Molecular Mechanisms Involved in the Chemical Instability of ONC201 and Methods to Counter Its Degradation in Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Conditions
2.3. Forced Degradation Test
2.4. Theorical Calculations
3. Results and Discussion
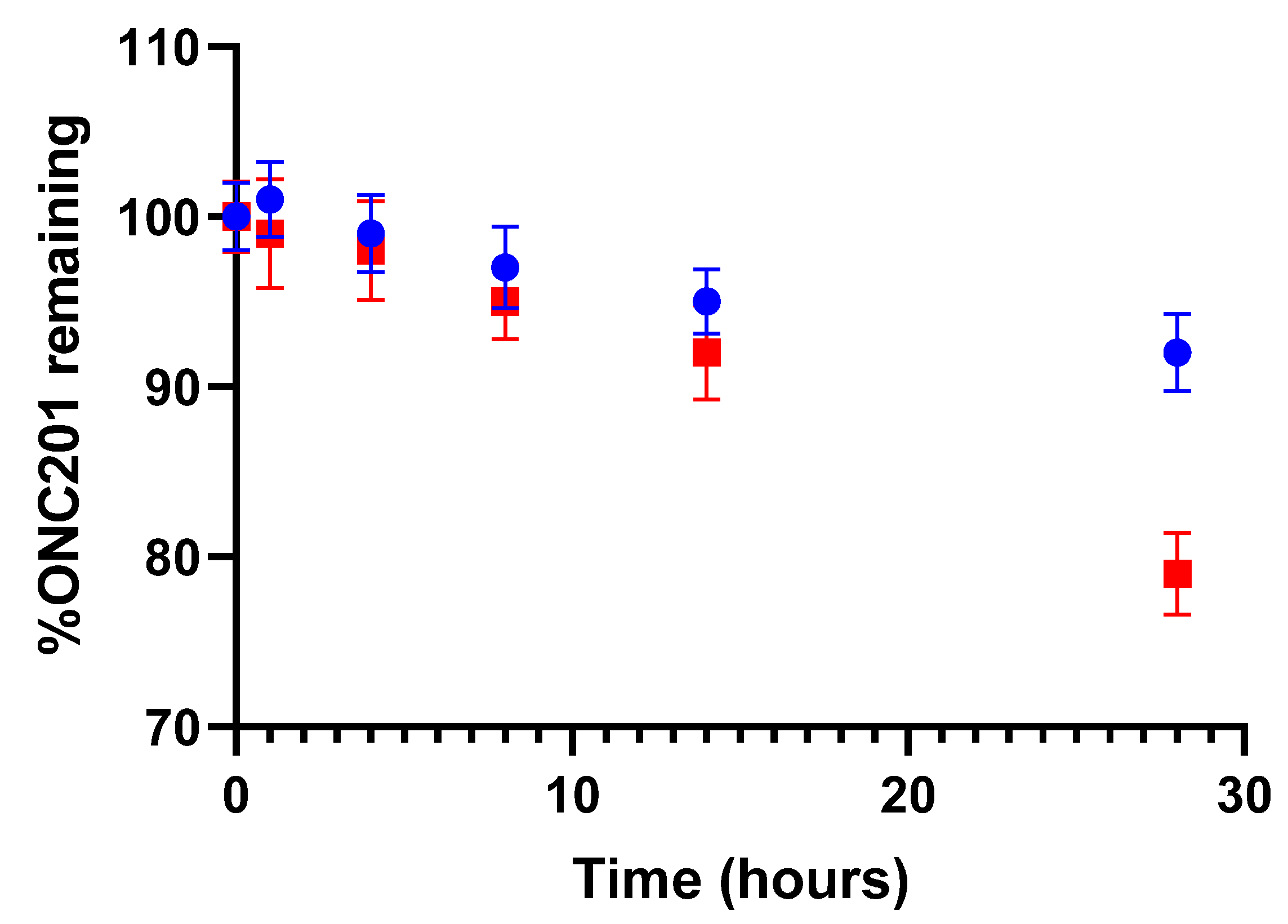
3.1. ONC201 Degradation under Light Exposure
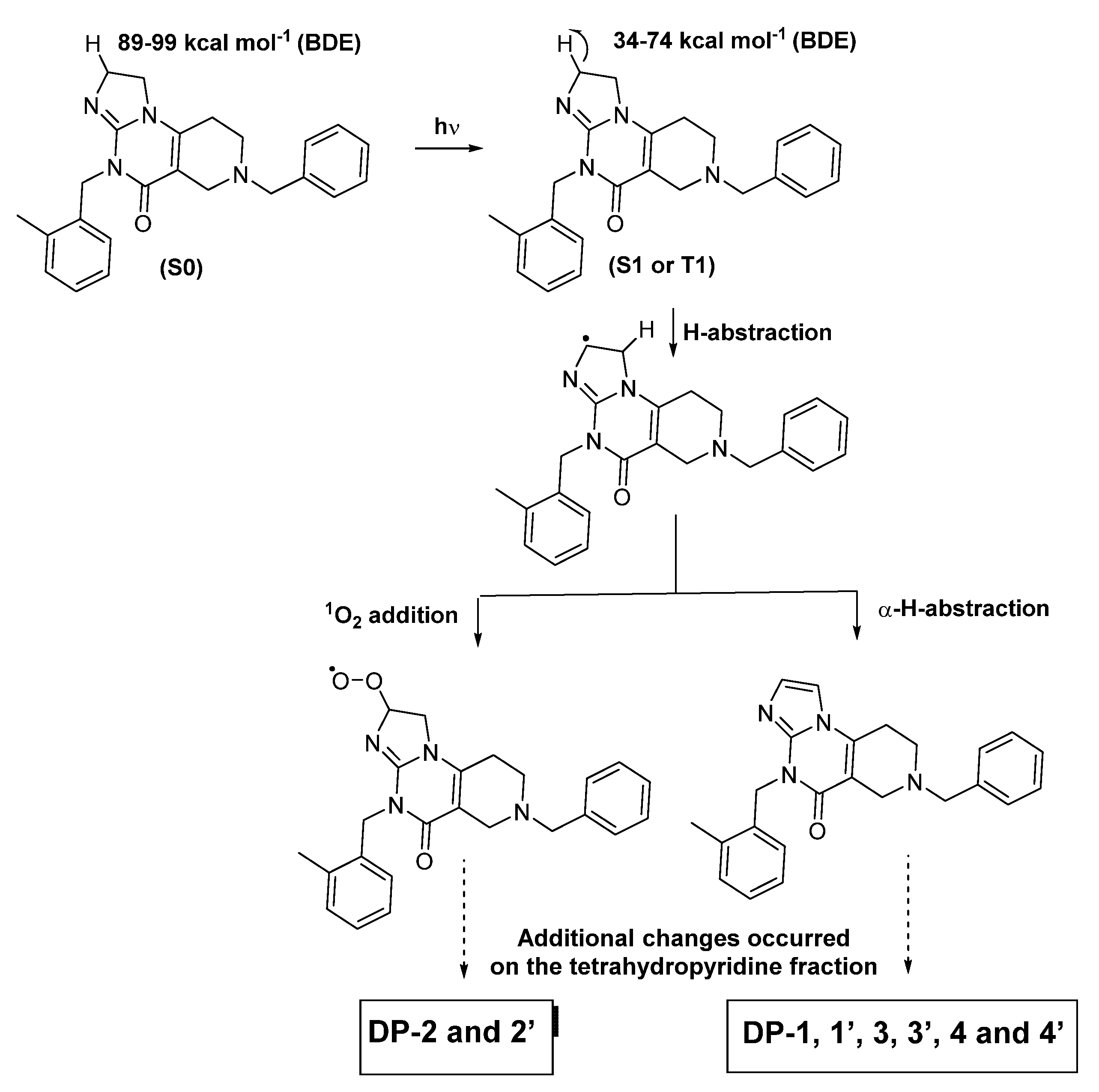
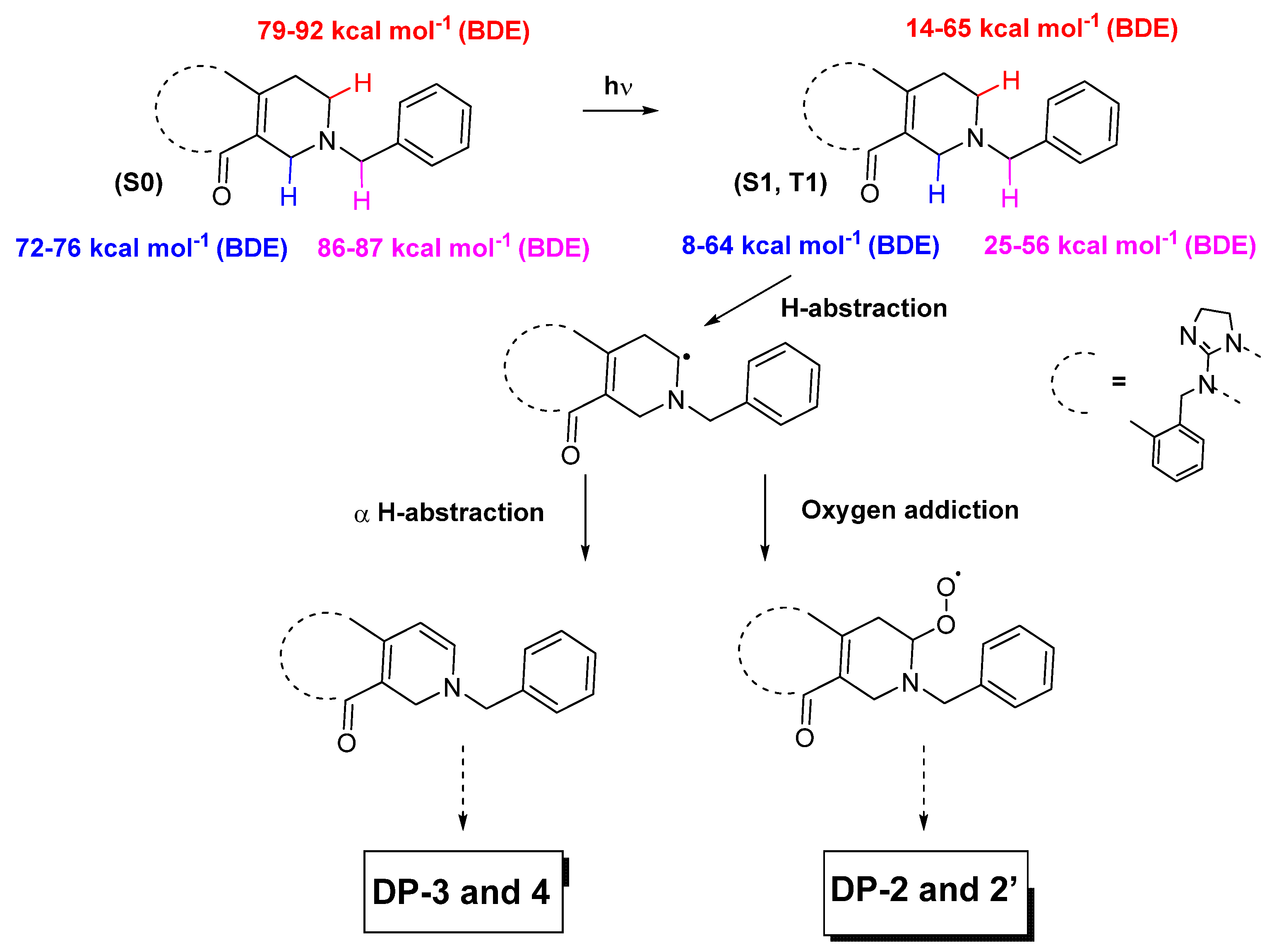
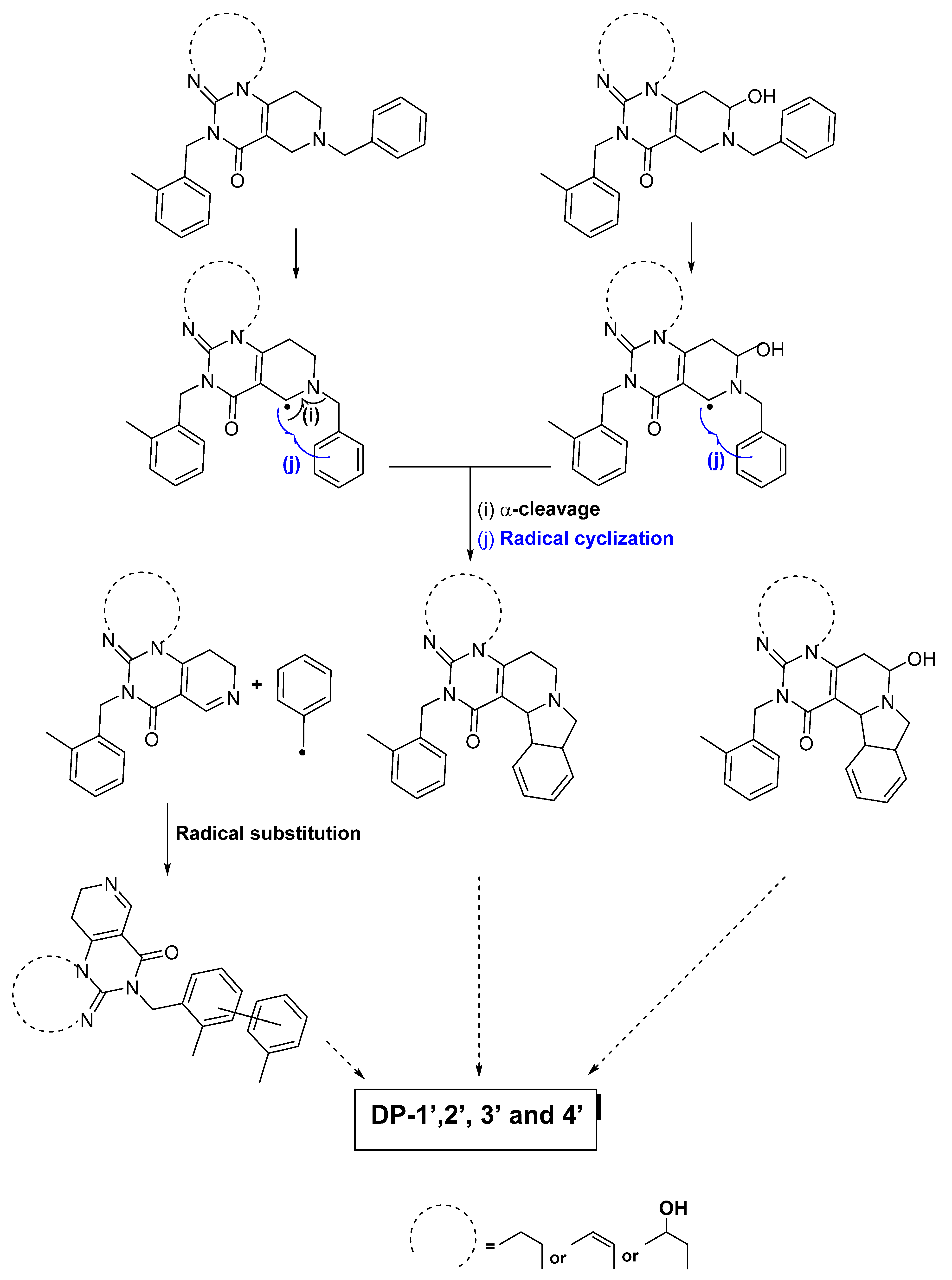
