Predicting Volume of Distribution in Neonates: Performance of Physiologically Based Pharmacokinetic Modelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. PBPK Substrate Models
2.2. Clinical Data
2.3. PBPK Simulations
2.4. Quantitative Analyses
3. Results
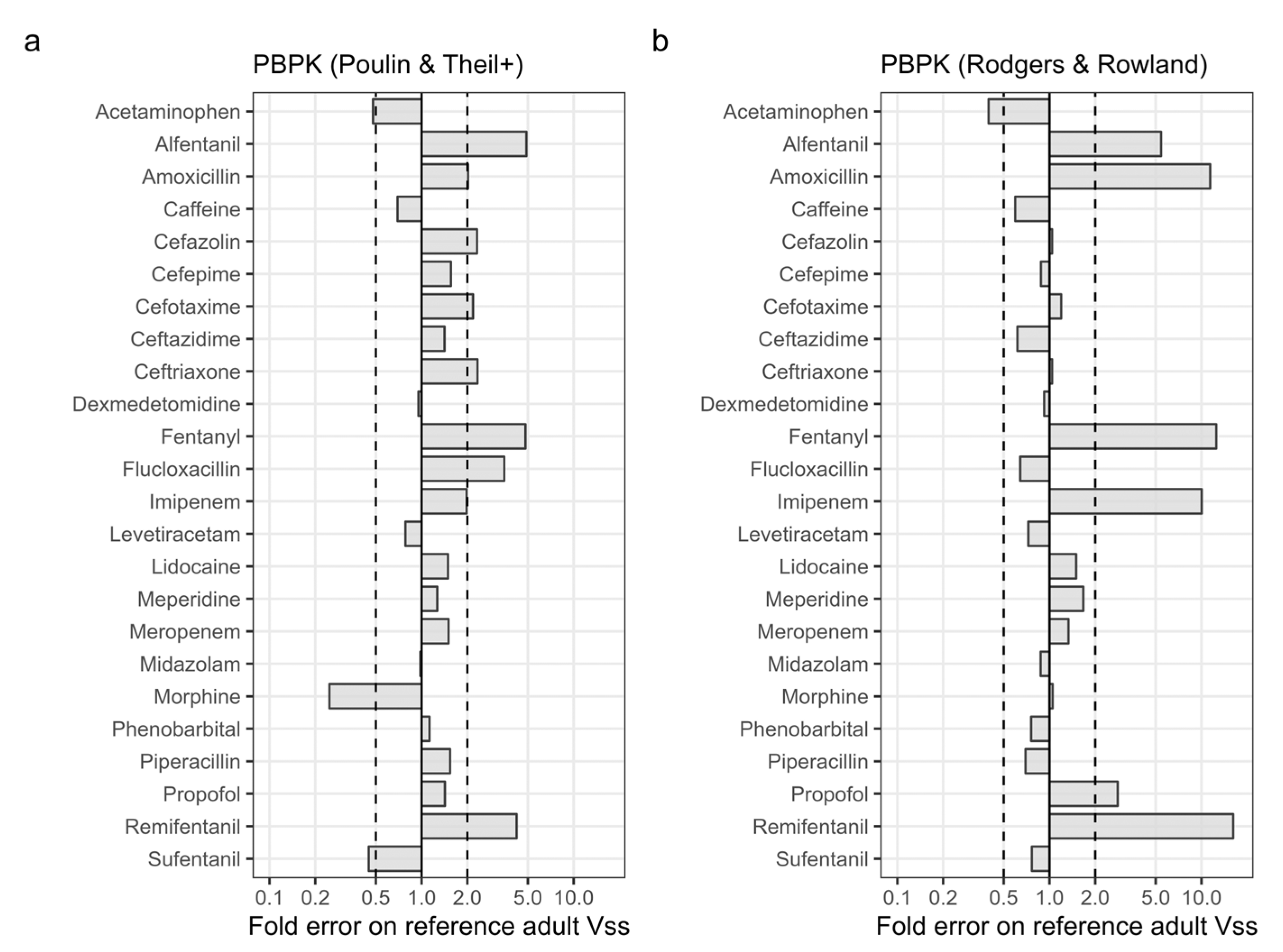
3.1. Accuracy of PBPK Predicted Adult Vss
3.2. Variability of PBPK Input Parameters
3.3. PBPK Predicted Changes in Neonatal Vss
3.4. Accuracy of Predicted Neonatal Vss
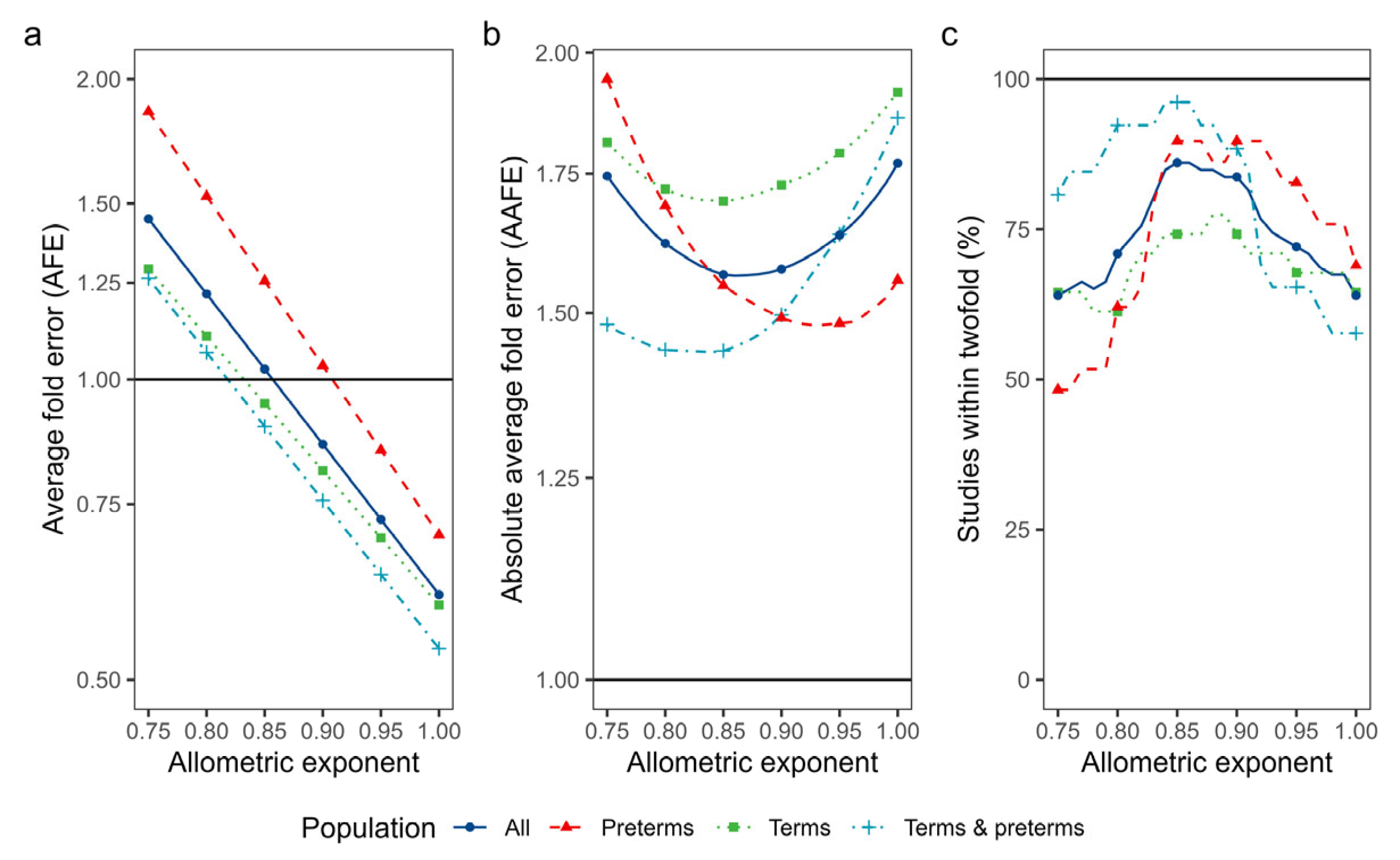
3.4.1. Isometric and Allometric Scaling of Neonatal Vss
3.4.2. PBPK Modelling of Vss
3.4.3. Covariate Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowland, M.; Tozer, T.N. Membranes and Distribution. In Clinical Pharmacokinetics and Pharmacodynamics. Concepts and Applications; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 73–110. ISBN 978-0-7817-5009-7. [Google Scholar]
- Lombardo, F.; Berellini, G.; Obach, R.S. Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds. Drug Metab. Dispos. 2018, 46, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, K.; Wright, I.M.R.; Schneider, J.J.; Jones, A.L.; Martin, J.H. Pharmacokinetics in Neonatal Prescribing: Evidence Base, Paradigms and the Future. Br. J. Clin. Pharmacol. 2015, 80, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Prediction of Clearance, Volume of Distribution, and Half-Life of Drugs in Extremely Low to Low Birth Weight Neonates: An Allometric Approach. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Mian, P.; van den Anker, J.N. Developmental Pharmacokinetics in Neonates: Maturational Changes and Beyond. Curr. Pharm. Des. 2017, 23, 5769–5778. [Google Scholar] [CrossRef]
- Sethi, P.K.; White, C.A.; Cummings, B.S.; Hines, R.N.; Muralidhara, S.; Bruckner, J.V. Ontogeny of Plasma Proteins, Albumin and Binding of Diazepam, Cyclosporine, and Deltamethrin. Pediatr. Res. 2016, 79, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.R.; Gonzalez, D.; Cohen-Wolkowiez, M.; Hornik, C.P.; Edginton, A.N. Improving Pediatric Protein Binding Estimates: An Evaluation of A1-Acid Glycoprotein Maturation in Healthy and Infected Subjects. Clin. Pharmacokinet. 2018, 57, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.; Kulo, A.; Verbesselt, R.; Naulaers, G.; de Hoon, J.; Vermeersch, P.; Allegaert, K. Cefazolin Plasma Protein Binding and Its Covariates in Neonates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3359–3365. [Google Scholar] [CrossRef]
- Michelet, R.; Bocxlaer, J.V.; Vermeulen, A. PBPK in Preterm and Term Neonates: A Review. Curr. Pharm. Des. 2017, 23, 5943–5954. [Google Scholar] [CrossRef]
- Poulin, P.; Theil, F.-P. Prediction of Pharmacokinetics Prior to in Vivo Studies. 1. Mechanism-Based Prediction of Volume of Distribution. J. Pharm. Sci. 2002, 91, 129–156. [Google Scholar] [CrossRef]
- Berezhkovskiy, L.M. Volume of Distribution at Steady State for a Linear Pharmacokinetic System with Peripheral Elimination. J. Pharm. Sci. 2004, 93, 1628–1640. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Mechanistic Approaches to Volume of Distribution Predictions: Understanding the Processes. Pharm. Res. 2007, 24, 918–933. [Google Scholar] [CrossRef]
- Johnson, T.N.; Small, B.G.; Yeo, K.R. Increasing Application of Pediatric Physiologically Based Pharmacokinetic Models across Academic and Industry Organizations. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Van Rongen, A.; Krekels, E.H.; Calvier, E.A.; de Wildt, S.N.; Vermeulen, A.; Knibbe, C.A. An Update on the Use of Allometric and Other Scaling Methods to Scale Drug Clearance in Children: Towards Decision Tables. Expert Opin. Drug Metab. Toxicol. 2022, 18, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Johnson, T.N.; Bui, K.H.; Cheung, S.Y.A.; Li, J.; Xu, H.; Al-Huniti, N.; Zhou, D. Predictive Performance of Physiologically Based Pharmacokinetic (PBPK) Modeling of Drugs Extensively Metabolized by Major Cytochrome P450s in Children. Clin. Pharmacol. Ther. 2018, 104, 188–200. [Google Scholar] [CrossRef]
- Zhou, W.; Johnson, T.N.; Xu, H.; Cheung, S.Y.A.; Bui, K.H.; Li, J.; Al-Huniti, N.; Zhou, D. Predictive Performance of Physiologically Based Pharmacokinetic and Population Pharmacokinetic Modeling of Renally Cleared Drugs in Children. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Van der Zanden, T.M.; de Wildt, S.N.; Liem, Y.; Offringa, M.; de Hoog, M. Developing a Paediatric Drug Formulary for the Netherlands. Arch. Dis. Child. 2017, 102, 357–361. [Google Scholar] [CrossRef]
- Williams, D.A.; Lemke, T.L. PKa Values for Some Drugs and Miscellaneous Organic Acids and Bases. In Foye’s Principles of Medicinal Chemistry; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2002; pp. 1070–1079. [Google Scholar]
