Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Transfection
2.2. Constructs and Peptides
2.3. Purification on STREP-Resin
2.4. MTT Assay
2.5. Western Blotting
2.6. RT-qPCR
2.7. Orthotopic Implantation of N2a Cells
2.8. Statistical Analysis and Guidelines
3. Results
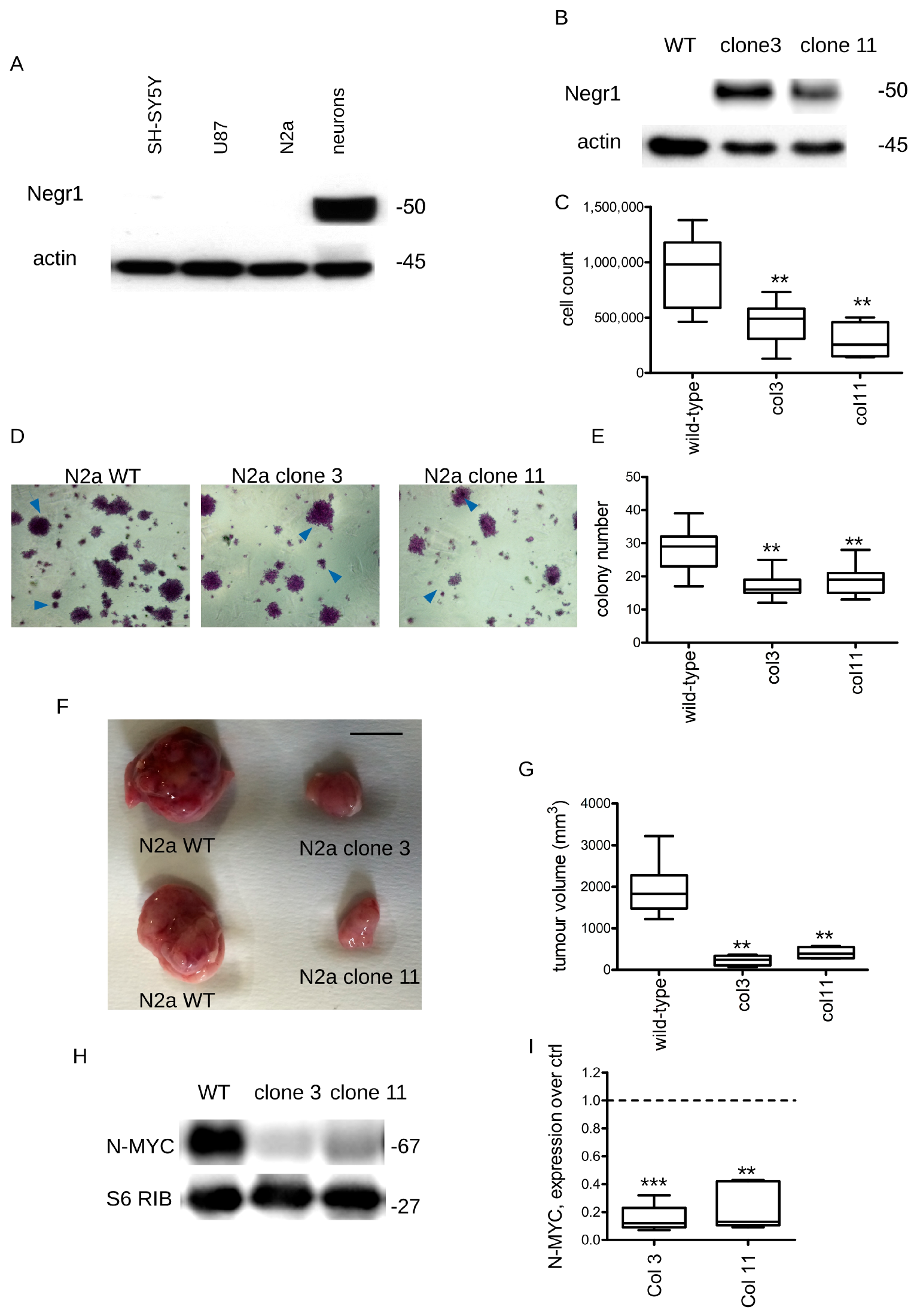
3.1. Negr1 Overexpression Halts Cancer Growth In Vitro and In Vivo
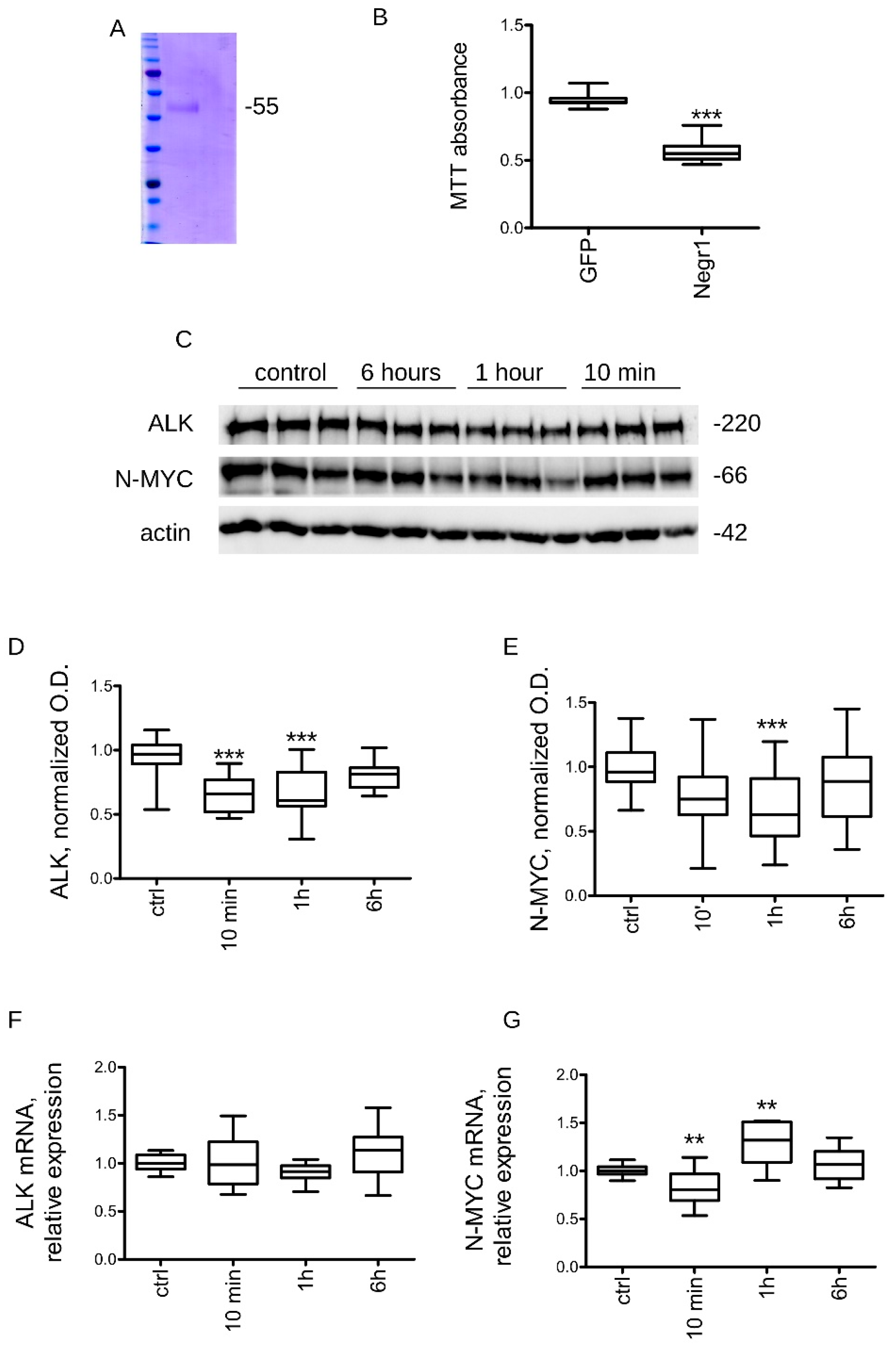
3.2. Negr1 Impacts ALK Protein Levels
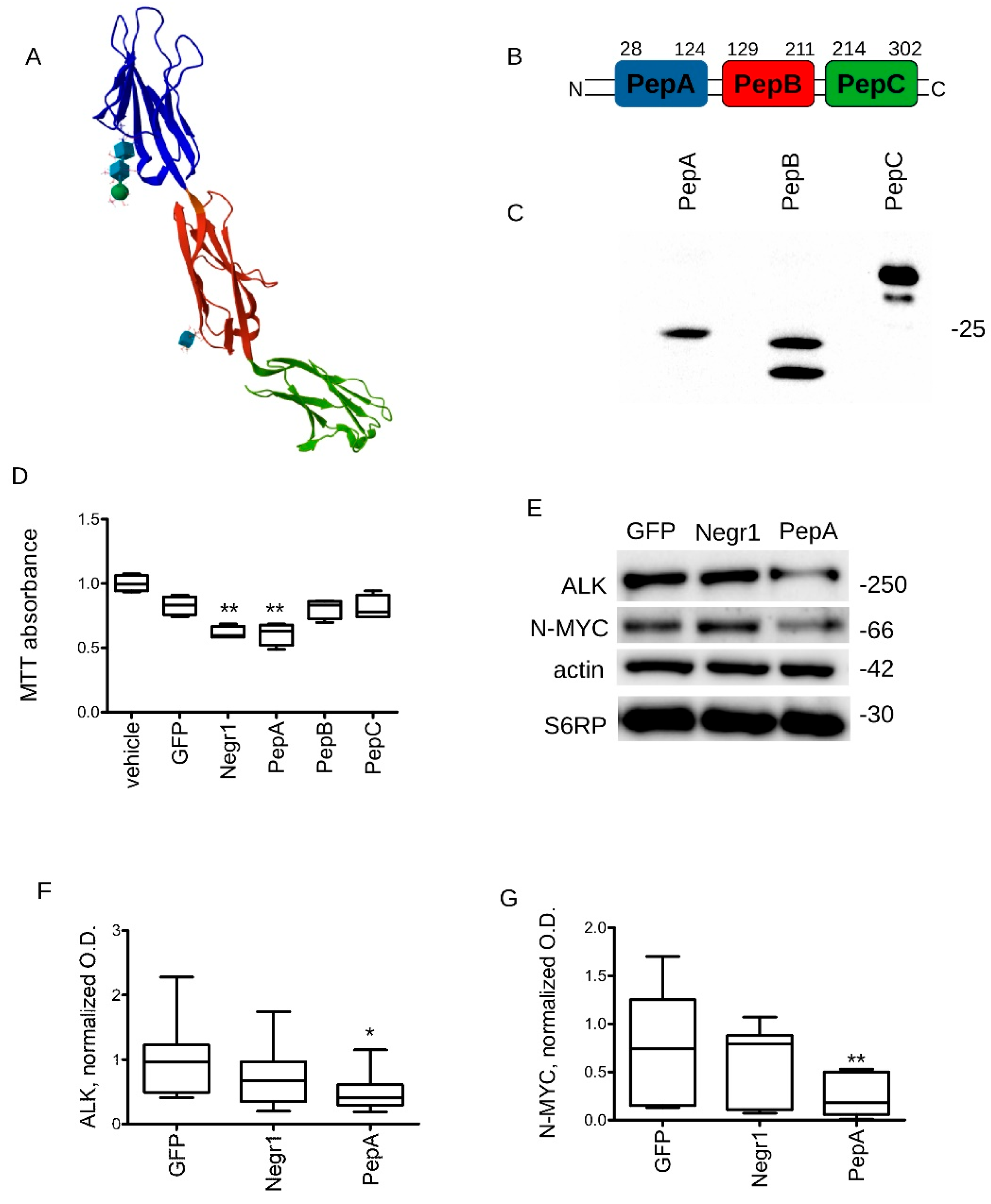
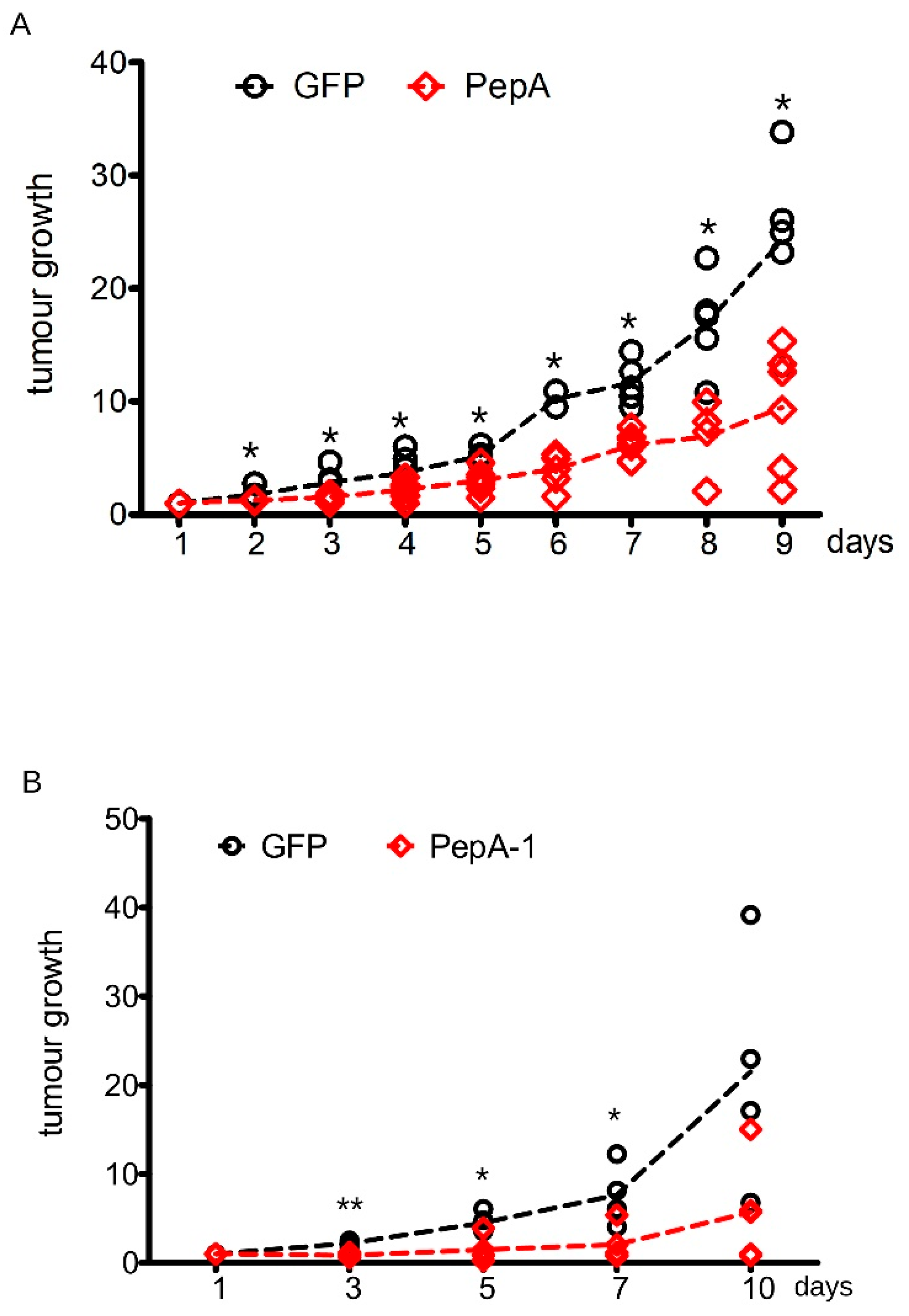
3.3. Negr1-Derived Peptides Halt Cancer Growth In Vitro
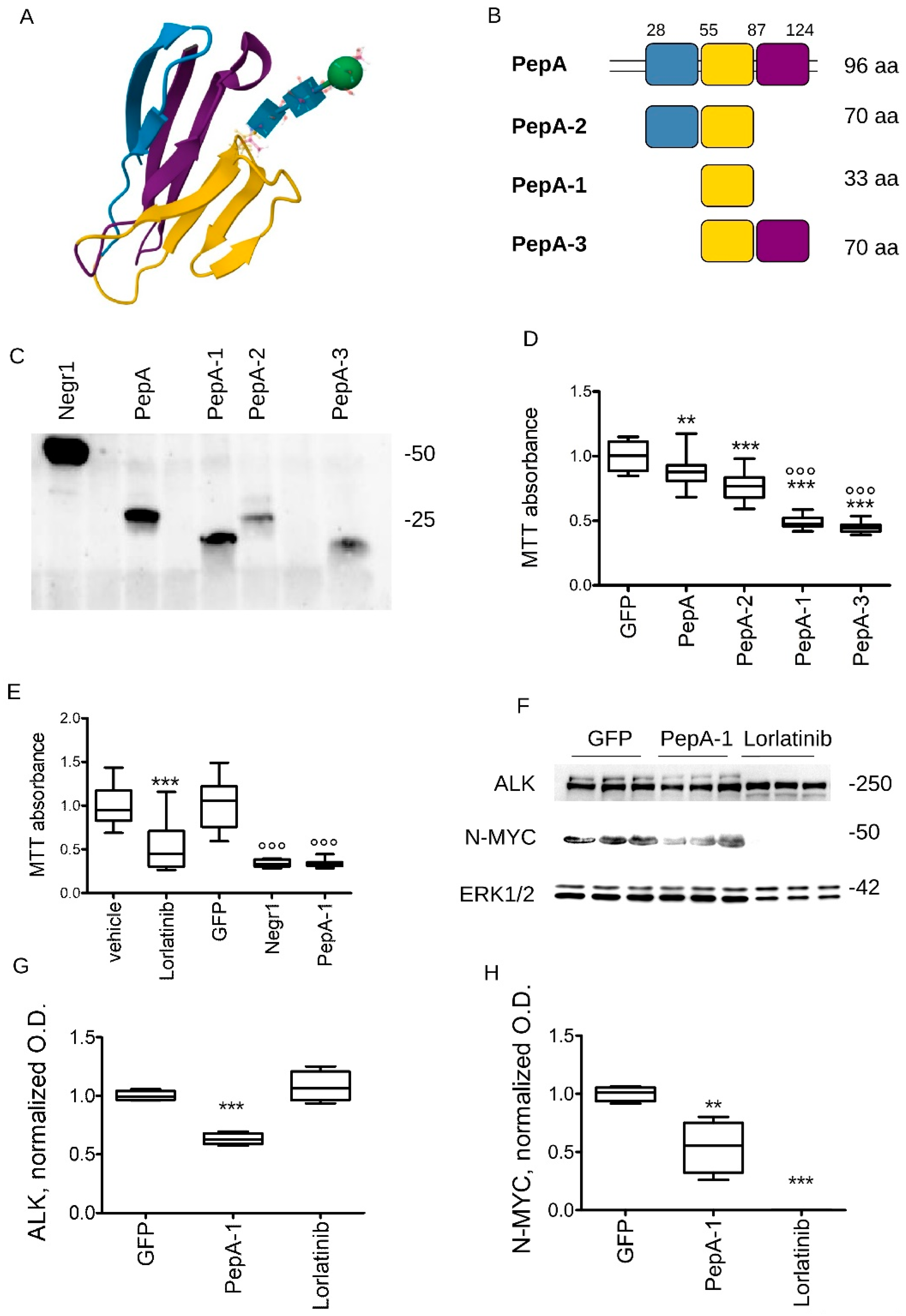
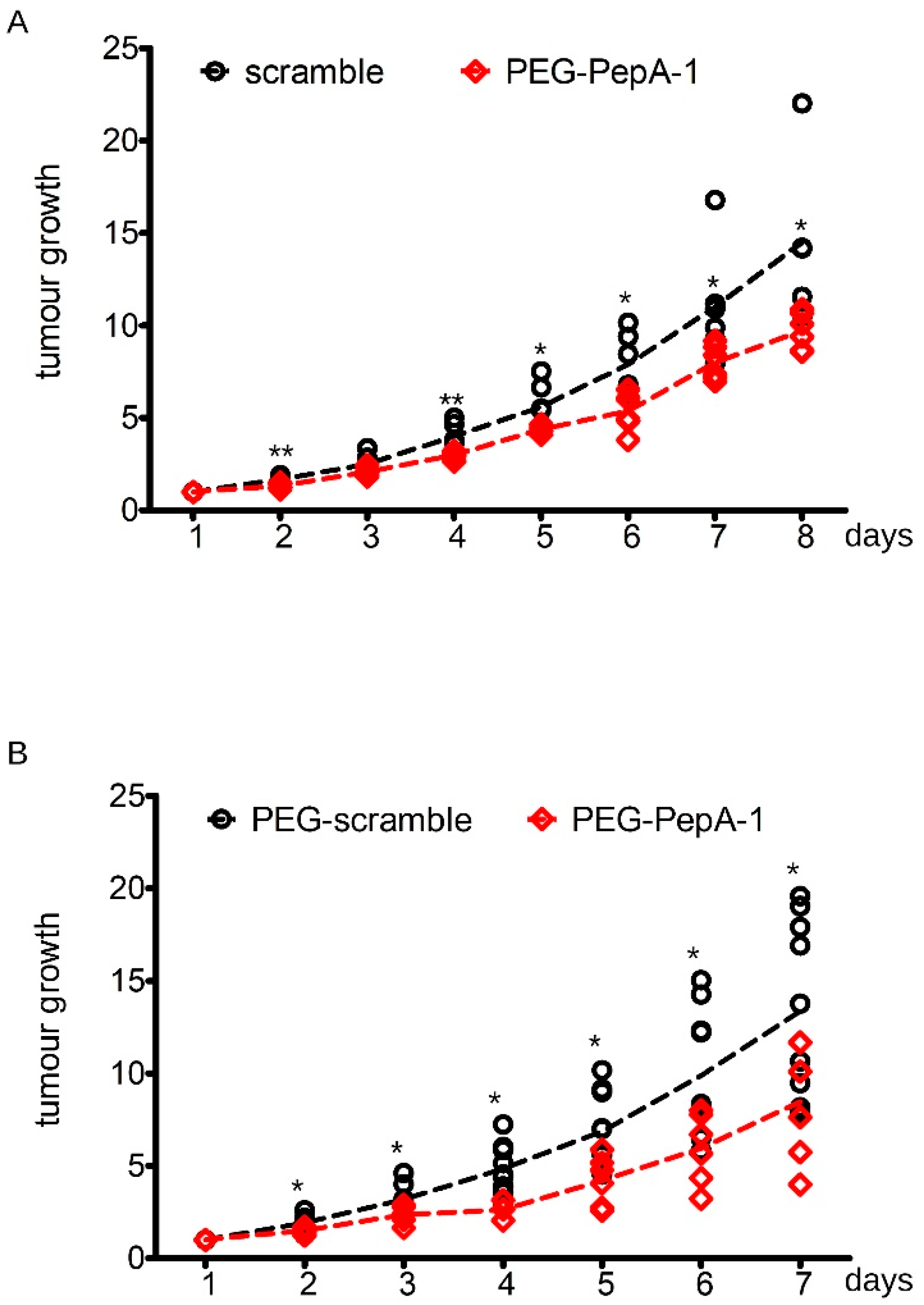
3.4. Soluble Negr1-Derived Peptides Halt Cancer Growth In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALK | Anaplastic lymphoma kinase |
| col | Colony |
| ctrl | Control |
| DIV | Day in vitro |
| IgLON | Immunoglobulin LSAMP, OBCAM, Neurotrimin family |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| rNegr1 | Recombinant Strep-FLAG Negr1 |
| RTK | Receptor tyrosine kinase |
| S.C. | Subcutaneous |
| WT | Wild-type |
References
- Mahapatra, S.; Challagundla, K.B. Neuroblastoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sokol, E.; Desai, A.V. The Evolution of Risk Classification for Neuroblastoma. Children 2019, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathi, P.; Mannangatti, P.; Emdad, L.; Das, S.K.; Fisher, P.B. The Quest to Develop an Effective Therapy for Neuroblastoma. J. Cell Physiol. 2021, 236, 7775–7791. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, D.; Montemurro, L.; Raieli, S.; Lampis, S.; Pession, A.; Hrelia, P.; Tonelli, R. MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment. Cancers 2022, 14, 4421. [Google Scholar] [CrossRef] [PubMed]
