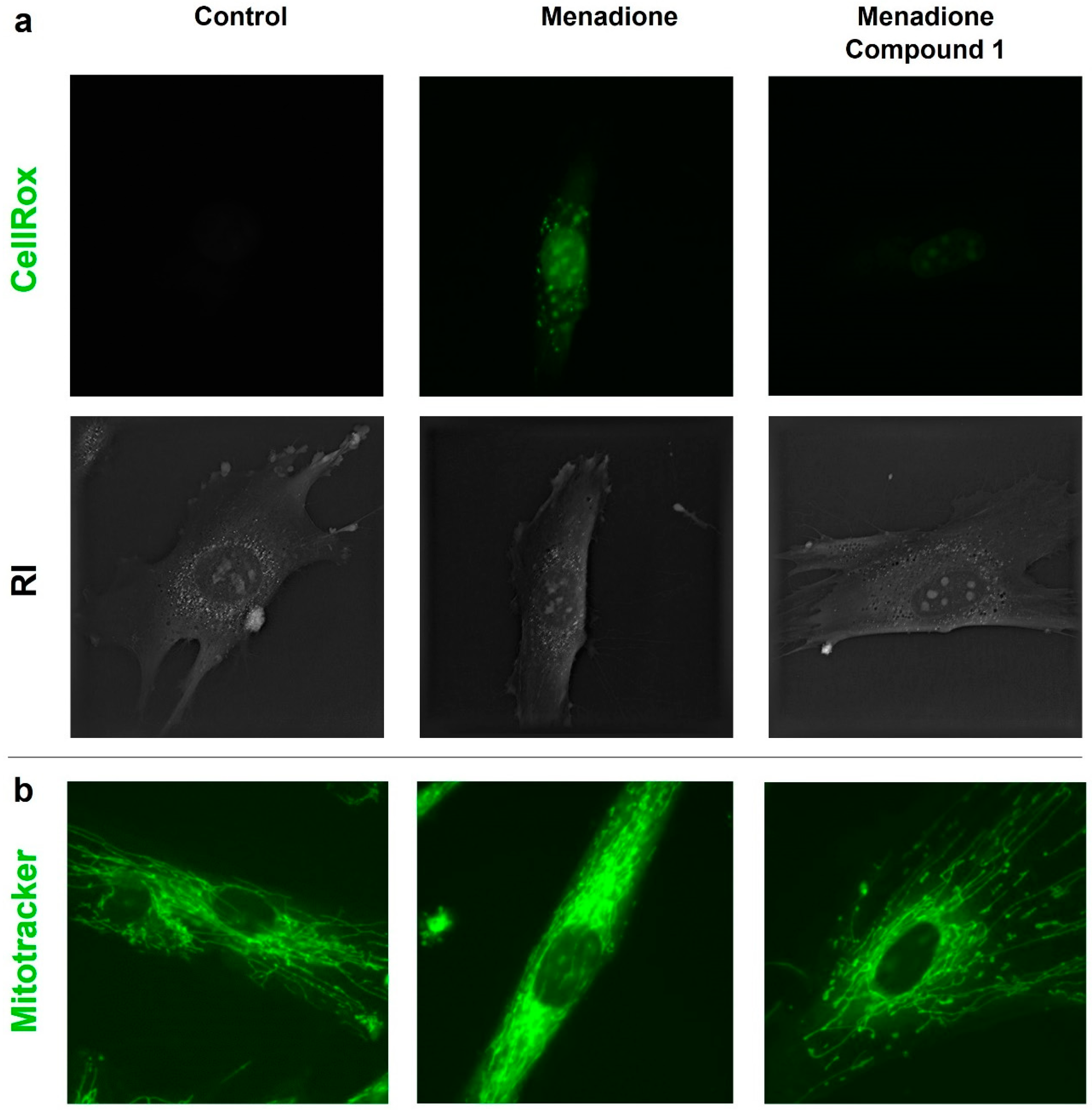
3.11. Antioxidant Activity
3.12. Skin Irritation
3.13. Reconstructed Human Pigmented Epidermis (RHPE) Depigmentation Efficacy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell. Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin pigmentation and pigmentary disorders: Focus on epidermal/dermal cross-talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, I.; Detsi, A. Recent developments on tyrosinase inhibitors based on the chalcone and aurone scaffolds. Curr. Enzym. Inhib. 2018, 14, 3–17. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorganic Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Elkanzi, N.A.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Rajendran, G.; Bhanu, D.; Aruchamy, B.; Ramani, P.; Pandurangan, N.; Bobba, K.N.; Oh, E.J.; Chung, H.Y.; Gangadaran, P.; Ahn, B.-C. Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry. Pharmaceuticals 2022, 15, 1250. [Google Scholar] [CrossRef]
- Shin, S.; Kum, H.; Ryu, D.; Kim, M.; Jung, E.; Park, D. Protective Effects of a New Phloretin Derivative against UVB-Induced Damage in Skin Cell Model and Human Volunteers. Int. J. Mol. Sci. 2014, 15, 18919–18940. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, M.; Liu, Y.; Zhang, M.; Yang, G. Tetramethoxychalcone, a Chalcone Derivative, Suppresses Proliferation, Blocks Cell Cycle Progression, and Induces Apoptosis of Human Ovarian Cancer Cells. PLoS ONE 2014, 9, e106206. [Google Scholar] [CrossRef] [PubMed]
- Okolo, E.N.; Ugwu, D.I.; Ezema, B.E.; Ndefo, J.C.; Eze, F.U.; Ezema, C.G.; Ezugwu, J.A.; Ujam, O.T. New chalcone derivatives as potential antimicrobial and antioxidant agents. Sci. Rep. 2021, 11, 21781. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Rolt, A.; Cox, L.S. Structural basis of the anti-ageing effects of polyphenolics: Mitigation of oxidative stress. BMC Chem. 2020, 14, 50. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Matoba, Y.; Kumagai, T.; Yamamoto, A.; Yoshitsu, H.; Sugiyama, M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J. Biol. Chem. 2006, 281, 8981–8990. [Google Scholar] [CrossRef]
- Holt, P.A.; Chaires, J.B.; Trent, J.O. Molecular docking of intercalators and groove-binders to nucleic acids using Autodock and Surflex. J. Chem. Inf. Model. 2008, 48, 1602–1615. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Buenrostro, J.D.; Giresi, P.G.; Zaba, L.C.; Chang, H.Y.; Greenleaf, W.J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins, and nucleosome position. Nat. Methods 2013, 10, 1213–1218. [Google Scholar] [CrossRef]
