Mechanistic of Vesicular Ethosomes and Elastic Liposomes on Permeation Profiles of Acyclovir across Artificial Membrane, Human Cultured EpiDerm, and Rat Skin: In Vitro-Ex Vivo Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Vesicular Formulation Containing ACV Probed with Rhodamine 123
2.2.2. Characterizations
2.2.3. In Vitro Drug Permeation across Artificial Membrane
2.2.4. In Vitro Drug Permeation across Human Culture EpiDerm Membrane
2.2.5. Ex Vivo Drug Permeation and Drug Deposition Using Rat Skin
2.2.6. In Vivo Transepidermal Water Loss (TEWL) Study Using Rat Skin
2.2.7. Confocal Laser Scanning Microscopy (CLSM)
2.2.8. Vesicle–Skin Interaction Study Using Scanning Electron Microscopy (SEM)
2.2.9. Topographical Study Using AFM Technique
2.2.10. Data Analysis for the Permeation Profile
3. Results and Discussion
3.1. Various Reformulated Products of ACV Probed with Rhodamine 123
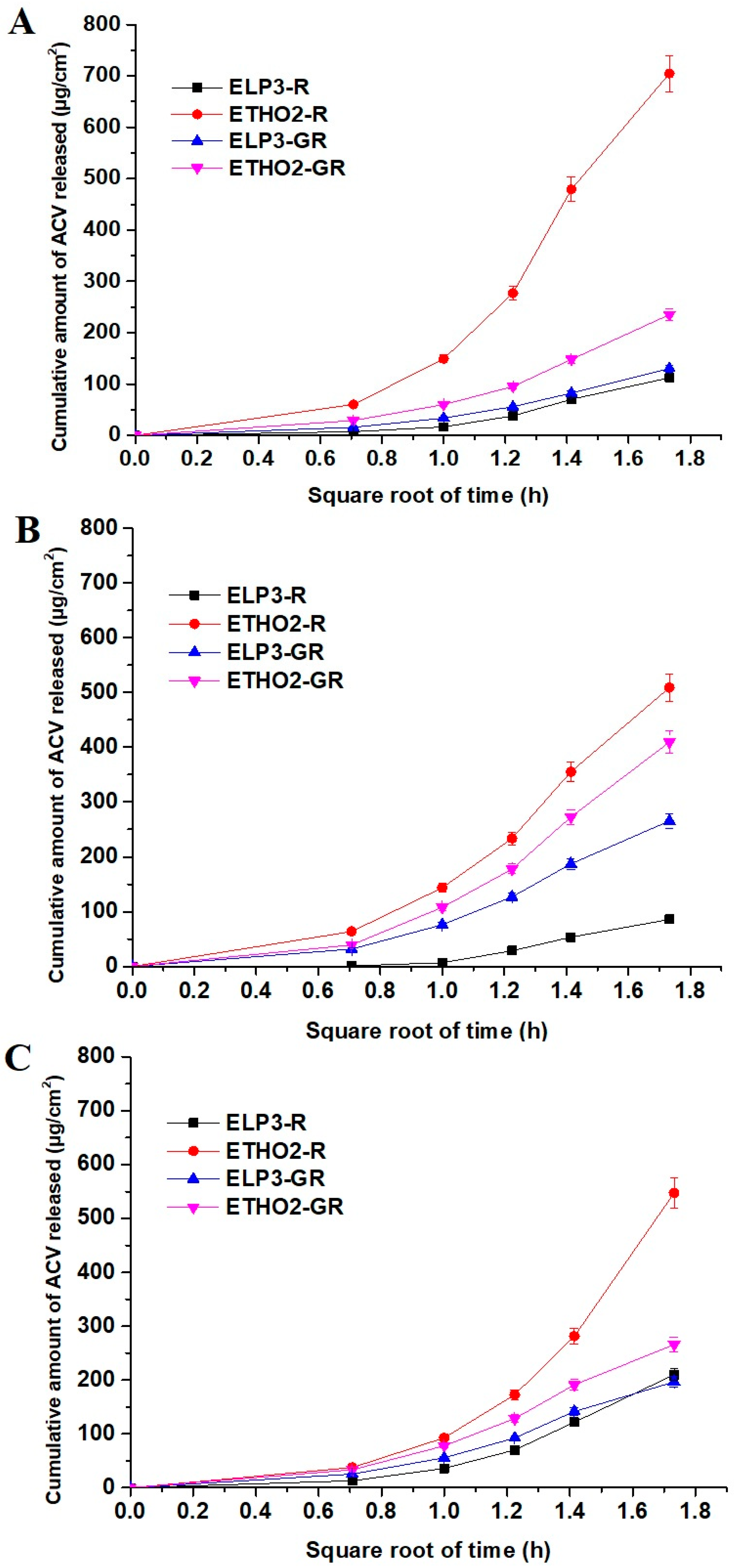
3.2. In Vitro Drug Permeation across Artificial Membrane
3.3. In Vitro Permeation Assessment Using EpiDerm
3.4. Ex Vivo Permeation of ACV across Rat Skin
3.5. In Vivo Transepidermal Water Loss (TEWL) Study Using Rat Skin
3.6. Confocal Laser Scanning Microscopy (CLSM)
3.7. Vesicle–Skin Interaction Study Using Scanning Electron Microscopy (SEM)
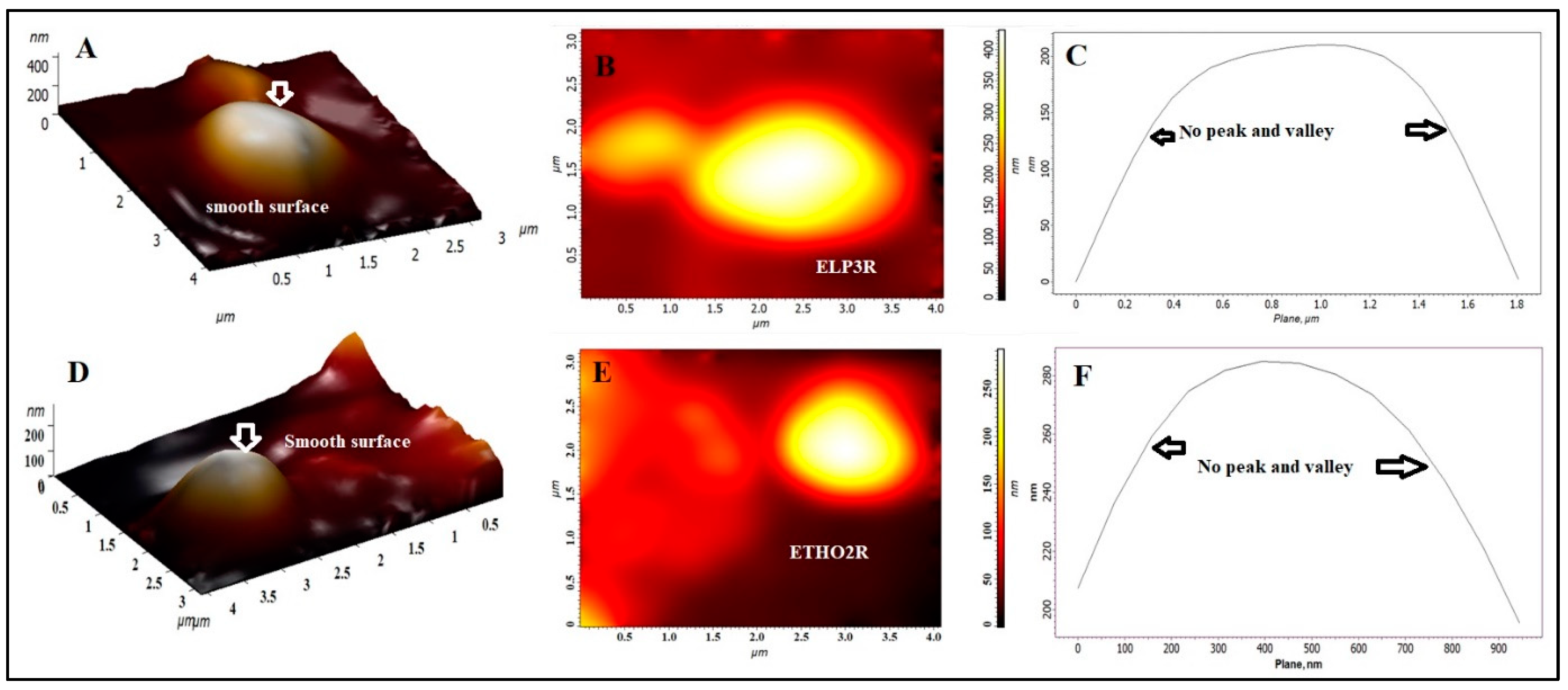
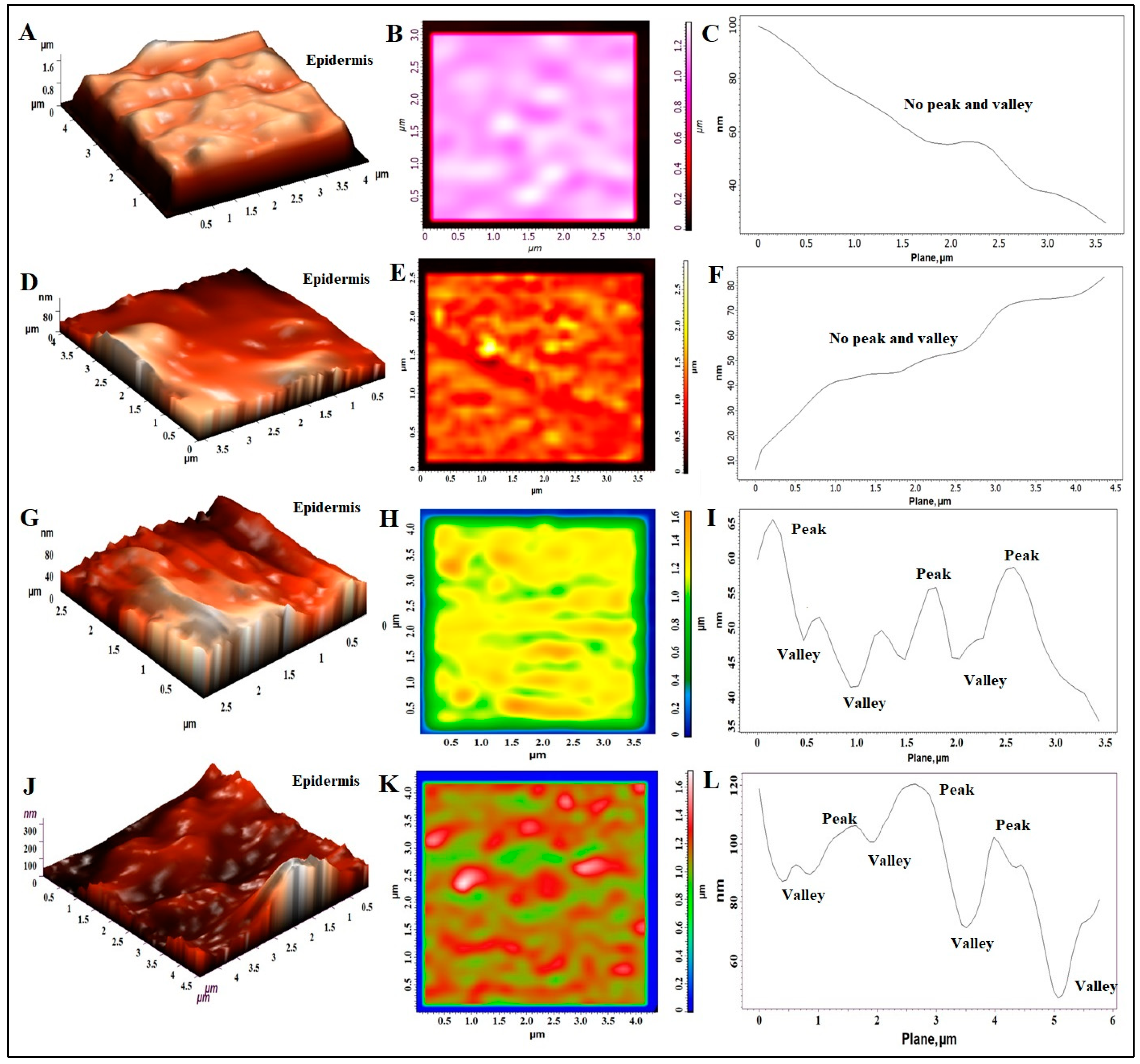
3.8. Atomic Force Microscopy (AFM) Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Looker, K.J.; Johnston, C.; Welton, N.J.; James, C.; Vickerman, P.; Turner, K.M.E.; Boily, M.-C.; Gottlieb, S.L. The global and regional burden of genital ulcer disease due to herpes simplex virus: A natural history modelling study. BMJ Glob. Health 2020, 5, e001875. [Google Scholar] [CrossRef]
