LC-MS Fingerprinting Development for Standardized Precipitate from Agastache mexicana, Which Induces Antihypertensive Effect through NO Production and Calcium Channel Blockade †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Drugs
2.2. Plant Material
2.3. Preparation of the Extract
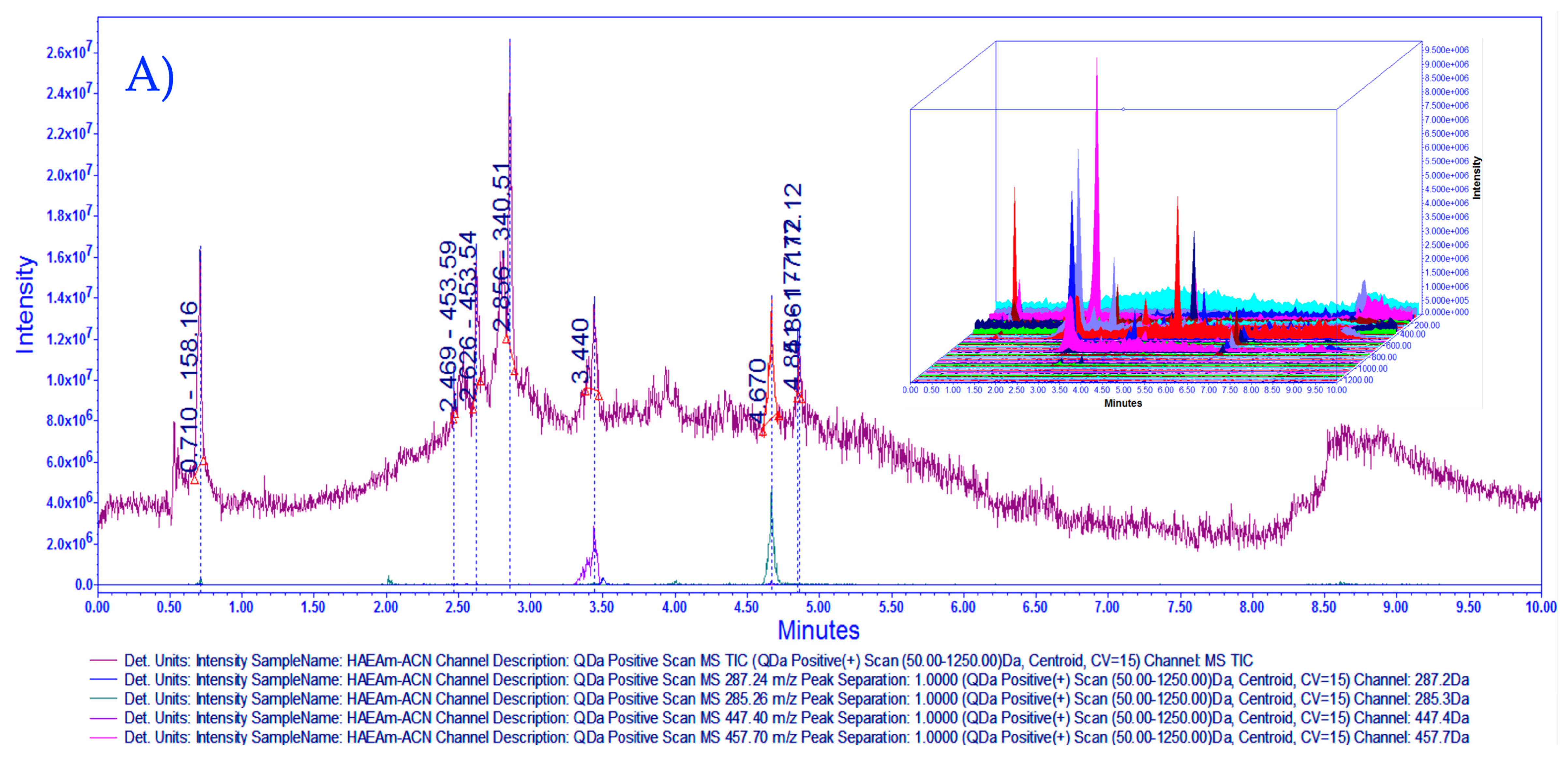
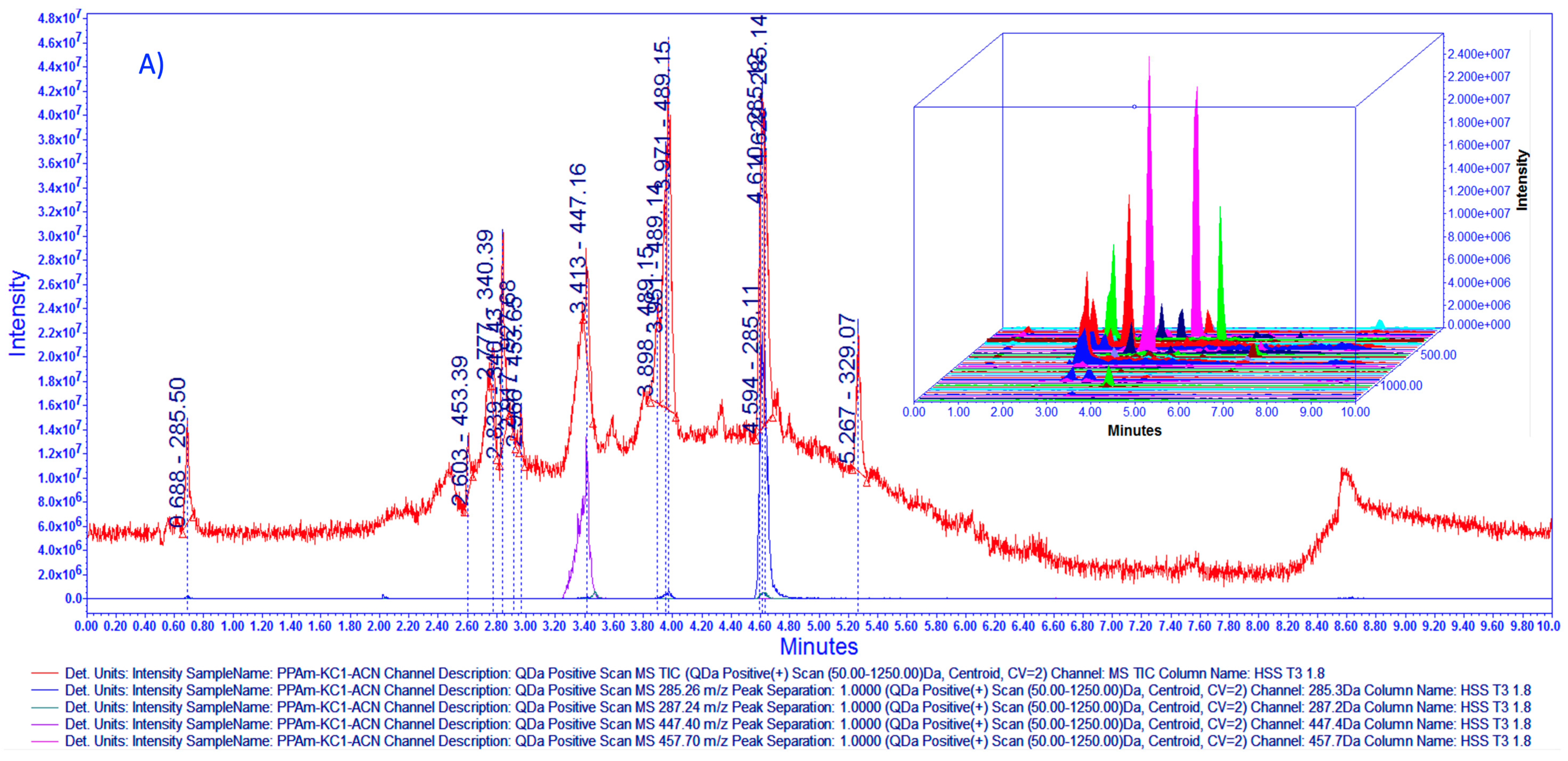
2.4. Chemical Fingerprinting Development by UPLC-MS Analysis
2.5. Rat Aorta Rings and Functional Studies
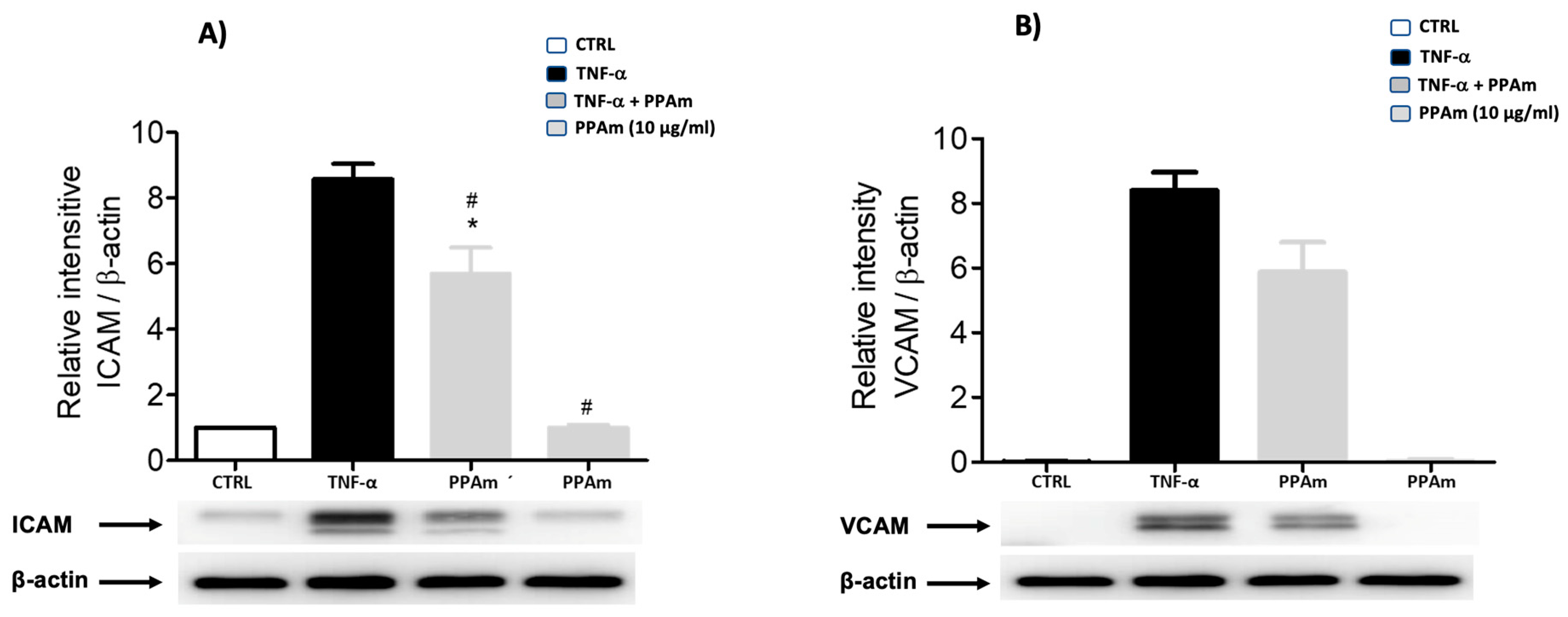
2.6. ICAM and VCAM Determination in HUVEC Cells
2.6.1. Cell Viability by Neutral Red Assay
2.6.2. Cytoplasmic and Nuclear Protein Extracts
2.6.3. Western Blotting
2.7. Acute and Subacute In Vivo Antihypertensive Experiments
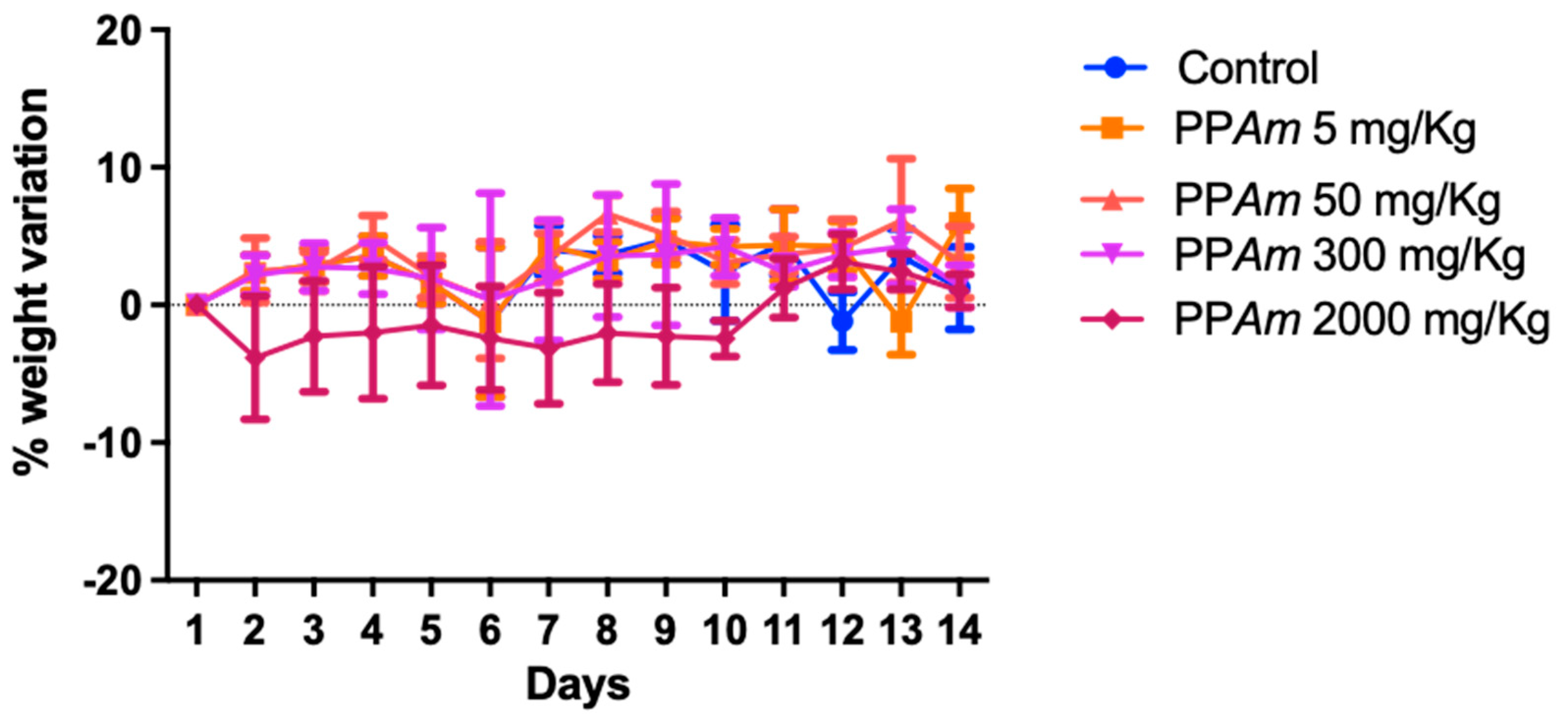
2.8. Acute Oral Toxicity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of HAEAm and PPAm by LC-MS
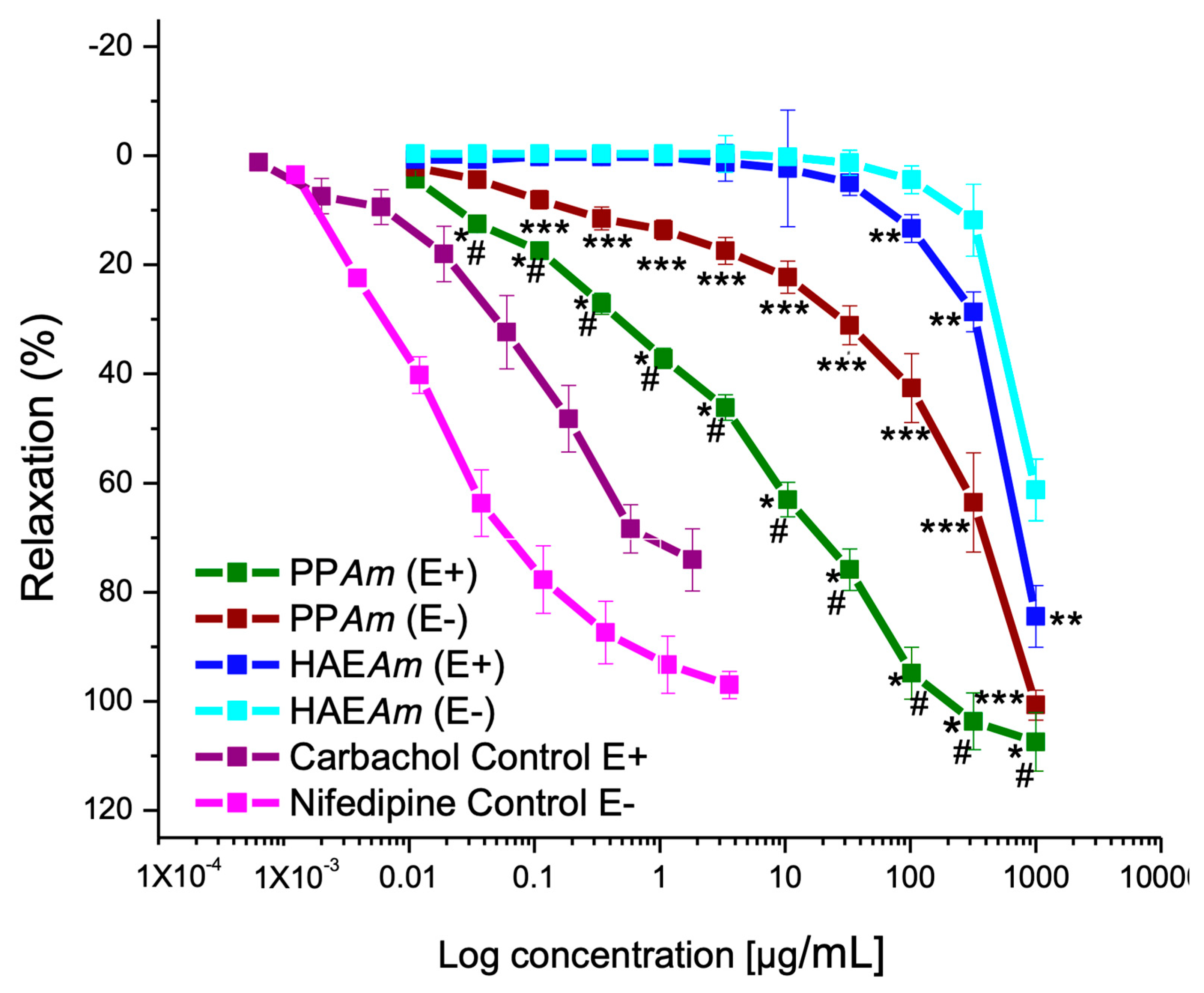
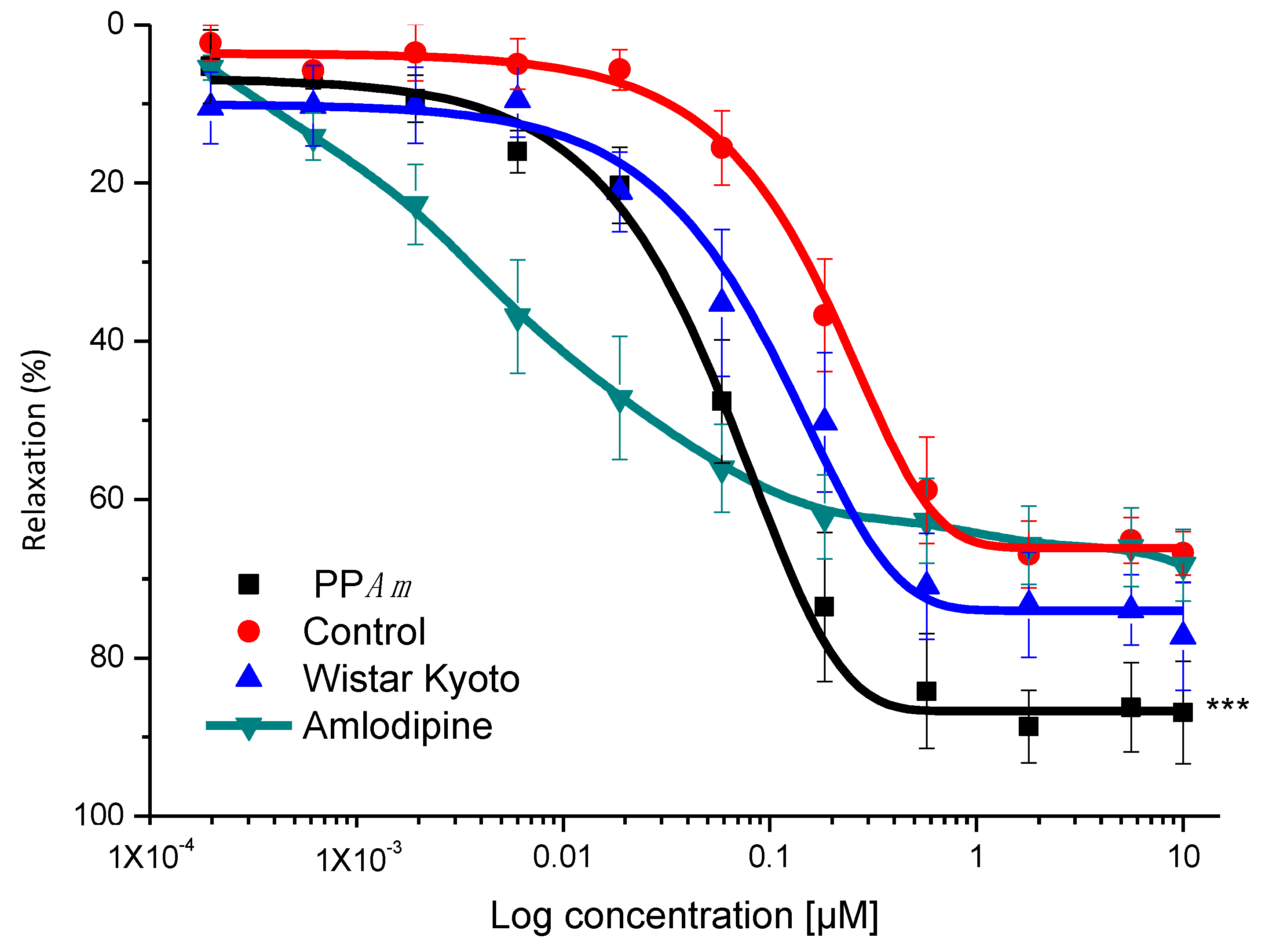
3.2. Vasorelaxant Effect of Hydroalcoholic Extract (HAEAm) and PPAm from Agastache mexicana
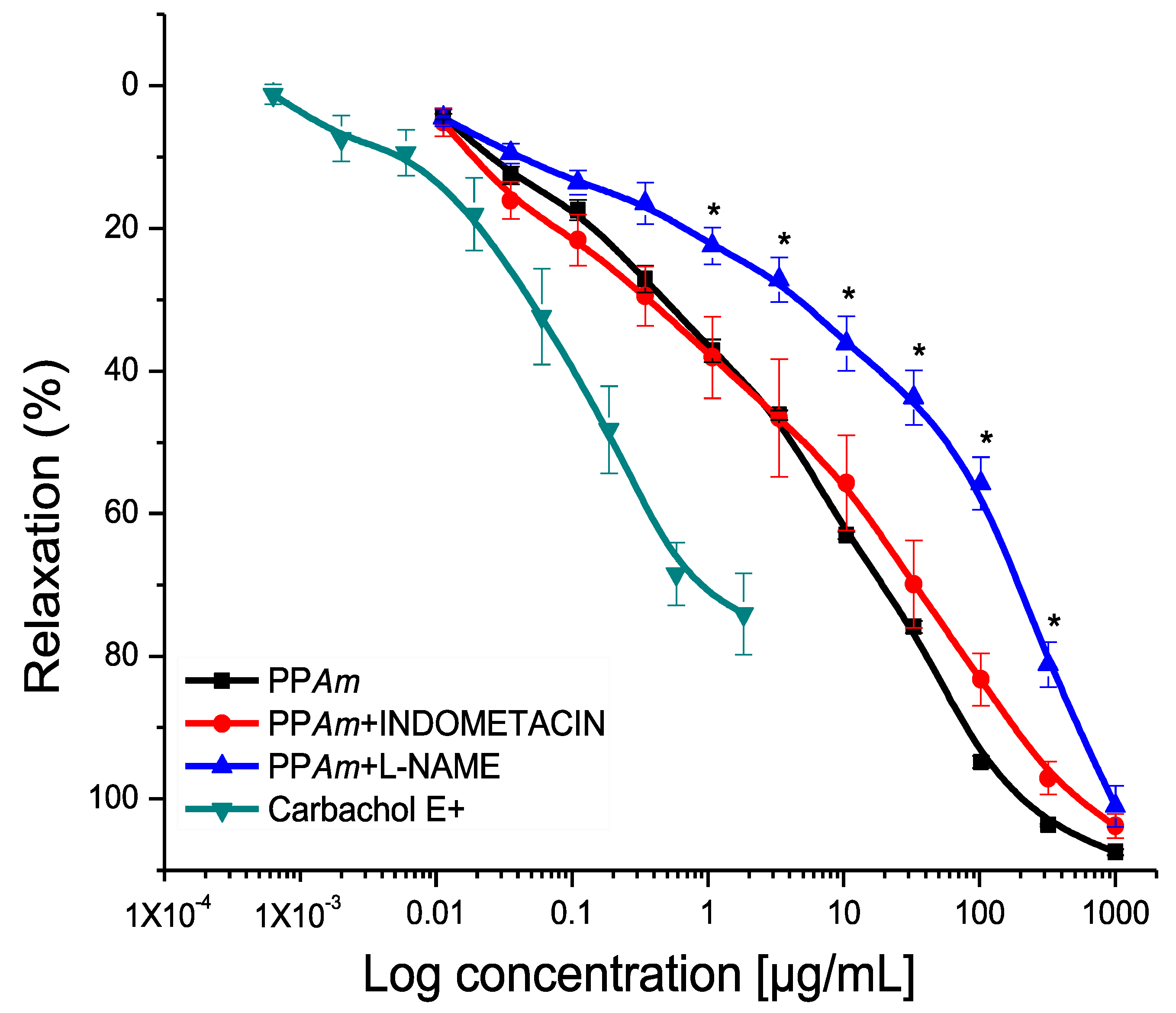
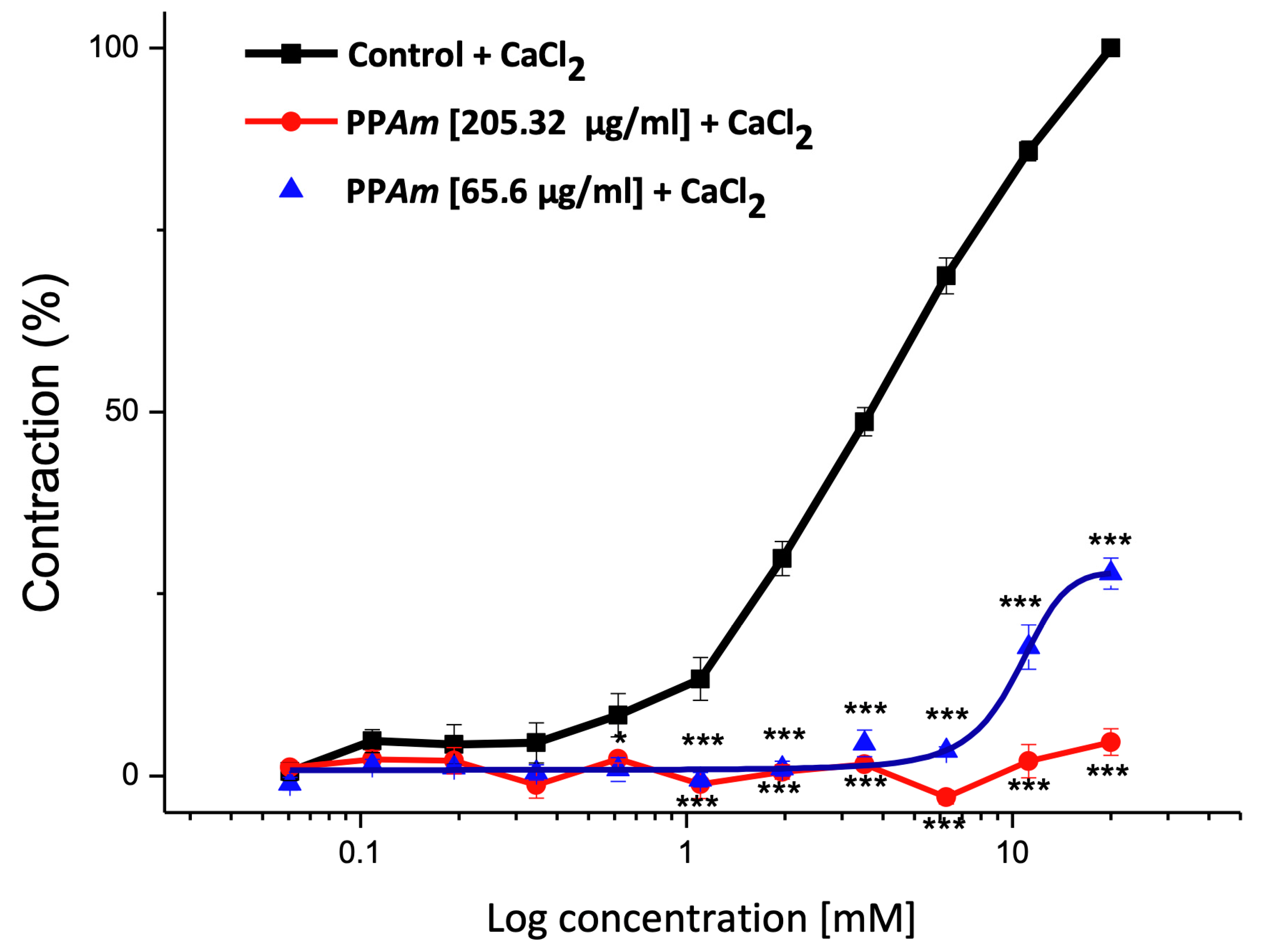
3.3. Functional Mechanism of Vasorelaxant Action of PPAm
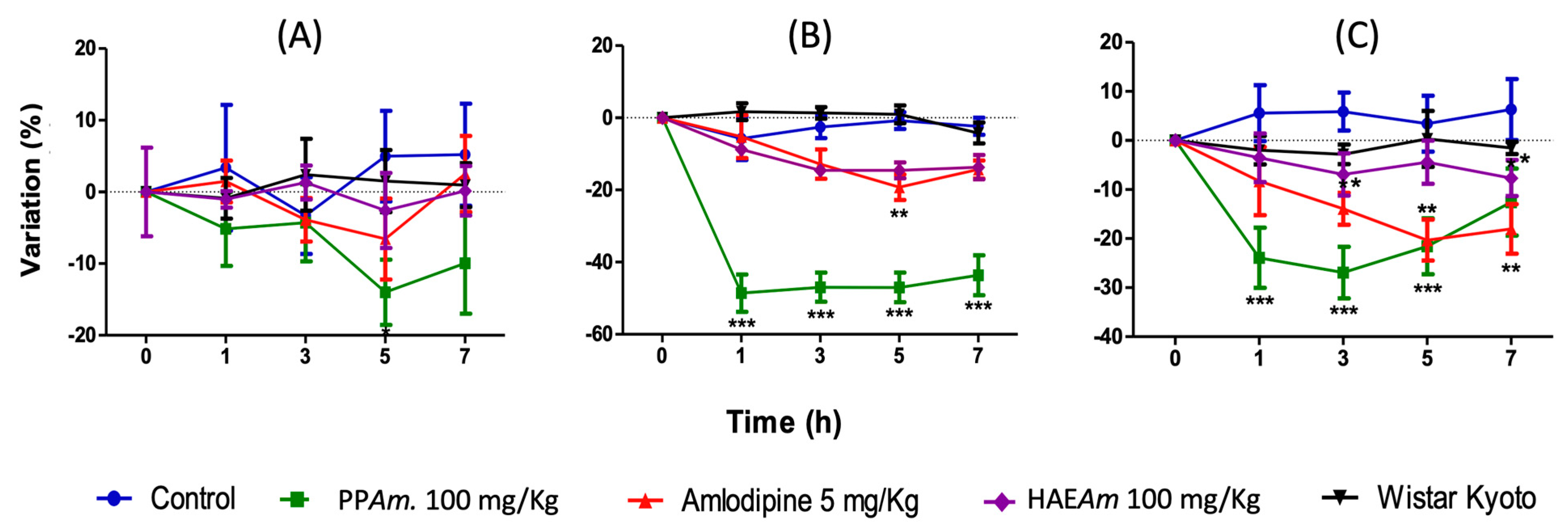
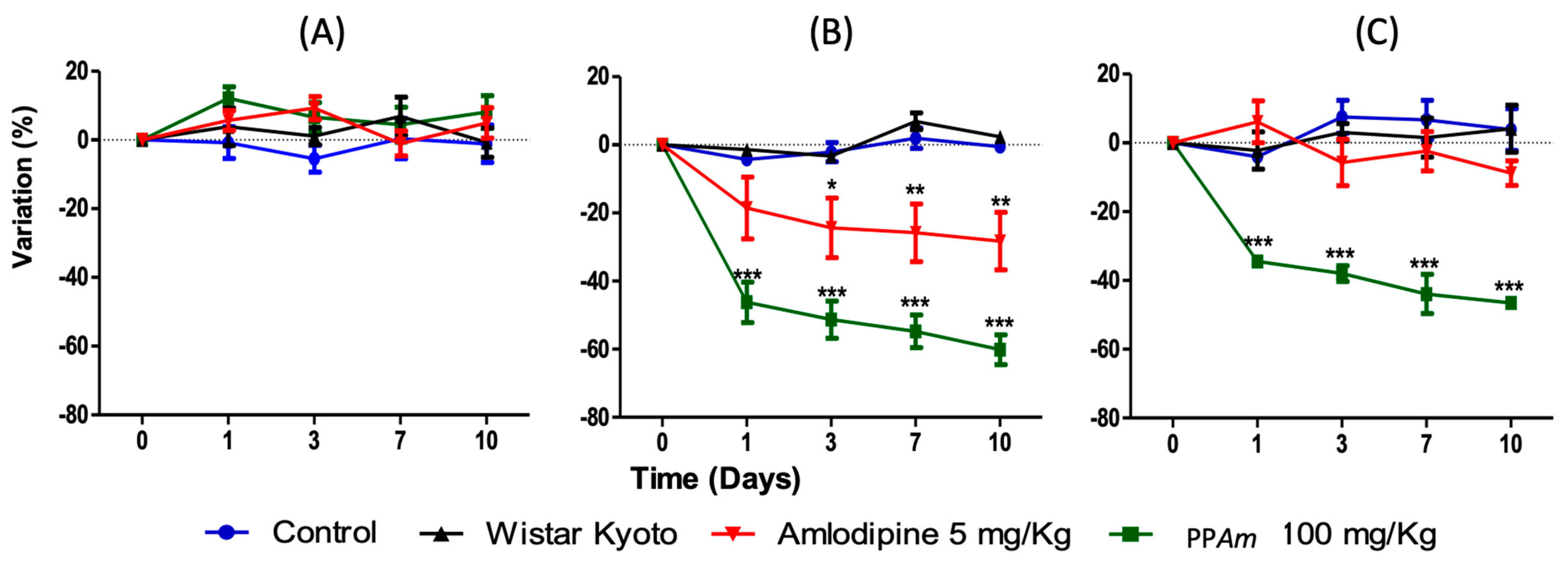
3.4. Acute and Subacute Antihypertensive Effects of Hydroalcoholic Extract (HAEAm) and PPAm from Agastache mexicana