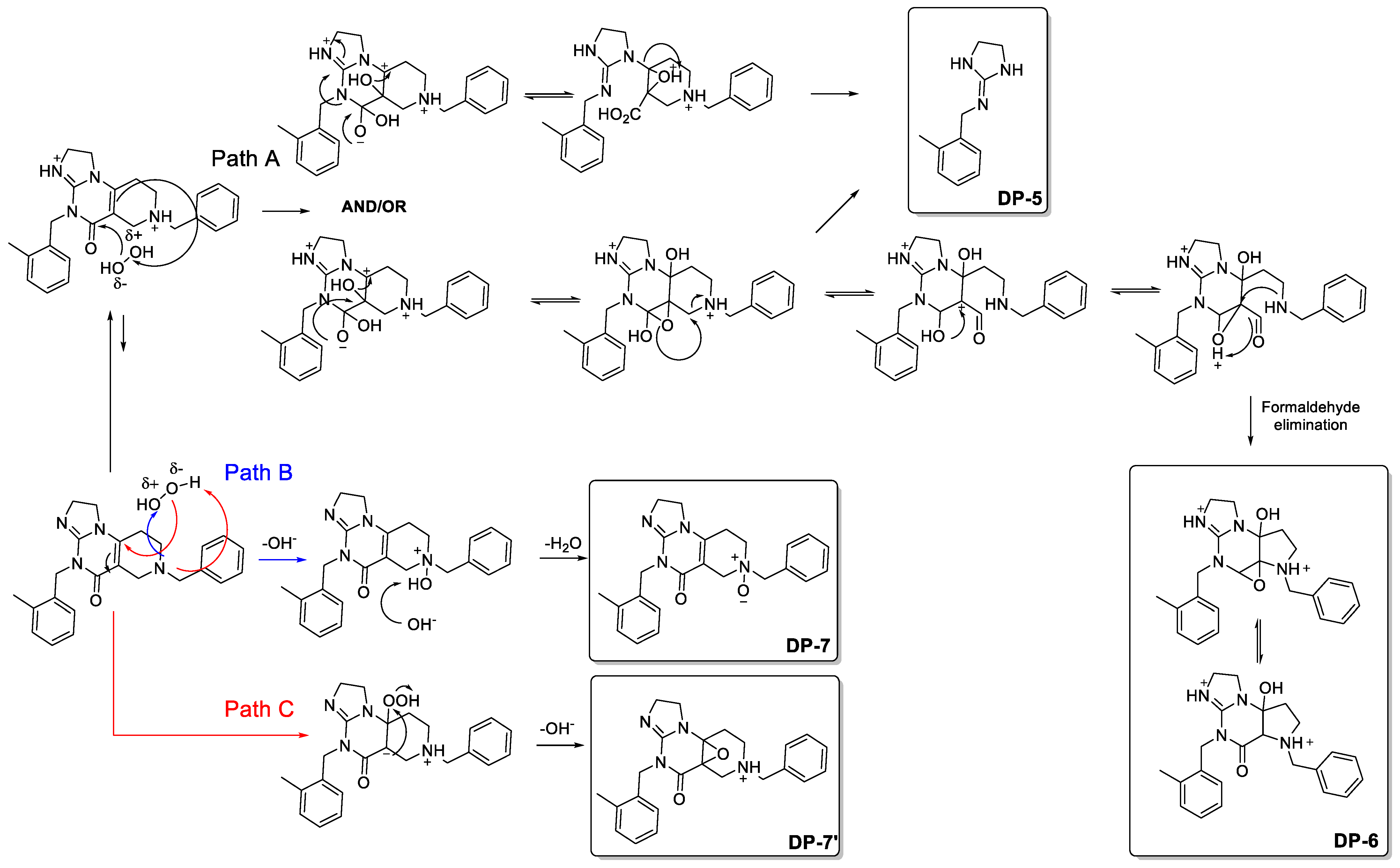
3.1.1. Proposed Degradation Mechanisms of ONC201 following Its Exposure to Light
3.1.2. Influence of pH on the Behavior of ONC201
3.2. ONC201 Degradation in the Presence of Hydrogen Peroxide
