Stability Study of Fosfomycin in Elastomeric Pumps at 4 °C and 34 °C: Technical Bases for a Continuous Infusion Use for Outpatient Parenteral Antibiotic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Fosfomycin Solution
2.3. Stability at 4 °C and 34 °C
2.4. Analytical Method
2.4.1. Chromatographic Conditions
2.4.2. Mass Conditions
2.4.3. Method Validation and Stress Testing
3. Results
3.1. Method Validation and Stress Test Results
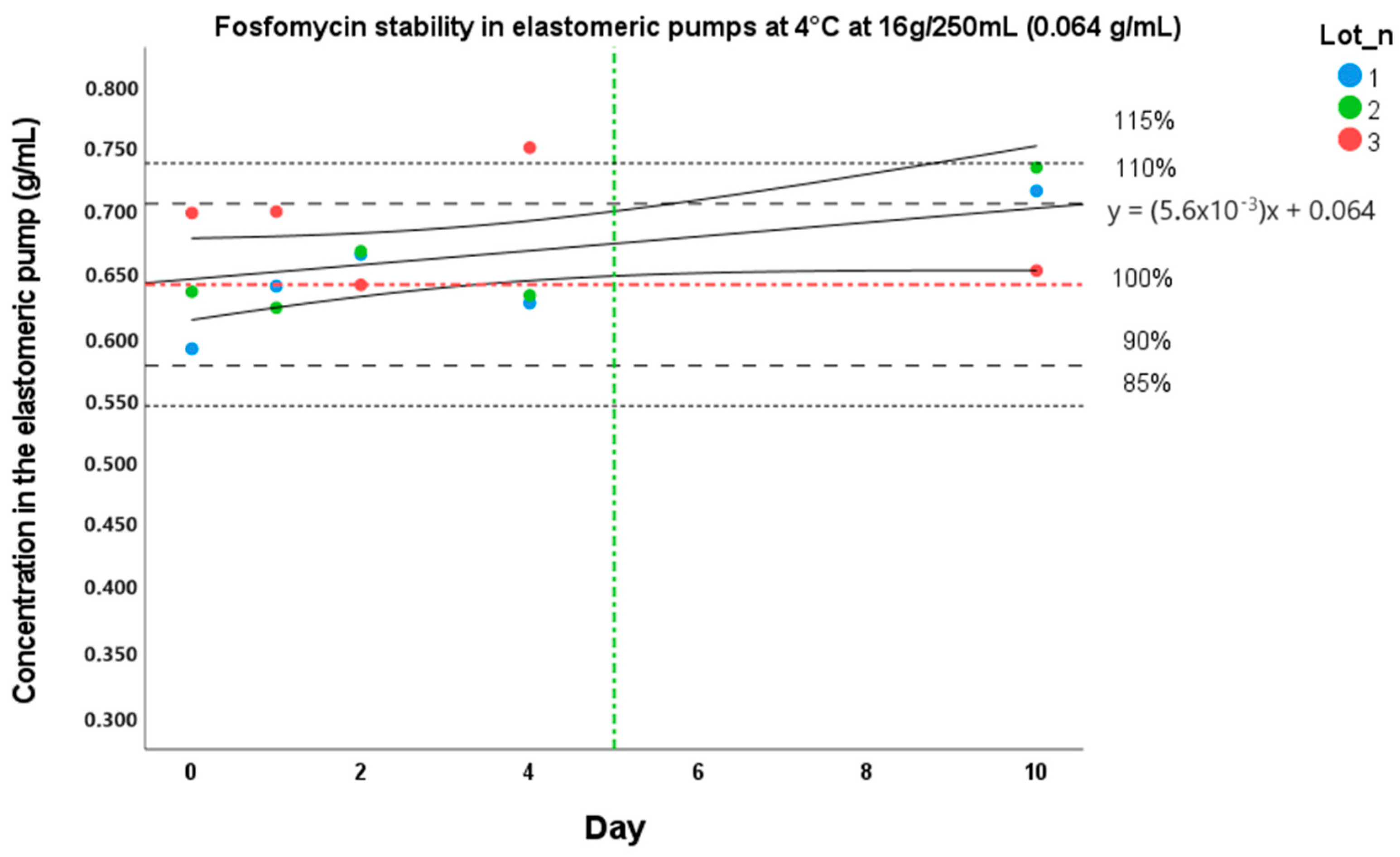
3.2. Pre-Administration Stability at 4 °C and In-Use/External Body Temperature Stability at 34 °C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalopoulos, A.S.; Livaditis, I.G.; Gougoutas, V. The revival of fosfomycin. Int. J. Infect. Dis. 2011, 15, e732–e739. [Google Scholar] [CrossRef]
- Raz, R. Fosfomycin: An old—New antibiotic. Clin. Microbiol. Infect. 2012, 18, 4–7. [Google Scholar] [CrossRef]
- Cao, Y.; Peng, Q.; Li, S.; Deng, Z.; Gao, J. The intriguing biology and chemistry of fosfomycin: The only marketed phosphonate antibiotic. RSC Adv. 2019, 9, 42204–42218. [Google Scholar] [CrossRef]
- Katayama, N.; Tsubotani, S.; Nozaki, Y.; Harada, S.; Ono, H. Fosfadecin and fosfocytocin, new nucleotide antibiotics produced by bacteria. J. Antibiot. 1990, 43, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gascon, A.; Canut-Blasco, A. Deciphering pharmacokinetics and pharmacodynamics of fosfomycin. Rev. Esp. Quimioter. 2019, 32 (Suppl. S1), 19–24. [Google Scholar] [PubMed]
- Roussos, N.; Karageorgopoulos, D.E.; Samonis, G.; Falagas, M.E. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int. J. Antimicrob. Agents 2009, 34, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2015, 29, 321–347. [Google Scholar] [CrossRef]
- Dijkmans, A.C.; Zacarias, N.V.O.; Burggraaf, J.; Mouton, J.W.; Wilms, E.B.; van Nieuwkoop, C.; Touw, D.J.; Stevens, J.; Kamerling, I.M.C. Fosfomycin: Pharmacological, Clinical and Future Perspectives. Antibiotics 2017, 6, 24. [Google Scholar] [CrossRef]
- Kirby, W.M. Pharmacokinetics of fosfomycin. Chemotherapy 1977, 23 (Suppl. S1), 141–151. [Google Scholar] [CrossRef]
- Parker, S.L.; Frantzeskaki, F.; Wallis, S.C.; Diakaki, C.; Giamarellou, H.; Koulenti, D.; Karaiskos, I.; Lipman, J.; Dimopoulos, G.; Roberts, J.A. Population Pharmacokinetics of Fosfomycin in Critically Ill Patients. Antimicrob. Agents Chemother. 2015, 59, 6471–6476. [Google Scholar] [CrossRef]
- Diez-Aguilar, M.; Canton, R. New microbiological aspects of fosfomycin. Rev. Esp. Quimioter. 2019, 32 (Suppl. S1), 8–18. [Google Scholar] [PubMed]
- Candel, F.J.; Matesanz David, M.; Barberan, J. New perspectives for reassessing fosfomycin: Applicability in current clinical practice. Rev. Esp. Quimioter. 2019, 32 (Suppl. S1), 1–7. [Google Scholar] [PubMed]
