Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents
Abstract
1. Introduction
2. Background and Principle
2.1. Principle of Photoacoustic Imaging
2.2. Contrast of Photoacoustic Imaging
2.3. Multispectral Photoacoustic Imaging
2.4. Photoacoustic Imaging Systems
3. Label-Free Photoacoustic Monitoring of Responses to Drug Delivery
4. Photoacoustic Monitoring of Pharmacokinetics and Biodistribution of Exogenous Agents
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, G.; Sanmugam, A.; Palem, V.V.; Sevanan, M.; Sairam, A.B.; Nachiappan, N.; Youn, B.; Lee, J.S.; Nallal, M.; Park, K.H. Nanomaterials for detection of biomolecules and delivering therapeutic agents in theragnosis: A review. Int. J. Biol. Macromol. 2024, 254, 127904. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Control. Release 2014, 190, 477–484. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, H.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-Targeting Multi-Functional Nanoparticles for Theragnosis: New Paradigm for Cancer Therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.-S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.-W.; Kim, I.-S. Tumor-Homing Multifunctional Nanoparticles for Cancer Theragnosis: Simultaneous Diagnosis, Drug Delivery, and Therapeutic Monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Oh, D.; Lee, D.; Heo, J.; Kweon, J.; Yong, U.; Jang, J.; Ahn, Y.J.; Kim, C. Contrast agent-free 3D Renal ultrafast doppler imaging reveals vascular dysfunction in acute and diabetic kidney diseases. Adv. Sci. 2023, 10, 2303966. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C. CuGeO3 Nanoparticles: An Efficient Photothermal Theragnosis Agent for CT Imaging-Guided Photothermal Therapy of Cancers. Front. Bioeng. Biotechnol. 2020, 8, 590518. [Google Scholar] [CrossRef] [PubMed]
- Curry, T.; Kopelman, R.; Shilo, M.; Popovtzer, R. Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol. Imaging 2014, 9, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Dreifuss, T.; Barnoy, E.; Motiei, M.; Popovtzer, R. Theranostic gold nanoparticles for CT imaging. In Design and Applications of Nanoparticles in Biomedical Imaging; Springer: Cham, Switzerland, 2017; pp. 403–427. [Google Scholar]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579. [Google Scholar] [CrossRef]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Eo, J.S.; Paeng, J.C.; Lee, D.S. Nuclear Imaging for Functional Evaluation and Theragnosis in Liver Malignancy and Transplantation. World J. Gastroenterol. 2014, 20, 5375. [Google Scholar] [CrossRef]
- Polyak, A.; Ross, T.L. Nanoparticles for SPECT and PET imaging: Towards personalized medicine and theranostics. Curr. Med. Chem. 2018, 25, 4328–4353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef]
- Pekkanen, A.M.; DeWitt, M.R.; Rylander, M.N. Nanoparticle enhanced optical imaging and phototherapy of cancer. J. Biomed. Nanotechnol. 2014, 10, 1677–1712. [Google Scholar] [CrossRef]
- Murar, M.; Albertazzi, L.; Pujals, S. Advanced optical imaging-guided nanotheranostics towards personalized cancer drug delivery. Nanomaterials 2022, 12, 399. [Google Scholar] [CrossRef]
- Juvekar, V.; Lee, D.J.; Park, T.G.; Samanta, R.; Kasar, P.; Kim, C.; Rotermund, F.; Kim, H.M. Two-photon excitation photosensitizers for photodynamic therapy: From small-molecules to nano-complex systems. Coord. Chem. Rev. 2024, 506, 215711. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Li, W.; Li, C. Real-time dual-modal photoacoustic and fluorescence small animal imaging. Photoacoustics 2024, 36, 100593. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Kim, H.-H.; Kim, C.-S.; Kim, J. Review on Optical Imaging Techniques for Multispectral Analysis of Nanomaterials. Nanotheranostics 2022, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Bell, A.G. The Photophone. Science 1880, 1, 130–134. [Google Scholar] [CrossRef]
