The Synergistic Influence of Polyflavonoids from Citrus aurantifolia on Diabetes Treatment and Their Modulation of the PI3K/AKT/FOXO1 Signaling Pathways: Molecular Docking Analyses and In Vivo Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Assay Kits
2.2. Preparation of Lemon Peel Extract (LPE)
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
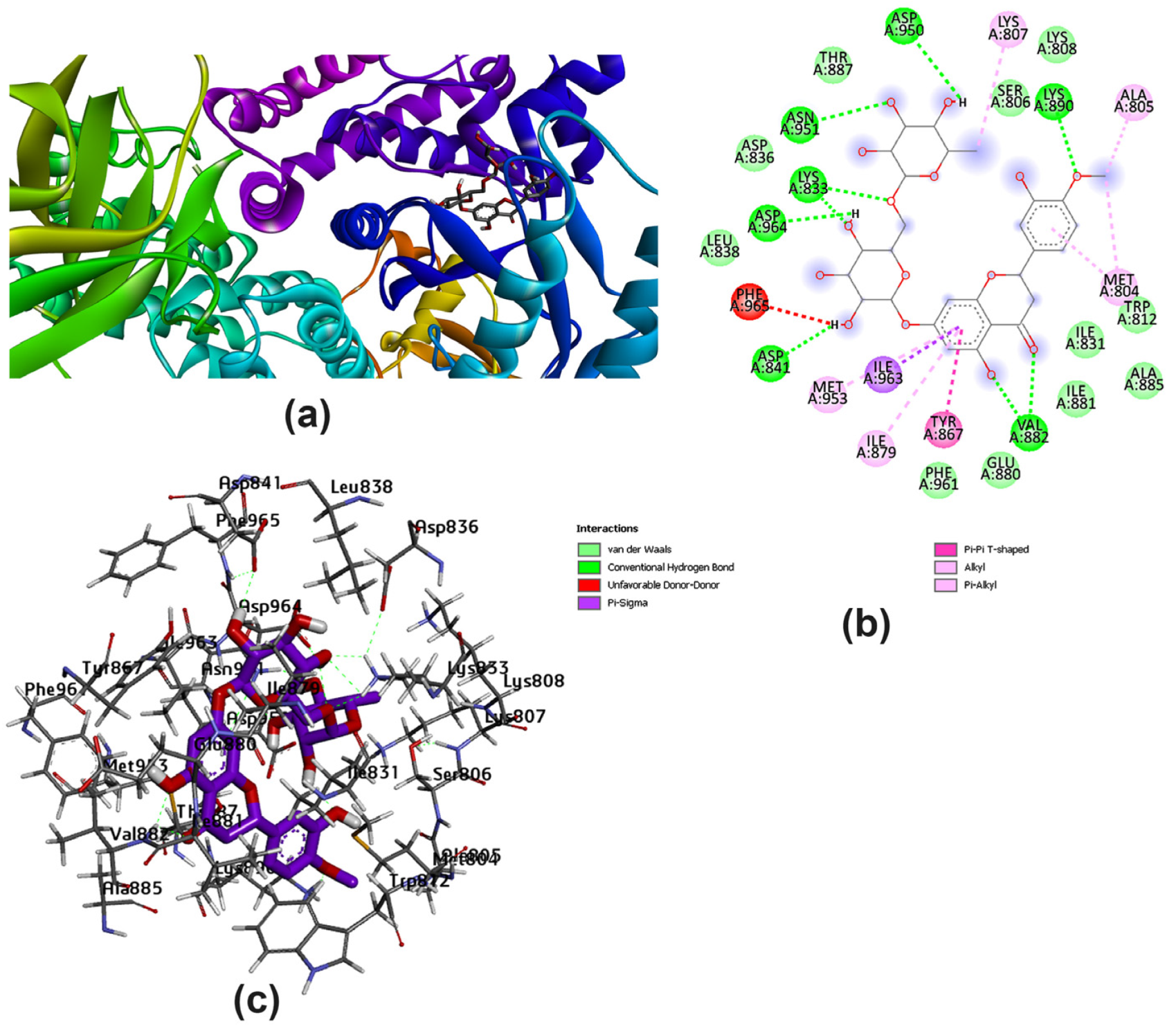
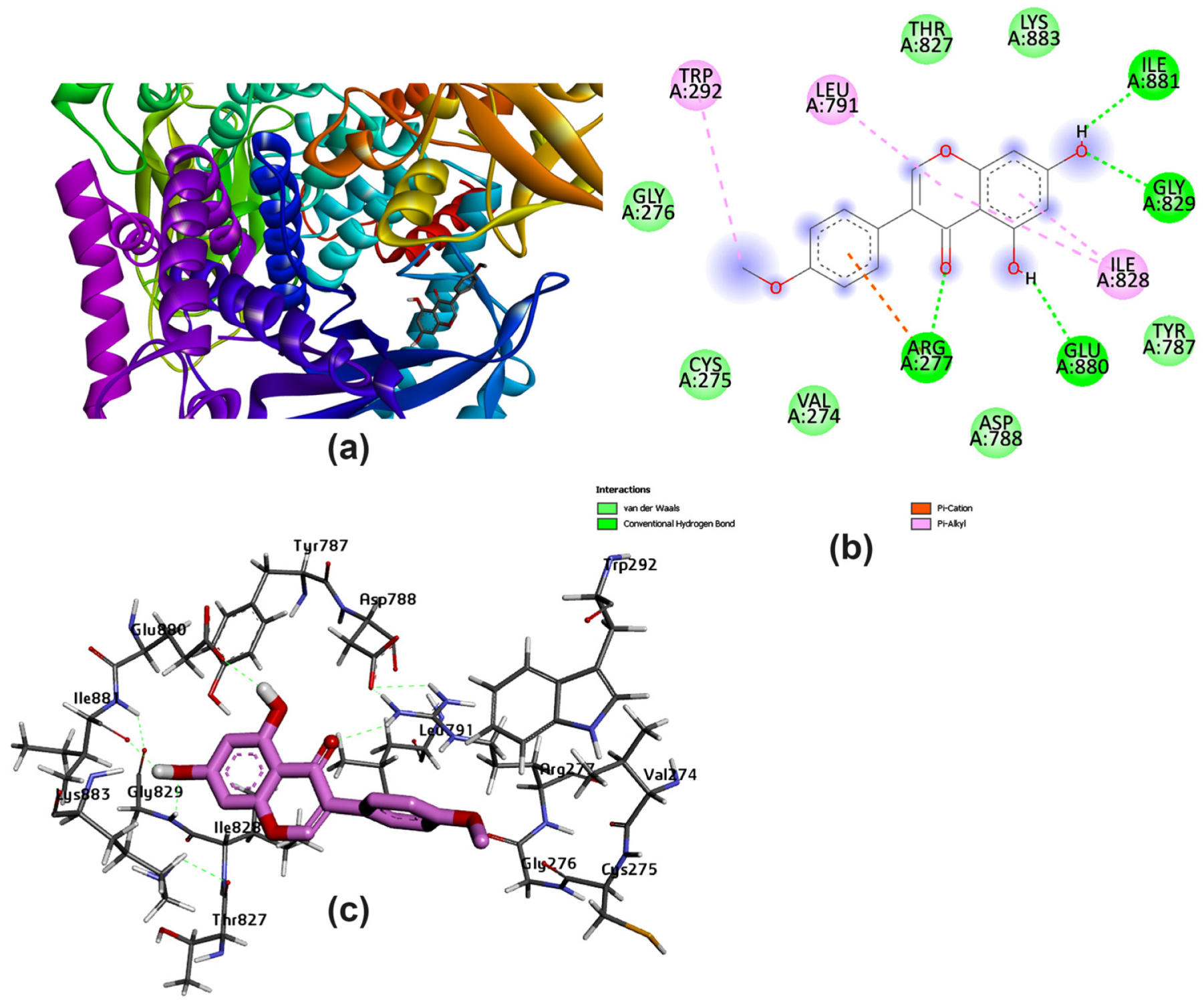
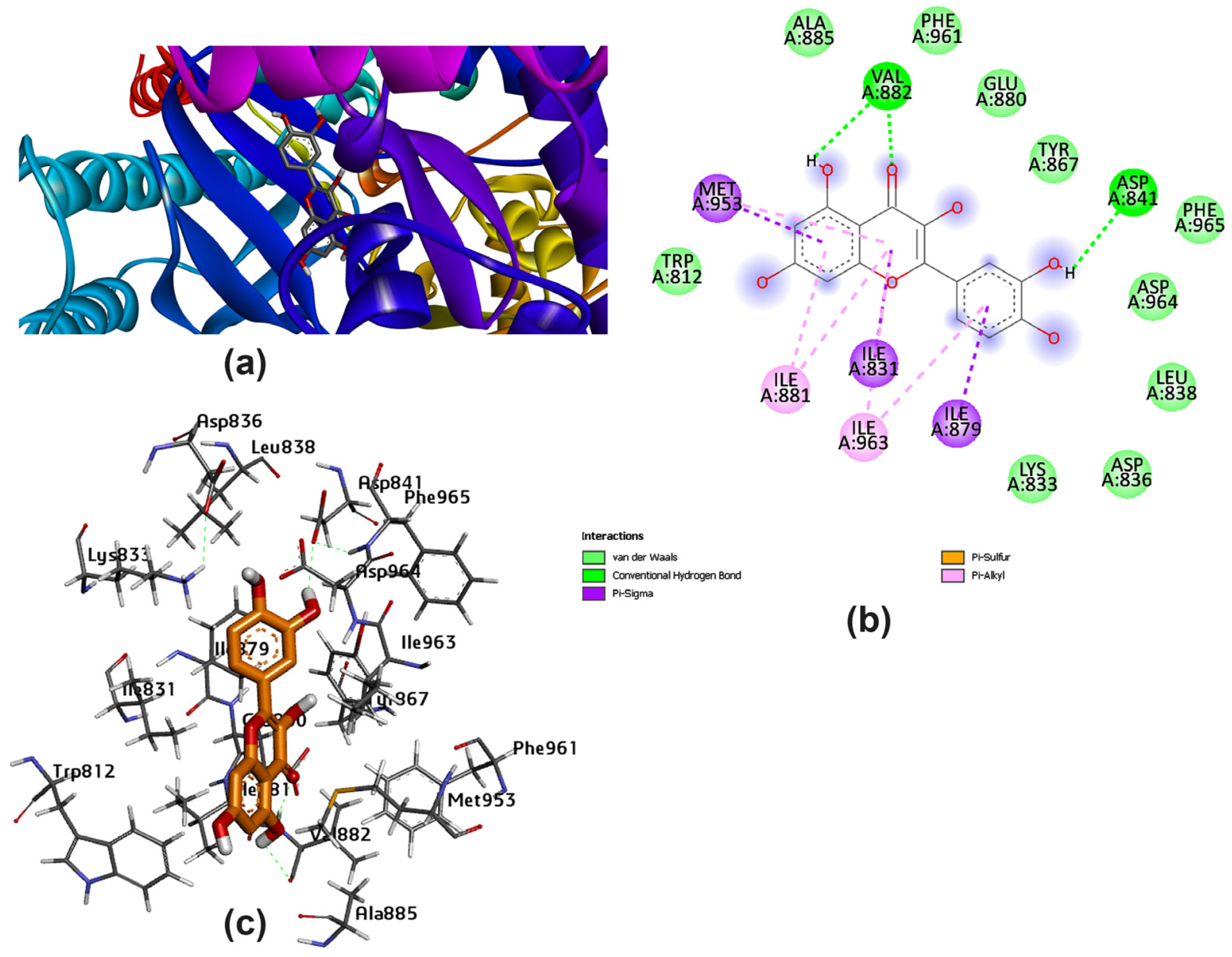
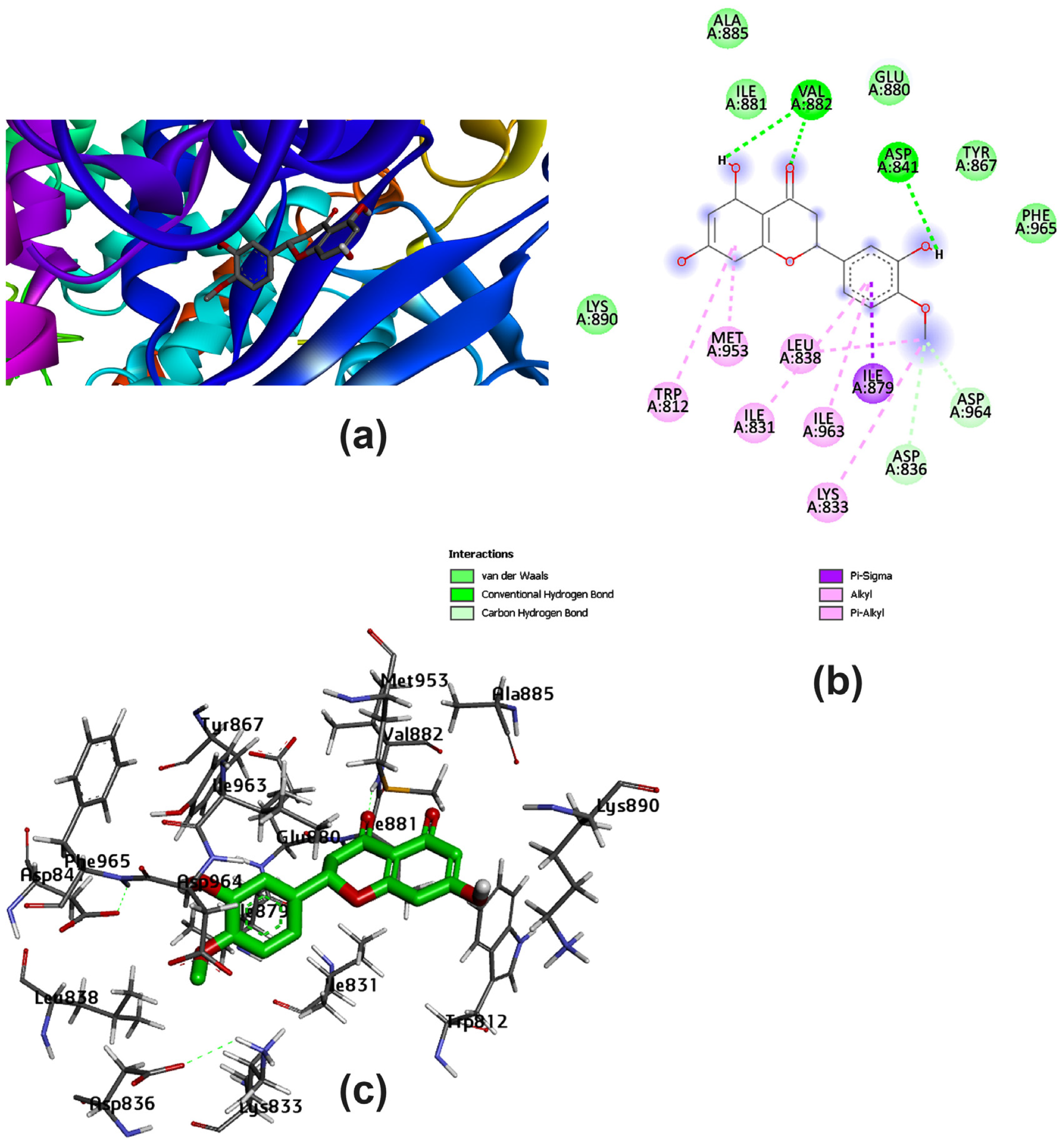
2.4. Molecular Docking Examination of Flavonoids in LPE
2.5. Physicochemical and Pharmacokinetic Protocol
2.6. In Vivo Investigations
Experimental Design and Induction of Diabetes
2.7. Blood Collection and Tissue Preparation
2.8. Biochemical Analysis
2.8.1. Lipid Profile Assay
2.8.2. Assessment of Oxidative Stress Biomarkers
2.8.3. Evaluation of Inflammatory Biomarkers
2.8.4. Glucose Transporter 2 (GLUT2) and GLUT4 and p-AKT
2.8.5. Quantitative Real-Time PCR (qRT-PCR) Analyses
2.9. Histopathological Examination
2.10. Statistical Analysis
3. Results
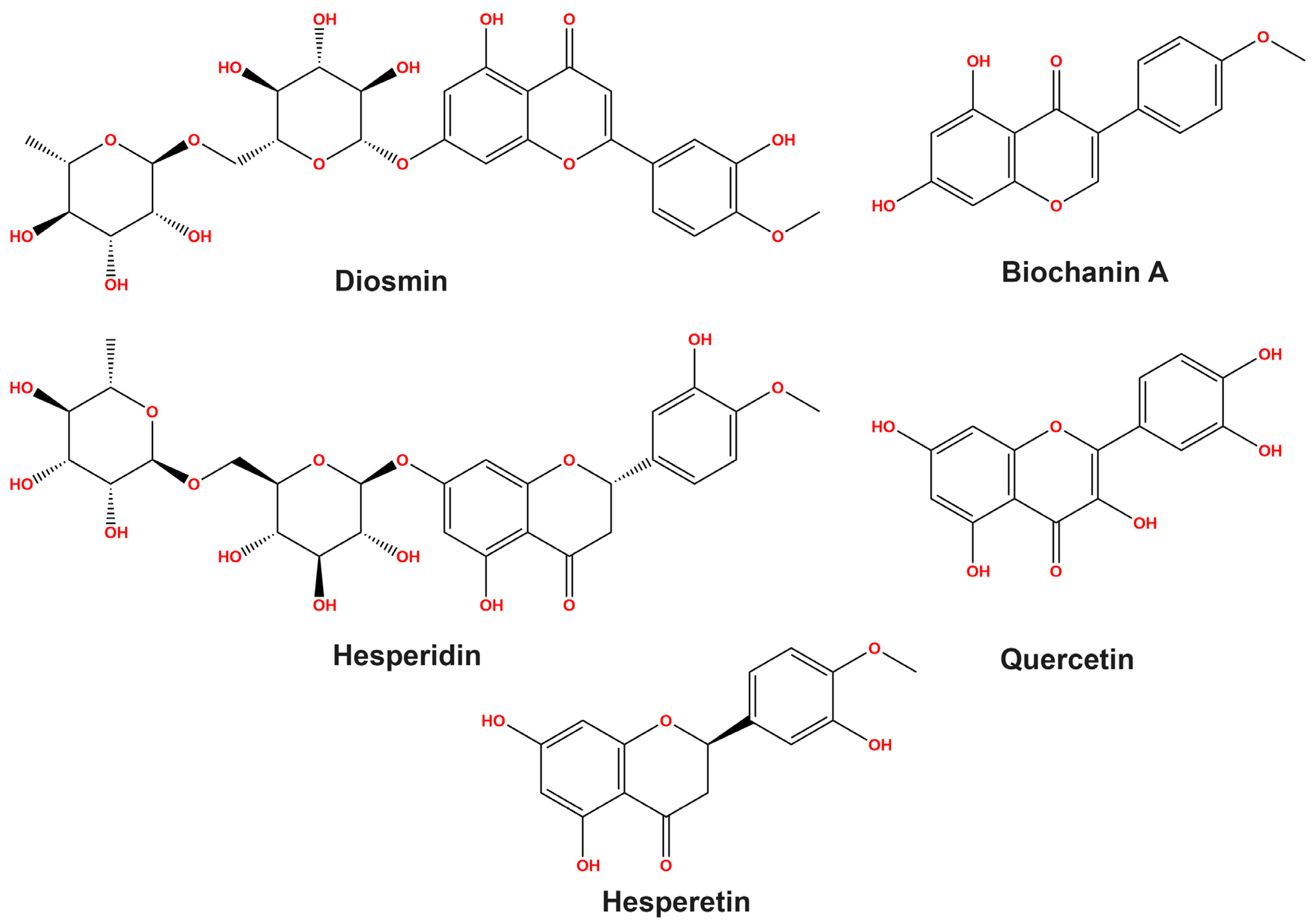
3.1. HPLC Study on Flavonoid Profiles
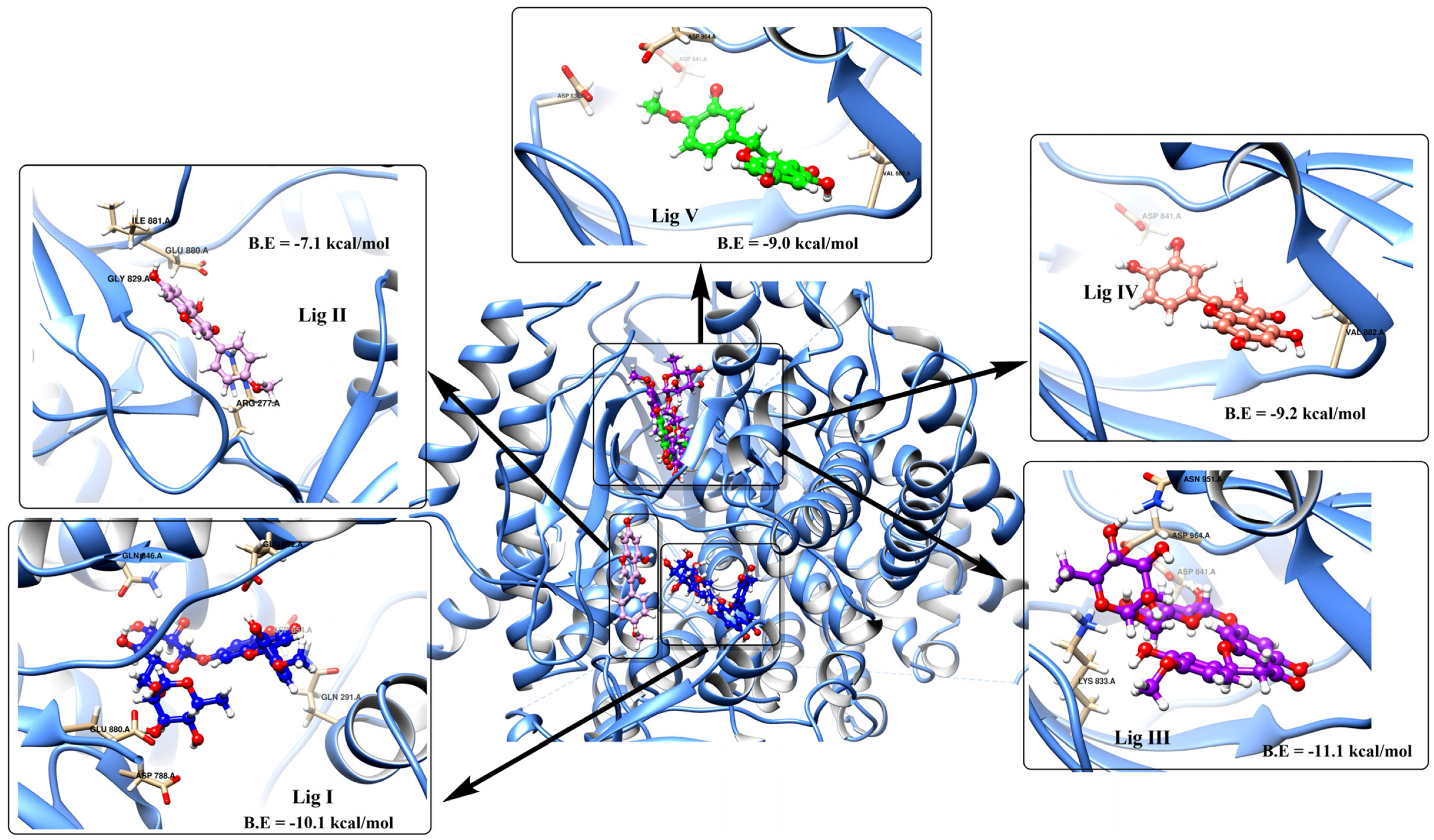
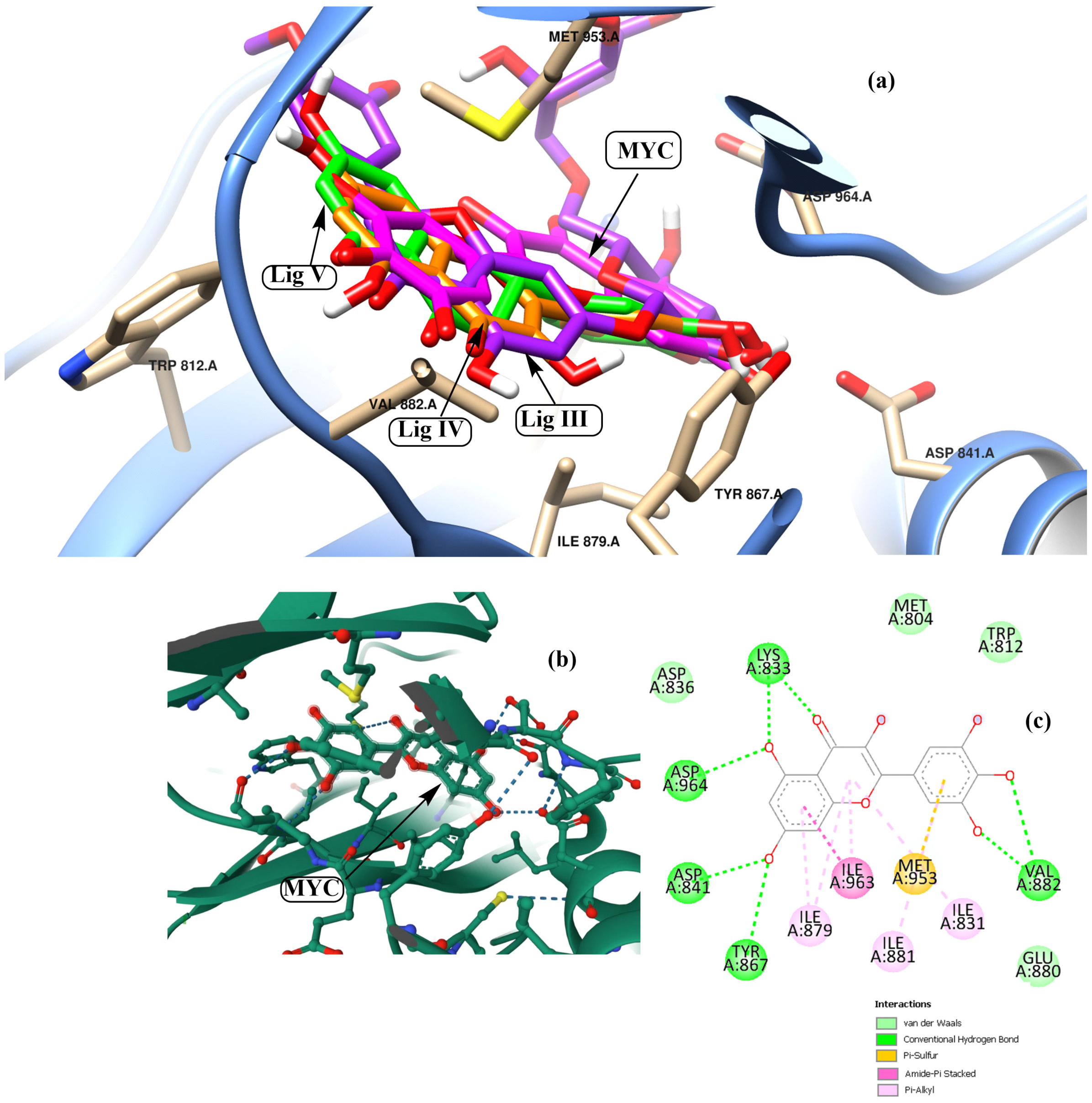
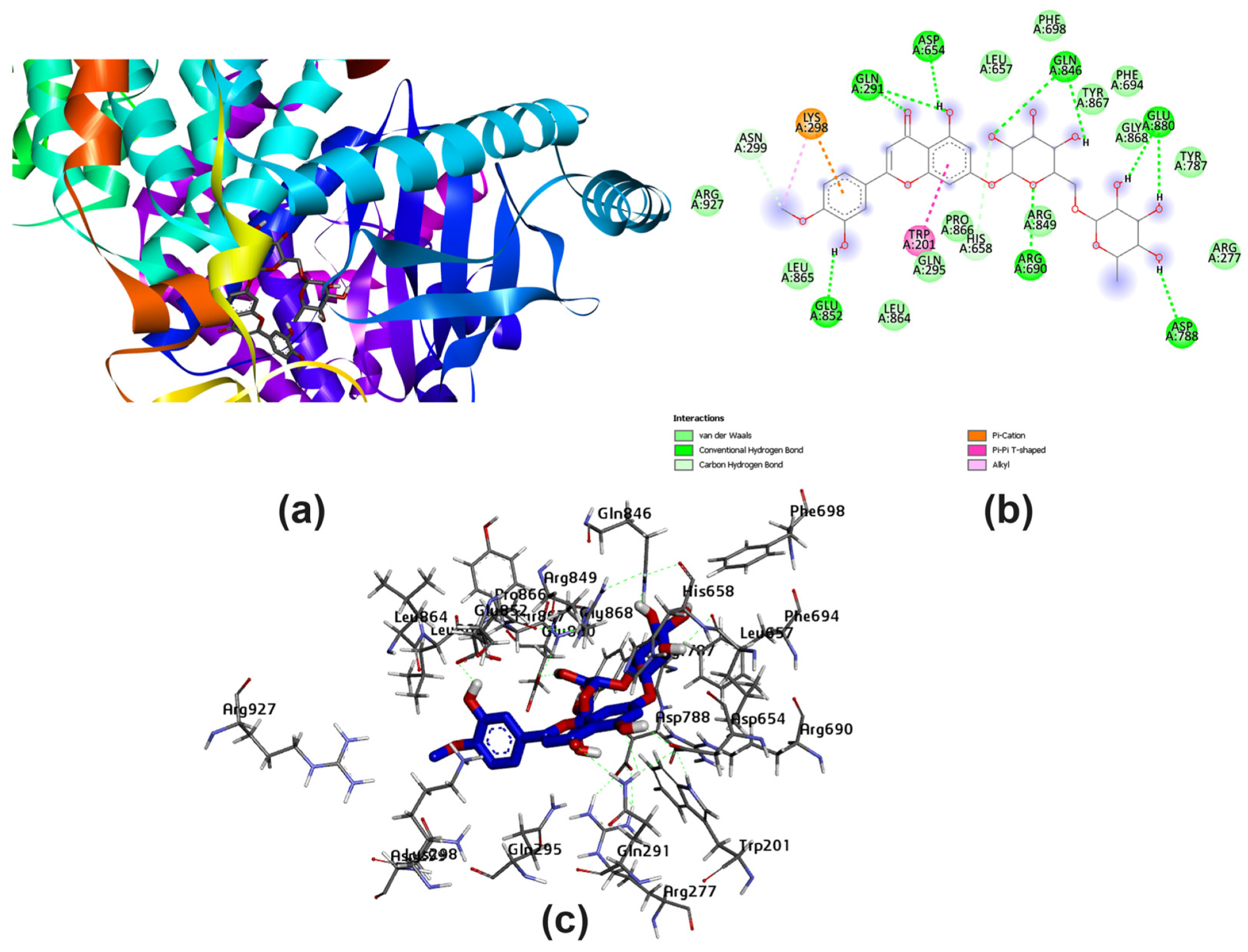
3.2. Potential Interaction of the PI3K Protein with LPE-Polyflavonoids
3.3. Pharmacokinetic Parameters and Drug-Likeness Prediction
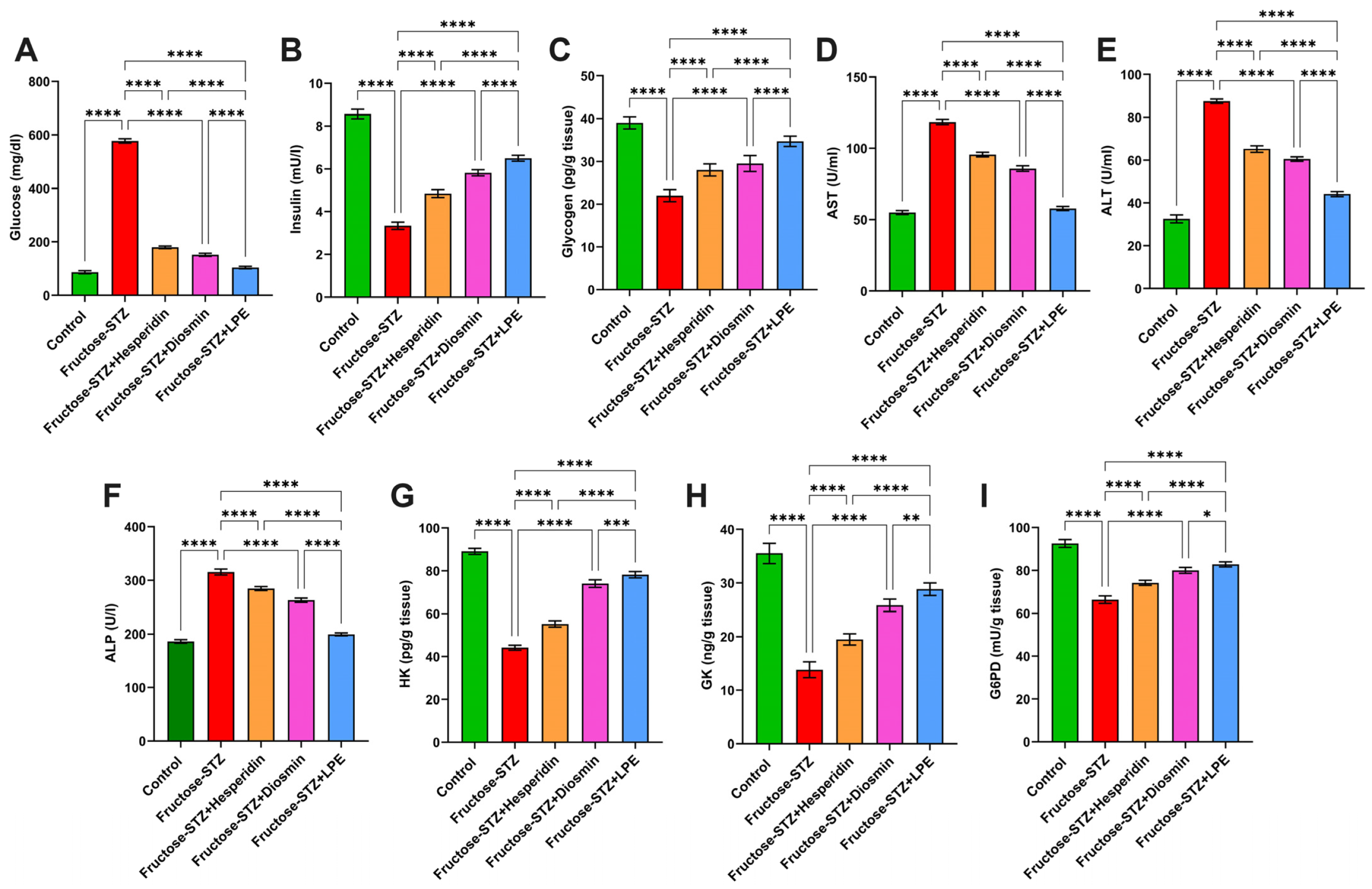
3.4. Impact of LPE-Polyflavonoids on Serum Glucose, Insulin, Glycogen, and Liver Enzymes
3.5. Influence of LPE-Polyflavonoids on Hepatic Carbohydrate-Metabolizing Enzyme Activities
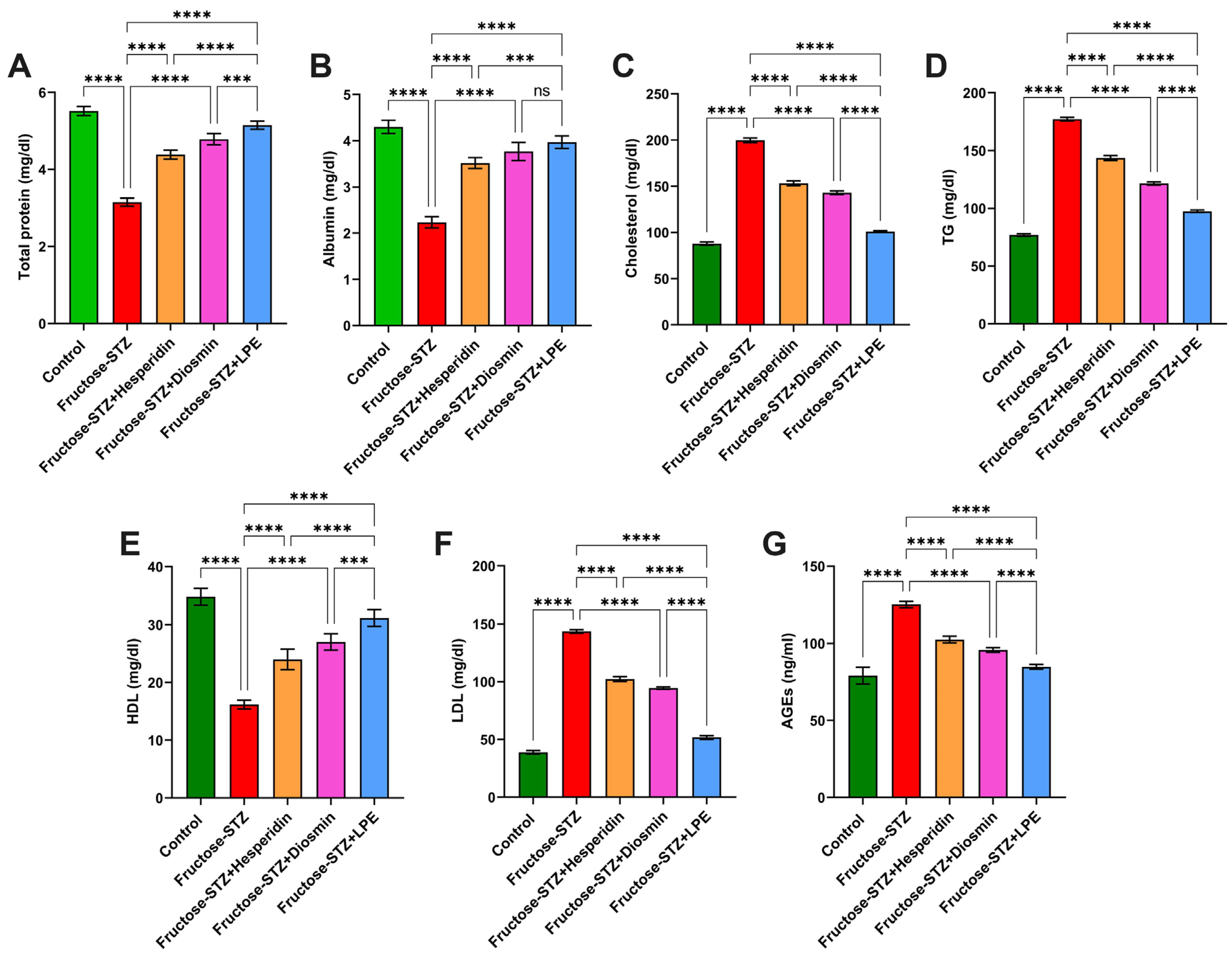
3.6. Effect of LPE-Polyflavonoids on Total Protein and Albumin Levels
3.7. Influence of LPE-Polyflavonoids on Serum Lipid Profile and AGEs
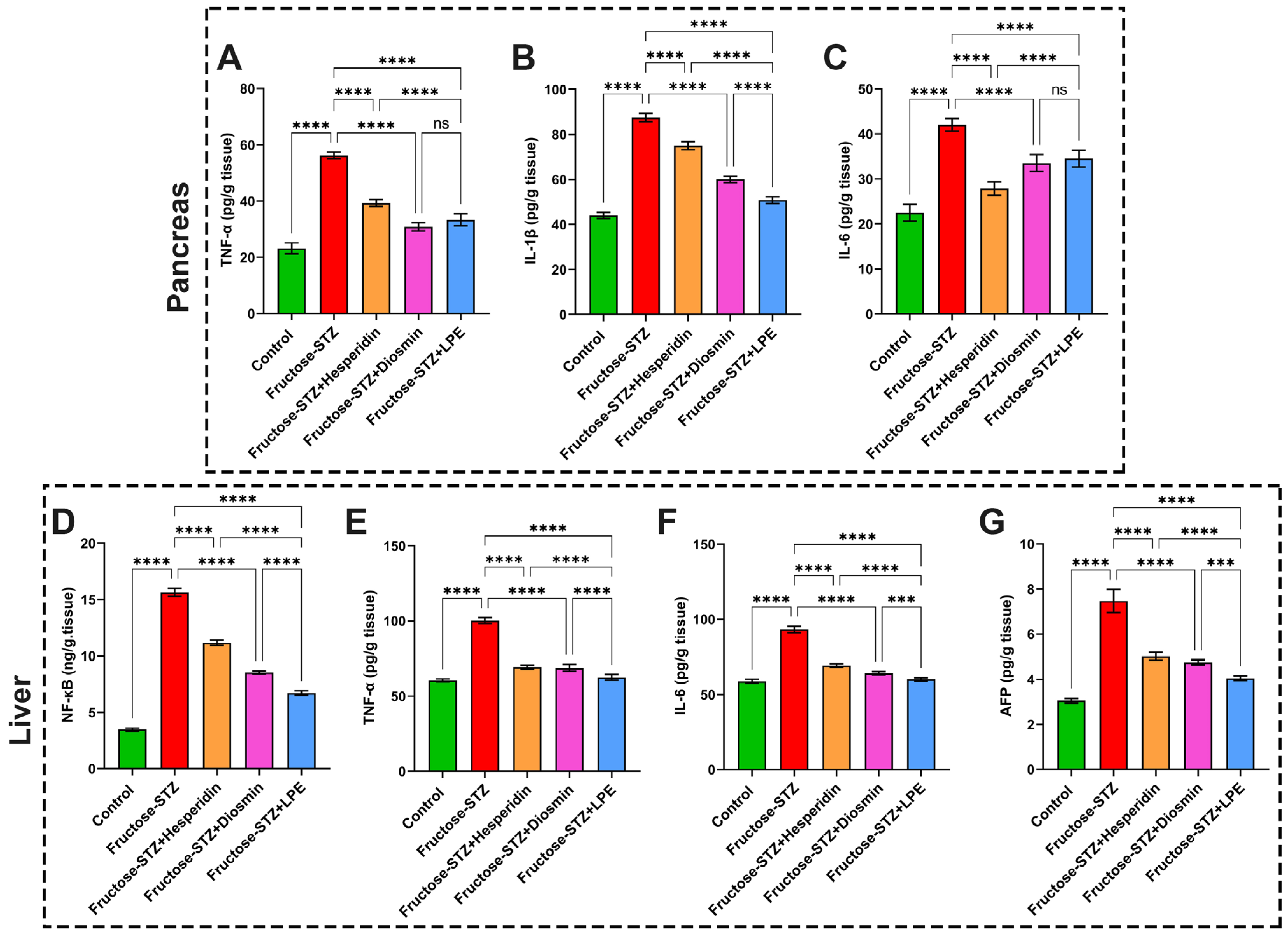
3.8. Effect of LPE-Polyflavonoids on Oxidative Stress Biomarkers in Pancreatic and Liver Tissues
3.9. Impact of LPE-Polyflavonoids on Liver and Pancreas Inflammatory Markers, NF-κB, and AFP
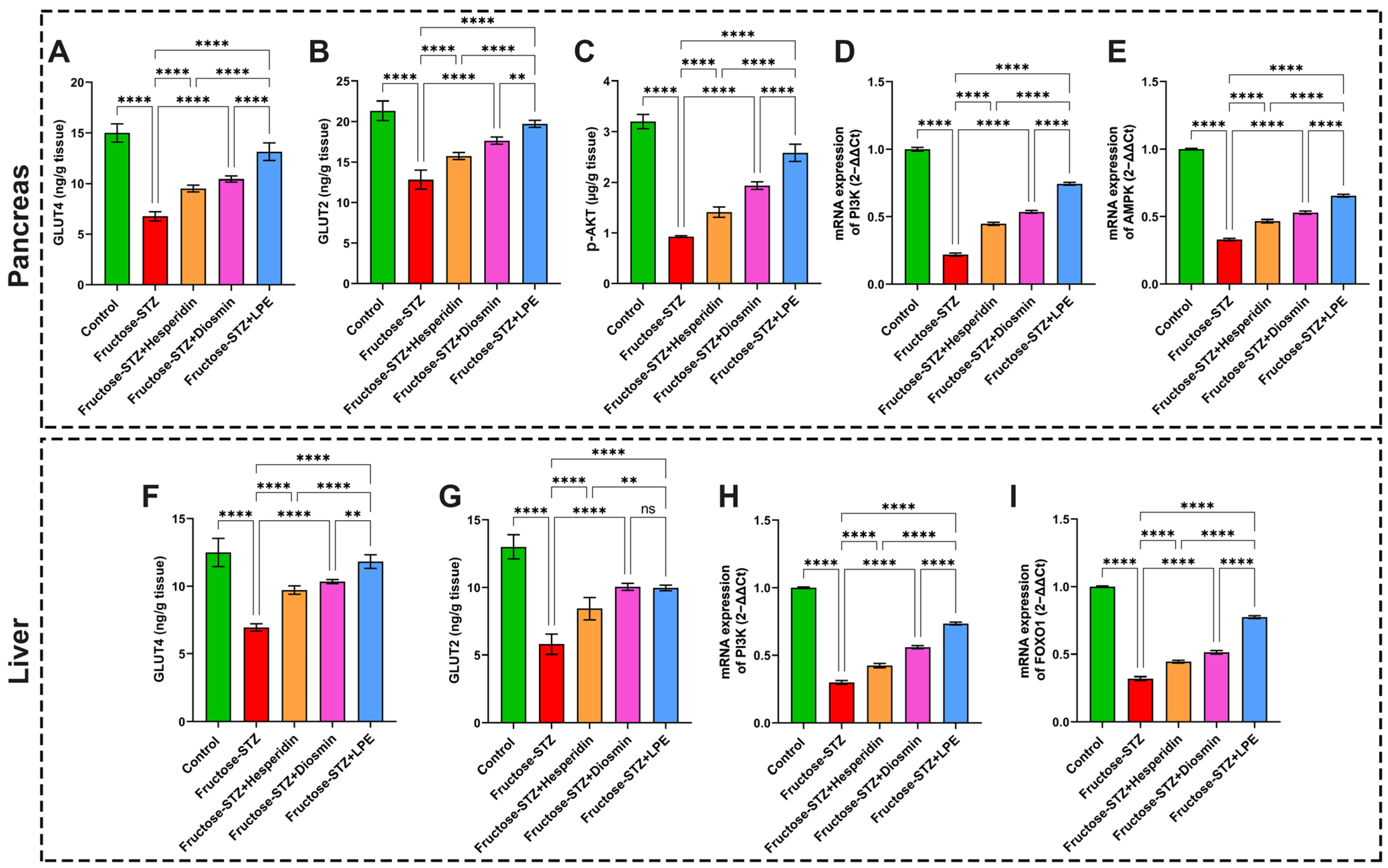
3.10. Effect of LPE-Polyflavonoids on GLUT4 and GLUT2 in Pancreas and Liver Tissues
3.11. Effect of LPE-Polyflavonoids on p-AKT in the Pancreas and Gene Expressions of PI3K, AMPK, and FOXO1 in Pancreatic and Hepatic Tissues
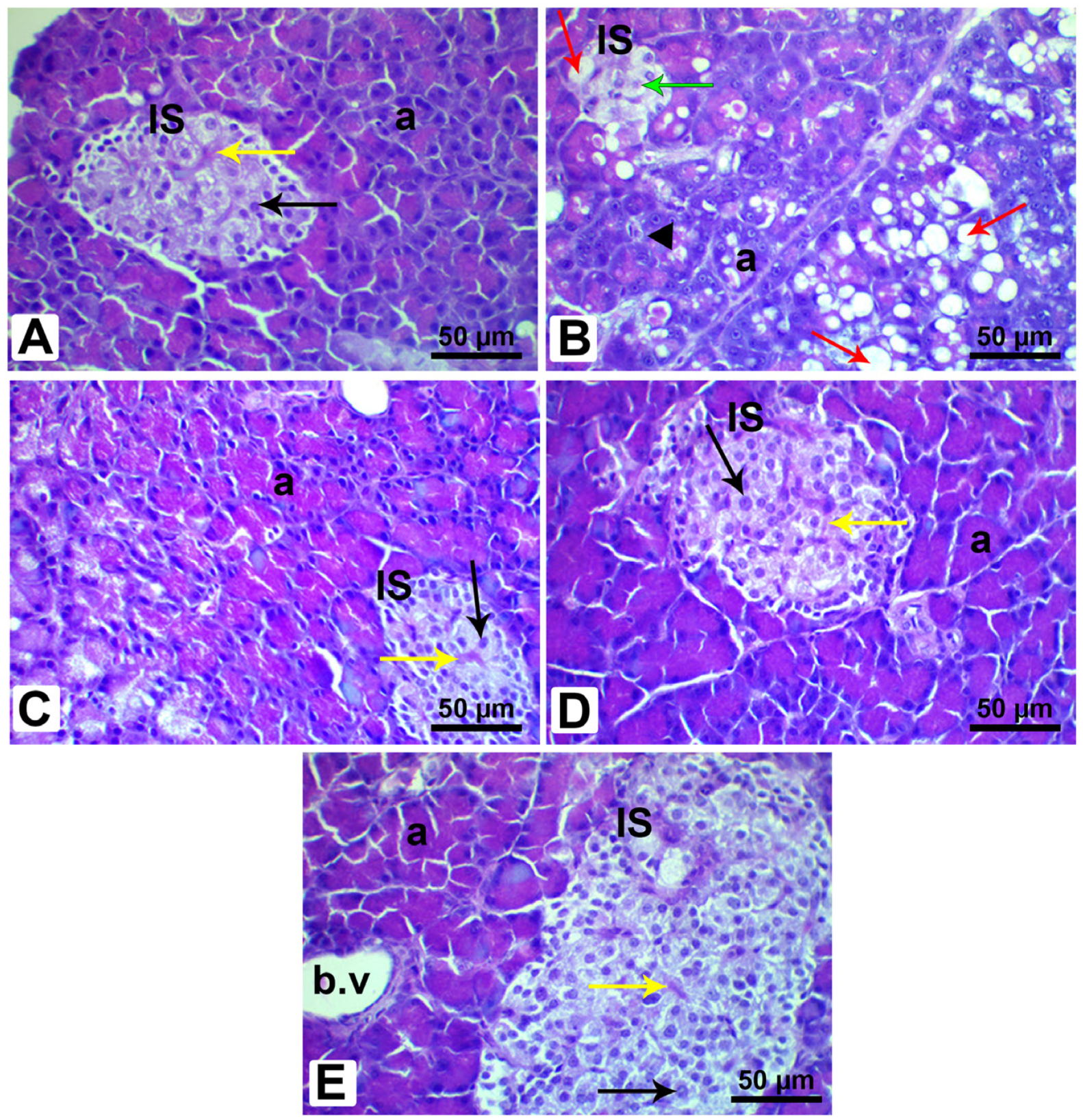
3.12. Histopathological Analysis of Pancreatic and Liver Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2022, 50, 638–643. [Google Scholar] [CrossRef]
- Hassan, M.A.; Tamer, T.M.; Rageh, A.A.; Abou-Zeid, A.M.; Abd El-Zaher, E.H.F.; Kenawy, E.-R. Insight into multidrug-resistant microorganisms from microbial infected diabetic foot ulcers. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Abd El-Aziz, S.; Elbadry, H.M.; El-Aassar, S.A.; Tamer, T.M. Prevalence, antimicrobial resistance profile, and characterization of multi-drug resistant bacteria from various infected wounds in North Egypt. Saudi J. Biol. Sci. 2022, 29, 2978–2988. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.; Abdelsalam, R.A.; Suddek, G.M.; Salem, H.A. Cinnamaldehyde ameliorates STZ-induced rat diabetes through modulation of IRS1/PI3K/AKT2 pathway and AGEs/RAGE interaction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Tamer, T.M.; Augustyniak, M.; El Wakil, A. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef]