- Mamada, H.; Iwamoto, K.; Nomura, Y.; Uesawa, Y. Predicting Blood-to-Plasma Concentration Ratios of Drugs from Chemical Structures and Volumes of Distribution in Humans. Mol. Divers. 2021, 25, 1261–1270. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Milligan, T.P.; Morris, H.C.; Hammond, P.M.; Price, C.P. Studies on Paracetamol Binding to Serum Proteins. Ann. Clin. Biochem. 1994, 31, 492–496. [Google Scholar] [CrossRef]
- Meuldermans, W.; Woestenborghs, R.; Noorduin, H.; Camu, F.; van Steenberge, A.; Heykants, J. Protein Binding of the Analgesics Alfentanil and Sufentanil in Maternal and Neonatal Plasma. Eur. J. Clin. Pharmacol. 1986, 30, 217–219. [Google Scholar] [CrossRef]
- Yasmeen, S.; Riyazuddeen; Rabbani, G. Calorimetric and Spectroscopic Binding Studies of Amoxicillin with Human Serum Albumin. J. Therm. Anal. Calorim. 2017, 127, 1445–1455. [Google Scholar] [CrossRef]
- Blanchard, J. Protein Binding of Caffeine in Young and Elderly Males. J. Pharm. Sci. 1982, 71, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Jongmans, C.; Muller, A.E.; Van Den Broek, P.; Cruz De Almeida, B.D.M.; Van Den Berg, C.; Van Oldenrijk, J.; Bos, P.K.; Koch, B.C.P. An Overview of the Protein Binding of Cephalosporins in Human Body Fluids: A Systematic Review. Front. Pharmacol. 2022, 13, 900551. [Google Scholar] [CrossRef] [PubMed]
- Weerink, M.A.S.; Struys, M.M.R.F.; Hannivoort, L.N.; Barends, C.R.M.; Absalom, A.R.; Colin, P. Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin. Pharmacokinet. 2017, 56, 893–913. [Google Scholar] [CrossRef] [PubMed]
- Bista, S.R.; Haywood, A.; Hardy, J.; Lobb, M.; Tapuni, A.; Norris, R. Protein Binding of Fentanyl and Its Metabolite Nor-Fentanyl in Human Plasma, Albumin and α-1 Acid Glycoprotein. Xenobiotica 2015, 45, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Wallenburg, E.; Brüggemann, R.J.M.; Roberts, J.A.; Jager, N.G.L.; Ulldemolins, M.; Wilkes, S.; Schouten, J.; Chin, P.K.L.; ter Heine, R. A Meta-Analysis of Protein Binding of Flucloxacillin in Healthy Volunteers and Hospitalized Patients. Clin. Microbiol. Infect. 2022, 28, 446.e1–446.e7. [Google Scholar] [CrossRef]
- Rehman, M.T.; Shamsi, H.; Khan, A.U. Insight into the Binding Mechanism of Imipenem to Human Serum Albumin by Spectroscopic and Computational Approaches. Mol. Pharm. 2014, 11, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Routledge, P.A.; Barchowsky, A.; Blornsson, T.D.; Kitchell, B.B.; Shand, D.G. Lidocaine Plasma Protein Binding. Clin. Pharmacol. Ther. 1980, 27, 347–351. [Google Scholar] [CrossRef]
- Wong, Y.C.; Chan, K.; Lau, O.W.; Aun, C.; Houghton, I.T. Protein Binding Characterization of Pethidine and Norpethidine and Lack of Interethnic Variability. Methods Find. Exp. Clin. Pharmacol. 1991, 13, 273–279. [Google Scholar]
- Halliday, N.J.; Dundee, J.W.; Collier, P.S.; Loughran, P.G.; Harper, K.W. Influence of Plasma Proteins on the Onset of Hypnotic Action of Intravenous Midazolam. Anaesthesia 1985, 40, 763–766. [Google Scholar] [CrossRef]
- Olsen, G.D. Morphine Binding to Human Plasma Proteins. Clin. Pharmacol. Ther. 1975, 17, 31–35. [Google Scholar] [CrossRef]
- Bailey, D.N.; Briggs, J.R. The Binding of Selected Therapeutic Drugs to Human Serum α-1 Acid Glycoprotein and to Human Serum Albumin In Vitro. Ther. Drug Monit. 2004, 26, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the General Anesthetics Propofol and Halothane to Human Serum Albumin: High Resolution Crystal Structures. J. Biol. Chem. 2000, 275, 38731–38738. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Donepudi, R.V.; Argoti, P.S.; Giezentanner, A.L.; Jain, R.; Boring, N.; Garcia, E.; Moise, K.J. Exploring the Pharmacokinetic Profile of Remifentanil in Mid-Trimester Gestations Undergoing Fetal Intervention Procedures. Front. Pharmacol. 2017, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Meistelman, C.; Benhamou, D.; Barre, J.; Levron, J.-C.; Mahe, V.; Mazoit, X.; Ecoffey, C. Effects of Age on Plasma Protein Binding of Sufentanil. Anesthesiology 1990, 72, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.D.; Jones, H.M.; Rowland, M.; Gibson, C.R.; Yates, J.W.T.; Chien, J.Y.; Ring, B.J.; Adkison, K.K.; Ku, M.S.; He, H.; et al. PhRMA CPCDC Initiative on Predictive Models of Human Pharmacokinetics, Part 2: Comparative Assessment of Prediction Methods of Human Volume of Distribution. J. Pharm. Sci. 2011, 100, 4074–4089. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; De Bruyn, T.; Wright, M.; Broccatelli, F. Comparing Mechanistic and Preclinical Predictions of Volume of Distribution on a Large Set of Drugs. Pharm. Res. 2018, 35, 87. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.A.; Santanna, J.; Schmid, C.H.; Szczech, L.A.; Feldman, H.I. Individual Patient- versus Group-Level Data Meta-Regressions for the Investigation of Treatment Effect Modifiers: Ecological Bias Rears Its Ugly Head. Stat. Med. 2002, 21, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental Pharmacology—Drug Disposition, Action, and Therapy in Infants and Children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef]