- Speleman, F.; Park, J.R.; Henderson, T.O. Neuroblastoma: A Tough Nut to Crack. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e548–e557. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jin, Y.; Yu, J.; Wang, J.; Yan, J.; Zhao, Q. Research Progress of Neuroblastoma Related Gene Variations. Oncotarget 2016, 8, 18444–18455. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.; Dyberg, C.; Pepich, A.; Johnsen, J.I. MYCN Function in Neuroblastoma Development. Front. Oncol. 2021, 10, 624079. [Google Scholar] [CrossRef]
- Trigg, R.M.; Turner, S.D. ALK in Neuroblastoma: Biological and Therapeutic Implications. Cancers 2018, 10, 113. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The Genetic Landscape of High-Risk Neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef]
- Fadeev, A.; Mendoza-Garcia, P.; Irion, U.; Guan, J.; Pfeifer, K.; Wiessner, S.; Serluca, F.; Singh, A.P.; Nüsslein-Volhard, C.; Palmer, R.H. ALKALs Are in Vivo Ligands for ALK Family Receptor Tyrosine Kinases in the Neural Crest and Derived Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E630–E638. [Google Scholar] [CrossRef]
- Mo, E.S.; Cheng, Q.; Reshetnyak, A.V.; Schlessinger, J.; Nicoli, S. Alk and Ltk Ligands Are Essential for Iridophore Development in Zebrafish Mediated by the Receptor Tyrosine Kinase Ltk. Proc. Natl. Acad. Sci. USA 2017, 114, 12027–12032. [Google Scholar] [CrossRef]
- Schönherr, C.; Ruuth, K.; Kamaraj, S.; Wang, C.-L.; Yang, H.-L.; Combaret, V.; Djos, A.; Martinsson, T.; Christensen, J.G.; Palmer, R.H.; et al. Anaplastic Lymphoma Kinase (ALK) Regulates Initiation of Transcription of MYCN in Neuroblastoma Cells. Oncogene 2012, 31, 5193–5200. [Google Scholar] [CrossRef]
- Claeys, S.; Denecker, G.; Durinck, K.; Decaesteker, B.; Mus, L.M.; Loontiens, S.; Vanhauwaert, S.; Althoff, K.; Wigerup, C.; Bexell, D.; et al. ALK Positively Regulates MYCN Activity through Repression of HBP1 Expression. Oncogene 2019, 38, 2690–2705. [Google Scholar] [CrossRef]