- Nakayama, T.; Kosugi, T.; Gersch, M.; Connor, T.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Roncal, C.; Perez-Pozo, S.E.; Johnson, R.J.; Nakagawa, T. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am. J. Physiol. Renal. Physiol. 2010, 298, F712–F720. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Abdelmageed, M.E.; Shehatou, G.S.G.; Suddek, G.M.; Salem, H.A. Protocatechuic acid improves hepatic insulin resistance and restores vascular oxidative status in type-2 diabetic rats. Environ. Toxicol. Pharmacol. 2021, 83, 103577. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Quinn, W.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, S.; Xu, L.; Li, B.; Chen, N. Metformin-induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1α signal pathway. Food Sci. Nutr. 2019, 7, 1695–1703. [Google Scholar] [CrossRef]
- Cheng, Z. The FoxO—Autophagy axis in health and disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Ding, X.; Wang, Y.; Gu, M.; Zhang, J.; Yan, S.; Li, N.; Song, Z.; Yin, J.; Lu, L. Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1α pathway in high-fat-diet-induced rats and insulin resistant cells. Int. J. Biol. Sci. 2019, 15, 2815. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Sabet, M.M.; Alhalili, Z.A.H.; Ismail, A.M.; Mohy-Eldin, M.S.; Hassan, M.A. Influence of Cedar Essential Oil on Physical and Biological Properties of Hemostatic, Antibacterial, and Antioxidant Polyvinyl Alcohol/Cedar Oil/Kaolin Composite Hydrogels. Pharmaceutics 2022, 14, 2649. [Google Scholar] [CrossRef]
- Tamer, T.M.; ElTantawy, M.M.; Brussevich, A.; Nebalueva, A.; Novikov, A.; Moskalenko, I.V.; Abu-Serie, M.M.; Hassan, M.A.; Ulasevich, S.; Skorb, E.V. Functionalization of chitosan with poly aromatic hydroxyl molecules for improving its antibacterial and antioxidant properties: Practical and theoretical studies. Int. J. Biol. Macromol. 2023, 234, 123687. [Google Scholar] [CrossRef]
- Nabil-Adam, A.; Ashour, M.L.; Tamer, T.M.; Shreadah, M.A.; Hassan, M.A. Interaction of Jania rubens Polyphenolic Extract as an Antidiabetic Agent with α-Amylase, Lipase, and Trypsin: In Vitro Evaluations and In Silico Studies. Catalysts 2023, 13, 443. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hegde, M.L.; Rao, K. Polyphenols as potential metal chelation compounds against Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 82, S335–S357. [Google Scholar] [CrossRef]
- Sundaram, R.; Naresh, R.; Shanthi, P.; Sachdanandam, P. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine 2013, 20, 577–584. [Google Scholar] [CrossRef]
- Mehl, F.; Marti, G.; Boccard, J.; Debrus, B.; Merle, P.; Delort, E.; Baroux, L.; Raymo, V.; Velazco, M.I.; Sommer, H. Differentiation of lemon essential oil based on volatile and non-volatile fractions with various analytical techniques: A metabolomic approach. Food Chem. 2014, 143, 325–335. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Tag, H.M.; Kelany, O.E.; Tantawy, H.M.; Fahmy, A.A. Potential anti-inflammatory effect of lemon and hot pepper extracts on adjuvant-induced arthritis in mice. J. Basic Appl. Zool. 2014, 67, 149–157. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Zhang, X.; Zhao, D.G.; Ma, Y.Y.; Li, D.; Ho, C.T.; Huang, Q. Aged citrus peel (chenpi) extract causes dynamic alteration of colonic microbiota in high-fat diet induced obese mice. Food Funct. 2020, 11, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- El-Dakhly, S.M.; Salama, A.A.A.; Hassanin, S.O.M.; Yassen, N.N.; Hamza, A.A.; Amin, A. Aescin and diosmin each alone or in low dose-combination ameliorate liver damage induced by carbon tetrachloride in rats. BMC Res. Notes 2020, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Mcharek, N.; Hanchi, B. Maturational effects on phenolic constituents, antioxidant activities and LC-MS/MS profiles of lemon (Citrus limon) peels. J. Appl. Bot. Food Qual. 2017, 90, 1–9. [Google Scholar]
- Abchir, O.; Yamari, I.; Nour, H.; Daoui, O.; Elkhattabi, S.; Errougui, A.; Chtita, S. Structure-Based Virtual Screening, ADMET analysis, and Molecular Dynamics Simulation of Moroccan Natural Compounds as Candidates α-Amylase Inhibitors. ChemistrySelect 2023, 8, e202301092. [Google Scholar] [CrossRef]
- Yamari, I.; Abchir, O.; Mali, S.N.; Errougui, A.; Talbi, M.; Kouali, M.E.; Chtita, S. The anti-SARS-CoV-2 activity of novel 9, 10-dihydrophenanthrene derivatives: An insight into molecular docking, ADMET analysis, and molecular dynamics simulation. Sci. Afr. 2023, 21, e01754. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Tamer, T.M.; Zhou, H.; Hassan, M.A.; Abu-Serie, M.M.; Shityakov, S.; Elbayomi, S.M.; Mohy-Eldin, M.S.; Zhang, Y.; Cheang, T. Synthesis and physicochemical properties of an aromatic chitosan derivative: In vitro antibacterial, antioxidant, and anticancer evaluations, and in silico studies. Int. J. Biol. Macromol. 2023, 240, 124339. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lasker, K.; Schneidman-Duhovny, D.; Webb, B.; Huang, C.C.; Pettersen, E.F.; Goddard, T.D.; Meng, E.C.; Sali, A.; Ferrin, T.E. UCSF Chimera, MODELLER, and IMP: An integrated modeling system. J. Struct. Biol. 2012, 179, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural Determinants of Phosphoinositide 3-Kinase Inhibition by Wortmannin, LY294002, Quercetin, Myricetin, and Staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Hassan, R.M.; Elsayed, M.; Kholief, T.E.; Hassanen, N.H.M.; Gafer, J.A.; Attia, Y.A. Mitigating effect of single or combined administration of nanoparticles of zinc oxide, chromium oxide, and selenium on genotoxicity and metabolic insult in fructose/streptozotocin diabetic rat model. Environ. Sci. Pollut. Res. Int. 2021, 28, 48517–48534. [Google Scholar] [CrossRef]
- Álvarez-Almazán, S.; Solís-Domínguez, L.C.; Duperou-Luna, P.; Fuerte-Gómez, T.; González-Andrade, M.; Aranda-Barradas, M.E.; Palacios-Espinosa, J.F.; Pérez-Villanueva, J.; Matadamas-Martínez, F.; Miranda-Castro, S.P.; et al. Anti-Diabetic Activity of Glycyrrhetinic Acid Derivatives FC-114 and FC-122: Scale-Up, In Silico, In Vitro, and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 12812. [Google Scholar] [CrossRef]
- Lopes-Virella, M.F.; Stone, P.; Ellis, S.; Colwell, J.A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin. Chem. 1977, 23, 882–884. [Google Scholar] [CrossRef]
