Chemical Characterization and In Vitro Evaluation of Glucans from Fermentation-Produced Nutraceutical Bionutri-AR1®: Antioxidant and Immunomodulatory Properties
Abstract
1. Introduction
2. Materials and Methods
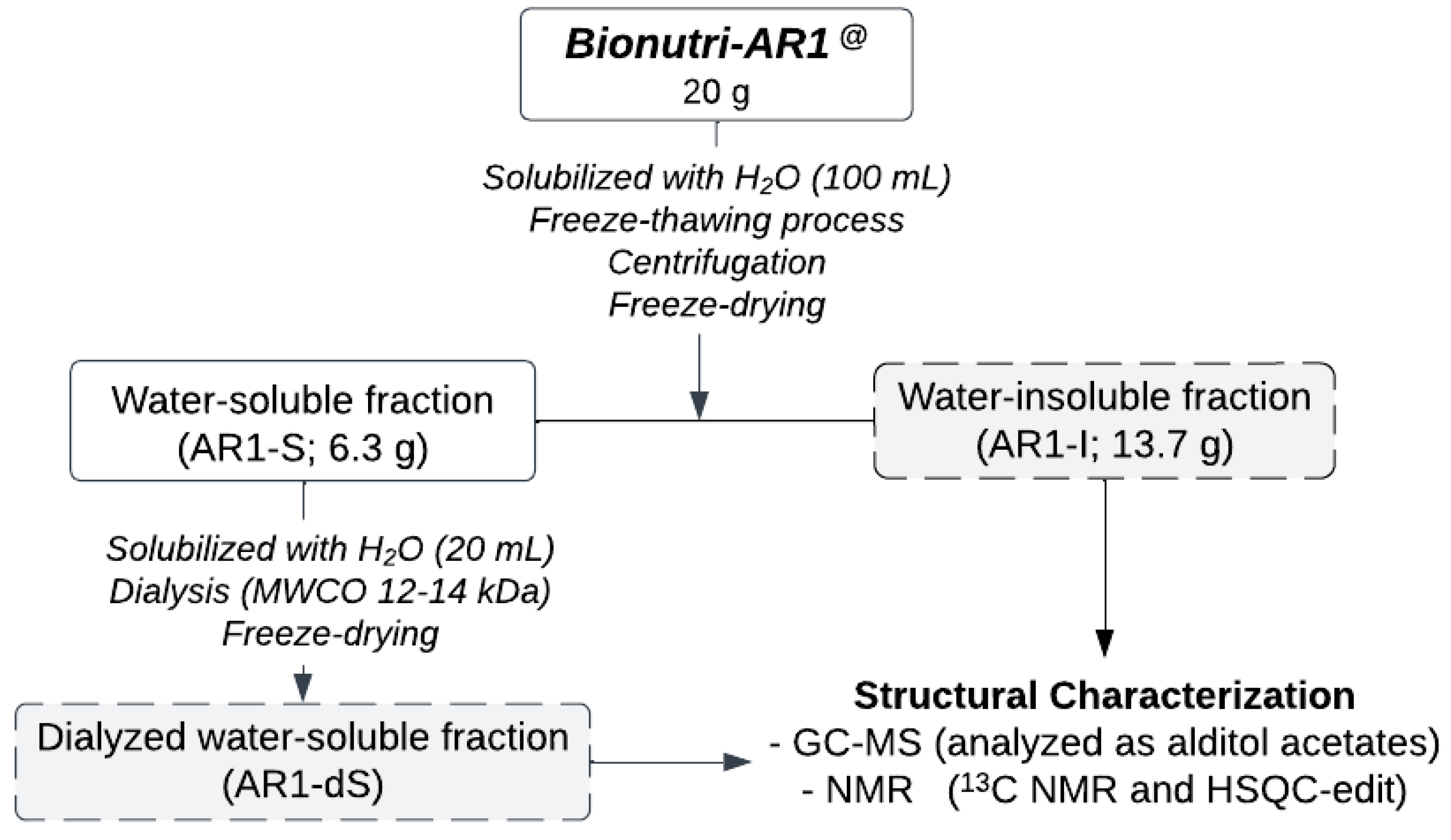
2.1. Purification of the Glucans from Bionutri AR1@
2.2. Monosaccharide Composition
2.3. Nuclear Magnetic Resonance Analysis (NMR)
2.4. Triple-Helix Analysis
2.5. Antioxidant Activity In Vitro
2.6. Enzymatic and Acid Hydrolysis of the α-Glucan from Bionutri AR1@
2.7. Immunomodulatory Assay
3. Results and Discussion
3.1. Chemical Characterization of the Polysaccharides from Bionutri AR1®
3.2. Antioxidant Activity of the Glucans from Bionutri AR1®
3.3. Immunomodulatory Effects of the Glucans from Bionutri AR1®
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Postigo, L.O.C.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current prospects of nutraceuticals: A review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Sut, S.; Dall’Acqua, S. Food-derived nutraceuticals for hypercholesterolemia management, mode of action and active ingredients. Food Biosci. 2023, 54, 102866. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals in hypercholesterolaemia: An overview. Br. J. Pharmacol. 2017, 174, 1450–1463. [Google Scholar] [CrossRef]
- Kamran, F.; Phillips, M.; Harman, D.; Reddy, N. Antioxidant activities of lupin (Lupinus angustifolius) protein hydrolysates and their potential for nutraceutical and functional foods. Food Chem. Adv. 2023, 2, 100297. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Yahaya, M.F.; Tooyama, I.; Damanhuri, H.A. A mechanistic evaluation of antioxidant nutraceuticals on their potential against age-associated neurodegenerative diseases. Antioxidants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Devi, P.V.; Islam, M.J.; Narzary, P.; Sharma, D.; Siltana, F. Bioactive compounds, nutraceutical values and its application in food product development of oyster mushroom. J. Future Foods 2024, 4, 335–342. [Google Scholar] [CrossRef]
- Minno, A.D.; Frigerio, B.; Spadarella, G.; Ravani, A.; Sansaro, D.; Amato, M.; Kitzmiller, J.P.; Pepi, M.; Tremoli, E.; Baldassarre, D. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017, 31, 193–203. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, D.S.E.; Fouad, M.A.; Elmeshad, A.N.; Nabarawi, M.A.; Elhabal, S.F. Anti-obesity nutraceuticals: Insights into mechanisms of action and potential use of biocompatible nanocarriers for delivery. Int. J. Appl. Pharm. 2024, 16, 57–65. [Google Scholar] [CrossRef]
- Medoro, A.; Davinelli, S.; Colletti, A.; Micoli, V.D.; Grandi, E.; Fogacci, F.; Scapagnini, G.; Cicero, A.F.G. Nutraceuticals as modulators of immune function: A review of potential therapeutic effects. Prev. Nutr. Food Sci. 2023, 28, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Caeiro, L.; Gandhay, D.; Anderson, L.J.; Garcia, J.M. A review of nutraceuticals in cancer cachexia. Cancers 2023, 15, 3884. [Google Scholar] [CrossRef] [PubMed]