3.5. Acute Oral Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Matsuzaki, M.; Matsuoka, H.; Shimamoto, K.; Shimada, K.; Rakugi, H.; Umemoto, S.; Kamiya, A.; Suzuki, N.; Kumagai, H.; et al. The combination therapy of hypertension to prevent cardiovascular events (COPE) trial: Rationale and design. Hypertens. Res. 2005, 28, 331–338. [Google Scholar] [CrossRef]
- Remiszewski, P.; Malinowska, B. Why multitarget vasodilatory (endo)cannabinoids are not effective as antihypertensive compounds after chronic administration: Comparison of their effects on systemic and pulmonary hypertension. Pharmaceuticals 2022, 15, 1119. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A.; Zúñiga, A. Epidemiologic changes and economic burden of hypertension in Latin America: Evidence from Mexico. Am. J. Hypertens. 2006, 19, 553–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staffileno, B.A. Treating hypertension with cardioprotective therapies: The role of ACE inhibitors, ARBs, and beta-blockers. J. Cardiovasc. Nurs. 2005, 20, 354–364. [Google Scholar] [CrossRef]
- Monroy-Ortiz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos, 2nd ed.; Universidad Autónoma del Estado de Morelos, Centro de Investigaciones Biotecnológicas: Morelos, Mexico, 2007. [Google Scholar]