3.2.1. Proposed Degradation Mechanisms
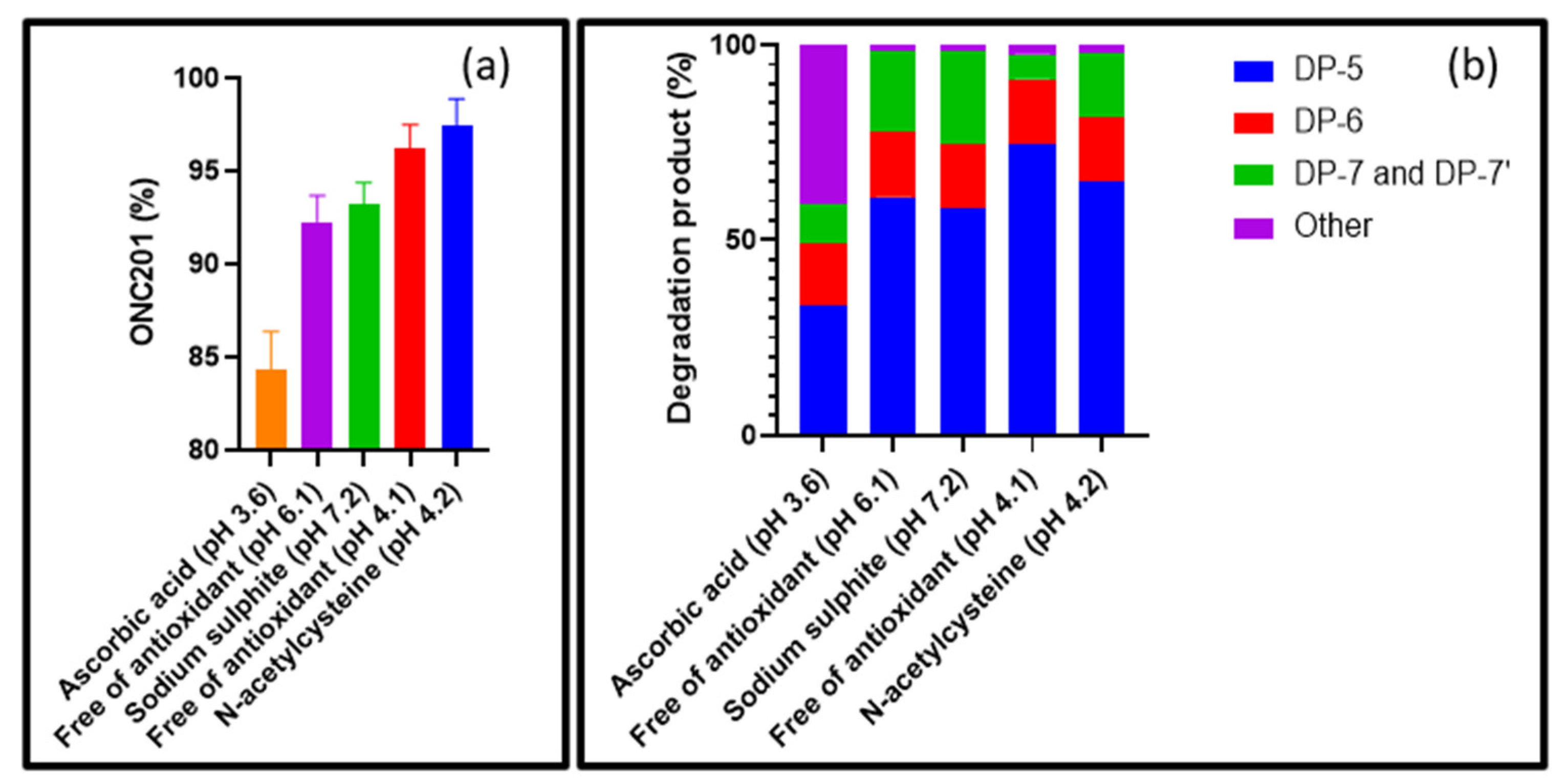
3.2.2. Assessing the Effect of Antioxidants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ralff, M.D.; Lulla, A.R.; Wagner, J.; El-Deiry, W.S. ONC201: A new treatment option being tested clinically for recurrent glioblastoma. Transl. Cancer Res. 2017, 6, S1239–S1243. [Google Scholar] [CrossRef] [PubMed]
- ONC201. Available online: https://www.gustaveroussy.fr/fr/onc201 (accessed on 1 August 2023).