- Aquila, G.; David, A.; Cecconi, R.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and exercise against muscle wasting during cancer cachexia. Cells 2020, 9, 2536. [Google Scholar] [CrossRef]
- Chopra, A.S.; Lordan, R.; Horbanczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbanczuk, J.O.; Józwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Otles, S.; Gokgunnec, L. Safety considerations in developing functional foods and nutraceuticals. In Encyclopedia of Food Safety, 2nd ed.; Smithers, G.W., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 223–230. [Google Scholar]
- Tsafrakidou, P.; Michaelidou, A.M.; Biliaderis, C.G. Fermented cereal-based products: Nutritional aspects, possible impact on gut microbiota and health implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Takemura, S.; Hirohashi, K.; Okada, S. A fermented grain food mixture, AOBTM, inhibits liver metastasis in the metastasis model of rat colon cancer. Biofactors 2004, 22, 67–69. [Google Scholar] [CrossRef]
- Cruz, E.M.S.; Fernandes Junior, H.J.; Tallo, F.S.; Pires-Oliveira, M.; Nicolau, A.L.D.; Carvalho, R.G.; Gehrke, F.S.; Caricati-Neto, A.; Taha, M.O.; Rodrigues, F.S.M. Benefits promoted by the use of a highly bioavailable fermentation-produced nutraceutical, rich in β-glucans and amino acids, for cancer patients treated with chemotherapy and radiotherapy. Res. Soc. Dev. 2022, 11, e92111536983. [Google Scholar] [CrossRef]
- Fernandes Junior, H.J.; Tallo, F.S.; Góes, R.B.; Oliveira, C.T.F.; Nicolau, L.A.D.; Arias, A.N.; Viana, B.L.A.; Menezes-Rodrigues, F.S. Potential clinical benefits and probable mechanisms of action promoted by a nutraceutical obtained by fermentation and rich in β-glucans and amino acids for oncologic patients. J. Med. Resid. Rev. 2024, 3, e055. [Google Scholar] [CrossRef]
- Alves Junior, R.S.; Silva, C.A.; Santos, C.; Nascimento, V.M.G.; Toledo, K.A. The impacts of the immunomodulatory effects of fungal beta-glucans on human health. Braz. J. Health Rev. 2024, 7, 1–20. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible mushrooms and beta-glucans: Impact on human health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, A.; Drywień, M.E.; Hamulka, J.; Sadkowski, T. Nutraceutical functions of beta-glucans in human nutrition. Rocz. Panstw. Zakl. Hig. 2019, 70, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Sengül, M.; Ufuk, S. Therapeutic and functional properties of beta-glucan and its effects on health. Eurasian J. Food Sci. Technol. 2022, 6, 29–41. [Google Scholar]
- Zhong, X.; Wang, G.; Li, F.; Fang, S.; Zhou, S.; Ishiwata, A.; Tonevitsky, A.G.; Shkurnikov, M.; Cai, H.; Ding, F. Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics 2023, 15, 1615. [Google Scholar] [CrossRef]
- Enshasy, H.A.E.; Kaul, R.H. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Jesus, S.; Costa, J.P.; Colaço, M.; Lebre, F.; Mateus, D.; Sebastião, A.I.; Cruz, M.T.; Alfaro-Moreno, E.; Borges, O. Exploring the immunomodulatory properties of glucan particles in human primary cells. Int. J. Pharm. 2024, 655, 123996. [Google Scholar] [CrossRef]