- Mirakhur, A.; Gallagher, M.J.; Ledson, M.J.; Hart, C.A.; Walshaw, M.J. Fosfomycin therapy for multiresistant Pseudomonas aeruginosa in cystic fibrosis. J. Cyst. Fibros 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.L.; Brown, S.D. Antibacterial spectrum of fosfomycin trometamol. J. Antimicrob. Chemother. 1995, 35, 228–230. [Google Scholar] [CrossRef]
- Sauermann, R.; Karch, R.; Langenberger, H.; Kettenbach, J.; Mayer-Helm, B.; Petsch, M.; Wagner, C.; Sautner, T.; Gattringer, R.; Karanikas, G.; et al. Antibiotic abscess penetration: Fosfomycin levels measured in pus and simulated concentration-time profiles. Antimicrob. Agents Chemother. 2005, 49, 4448–4454. [Google Scholar] [CrossRef]
- Matzi, V.; Lindenmann, J.; Porubsky, C.; Kugler, S.A.; Maier, A.; Dittrich, P.; Smolle-Juttner, F.M.; Joukhadar, C. Extracellular concentrations of fosfomycin in lung tissue of septic patients. J. Antimicrob. Chemother. 2010, 65, 995–998. [Google Scholar] [CrossRef]
- Falagas, M.E.; Athanasaki, F.; Voulgaris, G.L.; Triarides, N.A.; Vardakas, K.Z. Resistance to fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int. J. Antimicrob. Agents 2019, 53, 22–28. [Google Scholar] [CrossRef]
- Rinaldi, M.; Cojutti, P.G.; Zamparini, E.; Tedeschi, S.; Rossi, N.; Conti, M.; Giannella, M.; Pea, F.; Viale, P. Population pharmacokinetics and Monte Carlo simulation for dosage optimization of fosfomycin in the treatment of osteoarticular infections in patients without renal dysfunction. Antimicrob. Agents Chemother. 2021, 65, e02038-20. [Google Scholar] [CrossRef]
- Ballouz, T.; Zeenny, R.M.; Haddad, N.; Rizk, N.; Kanj, S.S. Retrospective evaluation of intravenous fosfomycin in multi-drug resistant infections at a tertiary care hospital in Lebanon. J. Infect. Dev. Ctries. 2021, 15, 1308–1313. [Google Scholar] [CrossRef]
- Grabein, B.; Graninger, W.; Rodriguez Bano, J.; Dinh, A.; Liesenfeld, D.B. Intravenous fosfomycin-back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Microbiol. Infect. 2016, 23, 363–372. [Google Scholar] [CrossRef]
- Linares, L.; Cervera, C.; Cofan, F.; Ricart, M.J.; Esforzado, N.; Torregrosa, V.; Oppenheimer, F.; Campistol, J.M.; Marco, F.; Moreno, A. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transpl. Proc. 2007, 39, 2222–2224. [Google Scholar] [CrossRef] [PubMed]
- Giamarellou, H.; Poulakou, G. Multidrug-resistant Gram-negative infections: What are the treatment options? Drugs 2009, 69, 1879–1901. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, L.; Quiles, M.; Rodriguez, A. A study of the levels of fosfomycin in the cerebrospinal fluid in adult meningitis. Chemotherapy 1977, 23 (Suppl. S1), 180–188. [Google Scholar] [CrossRef]
