Abstract
Mild photothermal therapy (PTT) shows great potential to treat cancers while avoiding unwanted damage to surrounding normal cells. However, the efficacy of mild PTT is normally moderate because of the low hyperthermia temperature and limited light penetration depth. Chemotherapy has unlimited penetration but often suffers from unsatisfactory efficacy in view of the occurrence of drug resistance, suboptimal drug delivery and release profile. As a result, the combinatory of chemotherapy and mild PTT would integrate their advantages and overcome the shortcomings. Herein, we synthesized an NIR-activatable and mild-temperature-sensitive nanoplatform (BDPII-gel@TSL) composed of temperature-sensitive liposomes (TSL), heat shock protein 90 (HSP90) inhibitor (geldanamycin) and photothermal agent (BDPII), for dual chemotherapy and mild PTT in cancer cells. BDPII, constructed with donor-acceptor moieties, acts as an excellent near-infrared (NIR) photothermal agent (PTA) with a high photothermal conversion efficiency (80.75%). BDPII-containing TSLs efficiently produce a mild hyperthermia effect (42 °C) under laser irradiation (808 nm, 0.5 W cm−2). Importantly, the phase transformation of TSL leads to burst release of geldanamycin from BDPII-gel@TSL, and this contributes to down-regulation of the overexpression of HSP90, ensuring efficient inhibition of cancer cell growth. This research provides a dual-sensitive synergistic therapeutic strategy for cancer cell treatment.
1. Introduction
Nowadays, treatment of malignant tumors is still a big challenge. This is a major cause of shorter life spans for patients around the world [1,2,3]. So far, chemotherapy is an effective therapeutic strategy to destroy tumor cells [4,5,6]. However, most reported chemotherapeutic agents are incapable of selectively anchoring at the tumor site; thus, they often induce unwanted destruction to normal tissue [7]. Moreover, tumor cells rapidly generate drug resistance after chemotherapy, which also leads to a limitation for this promising therapy [8,9]. Recently, a nano-sized tumor-anchored dual therapy system based on chemotherapeutics has demonstrated an advantage in the precise delivery of drugs to the tumor site and induced satisfactory tumor ablation with negligible drug resistance [7,10]. In particular, the positively triggered drug release system can overcome the shortage of inefficient drug release in a passive drug release system and is thus highly desired [11].
Recent studies have shown that light-controlled therapy with spatiotemporal controllability is a precision strategy with minimized side effects, and the light energy could also be a triggering source to induce drug release in light-stimulus drug delivery systems [12,13,14]. For instance, the active and controllable generation of ROS (reactive oxygen species) in PDT (photodynamic therapy) and hyperthermia in PTT (photothermal therapy) can be triggered by light irradiation and is guaranteed to be highly efficient at the spatiotemporal destruction of tumor cells [13,13,14,15,16,17,18,19,20,21,22,23]. The light irradiation for PTT can take advantage of NIR (near-infrared window, λ = 700–1100 nm) light, which can achieve much higher penetration than visible light sources [13,24,25,26,27,28]. Notably, most PTT is effective in surficial tumors and hardly shows efficacy in tumor cells far away from the light irradiation site owing to the intrinsically limited penetration depth for light in living tissues. Moreover, the NIR light in PTT also could be a stimulus source to positively trigger drug release in chemotherapy.
Normally, the NIR-triggered PTT strongly relies on hyperthermia (˃50 °C) to completely eradicate cancer cells, so that the resultant heating diffusion often brings in side-effects to surrounding normal tissues [20,23,29,30,31]. The emergence of mild PTT (~45 °C) enables a promising strategy to inhibit cancer cell growth without damage to healthy tissues through different activation of tumor and normal cells [32,33,34]. However, the low-temperature property in mild PTT may also produce insufficient and heterogeneous heat to the tumor, which causes subsequent survival and self-recurrence of tumor cells [34]. More importantly, the expression of the 90 KDa heat shock protein (HSP90) is sharply increased even with a temperature lower than 42 °C so that the therapeutic efficacy of mild PTT may be further limited [17,20,21,23,26,29,35,36,37,38]. Therefore, combinatory mild PTT and other therapeutics to overcome this drawback could be a better choice for optimal cancer treatment.
As one of the HSP families, HSP90 displays an indispensable role in cancer cell proliferation, apoptosis and differentiation [39,40,41,42]. Once the tumor cells are exposed to heat stimulation in PTT, HSP90 is overproduced in tumor cells to keep the cells alive. Therefore, it is also a promising targeting site to induce complete tumor cell ablation and boost the therapeutic efficacy in mild PTT [29,35,43]. In this contribution, we constructed an NIR-triggered dual-sensitized nanoplatform (BDPII-gel@TSL) based on a temperature-sensitive liposome (TSL), in which both HSP90 inhibitor (geldanamycin) and photothermal agent (aza-BODIPY) were encapsulated into TSL to realize a chemo–photothermal synergistic therapy to effectively inhibit tumor growth at mild temperature (42 °C) (as shown in Scheme 1). The construction of TSL is widely used to improve the drug release in thermotherapy through external local heat trigged by light irradiation of photothermal agent (PTA) [44,45,46,47]. A recent study indicates that TSL with a composition of DPPC/DSPC/DSPE-PEG-2000 is stable in biological media at 37 °C while undergoing a gel-to-liquid phased transition and inducing a 60% drug release at mild temperature (42 °C) (Scheme 1a). Thus it is a potential encapsulating shell applicable in mild PTT [11,47]. As an excellent PTA in this nano-system, aza-BODIPY displays strong NIR absorbance and near-infrared second window (NIR II) emission, which enables it to have a high photothermal conversion in PTT (as shown in Scheme 1b) [48,49,50,51,52]. In addition, geldanamycin can specifically bind to the HSP90 through the amine group to inhibit the overexpression of the protein and improve the heat sensitivity of cancer cells [40,53,54,55]. Additionally, the anticancer process of BDPII-gel@TSL NPs was turned on through the irradiation of 808 nm with a low power density (0.5 W cm−2), where the photothermal effect of BDPII in NPs was excited to generate a mild temperature (42 °C) and induce a phase transition of TSL to positive release the geldanamycin and BDPII, activating the chemo–photothermal dual synergistic therapy. Then, the heat sensitivity of cancer cells was enhanced by the inhibition of HSP90, which enables considerable tumor cell death at a mild temperature produced by BDPII.
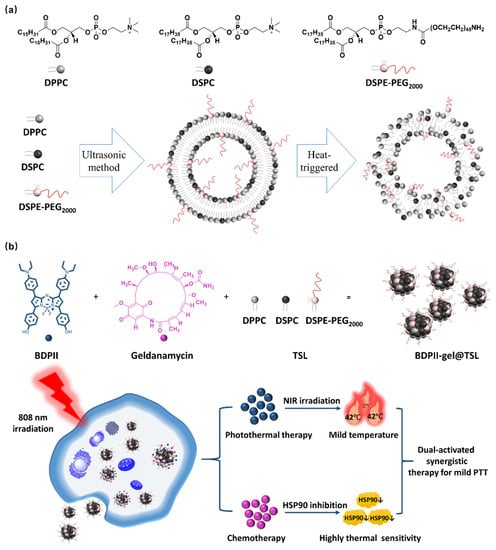
Scheme 1.