- Hasan, M.K.; Nafady, A.; Takatori, A.; Kishida, S.; Ohira, M.; Suenaga, Y.; Hossain, S.; Akter, J.; Ogura, A.; Nakamura, Y.; et al. ALK Is a MYCN Target Gene and Regulates Cell Migration and Invasion in Neuroblastoma. Sci. Rep. 2013, 3, 3450. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-H.; Lu, M.-Y.; Yang, Y.-L.; Chou, S.-W.; Lin, D.-T.; Lin, K.-H.; Hsu, W.-M.; Jeng, Y.-M.; Jou, S.-T. The Prognostic Roles of and Correlation between ALK and MYCN Protein Expression in Neuroblastoma. J. Clin. Pathol. 2020, 73, 154–161. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, J.-S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK Collaborates with MYCN in Neuroblastoma Pathogenesis. Cancer Cell 2012, 21, 362–373. [Google Scholar] [CrossRef]
- Szewczyk, K.; Wieczorek, A.; Młynarski, W.; Janczar, S.; Woszczyk, M.; Gamrot, Z.; Chaber, R.; Wysocki, M.; Pogorzała, M.; Bik-Multanowski, M.; et al. Unfavorable Outcome of Neuroblastoma in Patients With 2p Gain. Front. Oncol. 2019, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, M.; Yang, Y.; Du, C.; Zhou, H.; Liu, C.; Chen, Y.; Fan, L.; Ma, H.; Gong, Y.; et al. An Overview of PROTACs: A Promising Drug Discovery Paradigm. Mol. Biomed. 2022, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.J.; Crews, C.M. Proteolysis Targeting Chimeras (PROTACs) Come of Age: Entering the Third Decade of Targeted Protein Degradation. RSC Chem. Biol. 2021, 2, 725–742. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maekawa, S.; Miyata, S. IgLON Cell Adhesion Molecules Regulate Synaptogenesis in Hippocampal Neurons. Cell Biochem. Funct. 2009, 27, 496–498. [Google Scholar] [CrossRef]
- Takita, J.; Chen, Y.; Okubo, J.; Sanada, M.; Adachi, M.; Ohki, K.; Nishimura, R.; Hanada, R.; Igarashi, T.; Hayashi, Y.; et al. Aberrations of NEGR1 on 1p31 and MYEOV on 11q13 in Neuroblastoma. Cancer Sci. 2011, 102, 1645–1650. [Google Scholar] [CrossRef]