- Corces, M.R.; Trevino, A.E.; Hamilton, E.G.; Greenside, P.G.; Sinnott-Armstrong, N.A.; Vesuna, S.; Satpathy, A.T.; Rubin, A.J.; Montine, K.S.; Wu, B.; et al. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods 2017, 14, 959–962. [Google Scholar] [CrossRef]
- Li, S.; Zong, X.; Zhang, L.; Li, L.; Wu, J. A chromatin accessibility landscape during early adipogenesis of human adipose-derived stem cells. Adipocyte 2022, 11, 239–249. [Google Scholar] [CrossRef]
- Winder, A.J.; Harris, H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur. J. Biochem. 1991, 98, 317–326. [Google Scholar] [CrossRef]
- Qiao, Z.; Koizumi, Y.; Zhang, M.; Natsui, M.; Flores, M.J.; Gao, L.; Yusa, K.; Koyota, S.; Sugiyama, T. Anti-melanogenesis effect of Glechoma hederacea L. extract on B16 murine melanoma cells. Biosci. Biotechnol. Biochem. 2012, 76, 1877–1883. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Hasegawa, T.; Takahashi, H.; Ishibashi, T.; Itagaki, H.; Sugibayashi, K. Utility of MTT assay in three-dimensional cultured human skin model as an alternative for Draize skin irritation test: Approach using diffusion law of irritant in skin and toxicokinetics-toxicodynamics correlation. Pharm. Res. 2002, 19, 669–675. [Google Scholar] [CrossRef]
- Huxtable, R.J. Biochemistry of Sulfur; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew. Chem. Int. Ed. 2017, 56, 9812–9815. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Narsinghani, T.; Sharma, M.C.; Bhargav, S. Synthesis, docking studies and antioxidant activity of some chalcone and aurone derivatives. Med. Chem. Res. 2013, 22, 4059–4068. [Google Scholar] [CrossRef]
- Hushcha, Y.; Blo, I.; Oton-Gonzalez, L.; Di Mauro, G.; Martini, F.; Tognon, M.; De Mattei, M. MicroRNAs in the regulation of melanogenesis. Int. J. Mol. Sci. 2021, 22, 6104. [Google Scholar] [CrossRef]
- Brown, T.J.; Kollara, A.; Shathasivam, P.; Ringuette, M.J. Ventricular Zone Expressed PH Domain Containing 1 (VEPH1): An adaptor protein capable of modulating multiple signaling transduction pathways during normal and pathological development. Cell Commun. Signal. 2019, 17, 116. [Google Scholar] [CrossRef]