- Annereau, M.; Vignes, M.; Bouchema, T.S.E.; Denis, L.; Solgadi, A.; Vieillard, V.; Paul, M.; Rieutord, A.; Grill, J.; Secretan, P.-H.; et al. Identification of Degradation Products of the New Anticancer Drug Substance ONC201 by Liquid Chromatography–High-Resolution Multistage Mass Spectrometry. Chemosensors 2023, 11, 294. [Google Scholar] [CrossRef]
- Won, D.H.; Park, H.; Ha, E.-S.; Kim, Y.M.; Hwang, H.D.; Jang, S.W.; Kim, M.-S. Effect of Formulation Factors and Oxygen Levels on the Stability of Aqueous Injectable Solution Containing Pemetrexed. Pharmaceutics 2020, 12, 46. [Google Scholar] [CrossRef]
- Rosa, P.; Snovarski Salla, A.P.; De Bona Da Silva, C.; Bueno Rolim, C.M.; Horn Adams, A.I. Investigation of the Stabilizing Effects of Antioxidants and Benzophenone-3 on Desonide Photostability. AAPS PharmSciTech 2014, 15, 1155–1162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khattak, S.-R.; Shaikh, D.; Ahmad, I.; Usmanghani, K.; Sheraz, M.A.; Ahmed, S. Photodegradation and Stabilization of Betamethasone-17 Valerate in Aqueous/Organic Solvents and Topical Formulations. AAPS PharmSciTech 2013, 14, 177–182. [Google Scholar] [CrossRef][Green Version]
- Kryczyk-Poprawa, A.; Kwiecień, A.; Opoka, W. Photostability of Topical Agents Applied to the Skin: A Review. Pharmaceutics 2019, 12, 10. [Google Scholar] [CrossRef]
- Ang, Z.-Y.; Boddy, M.; Liu, Y.; Sunderland, B. Stability of apomorphine in solutions containing selected antioxidant agents. Drug Des. Devel. Ther. 2016, 10, 3253–3265. [Google Scholar] [CrossRef]
- He, L.; Sun, X.; Zhu, F.; Ren, S.; Wang, S. OH-initiated transformation and hydrolysis of aspirin in AOPs system: DFT and experimental studies. Sci. Total Environ. 2017, 592, 33–40. [Google Scholar] [CrossRef]
- Hadidi, S.; Shiri, F.; Norouzibazaz, M. A DFT study of the degradation mechanism of anticancer drug carmustine in an aqueous medium. Struct. Chem. 2019, 30, 1315–1321. [Google Scholar] [CrossRef]
- Abraham, C.S.; Muthu, S.; Prasana, J.C.; Armaković, S.; Armaković, S.J.; Rizwana, B.F.; Geoffrey, B.; David, R.H.A. Computational evaluation of the reactivity and pharmaceutical potential of an organic amine: A DFT, molecular dynamics simulations and molecular docking approach. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2019, 222, 117188. [Google Scholar] [CrossRef]
- Hazarika, R.; Kalita, B. Elucidating the therapeutic activity of selective curcumin analogues: DFT-based reactivity analysis. Struct. Chem. 2021, 32, 1701–1715. [Google Scholar] [CrossRef]
- Cacciari, R.D.; Reynoso, E.; Montejano, H.A.; Biasutti, M.A. Photodegradation of prednisolone under UVB solar irradiation. Role of photogenerated ROS in the degradation mechanism. Photochem. Photobiol. Sci. 2017, 16, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- EMA. ICH Q1A (R2) Stability Testing of New Drug Substances and Products—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products-scientific-guideline (accessed on 29 March 2023).
- EMA. ICH Q1B Photostability Testing of New Active Substances and Medicinal Products—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q1b-photostability-testing-new-active-substances-medicinal-products-scientific-guideline (accessed on 29 March 2023).