- Palma-Tenango, M.; Sánchez-Fernández, R.E.; Soto-Hernández, M. A Systematic Approach to Agastache mexicana Research: Biology, Agronomy, Phytochemistry, and Bioactivity. Molecules 2021, 26, 3751. [Google Scholar] [CrossRef]
- Flores-Flores, A.; Hernández-Abreu, O.; Rios, M.Y.; León-Rivera, I.; Aguilar-Guadarrama, B.; Castillo-España, P.; Perea-Arango, I.; Estrada-Soto, S. Vasorelaxant mode of action of dichloromethane-soluble extract from Agastache mexicana and its main bioactive compounds. Pharm. Biol. 2016, 54, 2807–2813. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Ventura-Martínez, R.; Chávez, M.; Díaz-Reval, I.; Pellicer, F. Spasmolytic and antinociceptive activities of ursolic acid and acacetin identified in Agastache mexicana. Planta Med. 2012, 78, 793–796. [Google Scholar] [CrossRef]
- Hernández-Abreu, O.; Castillo-España, P.; León-Rivera, I.; Ibarra-Barajas, M.; Villalobos-Molina, R.; González-Christen, J.; Vergara-Galicia, J.; Estrada-Soto, S. Antihypertensive and vasorelaxant effects of tilianin isolated from Agastache mexicana are mediated by NO/cGMP pathway and potassium channel opening. Biochem. Pharmacol. 2009, 78, 54–61. [Google Scholar] [CrossRef]
- Wei, J.; Cao, P.; Wang, J.; Kang, W. Analysis of Tilianin and Acacetin in Agastache rugosa by High-Performance Liquid Chromatography with Ionic Liquids-Ultrasound-Based Extraction. Chem. Cent. J. 2016, 10, 76. [Google Scholar] [CrossRef]