- Yoshida, H.; Okada, Y. Role of HYBID (Hyaluronan binding protein involved in hyaluronan depolymerization), alias KIAA1199/CEMIP, in hyaluronan degradation in normal and photoaged skin. Int. J. Mol. Sci. 2019, 20, 5804. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Aoki, M.; Komiya, A.; Endo, Y.; Kawabata, K.; Nakamura, T.; Sakai, S.; Sayo, T.; Okada, Y.; Takahashi, Y. HYBID (alias KIAA1199/CEMIP) and hyaluronan synthase coordinately regulate hyaluronan metabolism in histamine-stimulated skin fibroblasts. J. Biol. Chem. 2020, 295, 2483–2494. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.Y.; Moon, S.H.; Lee, J.D.; Kim, S. Autophagy in human skin fibroblasts: Impact of age. Int. J. Mol. Sci. 2018, 19, 2254. [Google Scholar] [CrossRef] [PubMed]
- Perrot, G.; Colin-Pierre, C.; Ramont, L.; Proult, I.; Garbar, C.; Bardey, V.; Jeanmaire, C.; Mine, S.; Danoux, L.; Berthélémy, N.; et al. Decreased expression of GPC1 in human skin keratinocytes and epidermis during ageing. Exp. Gerontol. 2019, 126, 110693. [Google Scholar] [CrossRef]
- Applegate, L.A.; Scaletta, C.; Panizzon, R. Evidence that ferritin is UV inducible in human skin: Part of a putative defense mechanism. J. Investig. Dermatol. 1998, 111, 159–163. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R. Examining the impact of skin lighteners in vitro. Oxid. Med. Cell. Longev. 2013, 2013, 702120. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.M. Thioredoxin Reductase—Its Role in Epidermal Redox Status. J. Photochem. Photobiol. B Biol. 2001, 64, 179–184. [Google Scholar] [CrossRef]
- Cadenas, C.; Franckenstein, D.; Schmidt, M.; Gehrmann, M.; Hermes, M.; Geppert, B.; Schormann, W.; Maccoux, L.J.; Schug, M.; Schumann, A.; et al. Role of thioredoxin reductase 1 and thioredoxin interacting protein in prognosis of breast cancer. Breast Cancer Res. 2010, 12, R44. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Sharma, A.; Ju, L.; Murai, J.; Umans, L.; Vermeire, L.; Pommier, Y.; Takeda, S.; Huylebroeck, D.; Caldecott, K.W.; et al. TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1. Nucleic Acids Res. 2012, 40, 8371–8380. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.; Ramsahoye, B. The relationship between chromatin structure and transcriptional activity in mammalian genomes. Brief. Funct. Genom. 2005, 4, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Wolffe, A.P. Relationships between chromatin organization and DNA methylation in determining gene expression. Semin. Cancer Biol. 1999, 9, 339–347. [Google Scholar] [CrossRef]