- Brown, P.; Elmasry, S.; Olagunju, A.; Garcia, S.; Sarihan, M. A Case of Disseminated Cutaneous Herpes Simplex Virus-1 as the First Manifestation of Human Immunodeficiency Virus Infection. J. Investig. Med. High Impact Case Rep. 2021, 9. [Google Scholar] [CrossRef]
- Jain, S.K.; Gupta, Y.; Jain, A.; Rai, K. Enhanced Transdermal Delivery of Acyclovir Sodium via Elastic Liposomes. Drug Deliv. 2008, 15, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, R.; Mateti, A.; Bandari, S.; Veerareddy, P.R. Transdermal delivery of acyclovir sodium via carbopol gels: Role of chemical permeation enhancers. Lett. Drug Des. Discov. 2011, 8, 381–389. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCrudden, M.T.C.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L.; et al. Hydrogel-Forming Microneedles Prepared from “Super Swelling” Polymers Combined with Lyophilised Wafers for Transdermal Drug Delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]
- Yoa-PU Hu, O. Acyclovir Transdermal Delivery System. 2000, 1–17. Available online: https://patents.google.com/patent/US6162459A/en (accessed on 17 May 2023).
- Almehmady, A.M.; Ali, S.A. Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions. Pharmaceutics 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
- Shekh, M.I.; Amirian, J.; Stadler, F.J.; Du, B.; Zhu, Y. Oxidized chitosan modified electrospun scaffolds for controllable release of acyclovir. Int. J. Bio-Log. Macromol. 2020, 151, 787–796. [Google Scholar] [CrossRef]
- Kazsoki, A.; Palcsó, B.; Alpár, A.; Snoeck, R.; Andrei, G.; Zelkó, R. Formulation of acy-clovir (core)-dexpanthenol (sheath) nanofibrous patches for the treatment of herpes labialis. Int. J. Pharm. 2022, 611, 121354. [Google Scholar] [CrossRef]