(a) The components (DPPC/DSPC/DSPE-PEG2000) of TSL and the schematic diagram for the phase transition when heat triggered. (b) Preparation of BDPII-gel@TSL (top), the schematic presentation of photothermally induced drug release, as well as the therapeutic strategy of the dual-activated photothermal and cemo synergistic therapy in mild PTT process (down).
2. Materials and Methods
2.1. Materials
The chemicals including 1-(4-hydroxyphenyl)ethan-1-one, 4-(diethylamino)benzalde hyde, Potassium hydro-xide (KOH), methanol (MeOH), Nitromethane (MeNO2), 1,2-diethylamine (DCE), ammonium acetate (NH4OAC), n-butanol (n-BuOH), boron (tri) fluoride etherate (BF3Et2O), N, N-Diisopropylethylamine (DIPEA), dichloromethane (DCM), petroleum ether (PE), ethyl acetate (EA), cyclohexane, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phospho ethanolamine-N-PEG2000 (DSPE-PEG2000), methylthiazolyldiphenyl-tetrazolium bro mide (MTT), Calcein-AM/PI assay kit, 4-[(E)-2-[(3Z)-2-chloro-3-[2-(2,6-diphenyl thiopyran-4-ylidene)ethylidene] cyclohexen-1-yl]ethenyl]-2,6 diphenylthiopyrylium, tetrafluorobo-rate (IR1061) were purchased from Sigma-Aldrich and used without further purification. Dulbecco’s modified essential medium (DMEM), Fetal bovine serum (FBS), Streptomycin-penicillin and phosphate buffer saline (PBS) were purchased from Gibco (Life Technologies, Carlsbad, CA, USA). Deionized water (18.2 MΩ·cm) was purified with a water purification system (WP-UP-UV-20) from Sichuan Water Technology Development Co. Ltd. (Chengdu, China). All other chemicals were used as received.
2.2. Synthesis and Characterization
The NMR and ESI-MS spectra for BDPII are measured by a Bruker AV-400 spectrometer and LTQ Orbit rap XL instruments, respectively. The synthetic routes and characterization results of BDPII are displayed in Figure 1a and Figures S1–S12.
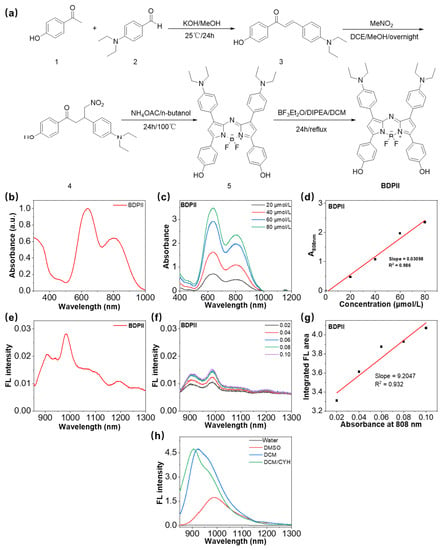
Figure 1.
(a) The synthetic routes for BDPII. (b) The absorbance spectra of BDPII in water with 1% DMSO. (c) The absorbance of BDPII in water with 1% DMSO at different concentrations. (d) The plot of absorbance at 808 nm of BDPII versus its concentration. (e) The emission spectra of BDPII in water with 1% DMSO upon 808 nm excitation. (f) The emission spectra of the BDPII solutions with different concentrations upon 808 nm excitation (Legend: the absorbance at 808 nm for the corresponding sample solution). (g) The integrated fluorescence emission area at 900–1300 nm as a function of absorbance at 808 nm of BDPII solutions. (h) The fluorescence intensity of BDPII in solvents with different polarity upon 808 nm excitation.
Synthesis of (E)-3-(4-(diethylamino)phenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one (Compound 3). 1-(4-hydroxyphenyl)ethan-1-one (compound 1, 5 g, 36.75 mM), 1-(4-(diethylamino)phenyl)ethan-1-one (compound 2, 6.15 g, 36.75 mM) and KOH (6.18 g, 110.25 mM) were dissolved in MeOH and stirred at room temperature for 4 h, followed with evaporation under reduced pressure and purification with silica gel column chromatography using PE/ DCM (v/v, 8:1) as the eluent to provide the bright red products with the yield of 86%. 1H NMR (400 MHz, DMSO) δ 8.01 (d, J = 8.7 Hz, 2H), 7.64 (d, J = 8.8 Hz, 2H), 7.58 (d, J = 3.6 Hz, 2H), 6.87 (d, J = 8.7 Hz, 2H), 6.69 (d, J = 8.9 Hz, 2H), 3.41 (q, J = 7.0 Hz, 4H), 1.11 (t, J = 7.0 Hz, 6H). 13C NMR (101 MHz, DMSO) δ 186.89 (s), 161.61 (d, J = 18.7 Hz), 149.32 (s), 144.04 (s), 130.91 (d, J = 18.1 Hz), 129.99 (s), 121.50 (s), 115.62 (s), 115.27 (d, J = 9.0 Hz), 111.22 (s), 43.90 (s), 12.59 (s). ESI (m/z): calculated for C19H21NO2+, [M+H]+ 296.1956, found 296.1953.
Synthesis of 3-(4-(diethylamino)phenyl)-1-(4-hydroxyphenyl)-4-nitrobutan-1-one (Compound 4). The compound 3 (2 g, 6.78 mM), MeNO2 (2.885 g, 33.90 mM) and DEA (3.76 g, 51.40 mM) were dissolved in EtOH and stirred at 40 °C. After 24 h, the EtOH were removed by evaporation, the crudes were washed by brine and extracted with DCM. The organic fraction was dried over Na2SO4, followed with evaporation under reduced pressure and purification with silica gel column chromatography using PE/ EA (v/v, 8:1) as the eluent to provide the red oil products with yield of 65%. 1H NMR (400 MHz, CDCl3) δ 7.88–7.79 (m, 2H), 7.13-7.03 (m, 2H), 6.88–6.80 (m, 2H), 6.63–6.54 (m, 2H), 4.76 (ddd, J = 12.1, 6.7, 1.7 Hz, 1H), 4.61 (ddd, J = 12.1, 7.9, 1.7 Hz, 1H), 4.20–4.01 (m, 2H), 3.33–3.27 (m, 5H), 1.16–1.08 (m, 7H). 13C NMR (101 MHz, CDCl3) δ 196.31 (s), 161.19 (s), 147.32 (s), 130.74 (s), 129.29 (s), 128.30 (s), 125.40 (s), 115.56 (s), 112.09 (s), 80.18 (s), 77.40 (s), 77.40–76.85 (m), 76.76 (s), 44.32 (s), 41.52 (s), 38.80 (s), 12.56 (s). ESI (m/z): calculated for C20H24N2O4+, [M+H]+ 357.2117, found 357.2121.