- Park, B.; Lee, K.M.; Park, S.; Yun, M.; Choi, H.-J.; Kim, J.; Lee, C.; Kim, H.; Kim, C. Deep tissue photoacoustic imaging of nickel (II) dithiolene-containing polymeric nanoparticles in the second near-infrared window. Theranostics 2020, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Han, M.; Park, J.; Kim, T.; Ryu, H.; Seo, Y.; Kim, W.J.; Kim, H.H.; Kim, C. A photoacoustic finder fully integrated with a solid-state dye laser and transparent ultrasound transducer. Photoacoustics 2021, 23, 100290. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Ahn, J.; Kim, Y.; Lim, J.; Park, J.; Kim, H.H.; Kim, W.J.; Kim, C. An ultrasensitive and broadband transparent ultrasound transducer for ultrasound and photoacoustic imaging in-vivo. Nat. Commun. 2024, 15, 1444. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Kim, C.; Favazza, C.; Wang, L.V. In vivo photoacoustic tomography of chemicals: High-resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, S.M.; Park, J.; Cho, S.-W.; Han, S.; Ahn, J.; Cho, S.; Kim, C.; Kim, C.-S.; Kim, J. Transportable Multispectral Optical-Resolution Photoacoustic Microscopy using Stimulated Raman Scattering Spectrum. IEEE Trans. Instrum. Meas. 2024, 73, 4502309. [Google Scholar] [CrossRef]
- Kye, H.; Song, Y.; Ninjbadgar, T.; Kim, C.; Kim, J. Whole-Body Photoacoustic Imaging Techniques for Preclinical Small Animal Studies. Sensors 2022, 22, 5130. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional photoacoustic imaging: From nano-and micro-to macro-scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Cho, S.-W.; Park, S.M.; Park, B.; Lee, T.G.; Kim, B.-M.; Kim, C.; Kim, J.; Lee, S.-W.; Kim, C.-S. High-speed photoacoustic microscopy: A review dedicated on light sources. Photoacoustics 2021, 24, 100291. [Google Scholar] [CrossRef]
- Xia, Q.; Lv, S.; Xu, H.; Wang, X.; Xie, Z.; Lin, R.; Zhang, J.; Shu, C.; Chen, Z.; Gong, X. Non-invasive evaluation of endometrial microvessels via in vivo intrauterine photoacoustic endoscopy. Photoacoustics 2024, 36, 100589. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, Y.; Chen, N.; Zeng, S.; Liu, L.; Gao, R.; Zhang, J.; Fang, C.; Song, L.; Liu, C. Visualizing tumor angiogenesis and boundary with polygon-scanning multiscale photoacoustic microscopy. Photoacoustics 2022, 26, 100342. [Google Scholar] [CrossRef]
- Gao, R.; Chen, T.; Ren, Y.; Liu, L.; Chen, N.; Wong, K.K.; Song, L.; Ma, X.; Liu, C. Restoring the imaging quality of circular transducer array-based PACT using synthetic aperture focusing technique integrated with 2nd-derivative-based back projection scheme. Photoacoustics 2023, 32, 100537. [Google Scholar] [CrossRef]
- Chen, N.; Yu, J.; Liu, L.; Xu, Z.; Gao, R.; Chen, T.; Song, L.; Zheng, W.; Liu, C. Video-rate high-resolution single-pixel nonscanning photoacoustic microscopy. Biomed. Opt. Express 2022, 13, 3823–3835. [Google Scholar] [CrossRef]
- Gao, R.; Liu, F.; Liu, W.; Zeng, S.; Chen, J.; Gao, R.; Wang, L.; Fang, C.; Song, L.; Sedgwick, A.C. Background-suppressed tumor-targeted photoacoustic imaging using bacterial carriers. Proc. Natl. Acad. Sci. USA 2022, 119, e2121982119. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Xu, Z.; Ren, Y.; Song, L.; Liu, C. Nonlinear mechanisms in photoacoustics—Powerful tools in photoacoustic imaging. Photoacoustics 2021, 22, 100243. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Xu, Z.; Song, L.; Liu, C. Breaking acoustic limit of optical focusing using photoacoustic-guided wavefront shaping. Laser Photonics Rev. 2021, 15, 2000594. [Google Scholar] [CrossRef]
- Olefir, I.; Tzoumas, S.; Restivo, C.; Mohajerani, P.; Xing, L.; Ntziachristos, V. Deep Learning-Based Spectral Unmixing for Optoacoustic Imaging of Tissue Oxygen Saturation. IEEE Trans. Med. Imaging 2020, 39, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic Tomography of Blood Oxygenation: A Mini Review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Kennedy, M.J.; Dixon, A.J.; Sun, N.; Cao, R.; Soetikno, B.T.; Chen, R.; Zhou, Q.; Shung, K.K.; Hossack, J.A. Simultaneous Photoacoustic Microscopy of Microvascular Anatomy, Oxygen Saturation, and Blood Flow. Opt. Lett. 2015, 40, 910–913. [Google Scholar] [CrossRef]
- Barulin, A.; Park, H.; Park, B.; Kim, I. Dual-wavelength UV-visible metalens for multispectral photoacoustic microscopy: A simulation study. Photoacoustics 2023, 32, 100545. [Google Scholar] [CrossRef]
- John, S.; Hester, S.; Basij, M.; Paul, A.; Xavierselvan, M.; Mehrmohammadi, M.; Mallidi, S. Niche preclinical and clinical applications of photoacoustic imaging with endogenous contrast. Photoacoustics 2023, 32, 100533. [Google Scholar] [CrossRef]