- Arafat, E.A.; El-Sayed, D.S.; Hussein, H.K.; Flaven-Pouchon, J.; Moussian, B.; El-Samad, L.M.; El Wakil, A.; Hassan, M.A. Entomotherapeutic Role of Periplaneta americana Extract in Alleviating Aluminum Oxide Nanoparticles-Induced Testicular Oxidative Impairment in Migratory Locusts (Locusta migratoria) as an Ecotoxicological Model. Antioxidants 2023, 12, 653. [Google Scholar] [CrossRef]
- Jollow, D.; Mitchell, J.; Zampaglione, N.A.; Gillette, J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Abei, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, L.M.; Bakr, N.R.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Hussein, H.K.; El Wakil, A.; Hassan, M.A. Silver nanoparticles instigate physiological, genotoxicity, and ultrastructural anomalies in midgut tissues of beetles. Chem.-Biol. Interact. 2022, 367, 110166. [Google Scholar] [CrossRef]
- El Sayed, D.S.; Abdelrehim, E.-S.M. Computational details of molecular structure, spectroscopic properties, topological studies and SARS-Cov-2 enzyme molecular docking simulation of substituted triazolo pyrimidine thione heterocycles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 261, 120006. [Google Scholar] [CrossRef]
- Cañizares-Carmenate, Y.; Mena-Ulecia, K.; Perera-Sardiña, Y.; Torrens, F.; Castillo-Garit, J.A. An approach to identify new antihypertensive agents using Thermolysin as model: In silico study based on QSARINS and docking. Arab. J. Chem. 2019, 12, 4861–4877. [Google Scholar] [CrossRef]
- Ouassaf, M.; Belaidi, S.; Mogren Al Mogren, M.; Chtita, S.; Ullah Khan, S.; Thet Htar, T. Combined docking methods and molecular dynamics to identify effective antiviral 2, 5-diaminobenzophenonederivatives against SARS-CoV-2. J. King Saud Univ.-Sci. 2021, 33, 101352. [Google Scholar] [CrossRef]
- Abchir, O.; Daoui, O.; Nour, H.; Yamari, I.; Elkhattabi, S.; Errougui, A.; Chtita, S. Exploration of Cannabis constituents as potential candidates against diabetes mellitus disease using molecular docking, dynamics simulations and ADMET investigations. Sci. Afr. 2023, 21, e01745. [Google Scholar] [CrossRef]
- Daoui, O.; Elkhattabi, S.; Chtita, S.; Elkhalabi, R.; Zgou, H.; Benjelloun, A.T. QSAR, molecular docking and ADMET properties in silico studies of novel 4,5,6,7-tetrahydrobenzo[D]-thiazol-2-Yl derivatives derived from dimedone as potent anti-tumor agents through inhibition of C-Met receptor tyrosine kinase. Heliyon 2021, 7, e07463. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The Antimicrobial and Antiviral Activity of Polyphenols from Almond (Prunus dulcis L.) Skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Abchir, O.; Daoui, O.; Belaidi, S.; Ouassaf, M.; Qais, F.A.; ElKhattabi, S.; Belaaouad, S.; Chtita, S. Design of novel benzimidazole derivatives as potential α-amylase inhibitors using QSAR, pharmacokinetics, molecular docking, and molecular dynamics simulation studies. J. Mol. Model. 2022, 28, 106. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.T.H.; Lawal, B.; Wei, L.; Wen, Y.-T.; Tzeng, D.T.W.; Lo, W.-C. Multiomics Identification of Potential Targets for Alzheimer Disease and Antrocin as a Therapeutic Candidate. Pharmaceutics 2021, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, D.H.; Vani, M.; Manikandan, S.; Vijayakumar, V. An immunohistochemical and biochemical evidences of pancreatic β-cell regeneration in type 2 diabetic rats after treated with Aegle marmelos leaf extract and Aegeline. Int. J. Res. Pharm. Sci. 2021, 12, 868–876. [Google Scholar] [CrossRef]