Synthesis of (Z)-4-(3-(4-(diethylamino)phenyl)-2-((3-(4-(diethylamino)phenyl)-5-(4-hydroxyphenyl)-1H-pyrrol-2-yl)imino)-2H-pyrrol-5-yl)phenol (Compound 5). Compound 4 (0.5 g, 1.40 mM) and NH4OAC (1.62 g, 21.05 mM) were dissolved into n-BuOH and reacted under reflux at 110 ℃ for 24 h. After cooling to the room temperature, the crude products were filtered, washed with EtOH and dried by evaporation under reduced pressure, then, followed by purification with silica gel column chromatography using PE/EA (v/v, 2:1) as eluent to provide the dark bule products with yield of 75%. 1H NMR (400 MHz, DMSO) δ 8.00 (d, J = 8.8 Hz, 4H), 7.86 (d, J = 8.6 Hz, 4H), 7.23 (s, 2H), 6.98 (d, J = 8.6 Hz, 4H), 6.73 (d, J = 8.9 Hz, 4H), 3.43 (dd, J = 13.8, 6.8 Hz, 8H), 1.15 (t, J = 7.0 Hz, 12H). 13C NMR (101 MHz, DMSO) δ 160.01 (s), 154.12 (s), 150.69 (s), 148.76 (s), 147.61 (s), 130.51 (s), 128.56 (s), 123.49 (s), 116.78 (s), 111.43 (s), 111.19 (s), 44.33 (s), 13.05 (s). ESI (m/z): calculated for C40H41N5O2+, [M+H]+ 624.3690, found 624.3694.
Synthesis of 4,4′-(1,9-bis(4-(diethylamino)phenyl)-5,5-difluoro-5H-4l4,5l4-dipyrrolo [1,2-c:2′,1′-f][1-3,5]triaza borinine-3,7-diyl)diphenol (BDPII). Compound 5 (0.5 g, 0.80 mM) and DIPEA (1.036 g, 8 mM) were dissolved in dry DCM and stirred for 30 min at 0 °C. Then, the BF3OEt2 was added into the mixture and reacted with room temperature for 24 h. The crudes were washed with water three times and dried over Na2SO4, followed by the purification with silica gel column chromatography using PE/ EA (v/v, 2:1) as eluent to provide the dark blue products with yield of 45%. 1H NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.7 Hz, 4H), 7.93 (d, J = 8.4 Hz, 4H), 6.84 (d, J = 8.4 Hz, 4H), 6.77–6.67 (m, 6H), 3.43 (dd, J = 13.6, 6.7 Hz, 8H), 1.22 (d, J = 7.1 Hz, 13H). 13C NMR (101 MHz, CDCl3) δ 157.97 (s), 156.76 (s), 148.37 (s), 145.08 (s), 142.92 (s), 131.20 (s), 129.96 (s), 124.82 (s), 120.57 (s), 115.66 (s), 114.36 (s), 111.36 (s), 29.75 (s), 12.76 (s). ESI (m/z): calculated for C40H40BF2N5O2+, [M]+ 671.3662, found 671.3664.
2.3. UV-Vis and Fluorescence Spectra Characterization
The UV-Vis and Fluorescence spectra were monitored with 1 cm quartz cuvette through Shimadzu (Suzhou, China) UV-1800 spectrophotometer and F-7000 spectrophotometer (HATACHI, Shizuoka, Japan), respectively.
2.4. Molar Extinction Coefficient Measurement
Initially, the absorbance of BDPII solution with different concentration were recorded using the UV-1800 spectrophotometer. the molar extinction coefficient (ε) for BDPII was calculated by the Beer-Lambert Law:
where A indicates the absorbance of BDPII at 808 nm, l and c represent the optical path (1 cm) and the concentration of BDPII solution, respectively.
2.5. Preparation of TSL Nanoparticles
The nanoparticles of BDPII@TSL and BDPII-gel@TSL were prepared with ultrasonic method [56]. Briefly, DPPC, DSPC and DSPE-PEG2000 (molar ratio, 80:15:5) were dissolved in chloroform and dried under vacuum to remove the chloroform [11]. Then, 10 mL BDPII, geldanamycin and BDPII/geldanamycin aqueous solution were added to form lipid film, flowed by a sonication for 40 min at 60 °C. After cooling to room temperature, these solutions were filtrated through syringe membrane with a pore size of 0.8 µm to remove the undesirable aggregates and stored at 4 °C for further measurement.
2.6. Quantum Yield Test
The quantum yield (Φ) of BDPII, BDPII@TSL and BDPII-gel@TSL NPs was determined by the previous reported method [57]. A typical NIR-II dye IR1061 was used as a standard with the NIR-II quantum yield of 0.75%. To obtain the value of QY, the IR-1061, BDPII, BDPII@TSL and BDPII-gel@TSL NPs were diluted through water with the 808 nm absorbance of 0.02, 0.04, 0.06, 0.08 and 0.10, after that, the emission spectra for these samples were detected to achieve the emission integration of peak area at the range of 900–1300 nm. Then, a slope of linear relation between the emission integration of peak area and the absorbance at 808 nm were determined to calculate the value of QY. The value of Φ for BDPII, BDPII@TSL and BDPII-gel@TSL were achieved by the following equation:
where the values of Slopesample and Sloperef are determined by the linear relationship between the integration of emission peak area and the absorbance at 808 nm. nsample and nref indicate the refractive index of the solvent in the sample and reference, respectively. Here they are both the refractive index of water.
2.7. Photothermal Conversion Efficiency Calculation
The photothermal conversion efficiency (PCE, η) for BDPII@TSL and BDPII-gel@TSL NPs in water were measured according to the reported works [28,58]. First, these NPs were irradiated by 808 nm laser with power of 1 W cm−2 for 15 min. Then, these samples were cooling down to room temperature with the temperature recorded by an infrared camera. The value of η for BDPII@TSL and BDPII-gel@TSL were calculated through the following equation:
where h and A indicate the heat transfer coefficient and surface area of the container, respectively. TMax and TSurr represent the maximum steady-state (62.73 °C for BDPII-gel@TSL and 62.51 °C for BDPII@TSL) and room temperature, I represents the laser power (1 W cm−2), A is absorbance of the specimen at 808 nm, QDis is the heat dissipation from the laser mediated by the solvent and the container, which could be calculated by Equation (2), the value of hA could be calculated through the Equation (3).
where TMax(water) is 28.9 °C, the value of τs for water is 297 s upon 808 nm irradiation.
where m is mass of the aqueous solution (1.0 g) and c is the heat capacity of water (4.2 J g−1), τs is the time constant which could be calculated by Equations (4) and (5):
TRT is the real temperature in this cooling process, θ is the driving force temperature.
2.8. Cell Incubation Experiment
HeLa (America Type Culture Collection) cells were incubated in a DMEM containing 10% FBS and 1% antibiotics composed by penicillin (100 units/mL)-streptomycin (100 μg/mL) using an incubator with a humidified 5% CO2 at 37 °C.
2.9. In vitro Confocal Laser Scanning Microscopy (CLSM)
In the live/dead cell co-staining assay, HeLa cells were seeded in 35 mm confocal chamber with a density of 7 × 104 for 24 h. The culture medium was placed with fresh medium containing 40 μM NPs to incubate for another 3 h. Then, the cells were irradiated under white light (0.5 W/cm2) for 15 min, followed by the addition of Calcein-AM (3 μM) and PI (4 μM) for 30 min, the cells were washed with PBS and immediately imaged using CLSM.