- Pedro, A.R.V.; Lima, T.; Fróis-Martins, R.; Leal, B.; Ramos, I.C.; Martins, E.G.; Cabrita, A.R.J.; Fonseca, A.J.M.; Maia, M.R.G.; Vilanova, M.; et al. Dectin-1-mediated production of pro-inflammatory cytokines induced by yeast β-glucans in bovine monocytes. Front. Immunol. 2021, 12, 689879. [Google Scholar] [CrossRef]
- Graaff, P.; Berrevoets, C.; Rösch, C.; Schols, H.A.; Verhoef, K.; Wichers, H.J.; Debets, R.; Govers, C. Curdlan, zymosan and a yeast-derived β-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol. Immunother. 2021, 70, 547–556. [Google Scholar] [CrossRef]
- He, S.; Yan, J.; Chen, L.; Chen, H.; Wan, W. Structure and in vitro antioxidant and immunomodulatory activity of a glucan from the leaves of Cyclocarya paliurus. J. Funct. Foods 2024, 113, 106016. [Google Scholar] [CrossRef]
- Wen, Y.; Bi, S.; Hu, X.; Yang, J.; Li, C.; Li, H.; Yu, D.B.; Zhu, J.; Song, L.; Yu, R. Structural characterization and immunomodulatory mechanisms of two novel glucans from Morchella importuna fruiting bodies. Int. J. Biol. Macromol. 2021, 183, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Jiang, Y.; Yang, F.; Wu, F.; Zheng, G.; Lin, Y.; Wang, C.; Xin, W.; Zhao, F. A homologous series of α-glucans from Hemicentrotus pulcherrimus and their immunomodulatory activity. Int. J. Biol. Macromol. 2024, 260, 129657. [Google Scholar] [CrossRef] [PubMed]
- Wold, C.W.; Christopoulos, P.F.; Arias, M.A.; Dzovor, D.E.; Øynebråten, I.; Corthay, A.; Inngjerdingen, K.T. Fungal polysaccharides from Inonotus obliquus are agonists for Toll-like receptors and induce macrophage anti-cancer activity. Commun. Biol. 2024, 7, 222. [Google Scholar] [CrossRef]
- Wolfrom, M.L.; Thompson, A. A reduction with sodium borohydride. Methods Carbohydr. Chem. 1963, 2, 65–67. [Google Scholar]
- Wolfrom, M.L.; Thompson, A. Acetylation. Methods Carbohydr. Chem. 1963, 2, 211–215. [Google Scholar]
- Guo, X.; Kang, J.; Xu, Z.; Guo, Q.; Zhang, L.; Ning, H.; Cui, S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydr. Polym. 2021, 262, 117962. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Silva, G.S.; Silva, D.A.; Guilhelmelli, F.; Jerônimo, M.S.; Cardoso-Miguel, M.R.D.; Bürgel, P.H.; Castro, R.J.A.; de Oliveira, S.A.M.; Silva-Pereira, I.; Bocca, A.L.; et al. Zymosan enhances in vitro phagocyte function and the immune response of mice infected with Paracoccidioides brasiliensis. Med. Mycol. 2021, 59, 749–762. [Google Scholar] [CrossRef]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.J.; Rössner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]
- Basso, A.M.M.; De Castro, R.J.A.; De Castro, T.B.; Guimarães, H.I.; Polez, V.L.P.; Carbonero, E.R.; Pomin, V.H.; Hoffmann, C.; Grossi-de-Sa, M.F.; Tavares, A.H.; et al. Immunomodulatory activity of β-glucan-containing exopolysaccharides from Auricularia auricular in phagocytes and mice infected with Cryptococcus neoformans. Med. Mycol. 2020, 58, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Falk, H.; Stanek, M. Two-dimensional 1H and 13C NMR spectroscopy and the structural aspects of amylose and amylopectin. Monatshefte Chem. 1997, 128, 777–784. [Google Scholar] [CrossRef]