- Hahn, M.; van Krieken, P.P.; Nord, C.; Alanentalo, T.; Morini, F.; Xiong, Y.; Eriksson, M.; Mayer, J.; Kostromina, E.; Ruas, J.L.; et al. Topologically selective islet vulnerability and self-sustained downregulation of markers for β-cell maturity in streptozotocin-induced diabetes. Commun. Biol. 2020, 3, 541. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Zdzieblo, D. Glucose transporters in pancreatic islets. Pflug. Arch. 2020, 472, 1249–1272. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Jin, J.; Zou, G.; Sui, Y.; Han, Y.; Zhao, D.; Liu, L. Hesperidin prevents hyperglycemia in diabetic rats by activating the insulin receptor pathway. Exp. Ther. Med. 2021, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus Flavonoids as Promising Phytochemicals Targeting Diabetes and Related Complications: A Systematic Review of In Vitro and In Vivo Studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Om, H.; El-Naggar, M.E.; El-Banna, M.; Fouda, M.M.G.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Combating atherosclerosis with targeted Diosmin nanoparticles-treated experimental diabetes. Investig. New Drugs 2020, 38, 1303–1315. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Abd El-Hameed, M.N.; Khamis, H.H.; Sharkawy, G.K.; Samir, A.E.-L.H. Antidiabetic and anti-inflammatory effects of two Fabaceae extracts against streptozotocin induced diabetic impairment in male rats. World J. Adv. Res. Rev. 2020, 6, 012–029. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Nwaogu, G.; Chukwuma, C.I.; Adeyemi, O.T.; Matsabisa, M.G.; Swain, S.S.; Aiyegoro, O.A. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: In silico studies of molecular interaction of quercetin with hexokinase and catalase. J. Food Biochem. 2020, 44, e13127. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Nandhakumar, E.; Haseena Banu, H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2019, 29, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Rabee, A.; Bennasir, H. Hesperidin an antioxidant flavonoid prevents carbon tetrachloride-induced hepatic toxicity in male albino rats. J. Innov. Pharm. Biol. 2018, 5, 127–132. [Google Scholar]
- Ali, F.E.; Azouz, A.A.; Bakr, A.G.; Abo-Youssef, A.M.; Hemeida, R.A. Hepatoprotective effects of diosmin and/or sildenafil against cholestatic liver cirrhosis: The role of Keap-1/Nrf-2 and P38-MAPK/NF-κB/iNOS signaling pathway. Food Chem. Toxicol. 2018, 120, 294–304. [Google Scholar] [CrossRef]
- Saravanan, R.; Vengatash Babu, K.; Ramachandran, V. Effect of Rebaudioside A, a diterpenoid on glucose homeostasis in STZ-induced diabetic rats. J. Physiol. Biochem. 2012, 68, 421–431. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Chung, S.S.M.; Xu, B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 1012–1017. [Google Scholar] [CrossRef]
- Wang, K.; Wang, H.; Liu, Y.; Shui, W.; Wang, J.; Cao, P.; Wang, H.; You, R.; Zhang, Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J. Funct. Foods 2018, 40, 261–271. [Google Scholar] [CrossRef]