- Hernández-Abreu, O.; Torres-Piedra, M.; García-Jiménez, S.; Ibarra-Barajas, M.; Villalobos-Molina, R.; Montes, S.; Rembao, D.; Estrada-Soto, S. Dose-dependent antihypertensive determination and toxicological studies of tilianin isolated from Agastache mexicana. J. Ethnopharmacol. 2013, 146, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Schiozer, A.L.; Cabral, E.C.; de Godoy, L.A.; Chaves, F.; Poppi, R.J.; Riveros, J.M.; Pereira, A.M.S.; de Souza, L.M.; Barata, L.E. Electrospray ionization mass spectrometry fingerprinting of extracts of the leaves of Arrabidaea chica. J. Braz. Chem. Soc. 2012, 23, 409–414. [Google Scholar] [CrossRef]
- Draper, J.; Lloyd, A.J.; Goodacre, R.; Beckmann, M. Flow infusion electrospray ionisation mass spectrometry for high throughput, non-targeted metabolite fingerprinting: A review. Metabolomics 2013, 9 (Suppl. S1), 4–29. [Google Scholar] [CrossRef]
- Alcântara, D.B.; Riceli, P.; Almeida, A.D.S.; Luz, L.R.; Nascimento, H.O.; Fernandes, T.S.M.; Dionísio, A.P.; Castro, A.C.R.; Nascimento, R.F.; Lopes, G.S.; et al. Development, optimization, and validation of an ultrasound-assisted liquid–liquid microextraction (UALLME) for selenomethionine analyses in cashew nut (Anacardium occidentale) by ultra-performance liquid chromatography coupled to electrospray ionization/single quadrupole mass spectrometer (UPLC-ESI/QDa). Food Anal. Methods 2022, 15, 3196–3208. [Google Scholar] [CrossRef]