- Zaccaria, M.; Ludovici, M.; Sanzani, S.M.; Ippolito, A.; Cigliano, R.A.; Sanseverino, W.; Scarpari, M.; Scala, V.; Fanelli, C.; Reverberi, M. Menadione-induced oxidative stress re-shapes the oxylipin profile of Aspergillus flavus and its lifestyle. Toxins 2015, 7, 4315–4329. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Katta, R. Sugar Sag: Glycation and the Role of Diet in Aging Skin. Ski. Ther. Lett. 2015, 20, 1–5. [Google Scholar]
- Alikhani, Z.; Alikhani, M.; Boyd, C.M.; Nagao, K.; Trackman, P.C.; Graves, D.T. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J. Biol. Chem. 2005, 280, 12087–12095. [Google Scholar] [CrossRef]
| Treatment | Tyrosinase Inhibition |
|---|---|
| Control | 0 ± 2% |
| Compound 1 | 56 ± 1% |
| Compound 2 | 22 ± 4% |
| Compound 3 | 16 ± 4% |
| Hydroquinone | 16 ± 1% |
| Kojic acid | 23 ± 1% |
| Treatment | Melanocytes | Fibroblasts | ||
|---|---|---|---|---|
| Cell Viability | Student’s t-Test | Cell Viability | Student’s t-Test | |
| Control | 100 ± 1% | - | 100 ± 5% | - |
| 3.125 µM | 96 ± 4% | 0.086 | 95 ± 11% | 0.290 |
| 31.25 µM | 101 ± 4% | 0.333 | 78 ± 14% | 0.134 |
| 156.25 µM | 103 ± 7% | 0.260 | 83 ± 16% | 0.259 |
| 312.5 µM | 89 ± 8% | 0.094 | 83 ± 14% | 0.111 |
| 1562.5 µM | 49 ± 3% | 0.003 ** | 60 ± 1% | 0.035 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Gutiérrez, A.; Bertran, A.; Noya, T.; Pena-Rodríguez, E.; Gómez-Escalante, S.; Pascual, S.; Luis, L.S.; González, M.C. New Chalcone-Derived Molecule for the Topical Regulation of Hyperpigmentation and Skin Aging. Pharmaceutics 2024, 16, 1405. https://doi.org/10.3390/pharmaceutics16111405
Martínez-Gutiérrez A, Bertran A, Noya T, Pena-Rodríguez E, Gómez-Escalante S, Pascual S, Luis LS, González MC. New Chalcone-Derived Molecule for the Topical Regulation of Hyperpigmentation and Skin Aging. Pharmaceutics. 2024; 16(11):1405. https://doi.org/10.3390/pharmaceutics16111405
Chicago/Turabian StyleMartínez-Gutiérrez, Alfredo, Alexandra Bertran, Teresa Noya, Eloy Pena-Rodríguez, Susana Gómez-Escalante, Sergio Pascual, Luis Shotze Luis, and Mari Carmen González. 2024. "New Chalcone-Derived Molecule for the Topical Regulation of Hyperpigmentation and Skin Aging" Pharmaceutics 16, no. 11: 1405. https://doi.org/10.3390/pharmaceutics16111405
APA StyleMartínez-Gutiérrez, A., Bertran, A., Noya, T., Pena-Rodríguez, E., Gómez-Escalante, S., Pascual, S., Luis, L. S., & González, M. C. (2024). New Chalcone-Derived Molecule for the Topical Regulation of Hyperpigmentation and Skin Aging. Pharmaceutics, 16(11), 1405. https://doi.org/10.3390/pharmaceutics16111405