- Kono, H.; Kondo, N.; Hirabayashi, K.; Ogata, M.; Totani, K.; Ikematsu, S.; Osada, M. NMR spectroscopic structural characterization of a water-soluble β-(1→3,1→6)-glucan from Aureobasidium pullulans. Carbohydr. Polym. 2017, 174, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tan, L.; Kong, L. Multiple levels of health benefits from resistant starch. J. Agric. Food Res. 2022, 10, 100380. [Google Scholar] [CrossRef]
- Li, C.; Dhital, S.; Gidley, M.J. High amylose wheat foods: A new opportunity to improve human health. Trends Food Sci. Technol. 2023, 135, 93–101. [Google Scholar] [CrossRef]
- Li, H.-T.; Zhang, W.; Zhu, H.; Chao, C.; Guo, Q. Unlocking the potential of high-amylose starch for gut health: Not all function the same. Fermentation 2023, 9, 134. [Google Scholar] [CrossRef]
- Albrecht, L.J.; Tauber, S.C.; Merres, J.; Kress, E.; Stope, M.B.; Jansen, S.; Pufe, T.; Brandenburg, L.O. Lack of proinflammatory cytokine interleukin-6 or tumor necrosis factor receptor-1 results in a failure of the innate immune response after bacterial meningitis. Mediators Inflamm. 2016, 2016, 7678542. [Google Scholar] [CrossRef]
- Chung, S.S.; Wu, Y.; Okobi, Q.; Adekoya, D.; Atefi, M.; Clarke, O.; Dutta, P.; Vadgama, J.V. Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation, and Withaferin A inhibited the signaling in colorectal cancer cells. Mediators Inflamm. 2017, 2017, 5958429. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Williams, D.L.; Hoving, J.C.; Engstad, R.; Netea, M.G.; Brown, G.D. Dectin-1 plays a redundant role in the immunomodulatory activities of β-glucan-rich ligands in vivo. Microbes Infect. 2013, 15, 511–515. [Google Scholar] [CrossRef]
- Cheng, Q.J.; Farrell, K.; Fenn, J.; Ma, A.; Makanani, S.K.; Siemsen, J. Dectin-1 ligands produce distinct training phenotypes in human monocytes through differential activation of signaling networks. Sci. Rep. 2024, 14, 1454. [Google Scholar] [CrossRef] [PubMed]
- Xisto, M.I.D.S.; Dias, L.S.; Bezerra, F.F.; Bittencourt, V.C.B.; Rollin-Pinheiro, R.; Cartágenes-Pinto, A.C.; Haido, R.M.T.; Mourão, P.A.D.S.; Barreto-Bergter, E. An alpha-glucan from Lomentospora prolificans mediates fungal–host interaction signaling through Dectin-1 and Mincle. J. Fungi 2023, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Damiano, S.; Montanino, C.; Buono, A.; Rosa, G.; Guida, B.; Santillo, M. Effect of beta- and alpha-glucans on immune modulating factors expression in enterocyte-like Caco-2 and goblet-like LS 174T cells. Int. J. Biol. Macromol. 2020, 153, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.J.; Wouters, I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.N. (1→3) β-D-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Geng, J.; Shi, Y.; Zhang, J.; Yang, B.; Wang, P.; Yuan, W.; Zhao, H.; Li, J.; Qin, F.; Hong, L.; et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 2021, 12, 3519. [Google Scholar] [CrossRef]