- Golestannejad, Z.; Khozeimeh, F.; Mehrasa, M.; Mirzaeei, S.; Sarfaraz, D. A novel drug delivery system using acyclovir nanofiber patch for topical treatment of recurrent herpes labialis: A randomized clinical trial. Clin. Exp. Dent. Res. 2022, 8, 184–190. [Google Scholar] [CrossRef]
- Alshehri, S.; Hussain, A.; Altamimi, M.A.; Ramzan, M. In vitro, ex vivo, and in vivo studies of binary ethosomes for transdermal delivery of acyclovir: A comparative assessment. J. Drug Deliv. Sci. Technol. 2021, 62, 102390. [Google Scholar] [CrossRef]
- Alqahtani, S.M.; Altharawi, A.; Altamimi, M.A.; Alossaimi, M.A.; Mahdi, W.A.; Ramzan, M.; Hussain, A. Method Development, Stability, and Pharmacokinetic Studies of Acyclovir-Loaded Topical Formulation in Spiked Rat Plasma. Processes 2022, 10, 2079. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.K.; Verma, P.R.P.; Ahsan, M.N. Formulation by design of felodipine loaded liquid and solid self-nanoemulsifying drug delivery systems using Box−Behnken design. Drug Dev. Ind. Pharm. 2014, 40, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Altamimi, M.A.; Hussain, A.; Imam, S.S.; Alshehri, S.; Singh, S.K.; Webster, T.J. Transdermal delivery of isoniazid loaded elastic liposomes to control cutaneous and systemic tuberculosis. J. Drug Deliv. Sci. Technol. 2020, 59, 101848. [Google Scholar] [CrossRef]
- Chatterjee, A.; Babu, R.J.; Klausner, M.; Singh, M. In vitro and in vivo comparison of dermal irritancy of jet fuel exposure using EpiDerm™ (EPI-200) cultured human skin and hairless rats. Toxicol. Lett. 2006, 167, 85–94. [Google Scholar] [CrossRef]