- McKie, A.B.; Vaughan, S.; Zanini, E.; Okon, I.S.; Louis, L.; de Sousa, C.; Greene, M.I.; Wang, Q.; Agarwal, R.; Shaposhnikov, D.; et al. The OPCML Tumor Suppressor Functions as a Cell Surface Repressor-Adaptor, Negatively Regulating Receptor Tyrosine Kinases in Epithelial Ovarian Cancer. Cancer Discov. 2012, 2, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Wu, K.-L.; Chang, Y.-Y.; Liu, Y.-W.; Huang, Y.-C.; Jian, S.-F.; Lin, Y.-S.; Tsai, P.-H.; Hung, J.-Y.; Tsai, Y.-M.; et al. The Downregulation of LSAMP Expression Promotes Lung Cancer Progression and Is Associated with Poor Survival Prognosis. J. Pers. Med. 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Ntougkos, E.; Rush, R.; Scott, D.; Frankenberg, T.; Gabra, H.; Smyth, J.F.; Sellar, G.C. The IgLON Family in Epithelial Ovarian Cancer: Expression Profiles and Clinicopathologic Correlates. Clin. Cancer Res. 2005, 11, 5764–5768. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.E.; Dunn, J.R.; du Plessis, D.G.; Shaw, E.J.; Reeves, P.; Gee, A.L.; Warnke, P.C.; Sellar, G.C.; Moss, D.J.; Walker, C. Expression of Cellular Adhesion Molecule “OPCML” Is down-Regulated in Gliomas and Other Brain Tumours. Neuropathol. Appl. Neurobiol. 2007, 33, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Szczurkowska, J.; Pischedda, F.; Pinto, B.; Managò, F.; Haas, C.A.; Summa, M.; Bertorelli, R.; Papaleo, F.; Schäfer, M.K.; Piccoli, G.; et al. NEGR1 and FGFR2 Cooperatively Regulate Cortical Development and Core Behaviours Related to Autism Disorders in Mice. Brain 2018, 141, 2772–2794. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Szczurkowska, J.; Cirnaru, M.D.; Giesert, F.; Vezzoli, E.; Ueffing, M.; Sala, C.; Francolini, M.; Hauck, S.M.; Cancedda, L.; et al. A Cell Surface Biotinylation Assay to Reveal Membrane-Associated Neuronal Cues: Negr1 Regulates Dendritic Arborization. Mol. Cell Proteom. 