2.10. In Vitro Dark & Light Cytotoxicity
The cell viability was estimated by MTT assay. The HeLa cells were seeded in 96-well plate with a density of 7 × 104 and incubated for 24 h. Then, the medium was replaced by fresh medium containing different concentration of NPs and the cells were cultured for another 24 h at 37 °C. The culture medium was then discarded, followed by an addition of MTT solution with the concentration of 0.5 mg/mL. After 4 h incubation, the cells were irradiated with white light (0.5 W/cm2, 20 min) and further incubated for 24 h at 37 °C. Finally, the MTT solution was gently removed, 100 μM DMSO was added to dissolve the crystals. Then, the absorbance for each plate at 540 nm was measured through a Bio-Rad microplate reader. The cell viability was calculated by the following equation [59]:
For the dark cytotoxicity sample, the irradiating step was absent and rest part of the experiments was unchanged.
3. Results and Discussion
3.1. Synthesis and Characterization of BDPII
PTAs with absorbance in NIR window are widely used in PTT for their high penetration ability and excellent optical performance, preferably at 808 nm for their high light harvesting and deep tissue penetration [24,25,26,27,28,60]. Unfortunately, NIR-triggered PTT is usually under the restriction of poor photostability, depressed light absorption capacity and low photothermal conversion efficiency (PCE) of PTAs [26,28,58,61]. Among numerous reported NIR photothermal agents, aza-boron-dipyrrome-thenes (aza-BODIPY) derivatives are photostable materials with a high molar absorption coefficient at the NIR window, a favorable non-radiative excitation decay pathway to efficiently produce photothermal energy, and adjustable photophysical properties and easy functionalization, all of which endow great potential for aza-BODIPY-based PTAs in PTT application [28,50,62,63]. The donor-acceptor (D-A) molecular engineering favors strong intramolecular charge transfer (ICT) and a narrow energy band gap [64], which facilitate a redshift of absorption to the NIR window and efficient fluorescent quenching to improve the PTT performance [65,66].
In this work, we used the electron-rich N,N-diethylaniline groups as electron donors and the classical aza-BODIPY electron-withdrawing cores as electron acceptors to construct the NIR photothermal agent (BDPII). The synthetic routes and characterization (NMR and ESI-Mass spectrum) of BDPII were shown in Figure 1a and Figures S1–S12, respectively. Briefly, BDPII was enlarged through the conjugation of N,N-diethylaniline and phenol groups on 3,5-position and 1,7-position of the aza-BODIPY core, respectively. The large conjugating system of BDPII enabled its high extinction coefficient (ε), and the strong charge transfer along the D-A backbone endowed a redshift to the NIR window as well as desirable PCE in the PTT application. As shown in Figure 1b and Table 1, the BDPII has two absorbance peaks visible in the NIR region, in which the short absorbance peak at about 639 nm is ascribed to the π-π* transition, and the long wavelength at about 808 nm is due to intramolecular charge transfer (ICT) effect of BDPII [64,67,68]. Additionally, the BDPII also exhibits an excellent extinction coefficient at 808 nm of 30,980 mol−1 cm−1 (Figure 1c,d). Benefiting from the strong ICT effect of BDPII, the emission spectrum is enlarged to the NIR II window in the range of 900–1000 nm (Figure 1e). However, it is impressive that it also demonstrates strong fluorescence quenching in water with a fluorescence quantum yield (Φ) of 0.56% (Figure 1f,g and Table 1) [69,70,71] in the measurements using a typical NIR II material IR 1061 (75%) as a standard (Figure S13). As indicated in Figure 1h, with the increase in solvent polarity, the fluorescent intensity of BDPII shows a notable decrease, and the emission wavelength presents a redshift, all of which demonstrate a strong solvatochromic effect for BDPII and result in its weak emission as shown in Figure 1e [72,73,74].

Table 1.
The optical performance of BDPII, BDPII@TSL and BDPII-gel@TSL.
3.2. Preparation of BDPII-gel@TSL NPs
Generally, a PTA with good water dispensability is required for biological application. Therefore, we used an ultrasonic method to formulate water-dispersible BDPII-gel@TSL with raw materials, including BDPII, geadanamycin and TSL, in which the TSL were prepared with the constituents of DPPC/DSPC/DSPE-PEG2000 by a molar ratio of 80:15:5 [11,56]. BDPII-gel@TSL was filtered through a syringe membrane with a pore size of 0.8 µm to remove the undesirable aggregates to achieve the relatively uniform-sized product. Owing to the hydrophobic core of BDPII and geldanamycin, as well as the hydrophilic shell of TSL, the BDPII and geldanamycin are inclined to locate in the phospholipid bilayer region (Figure 2a), in which the contents in TSL NPs were easily released upon heat triggering. Transmission electron microscopy (TEM) and dynamic light scattering (DLS) results showed spherical morphologies for BDPII-gel@TSL NPs with an average diameter of about 137 nm (Figure 2b and Table S1). According to the time-dependent diameter monitored by DLS shown in Figure 2c and Table S1, they remained stable in water, PBS and DMEM over two weeks of storage at 4 °C, indicating excellent stability for potential biological application. In order to investigate the synergistic effect between BDPII and geldanamycin in mild PTT conditions, BDPII was solely encapsulated in TSL to form BDPII@TSL, according to the same preparing protocol as for BDPII-gel@TSL. The results from DLS and TEM demonstrate spherical morphologies for BDPII@TSL NPs with an average diameter of approximately 116 nm in water (Table S1, Figure S14a). Furthermore, it also shows good stability in water, PBS and DMEM with 4 °C storage for 15 days (Table S1, Figure S14b).
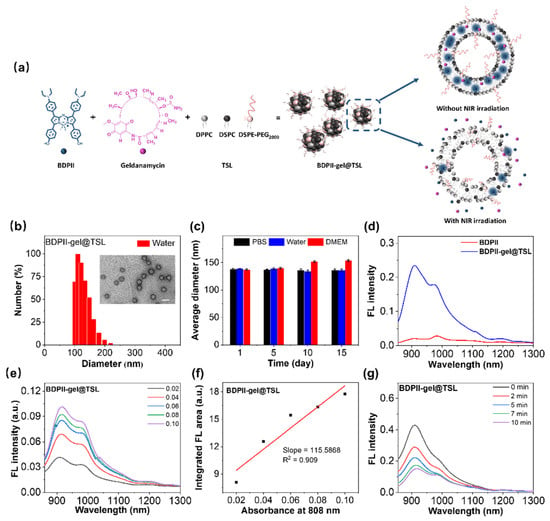
Figure 2.
(a) Illustration of preparation for BDPII-gel@TSL NPs and the condition of photothermal heating-triggered contents release without/with NIR irradiation. (b) The size distribution of BDPII-gel@TSL NPs measured by dynamic light scattering with a PDI of 0.144 and the particle morphology for BDPII-gel@TSL detected by transmission electron microscopy (inset) with a white scale bar of 200 nm. (c) The average diameter of BDPII-gel@TSL in water, PBS and DMEM with 4 °C storage for 15 days. (d) The emission spectra for BDPII and BDPII-gel@TSL upon 808 nm excitation. (e) The emission spectra of the BDPII-gel@TSL NPs with different concentrations upon 808 nm excitation (Legend: the absorbance at 808 nm for the corresponding sample solution). (f) The integrated fluorescence emission area at 900–1300 nm as a function of absorbance at 808 nm of BDPII-gel@TSL solutions. (g) The fluorescence intensity of BDPII-gel@TSL NPs with different irradiation times upon 808 nm excitation with the laser power of 0.5 W cm−2.