- Hamad, Y.; Dodda, S.; Frank, A.; Beggs, J.; Sleckman, C.; Kleinschmidt, G.; Lane, M.A.; Burnett, Y. Perspectives of Patients on Outpatient Parenteral Antimicrobial Therapy: Experiences and Adherence. Open. Forum Infect. Dis. 2020, 7, ofaa205. [Google Scholar] [CrossRef]
- Levack, A.E.; Turajane, K.; Yang, X.; Miller, A.O.; Carli, A.V.; Bostrom, M.P.; Wellman, D.S. Thermal Stability and In Vitro Elution Kinetics of Alternative Antibiotics in Polymethylmethacrylate (PMMA) Bone Cement. J. Bone Jt. Surg. Am. 2021, 103, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, S.; Rodante, F.; Tomassetti, M. Thermal stability of disodium and calcium phosphomycin and the effects of the excipients evaluated by thermal analysis. J. Pharm. Biomed. Anal. 2001, 24, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Wijma, R.A.; Bahmany, S.; Wilms, E.B.; van Gelder, T.; Mouton, J.W.; Koch, B.C.P. A fast and sensitive LC-MS/MS method for the quantification of fosfomycin in human urine and plasma using one sample preparation method and HILIC chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 263–269. [Google Scholar] [CrossRef]
- Papakondyli, T.A.; Gremilogianni, A.M.; Megoulas, N.C.; Koupparis, M.A. A novel derivatization method for the determination of Fosfomycin in human plasma by liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometric detection via phase transfer catalyzed derivatization. J. Chromatogr. A 2014, 1332, 1–7. [Google Scholar] [CrossRef]
- Shopova, T.; Huppe, T.; Wolf, B.; Sessler, D.I.; Volk, T.; Groesdonk, H.V.; Kreuer, S.; Maurer, F. Quantitative Determination of Fosfomycin in 10 muL of Plasma and Dialysate by Hydrophilic Interaction Liquid Chromatography Electrospray Ionization Mass Spectrometry. J. Chromatogr. Sci. 2020, 59, 165–174. [Google Scholar] [CrossRef]
- Parker, S.L.; Lipman, J.; Roberts, J.A.; Wallis, S.C. A simple LC-MS/MS method using HILIC chromatography for the determination of fosfomycin in plasma and urine: Application to a pilot pharmacokinetic study in humans. J. Pharm. Biomed. Anal. 2015, 105, 39–45. [Google Scholar] [CrossRef][Green Version]
- Martens-Lobenhoffer, J.; Bode-Boger, S.M. A validated method for the quantification of fosfomycin in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 990, 164–168. [Google Scholar] [CrossRef] [PubMed]
- EMA. ICH TOPIC Q1 E Evaluation of Stability Data: Note for Guidance on Evaluation of Stability Data; European Medicines Agency: Amsterdam, The Netherlands, 2003.
- EMA. Note for Guidance on In-Use Stability Testing of Human Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2001.
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.1_Breakpoint_Tables.pdf (accessed on 1 September 2023).
- AIFA. InfectoFos 40 mg/ml Polvere Per Soluzione Per Infusione. Fosfomicina. Informazioni Per L'utilizzatore. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_003703_043646_FI.pdf&sys=m0b1l3 (accessed on 1 September 2023).