- Li, X.; Yew, Y.W.; Ram, K.V.; Oon, H.H.; Thng, S.T.G.; Dinish, U.; Olivo, M. Structural and functional imaging of psoriasis for severity assessment and quantitative monitoring of treatment response using high-resolution optoacoustic imaging. Photoacoustics 2024, 38, 100611. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Park, S.; Cho, S.; Yoo, J.; Kim, C.; Kim, J. Ultrasound-Guided Breath-Compensation in Single-Element Photoacoustic Imaging for Three-Dimensional Whole-Body Images of Mice. Front. Phys. 2022, 10, 457. [Google Scholar] [CrossRef]
- Menger, M.M.; Körbel, C.; Bauer, D.; Bleimehl, M.; Tobias, A.L.; Braun, B.J.; Herath, S.C.; Rollmann, M.F.; Laschke, M.W.; Menger, M.D. Photoacoustic imaging for the study of oxygen saturation and total hemoglobin in bone healing and non-union formation. Photoacoustics 2022, 28, 100409. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; He, L.; Liang, Y.; Wang, L. Wide-field polygon-scanning photoacoustic microscopy of oxygen saturation at 1-MHz A-line rate. Photoacoustics 2020, 20, 100195. [Google Scholar] [CrossRef] [PubMed]
- Nemirova, S.; Orlova, A.; Kurnikov, A.; Litvinova, Y.; Kazakov, V.; Ayvazyan, I.; Liu, Y.-H.; Razansky, D.; Subochev, P. Scanning optoacoustic angiography for assessing structural and functional alterations in superficial vasculature of patients with post-thrombotic syndrome: A pilot study. Photoacoustics 2024, 38, 100616. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, B.; Ha, J.; Steinberg, I.; Hooper, S.M.; Jeong, C.; Park, E.-Y.; Choi, W.; Liang, T.; Bae, J.-S.; et al. Multiparametric Photoacoustic Analysis of Human Thyroid Cancers In Vivo. Cancer Res. 2021, 81, 4849–4860. [Google Scholar] [CrossRef]
- Schoustra, S.M.; Piras, D.; Huijink, R.; Op’t Root, T.J.; Alink, L.; Kobold, W.M.F.; Steenbergen, W.; Manohar, S. Twente Photoacoustic Mammoscope 2: System Overview and Three-Dimensional Vascular Network Images in Healthy Breasts. J. Biomed. Opt. 2019, 24, 121909. [Google Scholar] [CrossRef]
- Neuschler, E.I.; Butler, R.; Young, C.A.; Barke, L.D.; Bertrand, M.L.; Böhm-Vélez, M.; Destounis, S.; Donlan, P.; Grobmyer, S.R.; Katzen, J. A Pivotal Study of Optoacoustic Imaging to Diagnose Benign and Malignant Breast Masses: A New Evaluation Tool for Radiologists. Radiology 2017, 287, 398–412. [Google Scholar] [CrossRef]
- Diot, G.; Metz, S.; Noske, A.; Liapis, E.; Schroeder, B.; Ovsepian, S.V.; Meier, R.; Rummeny, E.; Ntziachristos, V. Multispectral Optoacoustic Tomography (MSOT) of Human Breast Cancer. Clin. Cancer Res. 2017, 23, 6912–6922. [Google Scholar] [CrossRef]
- Park, E.-Y.; Lee, H.; Han, S.; Kim, C.; Kim, J. Photoacoustic Imaging Systems Based on Clinical Ultrasound Platform. Exp. Biol. Med. 2022, 247, 551–560. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic Clinical Imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, D.; Mo, S.; Hong, X.; Xie, J.; Chen, Y.; Liu, L.; Song, D.; Tang, S.; Wu, H. Multimodal PA/US imaging in Rheumatoid Arthritis: Enhanced correlation with clinical scores. Photoacoustics 2024, 38, 100615. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, C.; Kim, J.Y.; Kim, C. Organic Nanostructures for Photoacoustic Imaging. ChemNanoMat 2015, 2, 156–166. [Google Scholar] [CrossRef]
- Choi, W.; Park, B.; Choi, S.; Oh, D.; Kim, J.; Kim, C. Recent advances in contrast-enhanced photoacoustic imaging: Overcoming the physical and practical challenges. Chem. Rev. 2023, 123, 7379–7419. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Lee, D.; Kim, S.; Kim, H.-H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Jiang, Z.; Ding, Y.; Lovell, J.F.; Zhang, Y. Design and Application of Organic Contrast Agents for Molecular Imaging in the Second Near Infrared (NIR-II) Window. Photoacoustics 2022, 28, 100426. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic imaging: Contrast agents and their biomedical applications. Adv. Mater. 2019, 31, 1805875. [Google Scholar] [CrossRef]