The fluorescence emission of BDPII-gel@TSL NPs was recorded and displayed in Figure 2d. Once BDPII molecules were encapsulated into TSL, the fluorescence intensity was notably enhanced, and the value of Φ for BDPII-gel@TSL (6.85%) was ten times higher than that for BDPII (0.56%) (Figure 2e,f and Table 1), which is attributed to the solvatochromic effect of BDPII as shown in Figure 1h. That is to say, with the BDPII molecules successfully loaded in TSL to form BDPII-gel@TSL NPs, the hydrophobic phospholipid bilayer region of TSL provides a non-polar environment for BDPII to exhibit strong emission. Therefore, the heat-triggered release of BDPII-gel@TSL NPs can be determined through fluorometry at 42 °C. As shown in Figure 2g, the fluorescence intensity of BDPII-gel@TSL NPs gradually decreases upon the irradiation for 0 min to 5 min (0.5 W cm−2, 808 nm), indicating the effective release of BDPII from the non-polar phospholipid bilayer of TSL into the polar medium (aqueous solution).
3.3. Photothermal Performance of BDPII-gel@TSL NPs
Encouraged by the excellent NIR absorption ability of BDPII, the photothermal performance for BDPII-gel@TSL NPs was further investigated upon 808 nm irradiation and shown in Figure 3. The temperature of BDPII-gel@TSL NPs in PBS solution displayed a dramatic increase upon 1 W cm−2 laser irradiation. Within 15 min of irradiation, the temperature of BDPII-gel@TSL NPs reached a temperature as high as 62.73 °C (Figure 3a). The corresponding infrared photos were recorded, as shown in Figure 3b. It was found that the temperature increase has a positive dependence on the concentration and laser power density (Figure 3c,d). Notably, the temperature could increase to 43 °C after 808 nm laser irradiation with 0.5 W cm−2 for 15 min (Figure 3d), which endows BDPII-gel@TSL NPs with potential in mild PTT application. The BDPII-gel@TSL NPs demonstrated no obvious photobleaching effect after multiple heating–cooling cycles with laser irradiation (Figure 3e), suggesting excellent photothermal stability. According to the temperature curve shown in Figure 3a, the photothermal conversion efficiency (PCE) of BDPII-gel@TSL NPs was calculated to be 80.75% (Figure 3f), which is quite high among previously reported PTAs [28,58,75], indicating its potential to be applied in mild PTT. The BDPII@TSL NPs exhibited an excellent PCE with a value of 78.70% (Figure S15), suggesting that the loading of geldanamycin hardly affects the optical properties of BDPII.
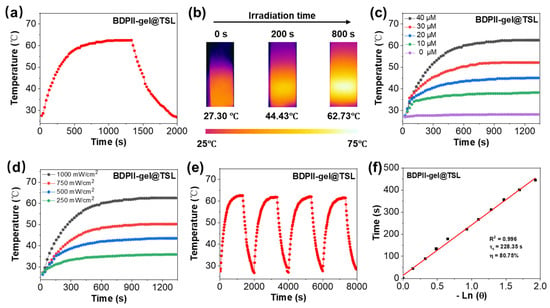
Figure 3.
(a) Photothermal heating effect for BDPII-gel@TSL in water (40 μM) upon 808 nm irradiation with 1 W cm−2 laser power and subsequent cooling to room temperature. (b) The infrared photos for BDPII-gel@TSL NPs at different irradiation times (1 W cm−2, 808 nm). The photothermal heating profiles of BDPII-gel@TSL NPs with different (c) concentrations (1 W cm−2, 808 nm) and (d) laser power densities (40 μM, 808 nm). (e) Photothermal stability for BDPII-gel@TSL NPs (40 μM) under 808 nm laser irradiation (1 W cm−2) for four on/off cycles. (f) The natural logarithm plot of the driving force temperature versus the cooling time for BDPII-gel@TSL in the photothermal heating process.
3.4. In Vitro Mild PTT Application
Given the good photothermal conversion capability and high photostability of the BDPII-gel@TSL NPs, the tests, including cytotoxicity and combinatory photothermal and chemotherapeutic efficacy, were examined via standard MTT assay. The experimental groups included the geldanamycin group, BDPII@TSL group and BDPII-gel@TSL group. Initially, upon NIR irradiation (808 nm) with a lower power density (0.5 W cm−2) for 30 min, HeLa cells in both the geldanamycin and BDPII@TSL groups displayed moderate cytotoxicity at mild temperature (42 °C), as shown in Figure 4a. It was calculated that the values of IC50 for the geldanamycin and BDPII@TSL groups were as high as 53.27 μM and 43.16 μM (Figure 4b), respectively. As compared to the geldanamycin and BDPII@TSL groups, the BDPII-gel@TSL group displayed much lower cell viability under the same conditions, with a low IC50 value of 24.36 μM (Figure 4b). Reasonably, after exposure to light irradiation, the moderate temperature (42 °C) produced by the BDPII would initially destroy the nanostructure of BDPII-gel@TSL and cause the burst release of BDPII and geldanamycin. This synergistic effect of photothermal therapy and chemotherapy with a burst release manner led to the high ablation efficacy on tumor cells (Figure 4a). Under dark conditions, as shown in Figure 4c, the cell viability for BDPII@TSL displayed negligible change and maintained a more than 90% survival rate, indicating good biocompatibility for BDPII@TSL. It was found that the cell viability for geldanamycin showed an apparent decrease with an increase in concentration, which is ascribed to the intrinsic chemotherapy of geldanamycin. It is known that geldanamycin, an HSP90 inhibitor, can cause proteasome-dependent degradation and simultaneously disrupt some critical signal ways to the growth of cancer cells through the bond between geldanamycin and N-terminal ATPase structural domain of HSP90 [76]. However, while the geldanamycin molecules were loaded into TSL to form BDPII-gel@TSL NPs, the bond of geldanamycin to HSP90 was restrained, in which the anticancer effect of geldanamycin in BDPII-gel@TSL NPs was significantly suppressed, and the decrease in cell viability of BDPII-gel@TSL was much minor. = In addition, the small loss of HeLa cell viability in Figure 4c was attributed to the slight leakage of geldanamycin from BDPII-gel@TSL NPs into the cells during endocytosis.
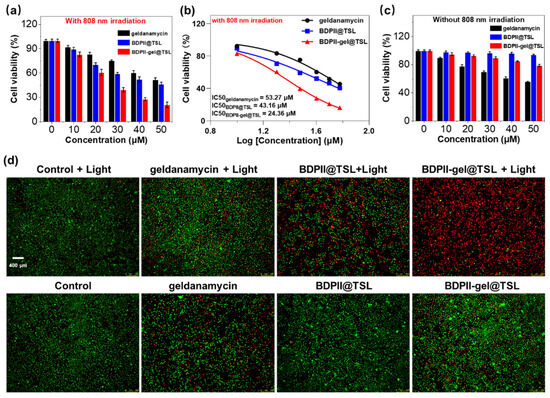
Figure 4.