- Lu, H.; Rosenbaum, S. Developmental Pharmacokinetics in Pediatric Populations. J. Pediatr. Pharmacol. Ther. 2014, 19, 262–276. [Google Scholar] [CrossRef]
- Yang, X.; Wu, H.; Mehta, D.; Sullivan, M.C.; Wang, J.; Burckart, G.J.; Troutman, J.A.; Fisher, J.W. Ontogeny Equations with Probability Distributions for Anthropomorphic Measurements in Preterm and Term Neonates and Infants for Use in a PBPK Model. Comput. Toxicol. 2019, 11, 101–117. [Google Scholar] [CrossRef]
- Abduljalil, K.; Pan, X.; Pansari, A.; Jamei, M.; Johnson, T.N. A Preterm Physiologically Based Pharmacokinetic Model. Part I: Physiological Parameters and Model Building. Clin. Pharmacokinet. 2020, 59, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Lingvall, M.; Reith, D.; Broadbent, R. The Effect of Sepsis upon Gentamicin Pharmacokinetics in Neonates. Br. J. Clin. Pharmacol. 2005, 59, 54–61. [Google Scholar] [CrossRef]
- Conil, J.M.; Georges, B.; Lavit, M.; Laguerre, J.; Samii, K.; Houin, G.; Saivin, S. A Population Pharmacokinetic Approach to Ceftazidime Use in Burn Patients: Influence of Glomerular Filtration, Gender and Mechanical Ventilation. Br. J. Clin. Pharmacol. 2007, 64, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Hochwald, O.; Jabr, M.; Osiovich, H.; Miller, S.P.; McNamara, P.J.; Lavoie, P.M. Preferential Cephalic Redistribution of Left Ventricular Cardiac Output during Therapeutic Hypothermia for Perinatal Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2014, 164, 999–1004.e1. [Google Scholar] [CrossRef] [PubMed]
- Gal, P.; Gilman, J.T. Drug Disposition in Neonates with Patent Ductus Arteriosus. Ann. Pharmacother. 1993, 27, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, M.P.H.; Groenendaal, F.; Egberts, A.C.G.; Rademaker, C.M.A. Effects of Hypothermia on Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2010, 49, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Korzekwa, K.; Radice, C.; Nagar, S. A Permeability- and Perfusion-based PBPK Model for Improved Prediction of Concentration-time Profiles. Clin. Transl. Sci. 2022, 15, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Benet, L.Z. Effects of Drug Transporters on Volume of Distribution. AAPS J. 2009, 11, 250–261. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.-F.; Saudrais, E.; Strazielle, N. Barriers to Drug Distribution into the Perinatal and Postnatal Brain. Pharm. Res. 2018, 35, 84. [Google Scholar] [CrossRef]
- Van Groen, B.D.; Nicolaï, J.; Kuik, A.C.; Cruchten, S.V.; van Peer, E.; Smits, A.; Schmidt, S.; de Wildt, S.N.; Allegaert, K.; Schaepdrijver, L.D.; et al. Ontogeny of Hepatic Transporters and Drug-Metabolizing Enzymes in Humans and in Nonclinical Species. Pharmacol. Rev. 2021, 73, 597–678. [Google Scholar] [CrossRef]
- Kilbaugh, T.J.; Himebauch, A.S.; Zaoutis, T.; Jobes, D.; Greeley, W.J.; Nicolson, S.C.; Zuppa, A.F. A Pilot and Feasibility Study of the Plasma and Tissue Pharmacokinetics of Cefazolin in an Immature Porcine Model of Pediatric Cardiac Surgery. Pediatr. Anesth. 2015, 25, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.A.H.; Clements, J.A.; Prescott, L.F. Clinical Pharmacokinetics of Paracetamol. Clin. Pharmacokinet. 1982, 7, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.; Nilsson, A.; Hartvig, P.; Tamsen, A. Pharmacokinetics of Midazolam in Total i.v. Anaesthesia. Br. J. Anaesth. 1987, 59, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A.; Koeppe, P. Comparative Pharmacokinetic Analysis of Amoxycillin Using Open Two and Three-Compartment Models. Eur. J. Clin. Pharmacol. 1982, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.J. The Pharmacology of Caffeine. In Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrès des Recherches Pharmaceutiques; Jucker, E., Meyer, U., Eds.; Birkhäuser: Basel, Switzerland, 1987; pp. 273–313. ISBN 978-3-0348-9289-6. [Google Scholar]
- Bergan, T. Comparative Pharmacokinetics of Cefazolin, Cephalothin, Cephacetril, and Cephapirine after Intravenous Administration. Chemotherapy 1977, 23, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Pais, G.M.; Chang, J.; Barreto, E.F.; Stitt, G.; Downes, K.J.; Alshaer, M.H.; Lesnicki, E.; Panchal, V.; Bruzzone, M.; Bumanglag, A.V.; et al. Clinical Pharmacokinetics and Pharmacodynamics of Cefepime. Clin. Pharmacokinet. 2022, 61, 929–953. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, R.; Blaser, J.; Bonetti, A.; Simmen, H.; Wise, R.; Siegenthaler, W. Comparative Multiple-Dose Pharmacokinetics of Cefotaxime, Moxalactam, and Ceftazidime. Rev. Infect. Dis. 1982, 4, S581–S584. [Google Scholar] [CrossRef]