- López, J.L.; Baltazar, C.; Torres, M.; Ruiz, A.; Esparza, R.; Rosas, G. Biosynthesis of silver nanoparticles using extracts of Mexican medicinal plants. In Characterization of Metals and Alloys; Pérez Campos, R., Contreras Cuevas, A., Esparza Muñoz, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 157–166. [Google Scholar] [CrossRef]
- El-Akhal, J.; Oliveira, A.P.; Bencheikh, R.; Valentão, P.; Andrade, P.B.; Morato, M. Vasorelaxant Mechanism of Herbal Extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats. Molecules 2022, 27, 8752. [Google Scholar] [CrossRef]
- Yorsin, S.; Sriwiriyajan, S.; Chongsa, W. Vasorelaxing Effect of Garcinia cowa Leaf Extract in Rat Thoracic Aorta and Its Underlying Mechanisms. J. Tradit. Complement. Med. 2022, 13, 219–225. [Google Scholar] [CrossRef]
- Jin, S.N.; Wen, J.F.; Li, X.; Kang, D.G.; Lee, H.S.; Cho, K.W. The Mechanism of Vasorelaxation Induced by Ethanol Extract of Sophora flavescens in Rat Aorta. J. Ethnopharmacol. 2011, 137, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kwon, Y.; Lee, S.; Lee, K.; Ham, I.; Choi, H.Y. Vasorelaxant Effects of Angelica decursiva Root on Isolated Rat Aortic Rings. BMC Complement. Altern. Med. 2017, 17, 474. [Google Scholar] [CrossRef]