(a) Viability of HeLa cells treated with geldanamycin, BDPII@TSL and BDPII-gel@TSL NPs upon 808 nm irradiation for 30 min. (b) Cell viability as a function of the concentration of geldanamycin, BDPII@TSL and BDPII-gel@TSL NPs. (c) Viability of HeLa cells treated with geldanamycin, BDPII@TSL and BDPII-gel@TSL NPs under dark conditions. (d) Fluorescence imaging for HeLa cells incubated with calcein-AM (green)/PI (red) and treated with no particles (control), BDPII@TSL, geldanamycin and BDPII-gel@TSL NPs without and with 808 nm irradiation for 30 min (calcein AM, live cell marker, λex = 473 nm, λem = 490–520 nm, Propidium iodide, dead cell marker, λex = 559 nm, λem = 575–615 nm).
In order to vividly examine the mild PTT efficacy in vitro, the calcein-AM and propidium iodide (PI) kit assay were used to visualize the HeLa cell mortality under NIR irradiation. Calcein-AM was used to label the living cells and can easily penetrate into the cell membrane and be hydrolyzed into the calcein structure to emit strong green fluorescence. Propidium iodide (PI) is a dead cell marker and can selectively be embedded into DNA dead cells and yield red fluorescence. As shown in Figure 4d, after exposure to NIR laser irradiation (808 nm, 0.5 W cm−2 for 30 min), the control group exhibited strong green emission with high cell activity, indicating that the low power density notably had no obvious influence on the cell viability. Owing to the sole therapeutic strategy of geldanamycin and BDPII as described in the MTT assay, the chemotherapy of geldanamycin and phototherapy of BDPII@TSL could merely compromise the cell viability less than 50%, and this means that the cells could be stained with both Calcein-AM and PI with green and red emission, respectively. In comparison, the cells incubated with BDPII-gel@TSL emit strong red fluorescence, indicating the synergistic photothermal and chemotherapeutic effects of the combination of BDPII and geldanamycin to effectively ablate cancer cells. Under dark conditions, the HeLa cells in both the control group and BDPII@TSL group exhibited strong green fluorescence, suggesting good biological compatibility of BDPII@TSL. The groups incubated with geldanamycin and BDPII-gel@TSL illustrate both green and red fluorescence without irradiation, indicating that the chemotherapy of geldanamycin partly inhibits tumor cell growth. Taken together, these results are consistent with the in vitro cytotoxicity results in Figure 4. Taken together, the NIR-activatable and mild-temperature-sensitive nanoplatform (BDPII-gel@TSL) is an efficient platform to ablate cancer cells in vitro.
4. Conclusions
In summary, we developed a TSL-coated dual-sensitized nanoplatform BDPII-gel@TSL loaded with PTA (BDPII) and HSP90 inhibitor (geldanamycin) for highly efficient combinatory chemotherapy and mild photothermal therapy of cancer cells. The synthesized BDPII exhibited functioned as an excellent PTA with NIR optical performance via donor-acceptor molecular engineering, while the PCE for BDPII could reach up to 80.73% upon the 808 nm irradiation. Importantly, BDPII nanoparticles could produce a mild temperature (42 °C) upon 0.5 W cm−2 of 808 nm laser irradiation to induce the phase transformation of TSL and stimulate the release of itself and geldanamycin from TSL. Furthermore, the triggered release of geldanamycin could initially down-regulate the overexpression of HSP90 and construct a photothermal-chemo synergistic therapy with BDPII to efficiently eradicate cancer cells under mild PTT conditions. This research points out new insights and directions for future improvement of mild PTT efficacy to effectively ablate tumor cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15092252/s1, Supporting Information is available from the Royal Society of Chemistry or from the author (Reagents and materials including the cultivation of cells; synthesis, NMR and ESI-MS of EAPVs; computations and details of the spectra and imaging measurements).
Author Contributions
Conceptualization, Y.P.; Software, H.J., B.L. and Y.L.; Supervision, B.G. and W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (21973022), the Guangdong Basic and Applied Basic Research Foundation (2023A1515012353, 2021A1515110086, 2022A1515011781), the Shenzhen Science and Technology Program (Grant No. ZDSYS20190902093220279, JCYJ20210324132816039), and Shenzhen Key Laboratory of Advanced Functional Carbon Materials Research and Comprehensive Application (ZDSYS20220527171407017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miller, W.; Isenberg, S.; Roberts, D. Molecular Regulation of Tumor Angiogenesis and Perfusion via Redox Signaling. Chem. Rev. 2009, 40, 3099–3124. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, M.; Du, R.; Zheng, X.; Tang, C.; Wu, Y.; He, J.; Huang, W.; Wang, Y.; Zhang, Z. A polyethyleneimine-driven self-assembled nanoplatform for fluorescence and MR dual-mode imaging guided cancer chemotherapy. Chem. Eng. J. 2018, 350, 69–78. [Google Scholar] [CrossRef]
- Lee, S.; O’Halloran, V.; Nguyen, T. Polymer-caged nanobins for synergistic cisplatin-doxorubicin combination chemotherapy. J. Am. Chem. Soc. 2010, 132, 17130–17138. [Google Scholar] [CrossRef]
- Chabner, A.; Roberts, G., Jr. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef]
- Cheng, J.; Qin, Y.; Liu, L.; Ma, H.; Chen, S.; Zhang, Q.; Zhang, Z. Dual-Targeting Photosensitizer-Peptide Amphiphile Conjugate for Enzyme-Triggered Drug Delivery and Synergistic Chemo-Photodynamic Tumor Therapy. Adv. Mater. Interfaces 2020, 7, 2000935–2000944. [Google Scholar] [CrossRef]
- Kim, J.; Yung, C.; Kim, J.; Chen, X. Combination of nitric oxide and drug delivery systems: Tools for overcoming drug resistance in chemotherapy. J. Control. Release 2017, 263, 223–230. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lu, K.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Tian, Y.; Xu, S.; Ren, E.; Bai, S.; Chen, X.; Chu, C.; Xu, Z.; Liu, G. Multi-responsive bottlebrush-like unimolecules self-assembled nano-riceball for synergistic sono-chemotherapy. Small Methods 2021, 5, 2000416–2000423. [Google Scholar] [CrossRef]
- Li, L.; Hagen, L.; Schipper, D.; Wijnberg, M.; Rhoon, C.; Eggermont, M.; Lindner, H.; Koning, A. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release 2010, 143, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aw, J.; Xing, B. Nanostructures for NIR light-controlled therapies. Nanoscale 2017, 9, 3698–3718. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv. Mater. 2018, 30, 1802591–1802600. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, Y.; Wang, C.; Jiang, X.; Wu, J. Light-controlled oxygen production and collection for sustainable photodynamic therapy in tumor hypoxia. Biomaterials 2020, 269, 120621–120634. [Google Scholar] [CrossRef]