2014, 13, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, F.; Piccoli, G. The IgLON Family Member Negr1 Promotes Neuronal Arborization Acting as Soluble Factor via FGFR2. Front. Mol. Neurosci. 2015, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Lee, H.; Choi, T.-Y.; Joo, Y.; Kim, S.-J.; Kim, H.; Kim, J.Y.; Jahng, J.W.; Lee, S.; Choi, S.-Y.; et al. Negr1 Controls Adult Hippocampal Neurogenesis and Affective Behaviors. Mol. Psychiatry 2019, 24, 1189–1205. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, J.-S.; Lee, B.; Hong, J.; Lee, S. Newly Identified Cancer-Associated Role of Human Neuronal Growth Regulator 1 (NEGR1). J. Cancer 2014, 5, 598–608. [Google Scholar] [CrossRef][Green Version]
- Corti, V.; Sanchez-Ruiz, Y.; Piccoli, G.; Bergamaschi, A.; Cannistraci, C.V.; Pattini, L.; Cerutti, S.; Bachi, A.; Alessio, M.; Malgaroli, A. Protein Fingerprints of Cultured CA3-CA1 Hippocampal Neurons: Comparative Analysis of the Distribution of Synaptosomal and Cytosolic Proteins. BMC Neurosci. 2008, 9, 36. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, A.; Zarrei, M.; Zhu, Y.; Hoffman, M.; Brenner, D.; Resnick, A.C.; Scherer, S.W.; Gallo, M. Ancestry and Frequency of Genetic Variants in the General Population Are Confounders in the Characterization of Germline Variants Linked to Cancer. BMC Med. Genet. 2020, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- de Larco, J.E.; Todaro, G.J. Growth Factors from Murine Sarcoma Virus-Transformed Cells. Proc. Natl. Acad. Sci. USA 1978, 75, 4001–4005. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Su, Y. Mouse Xenograft Models vs. GEM Models for Human Cancer Therapeutics. Dis. Model. Mech. 2008, 1, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.; Ferraro, G.B.; Fournier, A.E. IgLON Cell Adhesion Molecules Are Shed from the Cell Surface of Cortical Neurons to Promote Neuronal Growth. J. Biol. Chem. 2015, 290, 4330–4342. [Google Scholar] [CrossRef] [PubMed]