- Theilacker, K.; Arbuznikov, A.V.; Bahmann, H.; Kaupp, M. Evaluation of a Combination of Local Hybrid Functionals with DFT-D3 Corrections for the Calculation of Thermochemical and Kinetic Data. J. Phys. Chem. A 2011, 115, 8990–8996. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Broo, A.; Evertsson, E. Prediction of Drug Candidates’ Sensitivity Toward Autoxidation: Computational Estimation of C-H Dissociation Energies of Carbon-Centered Radicals. J. Pharm. Sci. 2014, 103, 1949–1955. [Google Scholar] [CrossRef]
- Iyer, J.; Brunsteiner, M.; Ray, A.; Davis, A.; Saraf, I.; Paudel, A. Theoretical and Experimental Investigation of Autoxidation Propensity of Selected Drugs in Solution State. Mol. Pharm. 2023, 20, 1768–1778. [Google Scholar] [CrossRef]
- Lienard, P.; Gavartin, J.; Boccardi, G.; Meunier, M. Predicting Drug Substances Autoxidation. Pharm. Res. 2015, 32, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.J.; Visana, N.M.; Patel, A.I.; Patel, A.B.; Patel, N.K.; Shah, S.R. Analytical Quality by Design in Stress Testing or Stability—Indicating Method. Asian J. Pharm. Anal. 2021, 11, 170–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, X.; Yu, Z.; Cheng, H. Influence of chemical speciation on photochemical transformation of three fluoroquinolones (FQs) in water: Kinetics, mechanism, and toxicity of photolysis products. Water Res. 2019, 148, 19–29. [Google Scholar] [CrossRef]
- Freed, A.L.; Strohmeyer, H.E.; Mahjour, M.; Sadineni, V.; Reid, D.L.; Kingsmill, C.A. pH control of nucleophilic/electrophilic oxidation. Int. J. Pharm. 2008, 357, 180–188. [Google Scholar] [CrossRef]
- Valdivia-Berroeta, G.A.; Gonnella, N.C. N-oxidation Regioselectivity and Risk Prediction Using DFT-ALIE Calculations. Pharm. Res. 2023, 40, 1873–1883. [Google Scholar] [CrossRef]
- Olsson, D.; Li, J.; Jonsson, M. Kinetic Effects of H 2 O 2 Speciation on the Overall Peroxide Consumption at UO2—Water Interfaces. ACS Omega 2022, 7, 15929–15935. [Google Scholar] [CrossRef] [PubMed]
- Grier, D.L.; Streitwieser, A. Electron density analysis of substituted carbonyl groups. J. Am. Chem. Soc. 1982, 104, 3556–3564. [Google Scholar] [CrossRef]
- Gabrič, A.; Hodnik, Ž.; Pajk, S. Oxidation of Drugs during Drug Product Development: Problems and Solutions. Pharmaceutics 2022, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Loubane, G.; Robert, G.; Firdaus, S.B.; Venne, P.; Comeau, C.; Boudreault, P.-L.; Komba, J.E.; Wagner, J.R.; Naylor, S.; Klarskov, K. Conundrum of dehydroascorbic acid and homocysteine thiolactone reaction products: Structural characterization and effect on peptide and protein N-homocysteinylation. Free Radic. Biol. Med. 2023, 206, 111–124. [Google Scholar] [CrossRef]