- Onyishi, C.U.; Desanti, G.E.; Wilkinson, A.L.; Lara-Reyna, S.; Frickel, E.M.; Fejer, G.; Christophe, O.D.; Bryant, C.E.; Mukhopadhyay, S.; Gordon, S.; et al. Toll-like receptor 4 and macrophage scavenger receptor 1 crosstalk regulates phagocytosis of a fungal pathogen. Nat Commun. 2023, 14, 4895. [Google Scholar] [CrossRef]
- Singh, R.P.; Bhardwaj, A. β-glucans: A potential source for maintaining gut microbiota and the immune system. Front. Nutr. 2023, 10, 1143682. [Google Scholar] [CrossRef]
- Varnosfaderani, S.M.N.; Ebrahimzadeh, F.; Oryani, M.A.; Khalili, S.; Almasi, F.; Heris, R.M.; Payandeh, Z.; Li, C.; Afjadi, M.N.; Bahrami, A.A. Potential promising anticancer applications of β-glucans: A review. Biosci. Rep. 2024, 44, BSR20231686. [Google Scholar] [CrossRef]







| Fractions | Units | 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|---|---|
| 6a | 6b | ||||||||
| AR1-I | →4)-α-Glcp-(1→ | 13C | 100.02 | 72.05 | 73.22 | 78.87 | 71.63 | 60.59 | |
| 1H | 5.12 | 3.33 | 3.69 | 3.37 | 3.63 | 3.68 | 3.60 | ||
| AR1-dS | →3,6)-β-Glcp-(1→ (A) | 13C | 102.90 | 72.79 | 86.16 | 68.47 | 74.85 | 68.49 | |
| 1H | 4.54 | 3.34 | 3.50 | 3.28 | 3.50 | 4.04 | 3.47 | ||
| →3)-β-Glcp-(1→ (B) | 13C | 102.82 | 72.47 | 86.97/86.75/86.60 | 68.68 | 76.25 | 60.84 | ||
| 1H | 4.52 | 3.32 | 3.51 | 3.26 | 3.28 | 3.69 | 3.47 | ||
| β-Glcp-(1→ (C) | 13C | 103.40 | 73.70 | 76.43 | 70.27 | 76.56 | 61.16 | ||
| 1H | 4.24 | 3.02 | 3.20 | 3.10 | 3.15 | 3.68 | 3.49 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonero, E.R.; Novikov, T.S.M.; Gomes, Y.G.S.; Brito, D.R.; Coelho, L.C.; Mendes, M.F.; Di Medeiros Leal, M.C.B.; Bocca, A.L.; Lião, L.M. Chemical Characterization and In Vitro Evaluation of Glucans from Fermentation-Produced Nutraceutical Bionutri-AR1®: Antioxidant and Immunomodulatory Properties. Pharmaceutics 2024, 16, 1404. https://doi.org/10.3390/pharmaceutics16111404
Carbonero ER, Novikov TSM, Gomes YGS, Brito DR, Coelho LC, Mendes MF, Di Medeiros Leal MCB, Bocca AL, Lião LM. Chemical Characterization and In Vitro Evaluation of Glucans from Fermentation-Produced Nutraceutical Bionutri-AR1®: Antioxidant and Immunomodulatory Properties. Pharmaceutics. 2024; 16(11):1404. https://doi.org/10.3390/pharmaceutics16111404
Chicago/Turabian StyleCarbonero, Elaine R., Tammara S. M. Novikov, Yagly G. S. Gomes, Dayane R. Brito, Luisa C. Coelho, Marcia F. Mendes, Maria Carolina B. Di Medeiros Leal, Anamélia L. Bocca, and Luciano M. Lião. 2024. "Chemical Characterization and In Vitro Evaluation of Glucans from Fermentation-Produced Nutraceutical Bionutri-AR1®: Antioxidant and Immunomodulatory Properties" Pharmaceutics 16, no. 11: 1404. https://doi.org/10.3390/pharmaceutics16111404
APA StyleCarbonero, E. R., Novikov, T. S. M., Gomes, Y. G. S., Brito, D. R., Coelho, L. C., Mendes, M. F., Di Medeiros Leal, M. C. B., Bocca, A. L., & Lião, L. M. (2024). Chemical Characterization and In Vitro Evaluation of Glucans from Fermentation-Produced Nutraceutical Bionutri-AR1®: Antioxidant and Immunomodulatory Properties. Pharmaceutics, 16(11), 1404. https://doi.org/10.3390/pharmaceutics16111404