- Xu, W.; Leskinen, J.; Sahlström, T.; Happonen, E.; Tarvainen, T.; Lehto, V.-P. Assembly of fluorophore J-aggregates with nanospacer onto mesoporous nanoparticles for enhanced photoacoustic imaging. Photoacoustics 2023, 33, 100552. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; Qin, D.; Anthony, M.Y.; Chung, E.; Jhunjhunwala, A.; Rose, J.A.; Emelianov, S.Y. pH-responsive ratiometric photoacoustic imaging of polyaniline nanoparticle-coated needle for targeted cancer biopsy. Photoacoustics 2023, 31, 100500. [Google Scholar] [CrossRef]
- Park, B.; Park, S.; Kim, J.; Kim, C. Listening to Drug Delivery and Responses via Photoacoustic Imaging. Adv. Drug Deliv. Rev. 2022, 184, 114235. [Google Scholar] [CrossRef]
- Guo, T.; Tang, Q.; Guo, Y.; Qiu, H.; Dai, J.; Xing, C.; Zhuang, S.; Huang, G. Boron Quantum Dots for Photoacoustic Imaging-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 13, 306–311. [Google Scholar] [CrossRef]
- Miao, Z.-H.; Wang, H.; Yang, H.; Li, Z.; Zhen, L.; Xu, C.-Y. Glucose-Derived Carbonaceous Nanospheres for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 15904–15910. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, N.; Kim, K.H.; Park, S.; Cho, Y.; Park, E.-Y.; Lim, J.; Çetindere, S.; Tümay, S.O.; Kim, W.J.; Li, X. Hexa-BODIPY-cyclotriphosphazene based nanoparticle for NIR fluorescence/photoacoustic dual-modal imaging and photothermal cancer therapy. Biosens. Bioelectron. 2022, 216, 114612. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, C.; Cheng, Y.; Cheng, Q. Photostability enhancement of silica-coated gold nanostars for photoacoustic imaging guided photothermal therapy. Photoacoustics 2021, 23, 100284. [Google Scholar] [CrossRef]
- Han, S.; Ninjbadgar, T.; Kang, M.; Kim, C.; Kim, J. Recent Advances in Photoacoustic Agents for Theranostic Applications. Nanomaterials 2023, 13, 695. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.-W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef]
- Ding, Y.; Park, B.; Ye, J.; Wang, X.; Liu, G.; Yang, X.; Jiang, Z.; Han, M.; Fan, Y.; Song, J. Surfactant-stripped semiconducting polymer micelles for tumor theranostics and deep tissue imaging in the NIR-II window. Small 2022, 18, 2104132. [Google Scholar] [CrossRef]
- Lee, H.; Park, B.; Lee, J.; Kang, Y.; Han, M.; Lee, J.; Kim, C.; Kim, W.J. Transcytosis-Inducing Multifunctional Albumin Nanomedicines with Deep Penetration Ability for Image-Guided Solid Tumor Treatment. Small 2023, 19, 2303668. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yang, J.; Lee, S.Y.; Kim, J.; Lee, J.; Kim, W.J.; Lee, S.; Kim, C. Deep Learning Enhances Multiparametric Dynamic Volumetric Photoacoustic Computed Tomography In Vivo (DL-PACT). Adv. Sci. 2023, 10, 2202089. [Google Scholar] [CrossRef]
- Kim, D.; Ahn, J.; Park, E.; Kim, J.Y.; Kim, C. In vivo quantitative photoacoustic monitoring of corticosteroid-induced vasoconstriction. J. Biomed. Opt. 2023, 28, 082805. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Q.; DiSpirito, A.; Vu, T.; Rong, Q.; Peng, X.; Sheng, H.; Shen, X.; Zhou, Q.; Jiang, L. Real-time whole-brain imaging of hemodynamics and oxygenation at micro-vessel resolution with ultrafast wide-field photoacoustic microscopy. Light Sci. Appl. 2022, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 vascular targeted photodynamic therapy effect monitoring by multispectral optoacoustic tomography (MSOT) in mice. Theranostics 2018, 8, 723. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, Q.; Jiang, L.; Nguyen, V.-T.; Vu, T.; Devlin, G.; Shaima, J.; Wang, X.; Chen, Y.; Ma, L. Longitudinal intravital imaging of mouse placenta. Sci. Adv. 2024, 10, eadk1278. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.; Chang, C.-C.; Chen, Y.-C.; Zhang, Q.; Zhang, X.-D.; Andreou, C.; Pang, J.; Liu, Z.-X.; Wang, D.-Y. Near-infrared phototheranostic iron pyrite nanocrystals simultaneously induce dual cell death pathways via enhanced Fenton reactions in triple-negative breast cancer. ACS Nano 2023, 17, 4261–4278. [Google Scholar] [CrossRef]