- Zhou, H.H.; Chan, Y.P.; Arnold, K.; Sun, M. Single-Dose Pharmacokinetics of Ceftriaxone in Healthy Chinese Adults. Antimicrob. Agents Chemother. 1985, 27, 192–196. [Google Scholar] [CrossRef]
- Ariano, R.E.; Duke, P.C.; Sitar, D.S. Population Pharmacokinetics of Fentanyl in Healthy Volunteers. J. Clin. Pharmacol. 2001, 41, 757–763. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Kirkpatrick, C.M.J.; Kinzig-Schippers, M.; Bulitta, J.B.; Holzgrabe, U.; Drusano, G.L.; Sörgel, F. Population Pharmacokinetics at Two Dose Levels and Pharmacodynamic Profiling of Flucloxacillin. Antimicrob. Agents Chemother. 2007, 51, 3290–3297. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Raungsri, N.; Punyo, J.; Sriwiriyajan, S. Pharmacokinetics of Imipenem in Healthy Volunteers Following Administration by 2 h or 0.5 h Infusion. J. Antimicrob. Chemother. 2005, 56, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N. Clinical Pharmacokinetics of Levetiracetam. Clin. Pharmacokinet. 2004, 43, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.N.; Aarons, L.J.; Bending, M.R.; Steiner, J.A.; Rowland, M. Pharmacokinetics of Lidocaine and Its Deethylated Metabolite: Dose and Time Dependency Studies in Man. J. Pharmacokinet. Biopharm. 1982, 10, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Mather, L.E.; Tucker, G.T.; Pflug, A.E.; Lindop, M.J.; Wilkerson, C. Meperidine Kinetics in Man; Intravenous Injection in Surgical Patients and Volunteers. Clin. Pharmacol. Ther. 1975, 17, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bax, R.P.; Bastain, W.; Featherstone, A.; Wilkinson, D.M.; Hutchison, M. The Pharmacokinetics of Meropenem in Volunteers. J. Antimicrob. Chemother. 1989, 24, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Hasselström, J.; Säwe, J. Morphine Pharmacokinetics and Metabolism in Humans. Clin. Pharmacokinet. 1993, 24, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.; Powell, J.R.; Conrad, K.; Likes, K.; Byers, J.; Baker, S.; Perrier, D. Phenobarbital Pharmacokinetics and Bioavailability in Adults. J. Clin. Pharmacol. 1982, 22, 141–148. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Kinzig, M.; Jakob, V.; Holzgrabe, U.; Sörgel, F.; Holford, N.H.G. Nonlinear Pharmacokinetics of Piperacillin in Healthy Volunteers—Implications for Optimal Dosage Regimens. Br. J. Clin. Pharmacol. 2010, 70, 682–693. [Google Scholar] [CrossRef]
- Eleveld, D.J.; Proost, J.H.; Cortínez, L.I.; Absalom, A.R.; Struys, M.M.R.F. A General Purpose Pharmacokinetic Model for Propofol. Anesth. Analg. 2014, 118, 1221–1237. [Google Scholar] [CrossRef]
- Bovill, J.G.; Sebel, P.S.; Blackburn, C.L.; Oei-Lim, V.; Heykants, J.J. The Pharmacokinetics of Sufentanil in Surgical Patients. Anesthesiology 1984, 61, 502–506. [Google Scholar] [CrossRef]
- Van Ganzewinkel, C.; Derijks, L.; Anand, K.; van Lingen, R.; Neef, C.; Kramer, B.; Andriessen, P. Multiple Intravenous Doses of Paracetamol Result in a Predictable Pharmacokinetic Profile in Very Preterm Infants. Acta Paediatr. 2014, 103, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.F.; Roberts, J.K.; Samiee-Zafarghandy, S.; Stockmann, C.; King, A.D.; Deutsch, N.; Williams, E.F.; Allegaert, K.; Wilkins, D.G.; Sherwin, C.M.T.; et al. Population Pharmacokinetics of Intravenous Paracetamol (Acetaminophen) in Preterm and Term Neonates: Model Development and External Evaluation. Clin. Pharmacokinet. 2016, 55, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Killian, A.; Davis, P.J.; Stiller, R.L.; Cicco, R.; Cook, D.R.; Guthrie, R.D. Influence of Gestational Age on Pharmacokinetics of Alfentanil in Neonates. Dev. Pharmacol. Ther. 1990, 15, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Pokela, M.-L.; Ryhanen, P.T.; Koivisto, M.E.; Olkkola, K.T.; Saukkonen, A.-L. Alfentanil-Induced Rigidity in Newborn Infants. Anesth. Analg. 1992, 75, 252. [Google Scholar] [CrossRef] [PubMed]
- Huisman-de Boer, J.J.; van den Anker, J.N.; Vogel, M.; Goessens, W.H.; Schoemaker, R.C.; de Groot, R. Amoxicillin Pharmacokinetics in Preterm Infants with Gestational Ages of Less than 32 Weeks. Antimicrob. Agents Chemother. 1995, 39, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Charles, B.G.; Preechagoon, Y.; Lee, T.C.; Steer, P.A.; Flenady, V.J.; Debuse, N. Population Pharmacokinetics of Intravenous Amoxicillin in Very Low Birth Weight Infants. J. Pharm. Sci. 1997, 86, 1288–1292. [Google Scholar] [CrossRef]
- Pullen, J.; Stolk, L.M.L.; Nieman, F.H.M.; Degraeuwe, P.L.J.; van Tiel, F.H.; Zimmermann, L.J.I. Population Pharmacokinetics and Dosing of Amoxicillin in (Pre)Term Neonates. Ther. Drug Monit. 2006, 28, 226. [Google Scholar] [CrossRef]