- Hong, W.; Jin, C.; Ming, S.; Wei, P.; Na, L. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem. Int. Ed. 2018, 58, 1057–1061. [Google Scholar]
- Huang, X.; El-Sayed, H.; Qian, W.; El-Sayed, A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Perez, M.; Pino, D.; Mitchell, G.; Moros, M.; Fuente, J. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 2014, 9, 52–61. [Google Scholar] [CrossRef]
- Hu, J.; Cheng, J.; Zhang, Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef]
- Jung, H.; Peter, S.; Amit, S.; Jinwoo, S.; Jonathan, L. Organic molecule-based photothermal agents: An expanding photothermal therapy universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Austin, A.; Hoover, E.; Layton, C. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779–793. [Google Scholar]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Rodrigues, F.; Moreira, F.; Correia, J. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater. Sci. 2020, 8, 2290–3020. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Sun, X.; Liang, G. Nanoagent-Promoted Mild-Temperature Photothermal Therapy for Cancer Treatment. Adv. Funct. Mater. 2021, 31, 2100738–2100752. [Google Scholar] [CrossRef]
- Day, S.; Morton, G.; West, L. Nanoparticles for thermal cancer therapy. J. Biomech. Eng. 2009, 131, 074001–074005. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; He, B.; Zeng, L.; Tan, T.; Cao, H.; He, X.; Zhang, Z.; Guo, S.; Li, Y. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 2016, 6, 762–772. [Google Scholar] [CrossRef]
- Shu, Z.; Hua, C.; Li, W.; Chun, L.; Li, L.; Yu, S.; Xing, S. A simple strategy for simultaneously enhancing photostability and mitochondrial-targeting stability of near-infrared fluorophores for multimodal imaging-guided photothermal therapy. J. Mater. Chem. B 2021, 9, 1089–1095. [Google Scholar]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, 2001806–2001812. [Google Scholar] [CrossRef]
- Mei, S.; Qiu, H.; Xiao, Y.; Yan, L.; Pei, L.; Wen, Z.; Qiang, Z. A Supramolecular Strategy to Engineering a Non-photobleaching and Near-Infrared Absorbing Nano-J-Aggregate for Efficient Photothermal Therapy. ACS Nano 2021, 15, 5032–5042. [Google Scholar]
- Sun, Q.; Tang, K.; Song, L.; Li, Y.; Pan, W.; Li, N.; Tang, B. Covalent organic framework based nanoagent for enhanced mild-temperature photothermal therapy. Biomater. Sci. 2021, 9, 7977–7983. [Google Scholar] [CrossRef]
- Gao, P.; Wang, H.; Cheng, Y. Strategies for efficient photothermal therapy at mild temperatures: Progresses and challenges. Chin. Chem. Lett. 2022, 33, 575–586. [Google Scholar] [CrossRef]
- Hu, H.; Li, D.; Dai, W.; Jin, Q.; Wang, D.; Ji, J.; Tang, B.Z.; Tang, Z. A NIR-II AIEgen-Based Supramolecular Nanodot for Peroxynitrite-Potentiated Mild-Temperature Photothermal Therapy of Hepatocellular Carcinoma. Adv. Funct. Mater. 2023, 33, 2213134–2213147. [Google Scholar] [CrossRef]
- Jung, B.-K.; Lee, K.; Hong, J.; Ghandehari, H.; Yun, C.-O. Mild hyperthermia induced by gold nanorod-mediated plasmonic photothermal therapy enhances transduction and replication of oncolytic adenoviral gene delivery. ACS Nano 2016, 10, 10533–10543. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, J. Nuclear-targeting gold nanorods for extremely low NIR activated photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 15952–15961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, J.; Guo, Z.; Yu, J.; Gao, Q.; Yin, W.; Zhu, S.; Gu, Z.; Zhao, Y. Efficient Near Infrared Light Triggered Nitric Oxide Release Nanocomposites for Sensitizing Mild Photothermal Therapy. Adv. Sci. 2018, 6, 1801122–1801132. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, X.; Wang, X.; Liu, S.; Liu, J.; Xie, Z. Enhanced efficacy of photothermal therapy by combining a semiconducting polymer with an inhibitor of a heat shock protein. Mater. Chem. Front. 2019, 3, 127–136. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-dimensional nanomaterials for photothermal therapy. Angew. Chem. Int. Ed. 2020, 132, 5943–5953. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Wang, S.; Liu, L.; Liu, K.; Ye, Y.; Wang, Z.; Wang, H.; Chen, B.; Jiang, J. Hyperthermia-Triggered On-Demand Biomimetic Nanocarriers for Synergetic Photothermal and Chemotherapy. Adv. Sci. 2020, 7, 1903642–1903651. [Google Scholar] [CrossRef]
- Yang, G.; Li, M.; Song, T.; Chen, X.; Zhang, H.; Wei, X.; Li, N.; Li, T.; Qin, X.; Li, S. Polydopamine-Engineered Theranostic Nanoscouts Enabling Intracellular HSP90 mRNAs Fluorescence Detection for Imaging-Guided Chemo–Photothermal Therapy. Adv. Healthc. Mater. 2022, 11, 22016152201628. [Google Scholar] [CrossRef]
- Liu, C.; Li, L.; Guo, Z.-P.; Lin, L.; Li, Y.-H.; Tian, H.-Y. PLG-g-mPEG Mediated Multifunctional Nanoparticles for Photoacoustic Imaging Guided Combined Chemo/Photothermal Antitumor Therapy. Chin. J. Polym. Sci. 2023, 41, 538–546. [Google Scholar] [CrossRef]
- Kang, H.; Plescia, J.; Song, Y.; Meli, M.; Colombo, G.; Beebe, K.; Scroggins, B.; Neckers, L.; Altieri, C. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J. Clin. Investig. 2009, 119, 454–464. [Google Scholar] [CrossRef]
- Jaeger, M.; Whitesell, L. HSP90: Enabler of cancer adaptation. Annu. Rev. Cancer Biol. 2019, 3, 275–297. [Google Scholar] [CrossRef]
- Poggio, P.; Sorge, M.; Seclì, L.; Brancaccio, M. Extracellular HSP90 machineries build tumor microenvironment and boost cancer progression. Front. Cell Dev. Biol. 2021, 9, 735529–735537. [Google Scholar] [CrossRef] [PubMed]
- Birbo, B.; Madu, E.; Madu, O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317–10336. [Google Scholar] [CrossRef]
- Yatvin, B.; Weinstein, N.; Dennis, H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef]
- Bassett, B.; Anderson, U.; Tacker, R. Use of temperature-sensitive liposomes in the selective delivery of methotrexate and cis-platinum analogues to murine bladder tumor. J. Urol. 1986, 135, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Unezaki, S.; Takahashi, N.; Iwatsuru, M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta (BBA)-Biomembr. 1993, 1149, 209–216. [Google Scholar] [CrossRef]
- Al-Ahmady, Z.; Kostarelos, K. Chemical components for the design of temperature-responsive vesicles as cancer therapeutics. Chem. Rev. 2016, 116, 3883–3918. [Google Scholar] [CrossRef]