- Javed, A.; Khan, S.; Salma, U.; Ahmad, T.; Khan, T.; Shah, A.J. Extract of Chenopodium album lowers blood pressure in rats through endothelium-dependent and -independent vasorelaxation. Ann. Pharm. Fr. 2023; in press. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development (OECD). Test No. 423: Acute oral toxicity-Acute Toxic Class Method. OECD Guidelines for the Testing of Chemicals; Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Mbosso Teinkela, J.E.; Oumarou, H.; Siwe Noundou, X.; Meyer, F.; Megalizzi, V.; Hoppe, H.C.; Macedo Krause, R.W.; Wintjens, R. Evaluation of in vitro antiplasmodial, antiproliferative activities, and in vivo oral acute toxicity of Spathodea campanulata flowers. Sci. Afr. 2023, 21, e01871. [Google Scholar] [CrossRef]
- Estrada-Soto, S.; Ornelas-Mendoza, K.; Navarrete-Vázquez, G.; Chávez-Silva, F.; Almanza-Pérez, J.C.; Villalobos-Molina, R.; Ortiz-Barragán, E.; Loza-Rodríguez, H.; Rivera-Leyva, J.C.; Flores-Flores, A.; et al. Insulin sensitization by PPARγ and GLUT-4 overexpression/translocation mediates the antidiabetic effect of Plantago australis. Pharmaceuticals 2023, 16, 535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic Triterpenes: New Tools to Fight Metabolic Syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Muruganathan, N.; Dhanapal, A.R.; Baskar, V.; Muthuramalingam, P.; Selvaraj, D.; Aara, H.; Shiek Abdullah, M.Z.; Sivanesan, I. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ullevig, S.L.; Short, J.D.; Wang, L.; Ahn, Y.J.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