- Song, J.; Kang, X.; Wang, L.; Ding, D.; Kong, D.; Li, W.; Qi, J. Near-infrared-II photoacoustic imaging and photo-triggered synergistic treatment of thrombosis via fibrin-specific homopolymer nanoparticles. Nat. Commun. 2023, 14, 6881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ke, Z.; Yang, F.; Li, K.; Chen, N.; Song, L.; Zheng, C.; Liang, D.; Liu, C. Deep learning enables superior photoacoustic imaging at ultralow laser dosages. Adv. Sci. 2021, 8, 2003097. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, L.; Ma, X.; Zhang, Y.; Liu, H.; Zheng, R.; Ren, J.; Zhou, H.; Ren, Y.; Gao, R. Dedicated photoacoustic imaging instrument for human periphery blood vessels: A new paradigm for understanding the vascular health. IEEE Trans. Biomed. Eng. 2021, 69, 1093–1100. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, N.; Li, T.; Zhang, J.; Lin, R.; Gong, X.; Song, L.; Liu, Z.; Liu, C. Motion correction in optical resolution photoacoustic microscopy. IEEE Trans. Med. Imaging 2019, 38, 2139–2150. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, G.; Lin, R.; Gong, X.; Song, L.; Li, T.; Wang, W.; Zhang, K.; Qian, X.; Zhang, H. Three-dimensional Hessian matrix-based quantitative vascular imaging of rat iris with optical-resolution photoacoustic microscopy in vivo. J. Biomed. Opt. 2018, 23, 046006. [Google Scholar] [CrossRef]
- Kim, D.; Park, E.; Park, J.; Perleberg, B.; Jeon, S.; Ahn, J.; Ha, M.; Kim, H.H.; Kim, J.Y.; Jung, C.K. An ultraviolet-transparent ultrasound transducer enables high-resolution label-free photoacoustic histopathology. Laser Photonics Rev. 2024, 18, 2300652. [Google Scholar] [CrossRef]
- Baik, J.W.; Kim, H.; Son, M.; Choi, J.; Kim, K.G.; Baek, J.H.; Park, Y.H.; An, J.; Choi, H.Y.; Ryu, S.Y. Intraoperative label-free photoacoustic histopathology of clinical specimens. Laser Photonics Rev. 2021, 15, 2100124. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Kim, T.Y.; Jung, S.; Choi, W.J.; Ahn, J.; Yoon, D.H.; Kim, J.; Jeon, S.; Lee, D. Quadruple ultrasound, photoacoustic, optical coherence, and fluorescence fusion imaging with a transparent ultrasound transducer. Proc. Natl. Acad. Sci. USA 2021, 118, e1920879118. [Google Scholar] [CrossRef]
- Park, B.; Bang, C.; Lee, C.; Han, J.; Choi, W.; Kim, J.; Park, G.; Rhie, J.; Lee, J.; Kim, C. 3D wide-field multispectral photoacoustic imaging of human melanomas in vivo: A pilot study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Tzoumas, S.; Ntziachristos, V. Spectral unmixing techniques for optoacoustic imaging of tissue pathophysiology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170262. [Google Scholar] [CrossRef]
- Yoon, C.; Park, E.; Misra, S.; Kim, J.Y.; Baik, J.W.; Kim, K.G.; Jung, C.K.; Kim, C. Deep learning-based virtual staining, segmentation, and classification in label-free photoacoustic histology of human specimens. Light Sci. Appl. 2024, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, J.; Lee, D.; Baik, J.W.; Kim, C. Review on practical photoacoustic microscopy. Photoacoustics 2019, 15, 100141. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Jeon, S.; Baik, J.W.; Cho, S.H.; Kim, C. Super-resolution localization photoacoustic microscopy using intrinsic red blood cells as contrast absorbers. Light Sci. Appl. 2019, 8, 156–166. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, J.; Li, L.; Du, L.; Zhang, Y.; Luo, Y.; Jiang, L.; Davis, S.; Zhou, Q.; de la Zerda, A.; et al. Optical-resolution photoacoustic microscopy with a needle-shaped beam. Nat. Photonics 2023, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, H.; Zheng, Z.; Sharma, A.; Wang, L.; Pramanik, M.; Zheng, Y. Deep and Domain Transfer Learning Aided Photoacoustic Microscopy: Acoustic Resolution to Optical Resolution. IEEE Trans. Med. Imaging 2022, 41, 3636–3648. [Google Scholar] [CrossRef]
- Moothanchery, M.; Dev, K.; Balasundaram, G.; Bi, R.; Olivo, M. Acoustic resolution photoacoustic microscopy based on microelectromechanical systems scanner. J. Biophotonics 2020, 13, e201960127. [Google Scholar] [CrossRef]
- Choi, W.; Oh, D.; Kim, C. Practical photoacoustic tomography: Realistic limitations and technical solutions. J. Appl. Phys. 2020, 127, 230903. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Tong, X.; Na, S.; Cao, R.; Yuan, X.; Garrett, D.C.; Shi, J.; Maslov, K.; Wang, L.V. High-speed three-dimensional photoacoustic computed tomography for preclinical research and clinical translation. Nat. Commun. 2021, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.C.; Chen, J.; Song, K.H.; Au, L.; Favazza, C.; Zhang, Q.; Cobley, C.M.; Gao, F.; Xia, Y. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano 2010, 4, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Baik, J.W.; Kim, D.; Choi, K.; Lee, S.; Park, S.-M.; Kim, J.Y.; Nam, S.H.; Kim, C. In vivo photoacoustic monitoring of vasoconstriction induced by acute hyperglycemia. Photoacoustics 2023, 30, 100485. [Google Scholar] [CrossRef]