- Zinatullina, K.M.; Kasaikina, O.T.; Kuz’min, V.A.; Khrameeva, N.P. Interaction of Glutathione with Hydrogen Peroxide: A Kinetic Model. Kinet. Catal. 2019, 60, 266–272. [Google Scholar] [CrossRef]
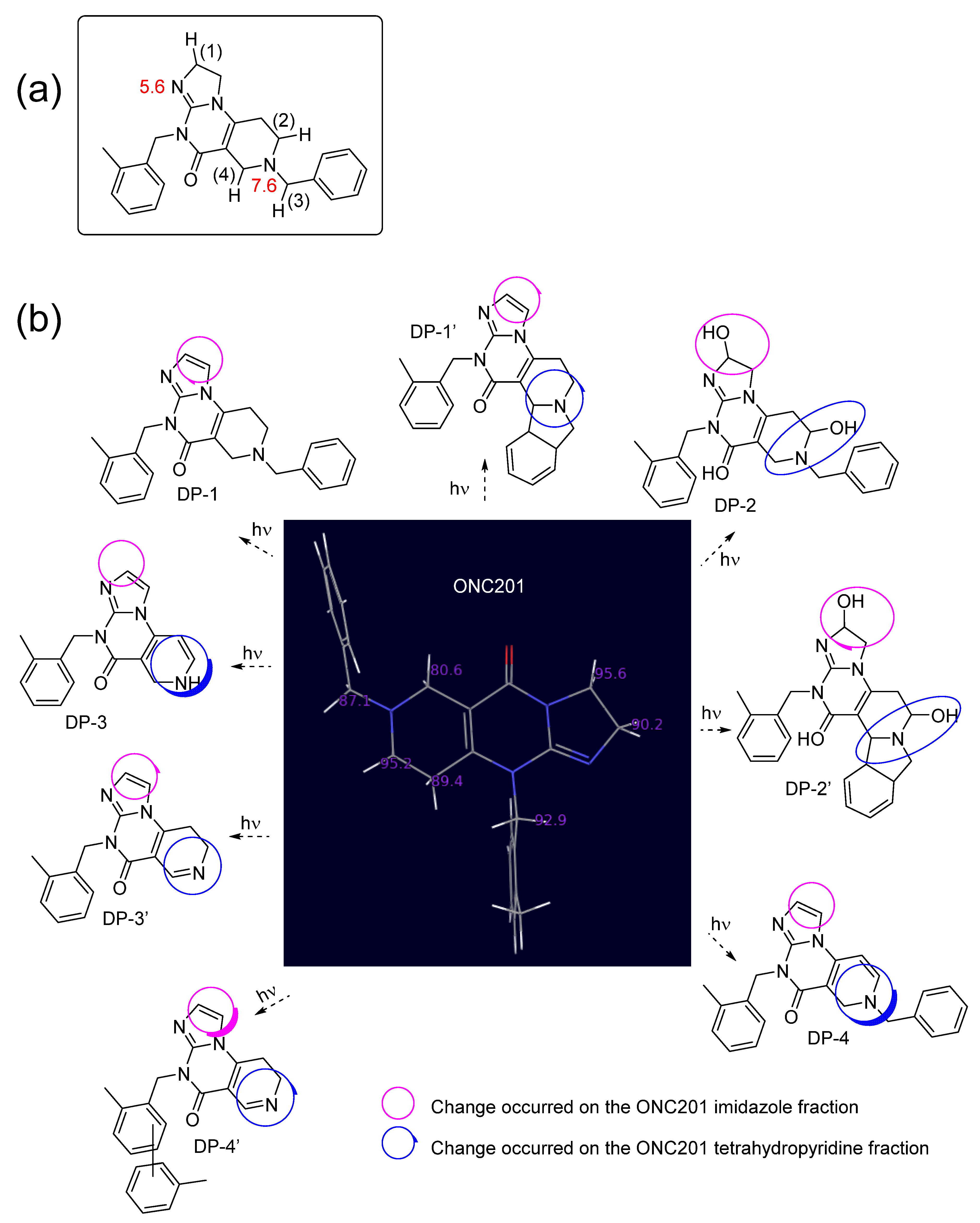


| BDE of Position (1) C–H Bond (Figure 1a) | BDE of Position (2) C–H Bond (Figure 1a) | BDE of Position (3) C–H Bond (Figure 1a) | BDE of Position (4) C–H Bond (Figure 1a) | |||||
|---|---|---|---|---|---|---|---|---|
| [ONC201+2H]2+ (pH 4) | ONC201 (pH 8) | [ONC201+2H]2+ (pH 4) | ONC201 (pH 8) | [ONC201+2H]2+ (pH 4) | ONC201 (pH 8) | [ONC201+2H]2+ (pH 4) | ONC201 (pH 8) | |
| S0 | 89 | 99 | 79 | 92 | 71 | 87 | 76 | 72 |
| S1 | 74 | 48 | 65 | 41 | 56 | 38 | 64 | 21 |
| T1 | 71 | 34 | 61 | 27 | 53 | 25 | 60 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annereau, M.; Vignes, M.; Denis, L.; Rieutord, A.; Legrand, F.-X.; Rioblanc, F.; Paul, M.; Grill, J.; Secretan, P.-H.; Do, B. Molecular Mechanisms Involved in the Chemical Instability of ONC201 and Methods to Counter Its Degradation in Solution. Pharmaceutics 2023, 15, 2371. https://doi.org/10.3390/pharmaceutics15102371
Annereau M, Vignes M, Denis L, Rieutord A, Legrand F-X, Rioblanc F, Paul M, Grill J, Secretan P-H, Do B. Molecular Mechanisms Involved in the Chemical Instability of ONC201 and Methods to Counter Its Degradation in Solution. Pharmaceutics. 2023; 15(10):2371. https://doi.org/10.3390/pharmaceutics15102371
Chicago/Turabian StyleAnnereau, Maxime, Marina Vignes, Lucas Denis, André Rieutord, François-Xavier Legrand, François Rioblanc, Muriel Paul, Jacques Grill, Philippe-Henri Secretan, and Bernard Do. 2023. "Molecular Mechanisms Involved in the Chemical Instability of ONC201 and Methods to Counter Its Degradation in Solution" Pharmaceutics 15, no. 10: 2371. https://doi.org/10.3390/pharmaceutics15102371
APA StyleAnnereau, M., Vignes, M., Denis, L., Rieutord, A., Legrand, F.-X., Rioblanc, F., Paul, M., Grill, J., Secretan, P.-H., & Do, B. (2023). Molecular Mechanisms Involved in the Chemical Instability of ONC201 and Methods to Counter Its Degradation in Solution. Pharmaceutics, 15(10), 2371. https://doi.org/10.3390/pharmaceutics15102371