- Christensen, J.M.; Chuong, M.C.; Le, H.; Pham, L.; Bendas, E. Hydrocortisone diffusion through synthetic membrane, mouse skin, and Epiderm™ cultured skin. Arch. Drug Inf. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Khuroo, T.; Khuroo, A.; Hussain, A.; Mirza, M.A.; Panda, A.K.; Iqbal, Z. Qbd based and Box-Behnken design assisted oral delivery of stable lactone (active) form of topotecan as polymeric nanoformulation: Cytotoxicity, pharmacokinetic, in vitro, and ex vivo gut permeation studies. J. Drug Deliv. Sci. Technol. 2022, 77, 103850. [Google Scholar] [CrossRef]
- Nava, G.; Piñon, E.; Mendoza, L.; Mendoza, N.; Quintanar, D.; Ganem, A. Formulation and in vitro, ex vivo and in vivo evaluation of elastic liposomes for transdermal delivery of ketorolac tromethamine. Pharmaceutics 2011, 3, 954–970. [Google Scholar] [CrossRef]
- Che, J.; Wu, Z.; Shao, W.; Guo, P.; Lin, Y.; Pan, W.; Zeng, W.; Zhang, G.; Wu, C.; Xu, Y. Synergetic skin targeting effect of hydroxypropyl-β-cyclodextrin combined with microemulsion for ketoconazole. Eur. J. Pharm. Biopharm. 2015, 93, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 642–647. [Google Scholar] [CrossRef]
- Chen, H.; Chang, X.; Du, D.; Liu, W.; Liu, J.; Weng, T.; Yang, Y.; Xu, H.; Yang, X. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J. Control. Release 2006, 110, 296–306. [Google Scholar] [CrossRef]
- Gavara, N. A beginner’s guide to atomic force microscopy probing for cell mechanics. Microsc. Res. Tech. 2016, 80, 75–84. [Google Scholar] [CrossRef]
- Kotova, S.L.; Shekhter, A.B.; Timashev, P.S.; Guller, A.E.; Mudrov, A.A.; Timofeeva, V.A.; Panchenko, V.Y.; Bagratashvili, V.N.; Solovieva, A.B. AFM study of the extracellular connective tissue matrix in patients with pelvic organ prolapse. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2014, 8, 754–760. [Google Scholar] [CrossRef]