- Venkannagari, H.; Kasper, J.M.; Misra, A.; Rush, S.A.; Fan, S.; Lee, H.; Sun, H.; Seshadrinathan, S.; Machius, M.; Hommel, J.D.; et al. Highly Conserved Molecular Features in IgLONs Contrast Their Distinct Structural and Biological Outcomes. J. Mol. Biol. 2020, 432, 5287–5303. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, Successful Approach to Drug Delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Jevsevar, S.; Kunstelj, M.; Porekar, V.G. PEGylation of Therapeutic Proteins. Biotechnol. J. 2010, 5, 113–128. [Google Scholar] [CrossRef]
- Antony, J.; Zanini, E.; Birtley, J.R.; Gabra, H.; Recchi, C. Emerging Roles for the GPI-Anchored Tumor Suppressor OPCML in Cancers. Cancer Gene Ther. 2021, 28, 18–26. [Google Scholar] [CrossRef]
- Yoo, A.; Lee, S. Neuronal Growth Regulator 1 May Modulate Interleukin-6 Signaling in Adipocytes. Front. Mol. Biosci. 2023, 10, 1148521. [Google Scholar] [CrossRef]
- Somasundaram, D.B.; Aravindan, S.; Gupta, N.; Yu, Z.; Baker, A.; Aravindan, N. ALK Expression, Prognostic Significance, and Its Association with MYCN Expression in MYCN Non-Amplified Neuroblastoma. World J. Pediatr. 2022, 18, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.Y.; Friboulet, L.; Kodack, D.P.; Engstrom, L.D.; Li, Q.; West, M.; Tang, R.W.; Wang, H.; Tsaparikos, K.; Wang, J.; et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015, 28, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Infarinato, N.R.; Park, J.H.; Krytska, K.; Ryles, H.T.; Sano, R.; Szigety, K.M.; Li, Y.; Zou, H.Y.; Lee, N.V.; Smeal, T.; et al. The ALK/ROS1 Inhibitor PF-06463922 Overcomes Primary Resistance to Crizotinib in ALK-Driven Neuroblastoma. Cancer Discov. 2016, 6, 96–107. [Google Scholar] [CrossRef]