- El-Najjar, N.; Jantsch, J.; Gessner, A. A rapid liquid chromatography-tandem mass spectrometry for the quantification of Fosfomycin in plasma, urine, and aqueous fluids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Matta, M.; Garimella, N.; Zere, T.; Weaver, J. Development and validation of a LC-MS/MS method for quantitation of fosfomycin—Application to in vitro antimicrobial resistance study using hollow-fiber infection model. Biomed. Chromatogr. 2018, 32, e4214. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.K.; Toh, W.G.; Hee, D.K.; Ting, E.Z.; Chua, N.G.S.; Zulkifli, F.I.B.; Sin, L.J.; Tan, T.T.; Kwa, A.L.; Lim, T.P. Quantification of Fosfomycin in Combination with Nine Antibiotics in Human Plasma and Cation-Adjusted Mueller-Hinton II Broth via LCMS. Antibiotics 2022, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 13485:2016; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. International Organization for Standardization: Geneva, Switzerland, 2016.
- FDA. Guidance for Industry: Bioanalytical Method Validation. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 4 March 2021).
- EMA. Guideline on Bioanalytical Method Validation. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (accessed on 4 March 2021).
- Muldoon, E.G.; Snydman, D.R.; Penland, E.C.; Allison, G.M. Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin. Infect. Dis. 2013, 57, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.D.; Czoski Murray, C.; Meads, D.; Minton, J.; Wright, J.; Twiddy, M. Clinical and cost-effectiveness, safety and acceptability of community intravenous antibiotic service models: CIVAS systematic review. BMJ Open 2017, 7, e013560. [Google Scholar] [CrossRef]
- Bugeja, S.J.; Stewart, D.; Vosper, H. Clinical benefits and costs of an outpatient parenteral antimicrobial therapy service. Res. Soc. Adm. Pharm. 2021, 17, 1758–1763. [Google Scholar] [CrossRef]
- Diamantis, S.; Longuet, P.; Lesprit, P.; Gauzit, R. Terms of use of outpatient parenteral antibiotic therapy. Infect. Dis. Now 2020, 51, 14–38. [Google Scholar] [CrossRef]
- Abe, T.; Matsuzaka, K.; Nakayama, T.; Otsuka, M.; Sagara, A.; Sato, F.; Yumoto, T. Impact of air temperature and drug concentration on liquid emission from elastomeric pumps. J. Pharm. Health Care Sci. 2021, 7, 1. [Google Scholar] [CrossRef]
- Voumard, R.; Van Neyghem, N.; Cochet, C.; Gardiol, C.; Decosterd, L.; Buclin, T.; de Valliere, S. Antibiotic stability related to temperature variations in elastomeric pumps used for outpatient parenteral antimicrobial therapy (OPAT). J. Antimicrob. Chemother. 2017, 72, 1462–1465. [Google Scholar] [CrossRef]