- Hadgraft, J.; Guy, R.H. Feasibility assessment in topical and transdermal delivery: Mathematical models and in vitro studies. In Transdermal Drug Delivery, 2nd ed.; Guy, R., Hadgraft, J., Eds.; Marcel Dekker: New York, NY, USA, 2003; pp. 1–23. [Google Scholar]
- Sinko, P.J. Diffusion. In Martin’s Physical Pharmacy and Pharmaceutical Sciences, 5th ed.; Sinko, P.J., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 301–335. [Google Scholar]
- Hansen, S.; Lehr, C.-M.; Schaefer, U.F. Improved input parameters for diffusion models of skin absorption. Adv. Drug Deliv. Rev. 2013, 65, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G.; Schätzlein, A.; Gebauer, D.; Paul, A. The skin: A pathway for systemic treatment with patches and lipid-based agent carriers. Adv. Drug Deliv. Rev. 1996, 18, 349–378. [Google Scholar] [CrossRef]
- Bamba, F.L.; Wepierre, J. Role of the appendageal pathway in the percutaneous absorption of pyridostigmine bromide in various vehicles. Eur. J. Drug Metab. Pharmacokinet. 2003, 18, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Niu, X.-Q.; Zhang, D.-P.; Bian, Q.; Feng, X.-F.; Li, H.; Rao, Y.-M.; Shen, Y.-F.; Geng, F.-N.; Yuan, A.-N.; Ying, X.-Y.; et al. Mechanism investigation of ethosomes transdermal permeation. Int. J. Pharm. X 2019, 1, 100027. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Abbott, S. An integrated approach to optimizing skin delivery of cosmetic and pharmaceutical actives. Int. J. Cosmet. Sci. 2012, 34, 217–222. [Google Scholar] [CrossRef]
- Ezati, N.; Robets, M.S.; Zhang, Q.; Moghimi, H.R. Measurement of Hansen Solubility Parameters of Human Stratum corneum. Iran. J. Pharm. Res. 2020, 19, 572–578. [Google Scholar]