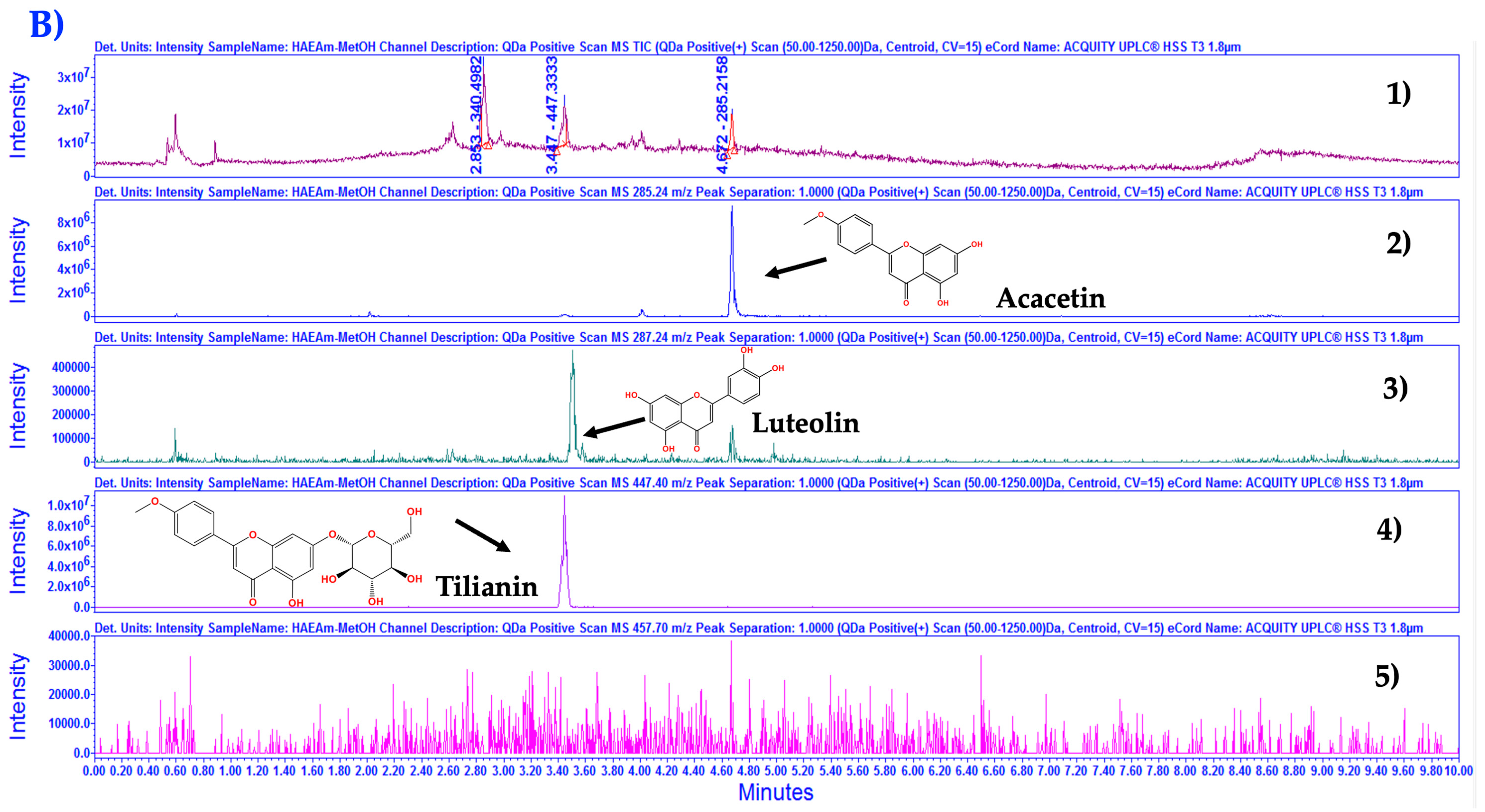
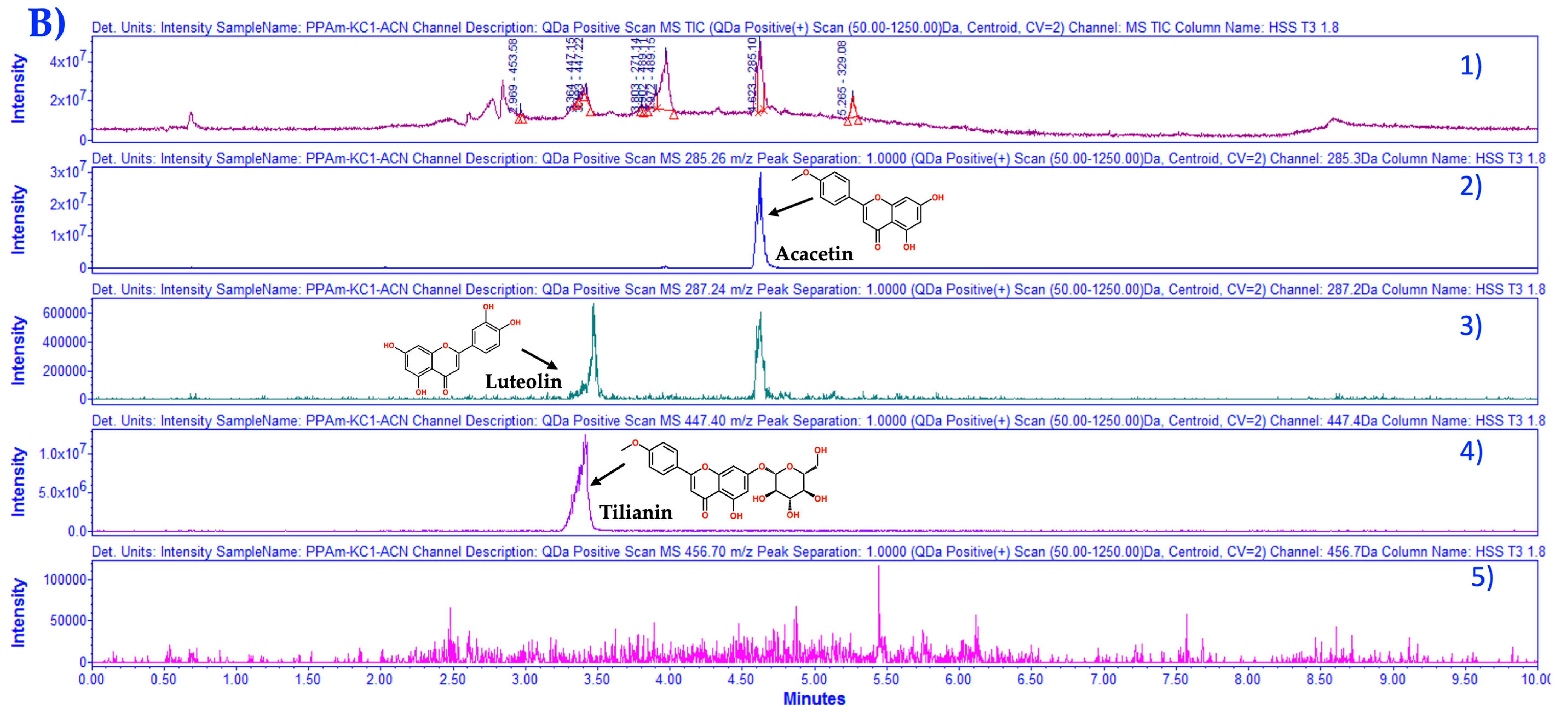
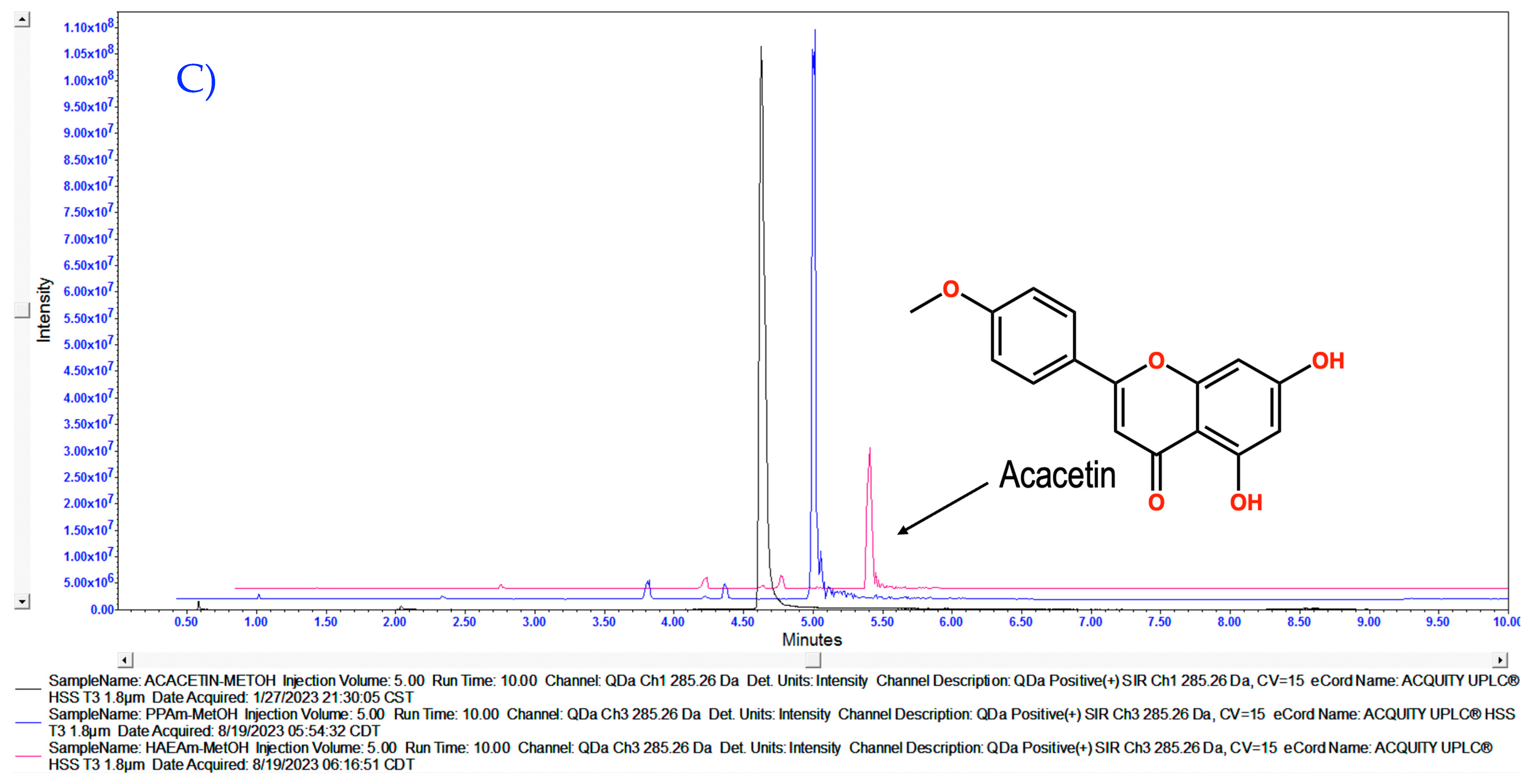
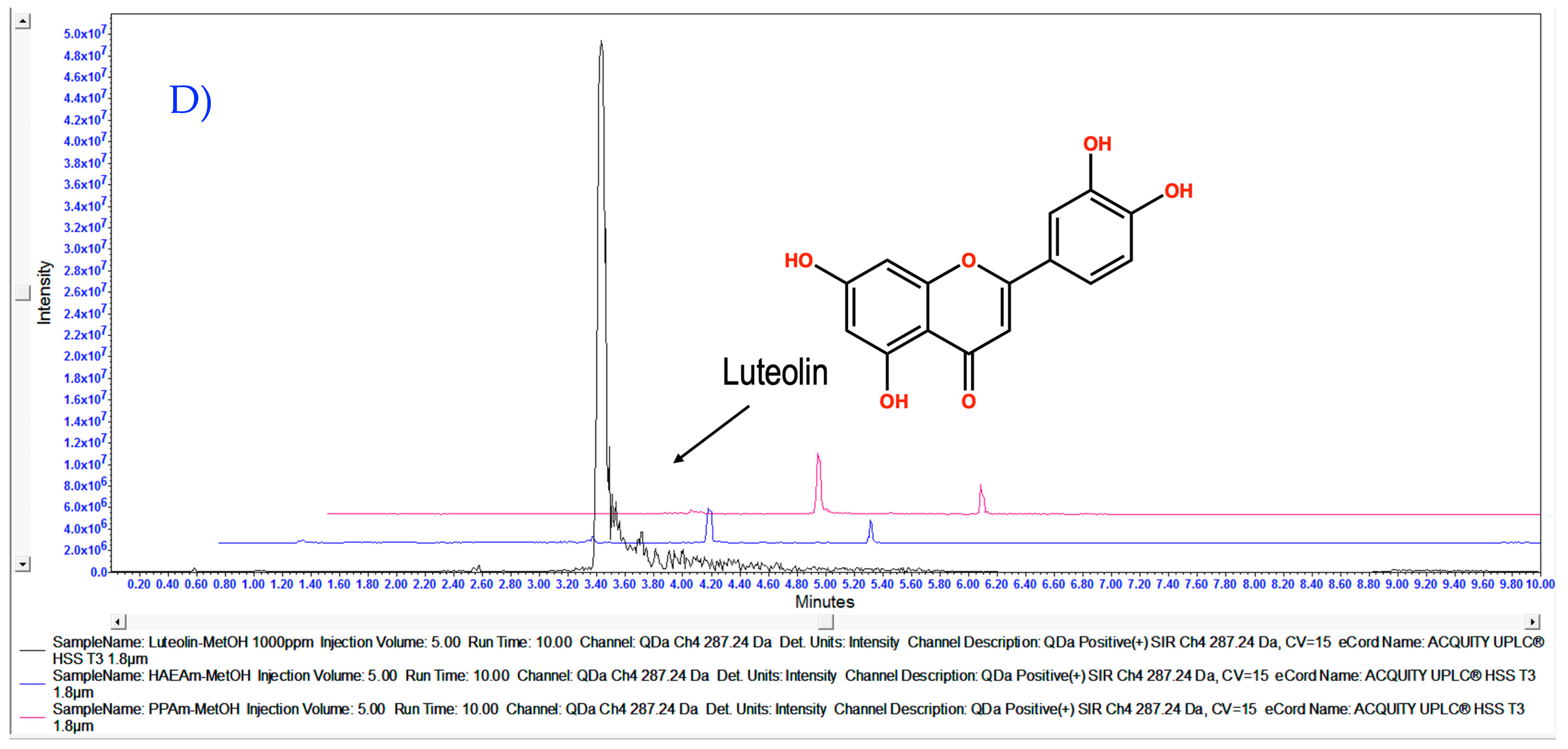
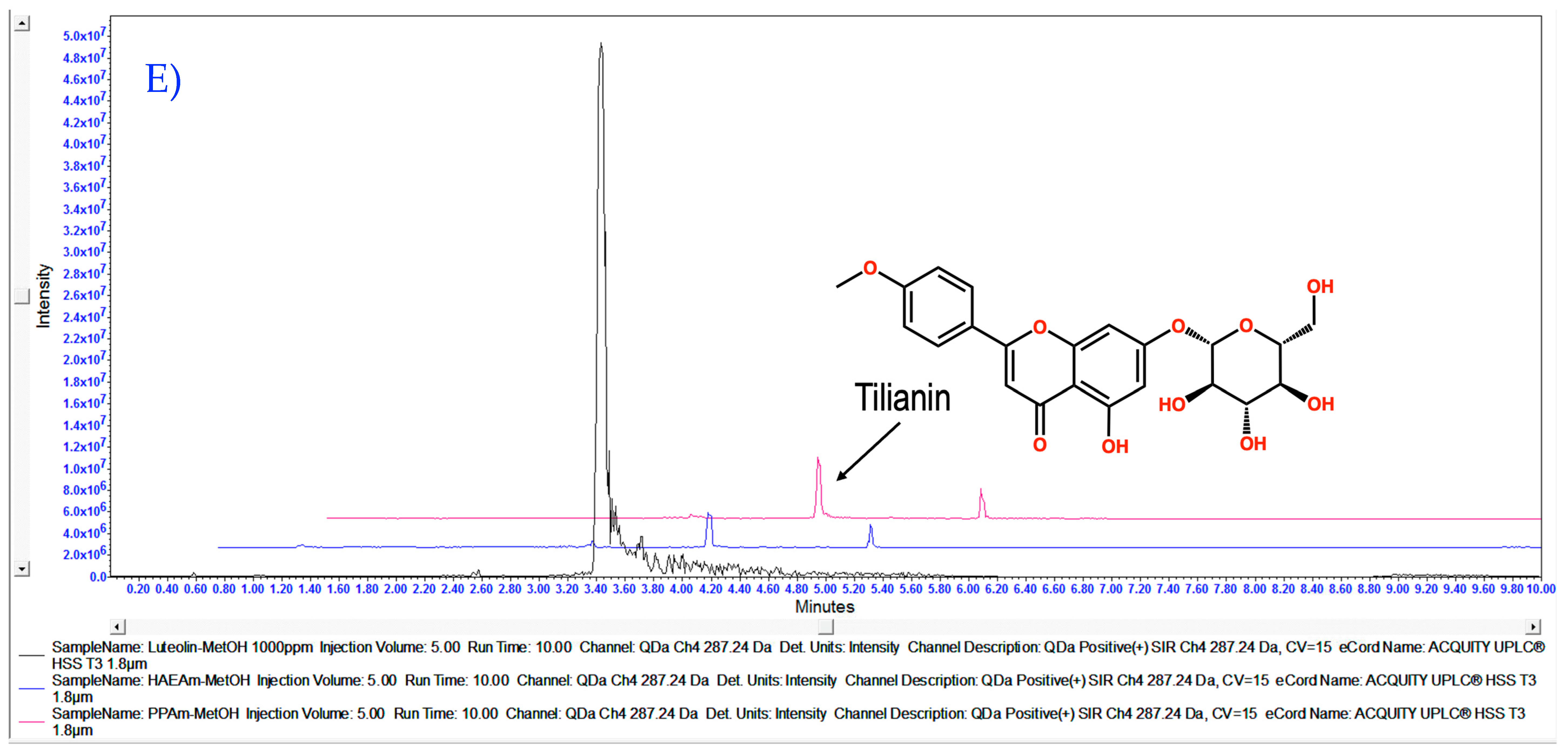
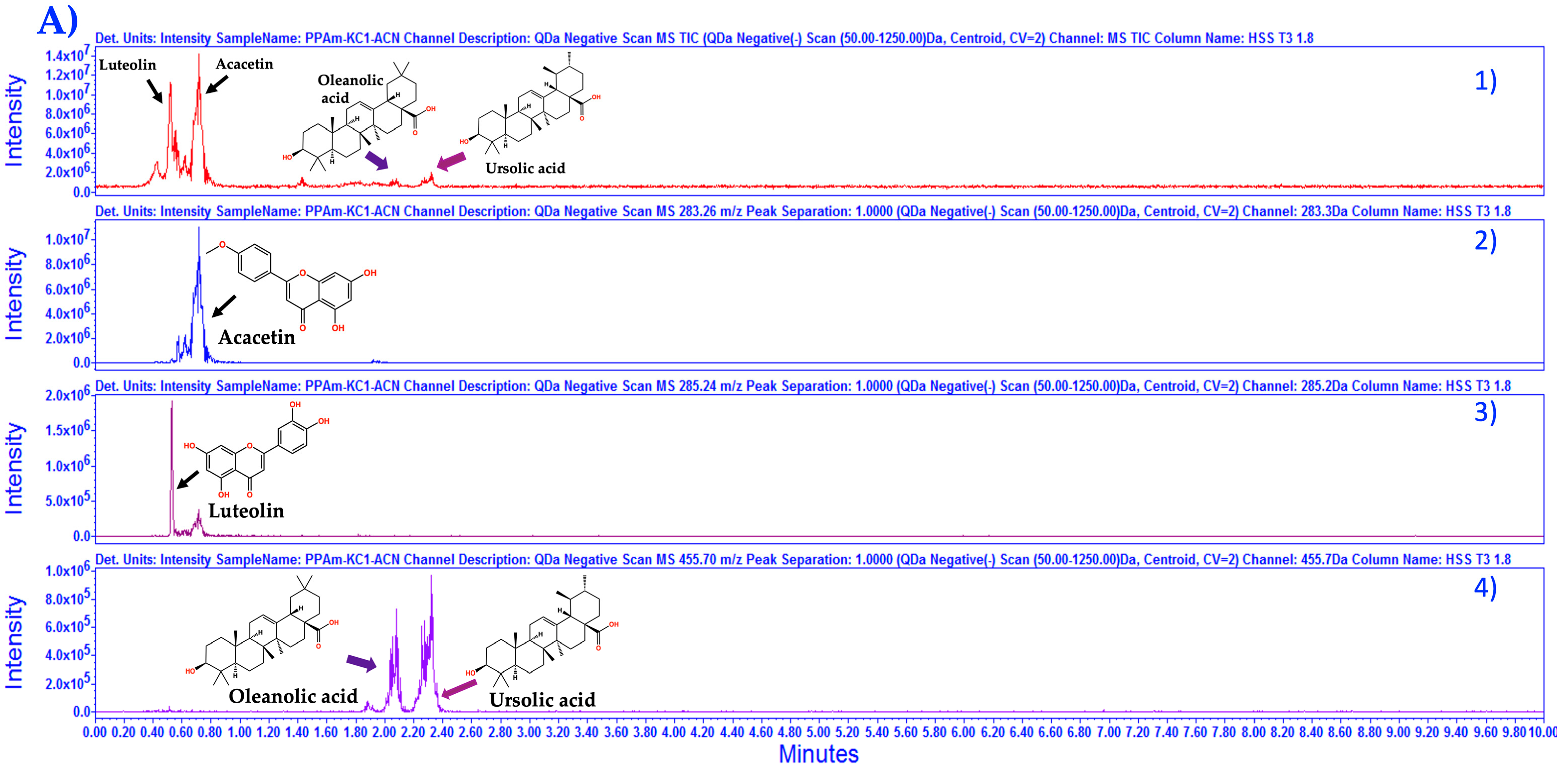
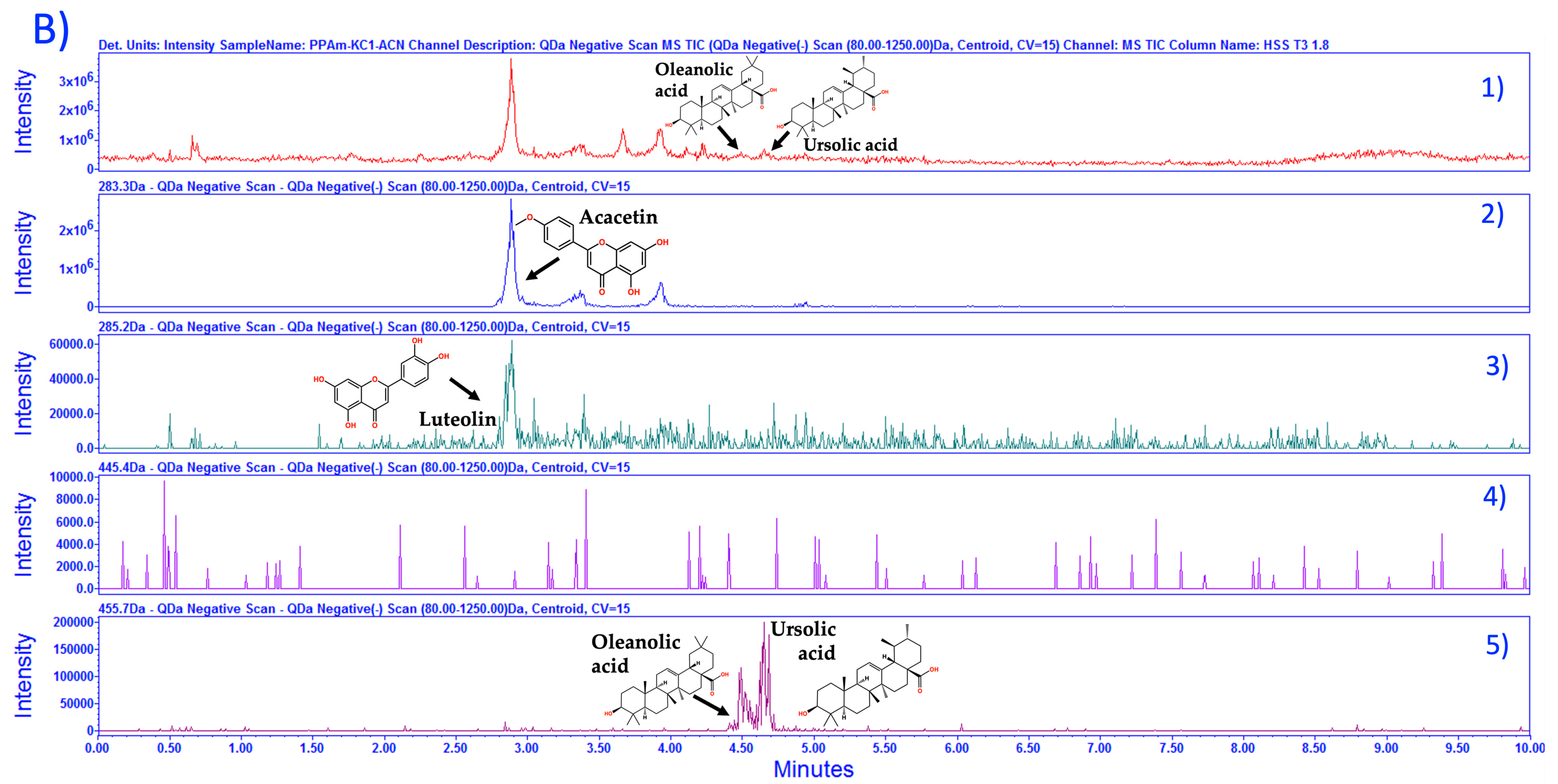
| Acacetin | Luteolin | Tilianin | |
|---|---|---|---|
| HAEAm | 19.20 | 3.88 | 6.36 |
| PPAm | 31.23 | 6.28 | 17.65 |
| HAEAm (E−) | HAEAm (E+) | PPAm (E−) | PPAm (E+) | Carbachol | Nifedipine | |
|---|---|---|---|---|---|---|
| Emax (%) | 61.5 ± 2.8 | 84.0 ± 3.6 | 100.0 ± 2.7 | 100.0 ± 0.4 | 74.1 ± 5.7 | 97 ± 2.5 |
| CE50 (µg/mL) | 503.9 ± 1.9 | 425.8 ± 3.6 | 157.3 ± 9.1 | 4.5 ± 0.7 | 0.082 ± 6.7 | 0.01 ± 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Torres, K.C.; Estrada-Soto, S.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.C.; Mora-Ramiro, B.; Perea-Arango, I.; Hernández-Núñez, E.; Villalobos-Molina, R.; Carmona-Castro, G.; et al. LC-MS Fingerprinting Development for Standardized Precipitate from Agastache mexicana, Which Induces Antihypertensive Effect through NO Production and Calcium Channel Blockade. Pharmaceutics 2023, 15, 2346. https://doi.org/10.3390/pharmaceutics15092346