- Akahane, M.; Enoki, Y.; Saiki, R.; Hayashi, Y.; Hiraoka, K.; Honma, K.; Itagaki, M.; Gotoda, M.; Shinoda, K.; Hanyu, S.; et al. Stability of antimicrobial agents in an elastomeric infusion pump used for outpatient parenteral antimicrobial therapy. Int. J. Infect. Dis. 2021, 103, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Sand, P.; Aladeen, T.; Kirkegaard, P.; LaChance, D.; Slover, C. Chemical Stability of Telavancin in Elastomeric Pumps. Curr. Ther. Res. Clin. Exp. 2015, 77, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rubio, B.; Del Valle-Moreno, P.; Herrera-Hidalgo, L.; Gutierrez-Valencia, A.; Luque-Marquez, R.; Lopez-Cortes, L.E.; Gutierrez-Urbon, J.M.; Luque-Pardos, S.; Fernandez-Polo, A.; Gil-Navarro, M.V. Stability of Antimicrobials in Elastomeric Pumps: A Systematic Review. Antibiotics 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]


| Day 0 | Day 1 | Day 2 | Day 4 | Day 10 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CV% | Stability (%) | CV% | Stability (%) | CV% | Stability (%) | CV% | Stability (%) | CV% | Stability (%) | |
| Batch 1 | 5.8 | 100 | 12.5 | 108 | 1.3 | 113 | 15.9 | 106 | 9.3 | 121 |
| Batch 2 | 5.7 | 100 | 10.8 | 98 | 1.9 | 105 | 16.3 | 100 | 5.9 | 115 |
| Batch 3 | 6.1 | 100 | 8.0 | 100 | 13.5 | 92 | 13.2 | 107 | 9.1 | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manca, A.; Palermiti, A.; Mula, J.; Cusato, J.; Maiese, D.; Simiele, M.; De Nicolò, A.; D’Avolio, A. Stability Study of Fosfomycin in Elastomeric Pumps at 4 °C and 34 °C: Technical Bases for a Continuous Infusion Use for Outpatient Parenteral Antibiotic Therapy. Pharmaceutics 2023, 15, 2347. https://doi.org/10.3390/pharmaceutics15092347
Manca A, Palermiti A, Mula J, Cusato J, Maiese D, Simiele M, De Nicolò A, D’Avolio A. Stability Study of Fosfomycin in Elastomeric Pumps at 4 °C and 34 °C: Technical Bases for a Continuous Infusion Use for Outpatient Parenteral Antibiotic Therapy. Pharmaceutics. 2023; 15(9):2347. https://doi.org/10.3390/pharmaceutics15092347
Chicago/Turabian StyleManca, Alessandra, Alice Palermiti, Jacopo Mula, Jessica Cusato, Domenico Maiese, Marco Simiele, Amedeo De Nicolò, and Antonio D’Avolio. 2023. "Stability Study of Fosfomycin in Elastomeric Pumps at 4 °C and 34 °C: Technical Bases for a Continuous Infusion Use for Outpatient Parenteral Antibiotic Therapy" Pharmaceutics 15, no. 9: 2347. https://doi.org/10.3390/pharmaceutics15092347
APA StyleManca, A., Palermiti, A., Mula, J., Cusato, J., Maiese, D., Simiele, M., De Nicolò, A., & D’Avolio, A. (2023). Stability Study of Fosfomycin in Elastomeric Pumps at 4 °C and 34 °C: Technical Bases for a Continuous Infusion Use for Outpatient Parenteral Antibiotic Therapy. Pharmaceutics, 15(9), 2347. https://doi.org/10.3390/pharmaceutics15092347