- Guan, J.; Tucker, E.R.; Wan, H.; Chand, D.; Danielson, L.S.; Ruuth, K.; El Wakil, A.; Witek, B.; Jamin, Y.; Umapathy, G.; et al. The ALK Inhibitor PF-06463922 Is Effective as a Single Agent in Neuroblastoma Driven by Expression of ALK and MYCN. Dis. Model. Mech. 2016, 9, 941–952. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Rooney, M.; Lin, J.J.; Nagy, R.J.; Yeap, B.Y.; Hubbeling, H.; Chin, E.; Ackil, J.; Farago, A.F.; Hata, A.N.; et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin. Cancer Res. 2019, 25, 6662–6670. [Google Scholar] [CrossRef]
- Yoda, S.; Lin, J.J.; Lawrence, M.S.; Burke, B.J.; Friboulet, L.; Langenbucher, A.; Dardaei, L.; Prutisto-Chang, K.; Dagogo-Jack, I.; Timofeevski, S.; et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov. 2018, 8, 714–729. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-Generation EGFR and ALK Inhibitors: Mechanisms of Resistance and Management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Röth, S.; Fulcher, L.J.; Sapkota, G.P. Advances in Targeted Degradation of Endogenous Proteins. Cell Mol. Life Sci. 2019, 76, 2761–2777. [Google Scholar] [CrossRef]
- Paudel, R.R.; Lu, D.; Roy Chowdhury, S.; Monroy, E.Y.; Wang, J. Targeted Protein Degradation via Lysosomes. Biochemistry 2022, 62, 564–579. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020, 181, 102–114. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC Targeted Protein Degraders: The Past Is Prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
| Peptide Name | Sequence |
|---|---|
| PepA | VDFPWAAVDNMLVRKGDTAVLRCYLEDGASKGAWLNRSSIIFAGGDKWSVDPRVSISTLNKRDYSLQIQNVDVTDDGPYTCSVQTQHTPRTMQVHL |
| PepB | PKIYDINMTINEGTNVTLTCLATGPEPVISWRHISPSAKPFENGQYLDIYGITRDQAGEYECSAENDVSFPDVKKVRVI |
| PepC | APTIQEIKSGTVTPGRSGLIRCEGAGVPPPAFEWYKGEKRLFNGQQGIIIQNFSTRSILTVTNVTQEHFGNYTCVAANKLGTTNASLP |
| PepA1 | DGASKGAWLNRSSIIFAGGDKWSVDPRVSISTL |
| PepA2 | VDFPWAAVDNMLVRKGDTAVLRCYLEDGASKGAWLNRSSIIFAGGDKWSVDPRVSISTL |