Cruz-Torres KC, Estrada-Soto S, Arias-Durán L, Navarrete-Vázquez G, Almanza-Pérez JC, Mora-Ramiro B, Perea-Arango I, Hernández-Núñez E, Villalobos-Molina R, Carmona-Castro G, et al. LC-MS Fingerprinting Development for Standardized Precipitate from Agastache mexicana, Which Induces Antihypertensive Effect through NO Production and Calcium Channel Blockade. Pharmaceutics. 2023; 15(9):2346. https://doi.org/10.3390/pharmaceutics15092346
Chicago/Turabian StyleCruz-Torres, Karla Catalina, Samuel Estrada-Soto, Luis Arias-Durán, Gabriel Navarrete-Vázquez, Julio César Almanza-Pérez, Beatriz Mora-Ramiro, Irene Perea-Arango, Emanuel Hernández-Núñez, Rafael Villalobos-Molina, Gabriela Carmona-Castro, and et al. 2023. "LC-MS Fingerprinting Development for Standardized Precipitate from Agastache mexicana, Which Induces Antihypertensive Effect through NO Production and Calcium Channel Blockade" Pharmaceutics 15, no. 9: 2346. https://doi.org/10.3390/pharmaceutics15092346
APA StyleCruz-Torres, K. C., Estrada-Soto, S., Arias-Durán, L., Navarrete-Vázquez, G., Almanza-Pérez, J. C., Mora-Ramiro, B., Perea-Arango, I., Hernández-Núñez, E., Villalobos-Molina, R., Carmona-Castro, G., Medina-Díaz, I.-M., & Ávila-Villarreal, G. (2023). LC-MS Fingerprinting Development for Standardized Precipitate from Agastache mexicana, Which Induces Antihypertensive Effect through NO Production and Calcium Channel Blockade. Pharmaceutics, 15(9), 2346. https://doi.org/10.3390/pharmaceutics15092346