- Pari, L.; Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010, 64, 477–481. [Google Scholar] [CrossRef]
- Chang, D.C.; Xu, X.; Ferrante, A.W.; Krakoff, J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol. Metab. Syndr. 2019, 11, 14. [Google Scholar] [CrossRef]
- Abdel-Sttar, A.R.; Khalaf, M.M.; Aboyoussef, A.M.; Abosaif, A. Ameliorative effect of hesperidin on carbon tetrachloride induced liver fibrosis in rats. Int. J. Pharm. Pharm. Sci. 2017, 9, 45–51. [Google Scholar] [CrossRef]
- Tahir, M.; Rehman, M.U.; Lateef, A.; Khan, R.; Khan, A.Q.; Qamar, W.; Ali, F.; O’Hamiza, O.; Sultana, S. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-α and NF-κB activation. Alcohol 2013, 47, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Omolola, S.-O.; Bukoye, O.H.O. Improvement of Oral Glucose Tolerance and Total Lipid Profile of Diabetic Rats Treated with Ficus exasperata Leaf-Based Diet. Covenant J. Phys. Life Sci. 2019, 7, 2354–3574. [Google Scholar]
- Abdel-Reheim, M.A.; Messiha, B.A.S.; Abo-Saif, A.A. Hepatoprotective effect of diosmin on iron-induced liver damage. Int. J. Pharmacol. 2017, 13, 529–540. [Google Scholar] [CrossRef]
- Yazdi, H.B.; Hojati, V.; Shiravi, A.; Hosseinian, S.; Vaezi, G.; Hadjzadeh, M.A. Liver Dysfunction and Oxidative Stress in Streptozotocin-Induced Diabetic Rats: Protective Role of Artemisia Turanica. J. Pharmacopunct. 2019, 22, 109–114. [Google Scholar] [CrossRef]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 453. [Google Scholar] [CrossRef]
- Eraslan, G.; Sarica, Z.S.; Bayram, L.C.; Tekeli, M.Y.; Kanbur, M.; Karabacak, M. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ. Sci. Pollut. Res. Int. 2017, 24, 27931–27941. [Google Scholar] [CrossRef]
- Caglayan, C.; Kandemir, F.M.; Darendelioğlu, E.; Küçükler, S.; Ayna, A. Hesperidin protects liver and kidney against sodium fluoride-induced toxicity through anti-apoptotic and anti-autophagic mechanisms. Life Sci. 2021, 281, 119730. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I., Jr.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxid. Med. Cell Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef]
- Hanchang, W.; Khamchan, A.; Wongmanee, N.; Seedadee, C. Hesperidin ameliorates pancreatic beta-cell dysfunction and apoptosis in streptozotocin-induced diabetic rat model. Life Sci. 2019, 235, 116858. [Google Scholar] [CrossRef]
- Uslu, S.; Alaca, N.; Kilic, K.D.; Uysal, A.; Kurtel, H. The effects of aerobic exercise frequencies on liver fibrosis, alpha-fetoprotein and cytokeratin 19 in experimental type 2 diabetes-induced rats: An immunohistochemistry study. Biotech. Histochem. 2018, 93, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xiao, J.; Chi, S. Piperlongumine attenuates oxidative stress, inflammatory, and apoptosis through modulating the GLUT-2/4 and AKT signaling pathway in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2021, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaqha, W.M.; Khan, M.; Salam, N.; Azzi, A.; Chaudhary, A.A. Anti-diabetic potential of Catharanthus roseus Linn. and its effect on the glucose transport gene (GLUT-2 and GLUT-4) in streptozotocin induced diabetic wistar rats. BMC Complement. Altern. Med. 2015, 15, 379. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Gabbar, M.A.; Abdel-Twab, S.M.; Fahmy, E.M.; Ebaid, H.; Alhazza, I.M.; Ahmed, O.M. Antidiabetic Potency, Antioxidant Effects, and Mode of Actions of Citrus reticulata Fruit Peel Hydroethanolic Extract, Hesperidin, and Quercetin in Nicotinamide/Streptozotocin-Induced Wistar Diabetic Rats. Oxid. Med. Cell Longev. 2020, 2020, 1730492. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, M.H.; Cheng, J.T.; Wu, M.C. Antihyperglycaemic action of diosmin, a citrus flavonoid, is induced through endogenous β-endorphin in type I-like diabetic rats. Clin. Exp. Pharmacol. Physiol. 2017, 44, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wang, J.; Liu, H.-Y.; Zhang, Y.-Q. Mulberry bark alleviates effect of STZ inducing diabetic mice through negatively regulating FoxO1. Evid.-Based Complement. Altern. Med. 2019, 2019, 2182865. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Kang, J.; Cao, Y.; Fan, S.; Yang, H.; An, Y.; Pan, Y.; Tie, L.; Li, X. Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic β-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. Apoptosis 2015, 20, 1420–1432. [Google Scholar] [CrossRef]
- Kinyua, A.W.; Ko, C.M.; Doan, K.V.; Yang, D.J.; Huynh, M.K.Q.; Moh, S.H.; Choi, Y.-H.; Kim, K.W. 4-hydroxy-3-methoxycinnamic acid regulates orexigenic peptides and hepatic glucose homeostasis through phosphorylation of FoxO1. Exp. Mol. Med. 2018, 50, e437. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A. Antithyroid effects of naringin, hesperidin and rutin in l-T4 induced hyperthyroid rats: Possible mediation through 5′ DI activity. Pharmacol. Rep. 2014, 66, 1092–1099. [Google Scholar] [CrossRef]
- Ekperikpe, U.S.; Owolabi, O.J.; Olapeju, B.I. Effects of Parkia biglobosa aqueous seed extract on some biochemical, haematological and histopathological parameters in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2019, 228, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Rashidi, R.; Shafiee-Nick, R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed. Pharmacother. 2019, 111, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; He, Y.; Ji, L.L.; Wang, K.Y.; Wang, Y.L.; Chen, D.F.; Geng, Y.; OuYang, P.; Lai, W.M. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 2017, 8, 101545–101559. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Zhao, Y.; Qiang, G.; Yang, X.; Xu, C.; Chen, X.; Liu, C.; Wang, X.; Zhang, L.; Du, G. Puerarin Mitigates Diabetic Hepatic Steatosis and Fibrosis by Inhibiting TGF-beta Signaling Pathway Activation in Type 2 Diabetic Rats. Oxid. Med. Cell Longev. 2018, 2018, 4545321. [Google Scholar] [CrossRef]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PI3K | 5′-CCTGGTAACTGCAACACTTC-3′ | 5′-AACGAATTCAAACCTACCCG-3′ |
| FOXO1 | 5′-TCATCCAATTGGTCTTGTGG-3′ | 5′-GTGTTTGCCTGTCTACCTTT-3′ |
| AMPK | 5′-TGTGAAGATCGGACACTACG-3′ | 5′-TAACTGCCACTTTATGGCCT-3′ |
| β-Actin | 5′-ATGTGGCTGAGGACTTTGATT-3′ | 5′-ATCTATGCCGTGGATACTTGG-3′ |
| Flavonoid Compounds | R.T./min | Con. (mg/kg) |
|---|---|---|
| Diosmin | 22.878 | 62.75 |
| Biochanin A | 17.477 | 62.35 |
| Hesperidin | 6.254 | 59.18 |
| Quercetin | 14.297 | 28.32 |
| Hesperetin | 16.147 | 9.31 |
| Lig I | Lig II | Lig III | Lig IV | Lig V | MYS Control | |