- Pullen, J.; Driessen, M.; Stolk, L.M.L.; Degraeuwe, P.L.J.; van Tiel, F.H.; Neef, C.; Zimmermann, L.J.I. Amoxicillin Pharmacokinetics in (Preterm) Infants Aged 10 to 52 Days: Effect of Postnatal Age. Ther. Drug Monit. 2007, 29, 376. [Google Scholar] [CrossRef]
- Bijleveld, Y.; Mathôt, R.; van der Lee, J.; Groenendaal, F.; Dijk, P.; van Heijst, A.; Simons, S.; Dijkman, K.; van Straaten, H.; Rijken, M.; et al. Population Pharmacokinetics of Amoxicillin in Term Neonates Undergoing Moderate Hypothermia. Clin. Pharmacol. Ther. 2018, 103, 458–467. [Google Scholar] [CrossRef]
- Tang, B.-H.; Wu, Y.-E.; Kou, C.; Qi, Y.-J.; Qi, H.; Xu, H.-Y.; Leroux, S.; Huang, X.; Zhou, Y.; Zheng, Y.; et al. Population Pharmacokinetics and Dosing Optimization of Amoxicillin in Neonates and Young Infants. Antimicrob. Agents Chemother. 2019, 63, e02336-18. [Google Scholar] [CrossRef]
- Charles, B.G.; Townsend, S.R.; Steer, P.A.; Flenady, V.J.; Gray, P.H.; Shearman, A. Caffeine Citrate Treatment for Extremely Premature Infants With Apnea: Population Pharmacokinetics, Absolute Bioavailability, and Implications for Therapeutic Drug Monitoring. Ther. Drug Monit. 2008, 30, 709. [Google Scholar] [CrossRef]
- Deguchi, Y.; Koshida, R.; Nakashima, E.; Watanabe, R.; Taniguchi, N.; Ichimura, F.; Tsuji, A. Interindividual Changes in Volume of Distribution of Cefazolin in Newborn Infants and Its Prediction Based on Physiological Pharmacokinetic Concepts. J. Pharm. Sci. 1988, 77, 674–678. [Google Scholar] [CrossRef] [PubMed]
- De Cock, R.F.W.; Smits, A.; Allegaert, K.; de Hoon, J.; Saegeman, V.; Danhof, M.; Knibbe, C.A.J. Population Pharmacokinetic Modelling of Total and Unbound Cefazolin Plasma Concentrations as a Guide for Dosing in Preterm and Term Neonates. J. Antimicrob. Chemother. 2014, 69, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Lima-Rogel, V.; Medina-Rojas, E.L.; Del Carmen Milán-Segovia, R.; Noyola, D.E.; Nieto-Aguirre, K.; López-Delarosa, A.; Romano-Moreno, S. Population Pharmacokinetics of Cefepime in Neonates with Severe Nosocomial Infections. J. Clin. Pharm. Ther. 2008, 33, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Bradley, J.S.; Reed, M.D.; van den Anker, J.N.; Domonoske, C.; Capparelli, E.V. Population Pharmacokinetic Assessment and Pharmacodynamic Implications of Pediatric Cefepime Dosing for Susceptible-Dose-Dependent Organisms. Antimicrob. Agents Chemother. 2016, 60, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, B.-F.; Kou, C.; Xu, H.-Y.; Tang, B.-H.; Wu, Y.-E.; Hao, G.-X.; Zhang, X.-P.; Zhao, W. Developmental Population Pharmacokinetics and Dosing Optimization of Cefepime in Neonates and Young Infants. Front. Pharmacol. 2020, 11, 14. [Google Scholar] [CrossRef]
- Kearns, G.L.; Jacobs, R.F.; Thomas, B.R.; Darville, T.L.; Trang, J.M. Cefotaxime and Desacetylcefotaxime Pharmacokinetics in Very Low Birth Weight Neonates. J. Pediatr. 1989, 114, 461–467. [Google Scholar] [CrossRef]
- Aujard, Y.; Brion, F.; Jacqz-Aigrain, E.; Kasse, M.C.; Chretien, P.; Criqui, C.; Mathieu, H. Pharmacokinetics of Cefotaxime and Desacetylcefotaxime in the Newborn. Diagn. Microbiol. Infect. Dis. 1989, 12, 87–91. [Google Scholar] [CrossRef]
- Shang, Z.-H.; Wu, Y.-E.; Lv, D.-M.; Zhang, W.; Liu, W.-Q.; van den Anker, J.; Xu, Y.; Zhao, W. Optimal Dose of Cefotaxime in Neonates with Early-Onset Sepsis: A Developmental Pharmacokinetic Model-Based Evaluation. Front. Pharmacol. 2022, 13, 916253. [Google Scholar] [CrossRef]
- Van Den Anker, J.N.; Van Der Heijden, B.J.; Hop, W.C.J.; Schoemaker, R.C.; Broerse, H.M.; Neijens, H.J.; Groot, R.D. The Effect of Asphyxia on the Pharmacokinetics of Ceftazidime in the Term Newborn. Pediatr. Res. 1995, 38, 808–811. [Google Scholar] [CrossRef]
- Van den Anker, J.N.; Schoemaker, R.C.; Hop, W.C.J.; van der Heijden, B.J.; Weber, A.; Sauer, P.J.J.; Neijens, H.J.; de Groot, R. Ceftazidime Pharmacokinetics in Preterm Infants: Effects of Renal Function and Gestational Age. Clin. Pharmacol. Ther. 1995, 58, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Van den Anker, J.N.; Schoemaker, R.C.; van der Heijden, B.J.; Broerse, H.M.; Neijens, H.J.; de Groot, R. Once-Daily versus Twice-Daily Administration of Ceftazidime in the Preterm Infant. Antimicrob. Agents Chemother. 1995, 39, 2048–2050. [Google Scholar] [CrossRef] [PubMed]
- Van den Anker, J.N.; Hop, W.C.; Schoemaker, R.C.; van der Heijden, B.J.; Neijens, H.J.; de Groot, R. Ceftazidime Pharmacokinetics in Preterm Infants: Effect of Postnatal Age and Postnatal Exposure to Indomethacin. Br. J. Clin. Pharmacol. 1995, 40, 439–443. [Google Scholar] [PubMed]