- Huda, K.; Lawrence, D.J.; Thompson, W.; Lindsey, S.H.; Bayer, C.L. In vivo noninvasive systemic myography of acute systemic vasoactivity in female pregnant mice. Nat. Commun. 2023, 14, 6286. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.A.; Sanaie, S.; Kuchaki Rafsanjani, M.; Hosseini, M.-S. Role of imaging in early diagnosis of acute ischemic stroke: A literature review. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 175. [Google Scholar] [CrossRef]
- Chiu, F.-Y.; Yen, Y. Imaging biomarkers for clinical applications in neuro-oncology: Current status and future perspectives. Biomark. Res. 2023, 11, 35. [Google Scholar] [CrossRef]
- Zhang, D.; Li, R.; Chen, M.; Vu, T.; Sheng, H.; Yang, W.; Hoffmann, U.; Luo, J.; Yao, J. Photoacoustic imaging of in vivo hemodynamic responses to sodium nitroprusside. J. Biophotonics 2021, 14, e202000478. [Google Scholar] [CrossRef]
- Shan, T.; Zhao, Y.; Jiang, S.; Jiang, H. In-vivo hemodynamic imaging of acute prenatal ethanol exposure in fetal brain by photoacoustic tomography. J. Biophotonics 2020, 13, e201960161. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, W.; Zhu, X.; Li, R.; Liu, W.; Chen, M.; Vu, T.; Jiang, L.; Zhou, Q.; Evans, C.L. Epinephrine-induced effects on cerebral microcirculation and oxygenation dynamics using multimodal monitoring and functional photoacoustic microscopy. Anesthesiology 2023, 139, 173–185. [Google Scholar] [CrossRef]
- Johnson, S.P.; Ogunlade, O.; Lythgoe, M.F.; Beard, P.; Pedley, R.B. Longitudinal photoacoustic imaging of the pharmacodynamic effect of vascular targeted therapy on tumors. Clin. Cancer Res. 2019, 25, 7436–7447. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, B.; Lim, H.G. Advances in photoacoustic imaging aided by nano contrast agents: Special focus on role of lymphatic system imaging for cancer theranostics. J. Nanobiotechnol. 2023, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, G.; Li, Q.; Liao, C.; Huang, L.; Ke, T.; Jiang, H.; Han, D. Photoacoustic imaging for the evaluation of early tumor response to antivascular treatment. Quant. Imaging Med. Surg. 2019, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, M.N.; Appak Baskoy, S.; Wang, Y.; Hysi, E.; Kolios, M.C. Use of photoacoustic imaging for monitoring vascular disrupting cancer treatments. J. Biophotonics 2023, 16, e202000209. [Google Scholar] [CrossRef]
- Sun, N.; Zheng, S.; Rosin, D.L.; Poudel, N.; Yao, J.; Perry, H.M.; Cao, R.; Okusa, M.D.; Hu, S. Development of a photoacoustic microscopy technique to assess peritubular capillary function and oxygen metabolism in the mouse kidney. Kidney Int. 2021, 100, 613–620. [Google Scholar] [CrossRef]
- Bunke, J.; Merdasa, A.; Sheikh, R.; Albinsson, J.; Erlöv, T.; Gesslein, B.; Cinthio, M.; Reistad, N.; Malmsjö, M. Photoacoustic imaging for the monitoring of local changes in oxygen saturation following an adrenaline injection in human forearm skin. Biomed. Opt. Express 2021, 12, 4084–4096. [Google Scholar] [CrossRef]
- Petri, M.; Stoffels, I.; Jose, J.; Leyh, J.; Schulz, A.; Dissemond, J.; Schadendorf, D.; Klode, J. Photoacoustic imaging of real-time oxygen changes in chronic leg ulcers after topical application of a haemoglobin spray: A pilot study. J. Wound Care 2016, 25, 87–91. [Google Scholar] [CrossRef]
- Capozza, M.; Blasi, F.; Valbusa, G.; Oliva, P.; Cabella, C.; Buonsanti, F.; Cordaro, A.; Pizzuto, L.; Maiocchi, A.; Poggi, L. Photoacoustic imaging of integrin-overexpressing tumors using a novel ICG-based contrast agent in mice. Photoacoustics 2018, 11, 36–45. [Google Scholar] [CrossRef]
- Wood, C.A.; Han, S.; Kim, C.S.; Wen, Y.; Sampaio, D.R.; Harris, J.T.; Homan, K.A.; Swain, J.L.; Emelianov, S.Y.; Sood, A.K. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat. Commun. 2021, 12, 5410. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Choi, S.; Lee, H.; Yang, J.; Jeon, H.; Sung, M.; Kim, W.J.; Kim, C. 3D Multiparametric Photoacoustic Computed Tomography of Primary and Metastatic Tumors in Living Mice. ACS Nano 2024, 18, 18176–18190. [Google Scholar] [CrossRef]