| PepA3 | DGASKGAWLNRSSIIFAGGDKWSVDPRVSISTLNKRDYSLQIQNVDVTDDGPYTCSVQTQHTPRTMQVHL |
| Primer | Sequence (5′-3′) |
|---|---|
| Negr1 FW | GTGCTCCTGGCGCAGGGC |
| Negr1 REV | TAAGATGCAGACCAGAAG |
| PepA FW | GTGGACTTCCCTTGG |
| PepA REV | GAGATGAACCTGC |
| PepB FW | CCGAAAATATACG |
| PepB REV | GATCACTCTCAC |
| PepC FW | GCGCCTACAATTCAG |
| PepC REV | GGGCAGGCTCGCGTTG |
| PepA1 FW | GATGGAGCATCAAAG |
| PepA1 REV | CAATGTGGAAATG |
| PepA2 FW | GTGGACTTCCCTTG |
| PepA2 REV | CAATGTGGAAATG |
| PepA3 FW | GATGGAGCATCAAAG |
| PepA3 REV | GAGATGAACCTGC |
| Primer | Sequence (5′-3′) |
|---|---|
| ALK1 FW | ACTGACACTCTCGCTTCTGAA |
| ALK1 REV | ATACGTTTCCTCTCAAAACCCC |
| ALK2 FW | GCTCCATGGAGTCACCCTC |
| ALK2 REV | CTCGAGGCCTCCTCGGAG |
| NMYC1 FW | GGTGGCTGCTCCTGCTCGTG |
| NMYC1 REV | TCCTCTTCATCTTCCTCCTCGT |
| NMYC2 FW | GCGGTCACTAGTGTGTCTG |
| ActinB FW | AATCGTGCGTGACATCAAAG |
| ActinB REV | AAGGAAGGCTGGAAAAGAGC |
| Gapdh FW | ACCACAGTCCATGCCATC |
| Gapdh REV | CAGGAAATGAGCTTGACAAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pischedda, F.; Ghirelli, A.; Tripathi, V.; Piccoli, G. Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo. Pharmaceutics 2023, 15, 2307. https://doi.org/10.3390/pharmaceutics15092307
Pischedda F, Ghirelli A, Tripathi V, Piccoli G. Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo. Pharmaceutics. 2023; 15(9):2307. https://doi.org/10.3390/pharmaceutics15092307
Chicago/Turabian StylePischedda, Francesca, Alessia Ghirelli, Vasvi Tripathi, and Giovanni Piccoli. 2023. "Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo" Pharmaceutics 15, no. 9: 2307. https://doi.org/10.3390/pharmaceutics15092307
APA StylePischedda, F., Ghirelli, A., Tripathi, V., & Piccoli, G. (2023). Negr1-Derived Peptides Trigger ALK Degradation and Halt Neuroblastoma Progression In Vitro and In Vivo. Pharmaceutics, 15(9), 2307. https://doi.org/10.3390/pharmaceutics15092307