- Wang, H.; Li, X.; Sun, S.; Mao, G.; Xiao, P.; Fu, C.; Liang, Z.; Zheng, M.; Huang, Y.; Tang, H.; et al. Population Pharmacokinetics and Dosing Simulations of Ceftazidime in Chinese Neonates. J. Pharm. Sci. 2018, 107, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- McCracken, G.H.; Siegel, J.D.; Threlkeld, N.; Thomas, M. Ceftriaxone Pharmacokinetics in Newborn Infants. Antimicrob. Agents Chemother. 1983, 23, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Koup, J.R.; Paravicini, U.; Stoeckel, K. Pharmacokinetics of Ceftriaxone in Neonates and Infants with Meningitis. J. Pediatr. 1984, 105, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Schaad, U.B.; Hayton, W.L.; Stoeckel, K. Single-Dose Ceftriaxone Kinetics in the Newborn. Clin. Pharmacol. Ther. 1985, 37, 522–528. [Google Scholar] [CrossRef]
- Mulhall, A.; de Louvois, J.; James, J. Pharmacokinetics and Safety of Ceftriaxone in the Neonate. Eur. J. Pediatr. 1985, 144, 379–382. [Google Scholar] [CrossRef]
- Chrysostomou, C.; Schulman, S.R.; Castellanos, M.H.; Cofer, B.E.; Mitra, S.; da Rocha, M.G.; Wisemandle, W.A.; Gramlich, L. A Phase II/III, Multicenter, Safety, Efficacy, and Pharmacokinetic Study of Dexmedetomidine in Preterm and Term Neonates. J. Pediatr. 2014, 164, 276–282.e3. [Google Scholar] [CrossRef]
- McAdams, R.M.; Pak, D.; Lalovic, B.; Phillips, B.; Shen, D.D. Dexmedetomidine Pharmacokinetics in Neonates with Hypoxic-Ischemic Encephalopathy Receiving Hypothermia. Anesthesiol. Res. Pract. 2020, 2020, e2582965. [Google Scholar] [CrossRef]
- Koehntop, D.E.; Rodman, J.H.; Brundage, D.M.; Hegland, M.G.; Buckley, J.J. Pharmacokinetics of Fentanyl in Neonates. Anesth. Analg. 1986, 65, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Gauntlett, I.S.; Fisher, D.M.; Hertzka, R.E.; Kuhis, E.; Spellman, M.J.; Rudolph, C. Pharmacokinetics of Fentanyl in Neonatal Humans and Lambs: Effects of Age. Anesthesiology 1988, 69, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Herngren, L.; Ehrnebo, M.; Broberger, U. Pharmacokinetics of Free and Total Flucloxacilin in Newborn Infants. Eur. J. Clin. Pharmacol. 1987, 32, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Pullen, J.; de Rozario, L.; Stolk, L.M.L.; Degraeuwe, P.L.J.; van Tiel, F.H.; Zimmermann, L.J.I. Population Pharmacokinetics and Dosing of Flucloxacillin in Preterm and Term Neonates. Ther. Drug Monit. 2006, 28, 351. [Google Scholar] [CrossRef] [PubMed]
- Gruber, W.C.; Rench, M.A.; Garcia-Prats, J.A.; Edwards, M.S.; Baker, C.J. Single-Dose Pharmacokinetics of Imipenem-Cilastatin in Neonates. Antimicrob. Agents Chemother. 1985, 27, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Freij, B.J.; McCracken, G.H.; Olsen, K.D.; Threlkeld, N. Pharmacokinetics of Imipenem-Cilastatin in Neonates. Antimicrob. Agents Chemother. 1985, 27, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Kliegman, R.M.; Yamashita, T.S.; Myers, C.M.; Blumer, J.L. Clinical Pharmacology of Imipenem and Cilastatin in Premature Infants during the First Week of Life. Antimicrob. Agents Chemother. 1990, 34, 1172–1177. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Ikawa, K.; Ikeda, K.; Ohge, H.; Morikawa, N. Population Pharmacokinetic–Pharmacodynamic Target Attainment Analysis of Imipenem Plasma and Urine Data in Neonates and Children. Pediatr. Infect. Dis. J. 2013, 32, 1208. [Google Scholar] [CrossRef]
- Merhar, S.L.; Schibler, K.R.; Sherwin, C.M.; Meinzen-Derr, J.; Shi, J.; Balmakund, T.; Vinks, A.A. Pharmacokinetics of Levetiracetam in Neonates with Seizures. J. Pediatr. 2011, 159, 152–154.e3. [Google Scholar] [CrossRef]
- Sharpe, C.M.; Capparelli, E.V.; Mower, A.; Farrell, M.J.; Soldin, S.J.; Haas, R.H. A Seven-Day Study of the Pharmacokinetics of Intravenous Levetiracetam in Neonates: Marked Changes in Pharmacokinetics Occur during the First Week of Life. Pediatr. Res. 2012, 72, 43–49. [Google Scholar] [CrossRef]
- Lima-Rogel, V.; López-López, E.J.; Medellín-Garibay, S.E.; Gómez-Ruiz, L.M.; Romero-Méndez, C.; Milán-Segovia, R.C.; Romano-Moreno, S. Population Pharmacokinetics of Levetiracetam in Neonates with Seizures. J. Clin. Pharm. Ther. 2018, 43, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, M.P.H.; Huitema, A.D.R.; van Hasselt, J.G.C.; Groenendaal, F.; Toet, M.C.; Egberts, T.C.G.; de Vries, L.S.; Rademaker, C.M.A. Lidocaine (Lignocaine) Dosing Regimen Based upon a Population Pharmacokinetic Model for Preterm and Term Neonates with Seizures. Clin. Pharmacokinet. 2011, 50, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Pokela, M.-L.; Olkkola, K.T.; Koivisto, M.; Ryhänen, P. Pharmacokinetics and Pharmacodynamics of Intravenous Meperidine in Neonates and Infants. Clin. Pharmacol. Ther. 1992, 52, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Van den Anker, J.N.; Pokorna, P.; Kinzig-Schippers, M.; Martinkova, J.; de Groot, R.; Drusano, G.L.; Sorgel, F. Meropenem Pharmacokinetics in the Newborn. Antimicrob. Agents Chemother. 2009, 53, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Padari, H.; Metsvaht, T.; Kõrgvee, L.-T.; Germovsek, E.; Ilmoja, M.-L.; Kipper, K.; Herodes, K.; Standing, J.F.; Oselin, K.; Lutsar, I. Short versus Long Infusion of Meropenem in Very-Low-Birth-Weight Neonates. Antimicrob. Agents Chemother. 2012, 56, 4760–4764. [Google Scholar] [CrossRef]