- Singh, S.; Giammanco, G.; Hu, C.-H.; Bush, J.; Cordova, L.S.; Lawrence, D.J.; Moran, J.L.; Chitnis, P.V.; Veneziano, R. Size-tunable ICG-based contrast agent platform for targeted near-infrared photoacoustic imaging. Photoacoustics 2023, 29, 100437. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Hu, Z.; Yu, P.; He, Z.; Chen, Y.; Chen, J.; Sun, H.; Wang, S.; Zhang, F. Ultra-photostable small-molecule dyes facilitate near-infrared biophotonics. Nat. Commun. 2024, 15, 2593. [Google Scholar] [CrossRef] [PubMed]
- Rathnamalala, C.S.; Hernandez, S.; Lucero, M.Y.; Swartchick, C.B.; Kalam Shaik, A.; Hammer, N.I.; East, A.K.; Gwaltney, S.R.; Chan, J.; Scott, C.N. Xanthene-Based Nitric Oxide-Responsive Nanosensor for Photoacoustic Imaging in the SWIR Window. Angew. Chem. Int. Ed. 2023, 62, e202214855. [Google Scholar] [CrossRef] [PubMed]
- Wi, J.-S.; Kim, J.; Kim, M.Y.; Choi, S.; Jung, H.J.; Kim, C.; Na, H.-K. Theoretical and experimental comparison of the performance of gold, titanium, and platinum nanodiscs as contrast agents for photoacoustic imaging. RSC Adv. 2023, 13, 9441–9447. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Sun, I.-C.; Dumani, D.S.; Emelianov, S.Y. Applications of the Photocatalytic and Photoacoustic Properties of Gold Nanorods in Contrast-Enhanced Ultrasound and Photoacoustic Imaging. ACS Nano 2024, 18, 3575–3582. [Google Scholar] [CrossRef]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef]
- St Lorenz, A.; Moses, A.S.; Mamnoon, B.; Demessie, A.A.; Park, Y.; Singh, P.; Taratula, O.; Taratula, O.R. A Photoacoustic Contrast Nanoagent with a Distinct Spectral Signature for Ovarian Cancer Management. Adv. Healthc. Mater. 2023, 12, 2202946. [Google Scholar] [CrossRef]
- Chen, Z.; Gezginer, I.; Augath, M.-A.; Ren, W.; Liu, Y.-H.; Ni, R.; Deán-Ben, X.L.; Razansky, D. Hybrid magnetic resonance and optoacoustic tomography (MROT) for preclinical neuroimaging. Light Sci. Appl. 2022, 11, 332. [Google Scholar] [CrossRef]
- Choi, W.; Park, E.-Y.; Jeon, S.; Yang, Y.; Park, B.; Ahn, J.; Cho, S.; Lee, C.; Seo, D.-K.; Cho, J.-H. Three-dimensional multistructural quantitative photoacoustic and US imaging of human feet in vivo. Radiology 2022, 303, 467–473. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, H.; Miao, Y.; Weng, J.; Huang, Z.; Fu, J.; Zhang, Y.; Lin, J.; Ye, D. Tailoring a near-infrared macrocyclization scaffold allows the control of in situ self-assembly for photoacoustic/PET bimodal imaging. Angew. Chem. Int. Ed. 2022, 61, e202200369. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Choi, S.; Kim, J.; Park, B.; Kim, C. Recent advances in deep-learning-enhanced photoacoustic imaging. Adv. Photonics Nexus 2023, 2, 054001. [Google Scholar] [CrossRef]
- Lan, H.; Jiang, D.; Yang, C.; Gao, F.; Gao, F. Y-Net: Hybrid deep learning image reconstruction for photoacoustic tomography in vivo. Photoacoustics 2020, 20, 100197. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, G.; Li, L.; Zhang, P.; Kim, J.Y.; Kim, Y.; Kim, H.H.; Wang, L.V.; Lee, S.; Kim, C. Deep learning acceleration of multiscale superresolution localization photoacoustic imaging. Light Sci. Appl. 2022, 11, 131. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, W.; Park, B.; Kim, C. A deep learning-based model that reduces speed of sound aberrations for improved in vivo photoacoustic imaging. IEEE Trans. Image Process. 2021, 30, 8773–8784. [Google Scholar] [CrossRef]

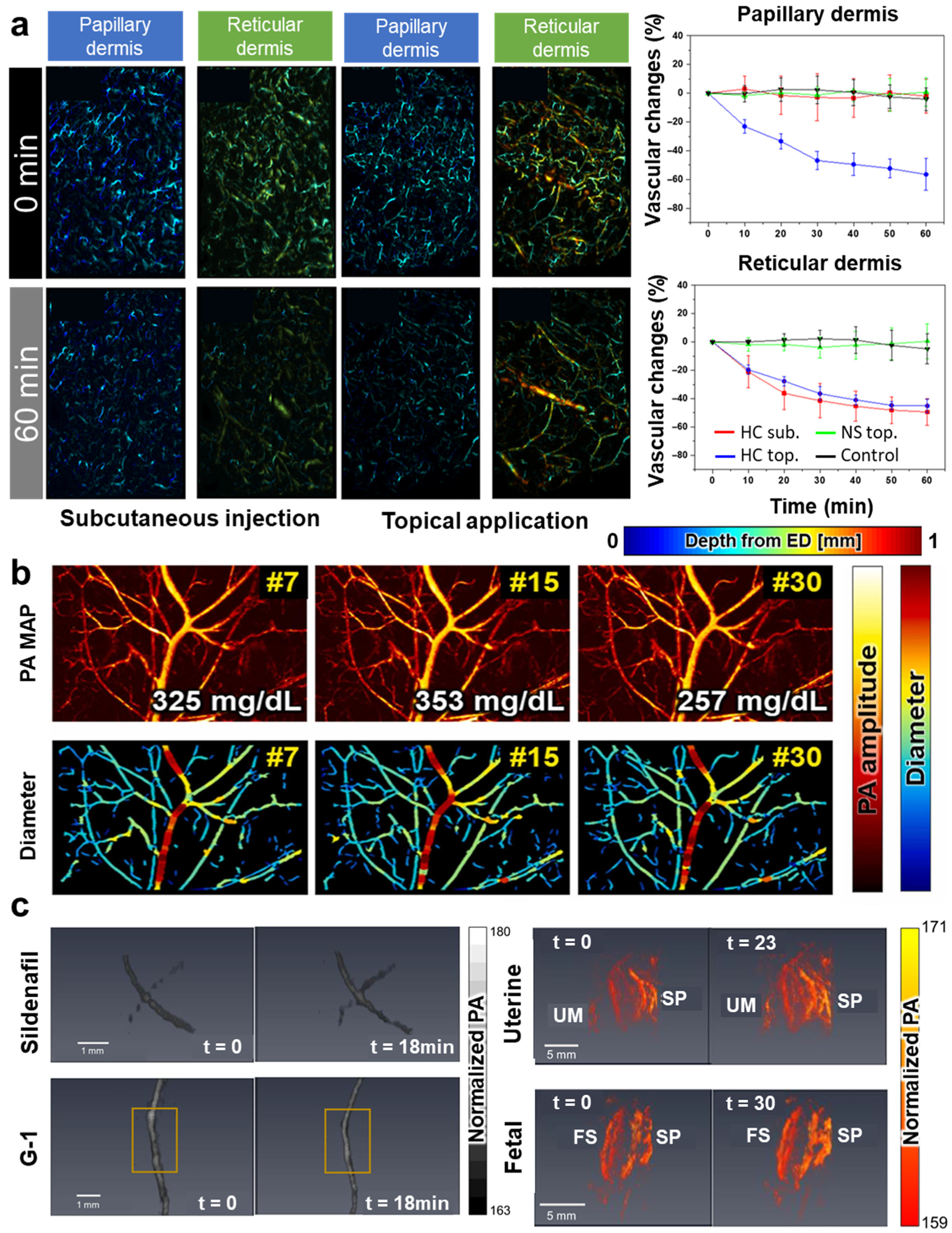
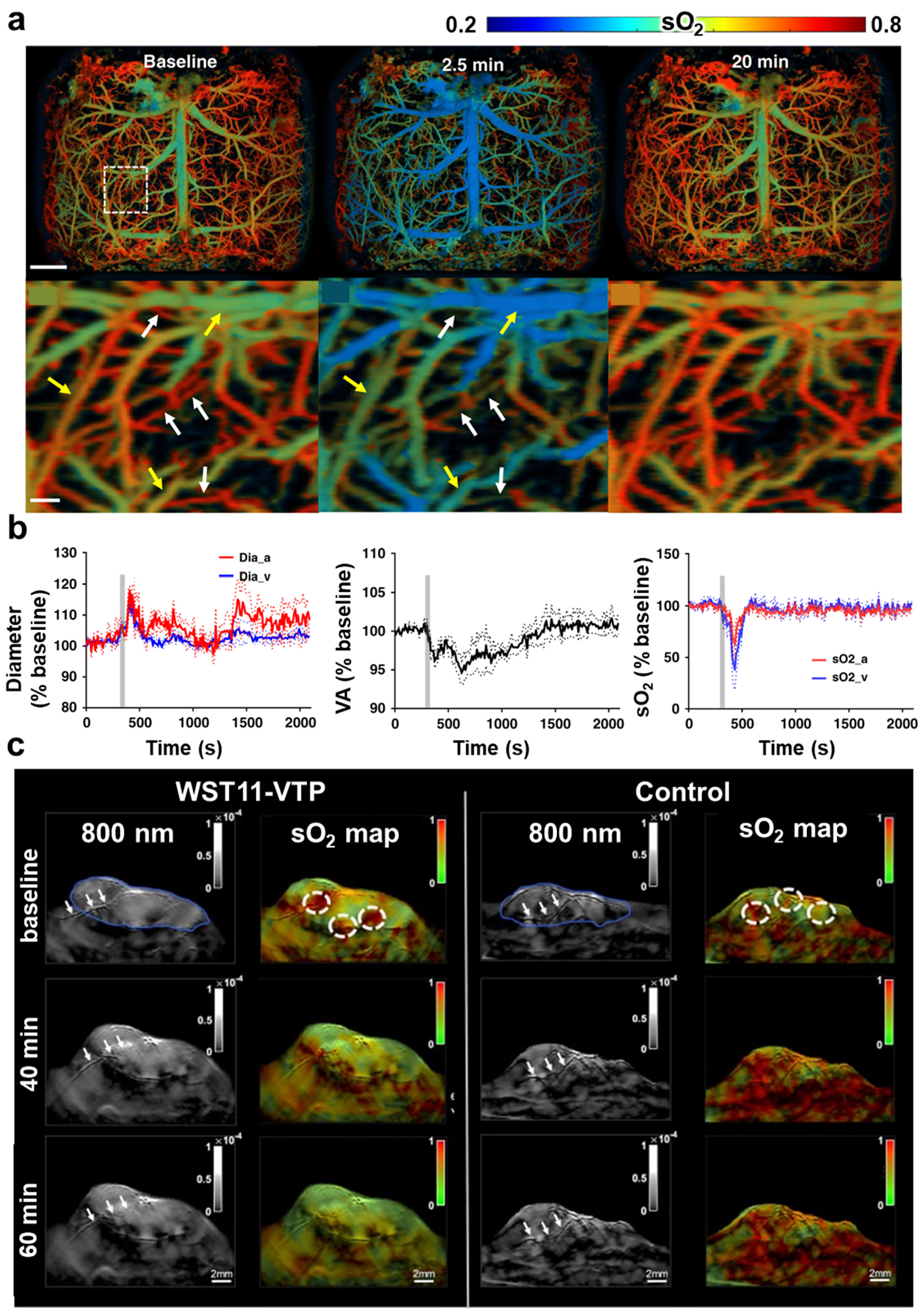
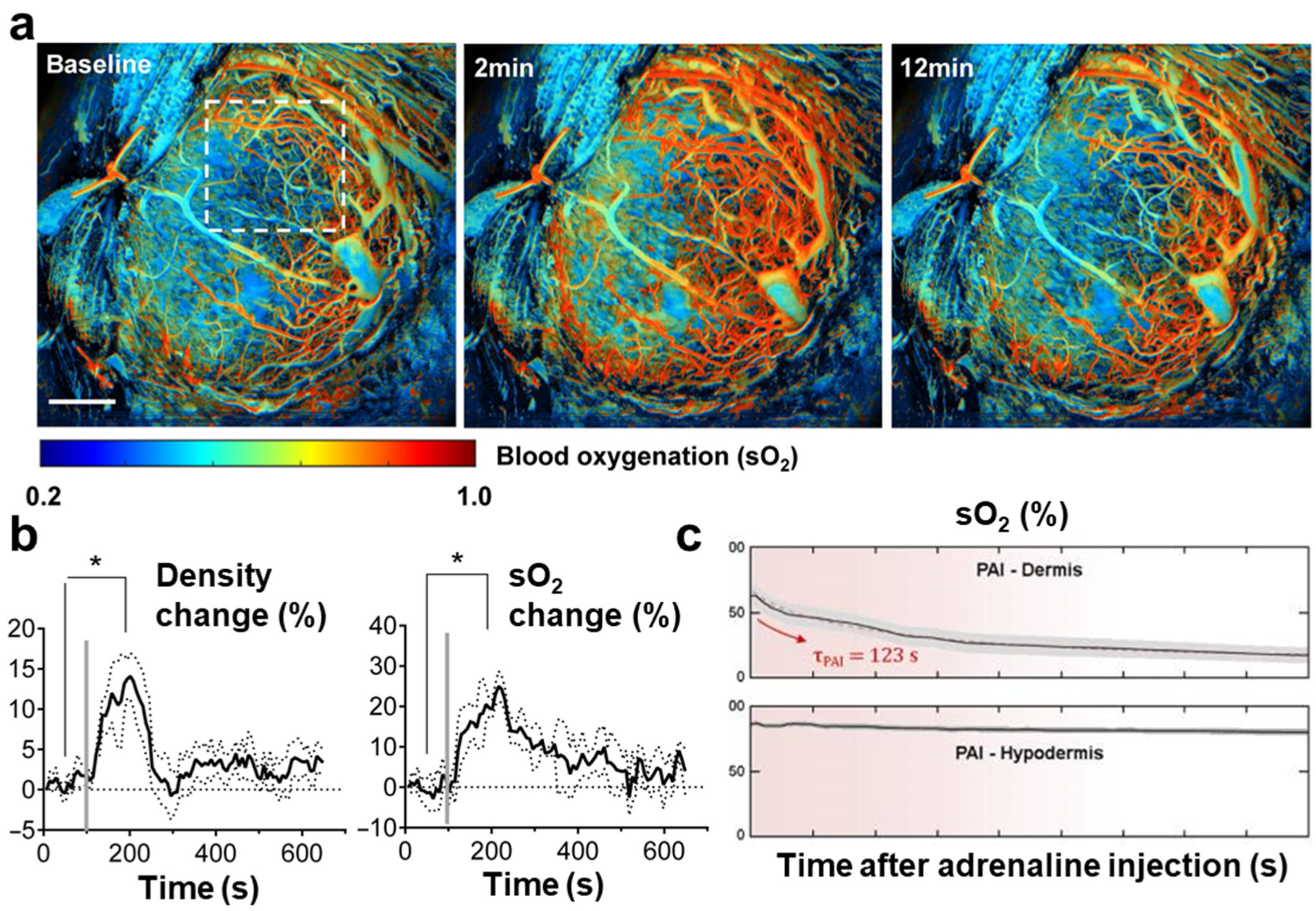

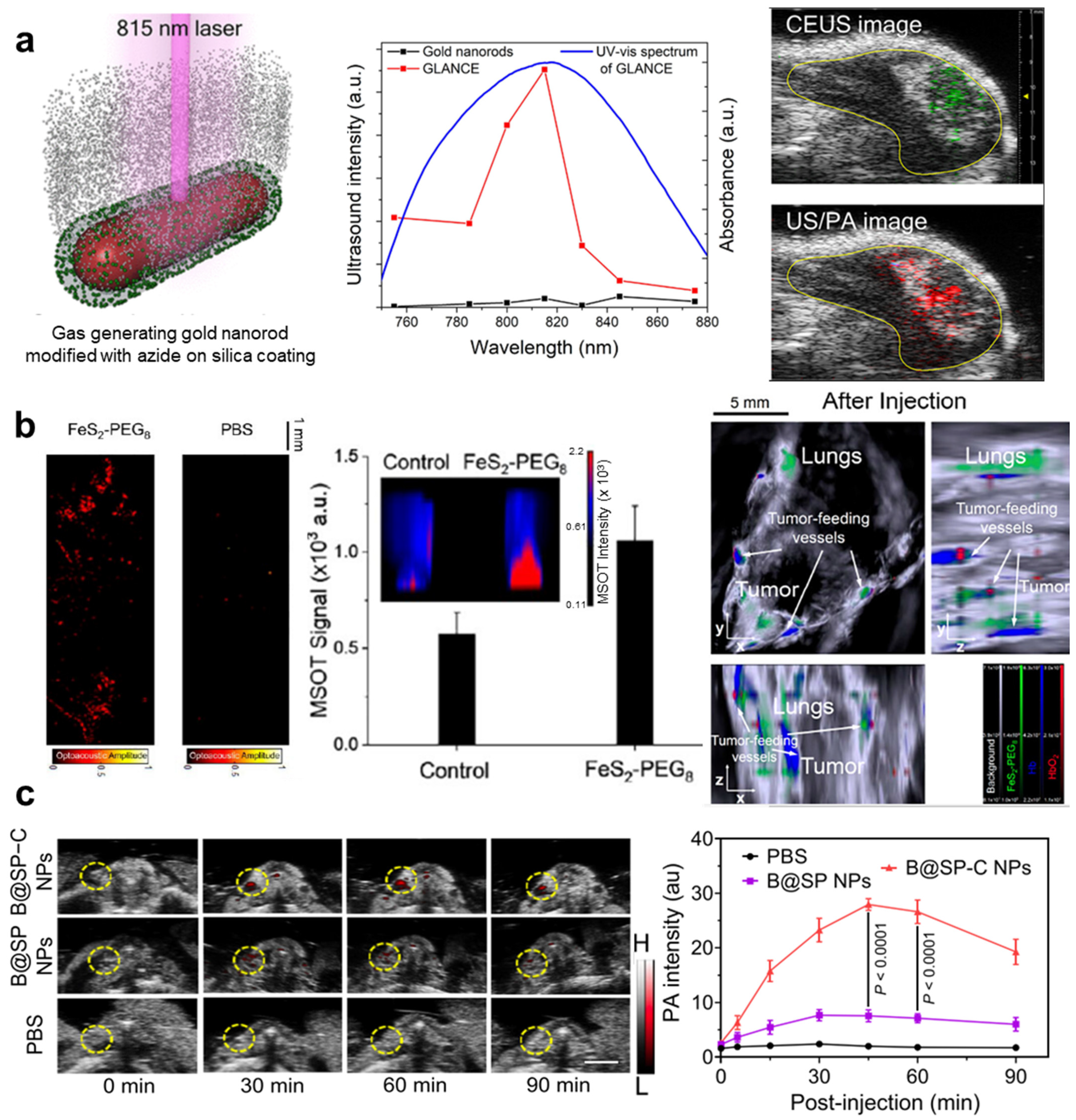
| Abbreviation | Explanation |
|---|---|
| HbO | Oxy-hemoglobin |
| HbR | Deoxy-hemoglobin |
| HbT | Total hemoglobin |
| sO2 | Oxygen saturation |
| MAP | Maximum amplitude projection |
| SNP | Sodium nitroprusside |
| DMXAA | 5,6-Dimethylxanthenone-4-acetic acid |
| VTP | Vascular-targeted photodynamic therapy |
| ICG | Indocyanine green |
| JA | J-aggregates |