- Cheng, H.; Kara, C.; Danielle, C.; Juan, G. Stable J-Aggregation of an aza-BODIPY-Lipid in a Liposome for Optical Cancer Imaging. Angew. Chem. Int. Ed. 2019, 58, 13394–13399. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Dai, H.; Lv, X.; Dong, X. Boosting O2 Photogeneration via Promoting Intersystem crossing and Electron donating Efficiency of Aza-BODIPY-Based Nanoplatforms for Hypoxicmild tumor Photodynamic Therapy. Small Methods 2020, 4, 2000013–2000024. [Google Scholar] [CrossRef]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef]
- Yw, A.; Dz, A.; Jie, W.B.; Dx, C.; Zx, B.; Xdj, A.; Jd, C. Near-infrared vinyl-containing aza-BODIPY nanoparticles as photosensitizer for phototherapy. Dye. Pigment. 2021, 198, 110026–110036. [Google Scholar]
- Zhao, M.; Zeng, Q.; Li, X.; Xing, D.; Zhang, T. Aza-BODIPY-based phototheranostic nanoagent for tissue oxygen auto-adaptive photodynamic/photothermal complementary therapy. Nano Res. 2022, 15, 716–727. [Google Scholar] [CrossRef]
- Qin, H.-L.; Panek, J.S. Total synthesis of the Hsp90 inhibitor geldanamycin. Org. Lett. 2008, 10, 2477–2479. [Google Scholar] [CrossRef]
- Pezzulo, A.; Tudas, A.; Stewart, G.; Buonfiglio, G.V.; Lindsay, D.; Taft, J.; Gansemer, D.; Zabner, J. HSP90 inhibitor geldanamycin reverts IL-13–and IL-17–induced airway goblet cell metaplasia. J. Clin. Investig. 2019, 129, 744–758. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, R.; Yang, H.; Xue, N.; Chen, X.; Yu, X. Rational design, synthesis, and biological evaluation of novel C6-modified geldanamycin derivatives as potent Hsp90 inhibitors and anti-tumor agents. Chin. Chem. Lett. 2023, 34, 107529–107535. [Google Scholar] [CrossRef]
- Yi, H.; Shun-Li, C.; Wei, G.; Xing, M.; Qunhui, Y. Understanding the Dynamic Behavior of an Anticancer Drug, Doxorubicin, on a Lipid Membrane Using Multiple Spectroscopic Techniques. J. Phys. Chem. B 2019, 23, 3756–3762. [Google Scholar]
- Yuan, Y.; Feng, Z.; Li, S.; Huang, Z.; Wan, Y.; Cao, C.; Lin, S.; Wu, L.; Zhou, J.; Liao, L. Molecular Programming of NIR-IIb-Emissive Semiconducting Small Molecules for In Vivo High—Contrast Bioimaging Beyond 1500 nm. Adv. Mater. 2022, 34, 2201263–2201272. [Google Scholar] [CrossRef]
- Feng, E.; Jiao, L.; Tang, S.; Chen, M.; Lv, S.; Liu, D.; Song, J.; Zheng, D.; Peng, X.; Song, F. Anti-photobleaching cyanine-based nanoparticles with simultaneous PET and ACQ effects for improved tumor photothermal therapy. Chem. Eng. J. 2022, 432, 134355–134363. [Google Scholar] [CrossRef]
- Yao, C.; Chen, Y.; Zhao, M.; Wang, S.; Wu, B.; Yang, Y.; Yin, D.; Yu, P.; Zhang, H.; Zhang, F. A Bright, Renal-Clearable NIR-II Brush Macromolecular Probe with Long Blood Circulation Time for Kidney Disease Bioimaging. Angew. Chem. Int. Ed. 2022, 61, e202114273–e202114280. [Google Scholar] [CrossRef]
- Bing, G.; Zong, S.; De, H.; Anran, L.; Shi, X.; Purima, N. Molecular Engineering of Conjugated Polymers for Biocompatible Organic Nanoparticles with Highly Efficient Photoacoustic and Photothermal Performance in Cancer Theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar]
- Macdonald, D.; Liu, W.; Zheng, G. An MRI-Sensitive, Non-Photobleachable Porphysome Photothermal Agent. Angew. Chem. Int. Ed. 2014, 53, 6956–6959. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Xie, M.; Zou, L.; Wang, Z.; Liu, S.; Zhao, Q. Halogenated Aza-BODIPY for imaging-guided synergistic photodynamic and photothermal tumor therapy. Adv. Healthc. Mater. 2018, 7, 1800606–1800616. [Google Scholar] [CrossRef]
- Chen, D.; Tang, Q.; Zou, J.; Yang, X.; Huang, W.; Zhang, Q.; Shao, J.; Dong, X. pH-Responsive PEG–Doxorubicin-Encapsulated Aza-BODIPY Nanotheranostic Agent for Imaging-Guided Synergistic Cancer Therapy. Adv. Healthc. Mater. 2018, 7, 1701272–1701282. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Li, P.; Li, B.; Jiang, H.; Guo, B.; Yuan, Q.; Gan, W. AIEgens for dual second harmonic generation and fluorescence “turn-on” imaging of membrane and photodynamic therapy in cancer cells. Mater. Chem. Front. 2023, 7, 502–513. [Google Scholar] [CrossRef]
- Bing, G.; Zong, S.; Kenry, K.; De, H.; Xiang, L.; Shi, X.; Cheng, L.; Hai, Z.; Bin, L. Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumors in the second near-infrared window. Mater. Horiz. 2017, 4, 1151–1156. [Google Scholar]
- Tao, D.; Kai, W.; Jun, X.; Wei, F.; Han, M.; Lei, Y.; Xiao, W.; Fu, X. Significant Enhancement of Photothermal and Photoacoustic Efficiencies for Semiconducting Polymer Nanoparticles through Simply Molecular Engineering. Adv. Funct. Mater. 2018, 28, 1800135–1800146. [Google Scholar]
- Shi, L.; Liu, H.; Li, K.; Sharma, A.; Yu, K.; Ji, S.; Li, L.; Zhou, Q.; Zhang, H.; Kim, S. An AIE-Based Probe for Rapid and Ultrasensitive Imaging of Plasma Membranes in Biosystems. Angew. Chem. Int. Ed. 2020, 59, 9962–9966. [Google Scholar] [CrossRef]
- Sun, J.; Cai, X.; Wang, C.; Du, K.; Chen, W.; Feng, F.; Wang, S. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J. Am. Chem. Soc. 2021, 143, 868–878. [Google Scholar] [CrossRef]
- Antaris, L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Zhuang, Z.; He, J.; Wen, W.; Qiu, P.; Wang, K. Visualizing astrocytes in the deep mouse brain in vivo. J. Biophotonics 2019, 12, e201800420–e201800430. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, T.; Lam, W.; Kwok, T.; Qian, J.; Tang, Z. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2020, 11, 1255–1265. [Google Scholar] [CrossRef]
- Han, C.; Duan, C.; Yang, W.; Xie, M.; Xu, H. Allochroic thermally activated delayed fluorescence diodes through field-induced solvatochromic effect. Sci. Adv. 2017, 3, e1700904–e1700914. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, Y.; Li, D.; Sun, C.; Lei, Z.; Lu, L.; Wang, T.; Zhang, F. Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10, 1058–1069. [Google Scholar] [CrossRef]
- Gao, D.; Liu, A.; Zhang, Y.; Zhu, Y.; Wei, D.; Sun, J.; Luo, H.; Fan, H. Temperature triggered high-performance carbon dots with robust solvatochromic effect and self-quenching-resistant deep red solid state fluorescence for specific lipid droplet imaging. Chem. Eng. J. 2021, 415, 128984–128997. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Ma, R.; Ji, C.; Müllen, K.; Yin, M. NIR-triggered dual sensitization of nanoparticles for mild tumor phototherapy. Nano Today 2022, 42, 101363–101372. [Google Scholar] [CrossRef]
- Lin, Z.; Peng, R.; Li, Z.; Wang, Y.; Lu, C.; Shen, Y.; Wang, J.; Shi, G. 17-ABAG, a novel geldanamycin derivative, inhibits LNCaP-cell proliferation through heat shock protein 90 inhibition. Int. J. Mol. Med. 2015, 36, 424–432. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