- Lima-Rogel, V.; Olguín-Mexquitic, L.; Kühn-Córdova, I.; Correa-López, T.; Romano-Aguilar, M.; Romero-Méndez, M.d.C.; Medellín-Garibay, S.E.; Romano-Moreno, S. Optimizing Meropenem Therapy for Severe Nosocomial Infections in Neonates. J. Pharm. Sci. 2021, 110, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Jacqz-Aigrain, E.; Wood, C.; Robieux, I. Pharmacokinetics of Midazolam in Critically Ill Neonates. Eur. J. Clin. Pharmacol. 1990, 39, 191–192. [Google Scholar] [CrossRef]
- Jacqz-Aigrain, E.; Daoud, P.; Burtin, P.; Maherzi, S.; Beaufils, F. Pharmacokinetics of Midazolam during Continuous Infusion in Critically Ill Neonates. Eur. J. Clin. Pharmacol. 1992, 42, 329–332. [Google Scholar] [CrossRef]
- Harte, G.; Gray, P.; Lee, T.; Steer, P.; Charles, B. Haemodynamic Responses and Population Pharmacokinetics of Midazolam Following Administration to Ventilated, Preterm Neonates. J. Paediatr. Child Health 1997, 33, 335–338. [Google Scholar] [CrossRef]
- Favié, L.M.A.; Groenendaal, F.; van den Broek, M.P.H.; Rademaker, C.M.A.; de Haan, T.R.; van Straaten, H.L.M.; Dijk, P.H.; van Heijst, A.; Simons, S.H.P.; Dijkman, K.P.; et al. Phenobarbital, Midazolam Pharmacokinetics, Effectiveness, and Drug-Drug Interaction in Asphyxiated Neonates Undergoing Therapeutic Hypothermia. Neonatology 2019, 116, 154–162. [Google Scholar] [CrossRef]
- Chay, P.C.W.; Duffy, B.J.; Walker, J.S. Pharmacokinetic-Pharmacodynamic Relationships of Morphine in Neonates. Clin. Pharmacol. Ther. 1992, 51, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Pokela, M.-L.; Olkkola, K.T.; Seppälä, T.; Koivisto, M. Age-Related Morphine Kinetics in Infants. Dev. Pharmacol. Ther. 1993, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Farrington, E.A.; McGuinness, G.A.; Johnson, G.F.; Erenberg, A.; Leff, R.D. Continuous Intravenous Morphine Infusion in Postoperative Newborn Infants. Am. J. Perinatol. 1993, 10, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.J.S.; Anderson, B.J.; Holford, N.H.G.; Hall, R.W.; Young, T.; Shephard, B.; Desai, N.S.; Barton, B.A. Morphine Pharmacokinetics and Pharmacodynamics in Preterm and Term Neonates: Secondary Results from the NEOPAIN Trial. Br. J. Anaesth. 2008, 101, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Heimann, G.; Gladtke, E. Pharmacokinetics of Phenobarbital in Childhood. Eur. J. Clin. Pharmacol. 1977, 12, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Grasela, T.H.; Donn, S.M. Neonatal Population Pharmacokinetics of Phenobarbital Derived from Routine Clinical Data. Dev. Pharmacol. Ther. 1985, 8, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Donn, S.M.; Grasela, T.H.; Goldstein, G.W. Safety of a Higher Loading Dose of Phenobarbital in the Term Newborn. Pediatrics 1985, 75, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Šíma, M.; Pokorná, P.; Hartinger, J.; Slanař, O. Estimation of Initial Phenobarbital Dosing in Term Neonates with Moderate-to-Severe Hypoxic Ischaemic Encephalopathy Following Perinatal Asphyxia. J. Clin. Pharm. Ther. 2018, 43, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, P.; Posch, L.; Šíma, M.; Klement, P.; Slanar, O.; van den Anker, J.; Tibboel, D.; Allegaert, K. Severity of Asphyxia Is A Covariate of Phenobarbital Clearance in Newborns Undergoing Hypothermia. J. Matern. Fetal Neonatal Med. 2019, 32, 2302–2309. [Google Scholar] [CrossRef]
- Kacet, N.; Roussel-Delnallez, M.; Gremillet, C.; Dubos, J.P.; Storme, L.; Lequien, P. Pharmacokinetic Study of Piperacillin in Newborns Relating to Gestational and Postnatal Age. Pediatr. Infect. Dis. J. 1992, 11, 365. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Li, Q.; Cao, D.; Shi, W.; Cao, Y.; Wu, D.; Zhu, Y.; Wang, Y.; Chen, C. Population Pharmacokinetics of Piperacillin/Tazobactam in Neonates and Young Infants. Eur. J. Clin. Pharmacol. 2013, 69, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Peeters, M.Y.; Verbesselt, R.; Tibboel, D.; Naulaers, G.; de Hoon, J.N.; Knibbe, C.A. Inter-Individual Variability in Propofol Pharmacokinetics in Preterm and Term Neonates. Br. J. Anaesth. 2007, 99, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; Hoon, J.D.; Verbesselt, R.; Naulaers, G.; Murat, I. Maturational Pharmacokinetics of Single Intravenous Bolus of Propofol. Pediatr. Anesth. 2007, 17, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.K.; Davis, P.J.; Dear, G.d.L.; Ginsberg, B.; McGowan, F.X.; Stiller, R.D.; Henson, L.G.; Huffman, C.; Muir, K.T. Pharmacokinetics of Remifentanil in Anesthetized Pediatric Patients Undergoing Elective Surgery or Diagnostic Procedures. Anesth. Analg. 2001, 93, 1393. [Google Scholar] [CrossRef] [PubMed]