|---|---|---|---|---|---|---|
| Physicochemical properties | ||||||
| Molecular weight (Da) | 608.54 | 284.26 | 610.56 | 302.24 | 302.28 | 318.24 |
| Log Po/w (MLOGP) | −3.23 | 0.77 | −3.04 | −0.56 | 0.41 | |
| Number of H-bond acceptors | 15 | 5 | 15 | 7 | 6 | 8 |
| Number of H-bond donors | 8 | 2 | 8 | 5 | 3 | 6 |
| Molar refractivity | 143.82 | 78.46 | 141.41 | 78.04 | 78.06 | 80.06 |
| Number of rotatable bonds | 7 | 2 | 7 | 1 | 2 | 1 |
| TPSA (Å2) | 238.2 | 79.9 | 234.29 | 131.36 | 151.59 | |
| Pharmacokinetics | ||||||
| Gastrointestinal (GI) absorption | Low | High | Low | High | High | Low |
| Blood–brain barrier (BBB) permeant | No | No | No | No | No | No |
| P-glycoprotein substrate | Yes | No | Yes | No | Yes | No |
| Log Kp (skin permeation) | −9.91 | −5.91 | −10.12 | −7.05 | −6.3 | −7.4 |
| Drug likeness | ||||||
| Log S (ESOL) | −3.51 | −3.92 | −4.33 | −3.16 | −3.62 | −3.01 |
| Water solubility class | Moderately soluble | Moderately soluble | Moderately soluble | Soluble | Soluble | Soluble |
| Lipinski rule | No | Yes | No | Yes | Yes | Yes |
| Ghose | No | Yes | No | Yes | Yes | Yes |
| Veber | No | Yes | No | Yes | Yes | No |
| Egan | No | Yes | No | Yes | Yes | No |
| PAINs | 0 alerts | 0 alerts | 0 alerts | 1 alert: catechol_A | 0 alerts | 1 alert: catechol_A |
| Bioavailability score | 0.17 | 0.55 | 0.17 | 0.55 | 0.55 | 0.55 |
| Metabolism | ||||||
| CYP1A2 inhibitor | No | Yes | No | Yes | Yes | Yes |
| CYP2C19 inhibitor | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | Yes | No | Yes | No | No |
| CYP3A4 inhibitor | No | Yes | No | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.A.; Elmageed, G.M.A.; El-Qazaz, I.G.; El-Sayed, D.S.; El-Samad, L.M.; Abdou, H.M. The Synergistic Influence of Polyflavonoids from Citrus aurantifolia on Diabetes Treatment and Their Modulation of the PI3K/AKT/FOXO1 Signaling Pathways: Molecular Docking Analyses and In Vivo Investigations. Pharmaceutics 2023, 15, 2306. https://doi.org/10.3390/pharmaceutics15092306
Hassan MA, Elmageed GMA, El-Qazaz IG, El-Sayed DS, El-Samad LM, Abdou HM. The Synergistic Influence of Polyflavonoids from Citrus aurantifolia on Diabetes Treatment and Their Modulation of the PI3K/AKT/FOXO1 Signaling Pathways: Molecular Docking Analyses and In Vivo Investigations. Pharmaceutics. 2023; 15(9):2306. https://doi.org/10.3390/pharmaceutics15092306
Chicago/Turabian StyleHassan, Mohamed A., Ghada M. Abd Elmageed, Ibtehal G. El-Qazaz, Doaa S. El-Sayed, Lamia M. El-Samad, and Heba M. Abdou. 2023. "The Synergistic Influence of Polyflavonoids from Citrus aurantifolia on Diabetes Treatment and Their Modulation of the PI3K/AKT/FOXO1 Signaling Pathways: Molecular Docking Analyses and In Vivo Investigations" Pharmaceutics 15, no. 9: 2306. https://doi.org/10.3390/pharmaceutics15092306
APA StyleHassan, M. A., Elmageed, G. M. A., El-Qazaz, I. G., El-Sayed, D. S., El-Samad, L. M., & Abdou, H. M. (2023). The Synergistic Influence of Polyflavonoids from Citrus aurantifolia on Diabetes Treatment and Their Modulation of the PI3K/AKT/FOXO1 Signaling Pathways: Molecular Docking Analyses and In Vivo Investigations. Pharmaceutics, 15(9), 2306. https://doi.org/10.3390/pharmaceutics15092306