| SWIR | Shortwave infrared |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| NIR | Near infrared |
| System | Detector Specification (Center Frequency) | Lateral Resolution | Imaging Depth | Approximate Imaging Area (Target) | Ref. |
|---|---|---|---|---|---|
| OR-PAM | Single element (50 MHz) | 0.4–0.7 µm | 0.76 mm | 15 × 10 mm2 (Mouse ear) | [98] |
| Single element (n/a) | 1.2 µm | 1 mm | 6 × 10 mm2 (Mouse brain) | [99] | |
| AR-PAM | Single element (50 MHz) | 52.5 µm | 3–6 mm | 30 × 35 mm2 (Mouse ear) | [100] |
| Single element (50/75 MHz) | 84/54 µm | 2.7/1.8 mm | 9 × 10 mm2 (Mouse ear) | [101] | |
| PACT | 1024 elements Hemispherical (2 MHz) | 380 µm | 10 mm | 65 × 85 mm2 (Mouse whole body) | [80] |
| 1024 elements Arc (2.25 MHz) | 370–390 µm | 40 mm | 75 × 85 mm2 (Human breast) | [103] |
| Target Tissue | Image Modality | Detector Spec. (Center F) | Imaging Performance | Detection | Drug or Contrast Agents | Ref. |
|---|---|---|---|---|---|---|
| Skin | OR-PAM | Single (50 MHz) | LRes: 5 µm ARes: 30 µm ID: 1 mm | Vasoconstriction | Corticosteroid | [81] |
| Ear | PAM | Single (50 MHz) | LRes: 5 µm IDes: 1 mm | Vasoconstriction | Glucose | [105] |
| Placenta | PACT | 96 el. Arc (6 MHz) | LRes: 390 µm ARes: 370 µm | Vasodilation | Sildenafil and G protein-coupled receptor G-1 | [106] |
| UFF-PAM | Single (40 MHz) | LRes: 10 µm | Vasodilation Vascular structure Oxygenation | Alcohol | [84] | |
| Brain | UFF-PAM | Single (40 MHz) | LRes: 10 µm IDes: 1.5 mm | Vasoconstriction Vasodilation Oxygenation | SNP | [82] |
| OR-PAM | Single (30 MHz) | LRes: 3 µm ARes: 25 µm | Vasodilation Oxygenation | SNP | [109] | |
| PACT | 256 el. Cylinder (4 MHz) | n/a | Vascular structure Oxygenation | Alcohol | [110] | |
| PAM | n/a | ID: several millimeters | Vasoconstriction Oxygenation | Epinephrine | [111] | |
| PACT | 256 el. Linear (n/a) | n/a | Agent’s biodistribution Oxygenation | AF-based dye | [123] | |
| Tumor | All-optical PA | Fabry–Perot sensor | LRes: 50–150 µm ARes: 50–150 µm ID: 10 mm | Vascular structure | OXi4503 | [112] |
| AR-PAM | Single (25 MHz) | LRes: 130 µm ARes: 60 µm | Vascular structure Oxygenation | Bevacizumab | [114] | |
| PACT | Linear (15 MHz) | n/a | Oxygenation | DMXAA | [115] | |
| PACT | 256 el. Arc (4 MHz) | Res: 200 µm | Vascular structure Oxygenation | WST11 | [83] | |
| PACT | Array (40 MHz) | n/a | Agent’s biodistribution | Gas-generating laser-activatable nanorods | [127] | |
| PACT | 256 el. Array (5 MHz) | n/a | Agent’s biodistribution | FeS₂ nanocrystals | [85] | |
| PACT | Array (40 MHz) | ARes: 40 µm | Agent’s biodistribution | Polymeric nanoparticle | [129] | |
| Human forearm skin | PACT | Array (30 MHz) | LRes: 50 µm ARes: 110 µm IDes: 20 µm | Vasoconstriction Oxygenation | Adrenaline | [117] |
| Ceritubular capillary | PAM | Single (35 MHz) | IDes: 200 µm | Vascular structure Oxygenation | Lipopolysaccharide | [116] |
| Human leg | PACT | Linear array (21 MHz) | n/a | Oxygenation | Hemoglobin spray | [118] |
| Whole body | PACT | 1024 el. Hemispherical (2 MHz) | Isotropic R: 380 µm | Agent’s biodistribution | ICG | [80] |
| Liver and spleen | PACT | Single (5, 35 MHz) | ID: 5 mm | Agent’s biodistribution | JAAZs | [122] |
| PACT | 128 el. Array (5 MHz) | n/a | Agent’s biodistribution | Xanthene-based NO-responsive nanosensors | [124] | |
| Carotid artery | PACT | Array (n/a) | n/a | Agent’s biodistribution | Semiconducting homopolymer nanoplatform | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, S.; Kim, C.; Kim, J.; Park, B. Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics 2024, 16, 1240. https://doi.org/10.3390/pharmaceutics16101240
Kim J, Choi S, Kim C, Kim J, Park B. Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics. 2024; 16(10):1240. https://doi.org/10.3390/pharmaceutics16101240
Chicago/Turabian StyleKim, Jiwoong, Seongwook Choi, Chulhong Kim, Jeesu Kim, and Byullee Park. 2024. "Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents" Pharmaceutics 16, no. 10: 1240. https://doi.org/10.3390/pharmaceutics16101240
APA StyleKim, J., Choi, S., Kim, C., Kim, J., & Park, B. (2024). Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics, 16(10), 1240. https://doi.org/10.3390/pharmaceutics16101240