- Greeley, W.J.; de Bruijn, N.P.; Davis, D.P. Sufentanil Pharmacokinetics in Pediatric Cardiovascular Patients. Anesth. Analg. 1987, 66, 1067. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, P.; Šíma, M.; Koch, B.; Tibboel, D.; Slanař, O. Sufentanil Disposition and Pharmacokinetic Model-Based Dosage Regimen for Sufentanil in Ventilated Full-Term Neonates. Pharmacology 2021, 106, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.P.; Nilsson, A.; Hartvig, P. Pharmacokinetics of alfentanil in total i.v. anaesthesia. Br. J. Anaesth. 1988, 60, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.D.; Lemmens, H.J.; Fiset, P.; Hermann, D.J.; Muir, K.T.; Stanski, D.R.; Shafer, S.L. The Pharmacokinetics of the New Short-acting Opioid Remifentanil (GI87084B) in Healthy Adult Male Volunteers. Anesthesiology 1993, 79, 881–892. [Google Scholar] [CrossRef]



| Drug | MWT (g/mol) a | LogP a | Ionization Type (pKa) | fu a | Main Binding Protein | BP |
|---|---|---|---|---|---|---|
| Acetaminophen | 151 | 0.76 | Monoprotic acid (9.7) [18] | 0.520 | HSA [21] | 1.04 [19] |
| Alfentanil | 417 | 2.80 | Monoprotic base (6.5) [18] | 0.086 | AGP [22] | 0.63 [19] |
| Amoxicillin | 365 | 0.42 | Ampholyte (2.4, 9.6) [18] | 0.850 | HSA [23] | 1.04 [19] |
| Caffeine | 194 | 0.08 | Neutral | 0.640 | HSA [24] | 1.00 [19] |
| Cefazolin | 455 | −1.20 | Monoprotic acid (2.1) [18] | 0.180 | HSA [25] | 0.60 [19] |
| Cefepime | 481 | −3.60 | Monoprotic acid (2.8) b | 0.780 | HSA [25] | 0.74 [19] |
| Cefotaxime | 455 | −0.05 | Monoprotic acid (3.4) [18] | 0.600 | HSA [25] | 1.00 c |
| Ceftazidime | 547 | −1.70 | Diprotic acid (1.8, 2.7) [18] | 0.790 | HSA [25] | 0.72 [19] |
| Ceftriaxone | 555 | −3.20 | Diprotic acid (3.2, 4.1) [18] | 0.100 | HSA [25] | 1.00 c |
| Dexmedetomidine | 200 | 2.80 | Monoprotic base (6.5) b | 0.060 | HSA [26] | 0.80 d |
| Fentanyl | 336 | 4.10 | Monoprotic base (8.4) [18] | 0.160 | HSA [27] | 0.99 [19] |
| Flucloxacillin | 454 | 2.80 | Monoprotic acid (2.7) [18] | 0.043 | HSA [28] | 1.00 c |
| Imipenem | 299 | −0.81 | Ampholyte (3.4, 10.9) b | 0.860 | HSA [29] | 1.00 c |
| Levetiracetam | 170 | −0.48 | Neutral | 0.900 | HSA e | 0.69 d |
| Lidocaine | 234 | 2.70 | Monoprotic base (7.8) [18] | 0.330 | AGP [30] | 0.78 [19] |
| Meperidine | 247 | 3.00 | Monoprotic base (8.7) [18] | 0.420 | HSA [31] | 1.01 [19] |
| Meropenem | 383 | −1.00 | Ampholyte (3.3, 9.4) b | 0.870 | HSA e | 0.507 [19] |
| Midazolam | 326 | 3.10 | Monoprotic base (6.2) [18] | 0.017 | HSA [32] | 0.68 [19] |
| Morphine | 285 | 1.10 | Monoprotic base (8.0) [18] | 0.650 | HSA [33] | 1.00 [19] |
| Phenobarbital | 232 | 1.30 | Monoprotic acid (7.4) [18] | 0.490 | HSA [34] | 0.99 [19] |
| Piperacillin | 518 | 0.84 | Monoprotic acid (3.5) b | 0.500 | HSA e | 0.65 [19] |
| Propofol | 178 | 4.20 | Neutral | 0.016 | HSA [35] | 1.25 [19] |
| Remifentanil | 376 | 2.30 | Monoprotic base (7.5) b | 0.300 | AGP [36] | 0.90 d |
| Sufentanil | 387 | 3.90 | Monoprotic base (8.0) [18] | 0.075 | AGP [37] | 0.74 [19] |
| Prediction Method | AFE | AAFE | Studies within Two-Fold (%) | |
|---|---|---|---|---|
| Predicting adult Vss (L/kg): | ||||
| PBPK (Poulin & Theil+) | 1.45 | 1.95 | 54 | |
| PBPK (Rodgers & Rowland) | 1.54 | 2.14 | 71 | |
| Predicting neonatal Vss (L/kg): | ||||
| Isometric scaling | 0.61 | 1.77 | 64 | |
| PBPK (Poulin & Theil+) | 0.82 | 1.68 | 76 | |
| PBPK (Rodgers & Rowland) | 0.83 | 2.03 | 55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sutter, P.-J.; Rossignol, P.; Breëns, L.; Gasthuys, E.; Vermeulen, A. Predicting Volume of Distribution in Neonates: Performance of Physiologically Based Pharmacokinetic Modelling. Pharmaceutics 2023, 15, 2348. https://doi.org/10.3390/pharmaceutics15092348
De Sutter P-J, Rossignol P, Breëns L, Gasthuys E, Vermeulen A. Predicting Volume of Distribution in Neonates: Performance of Physiologically Based Pharmacokinetic Modelling. Pharmaceutics. 2023; 15(9):2348. https://doi.org/10.3390/pharmaceutics15092348
Chicago/Turabian StyleDe Sutter, Pieter-Jan, Phebe Rossignol, Lien Breëns, Elke Gasthuys, and An Vermeulen. 2023. "Predicting Volume of Distribution in Neonates: Performance of Physiologically Based Pharmacokinetic Modelling" Pharmaceutics 15, no. 9: 2348. https://doi.org/10.3390/pharmaceutics15092348
APA StyleDe Sutter, P.-J., Rossignol, P., Breëns, L., Gasthuys, E., & Vermeulen, A. (2023). Predicting Volume of Distribution in Neonates: Performance of Physiologically Based Pharmacokinetic Modelling. Pharmaceutics, 15(9), 2348. https://doi.org/10.3390/pharmaceutics15092348