- Ågren, J.; Zelenin, S.; Håkansson, M.; Eklöf, A.-C.; Aperia, A.; Nejsum, L.N.; Nielsen, N.; Sedin, G. Transepidermal water loss in developing rats: Role of aquaporins in the immature skin. Pediatr. Res. 2003, 53, 558–565. [Google Scholar] [CrossRef]
- Kocsis, D.; Kichou, H.; Döme, K.; Varga-Medveczky, Z.; Révész, Z.; Antal, I.; Erdő, F. Structural and Functional Analysis of Excised Skins and Human Reconstructed Epidermis with Confocal Raman Spectroscopy and in Microfluidic Diffusion Chambers. Pharmaceutics 2022, 14, 1689. [Google Scholar] [CrossRef] [PubMed]
- Bronaugh, R.L.; Stewart, R.F.; Congdon, E.R. Methods for in vitro percutaneous absorption studies II: Animal models for human skin. Toxicol. Appl. Pharmacol. 1982, 62, 481–488. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Schätzlein, A.; Richardsen, H. Ultradeformable lipid vesicles can penetrate the skin and other semi-permeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1564, 21–30. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rhodamine-123-free-base (accessed on 10 May 2023).
- Fredonnet, J.; Gasc, G.; Serre, G.; Séverac, C.; Simon, M. Topographical and nano-mechanical characterization of native corneocytes using atomic force microscopy. J. Dermatol. Sci. 2014, 75, 63–65. [Google Scholar] [CrossRef]
- Ghidossi, P.; Mel, M.; Pierron, F. Edge machining effects on the failure of polymer matrix composite coupons. Compos. Part A Appl. Sci. Manuf. 2004, 35, 989–999. [Google Scholar] [CrossRef]
- Duboust, N.; Ghadbeigi, H.; Pinna, C.; Ayvar-Soberanis, S.; Collis, A.; Scaife, R.; Kerrigan, K. An optical method for measuring surface roughness of machined carbon fibre-reinforced plastic composites. J. Compos. Mater. 2016, 51, 289–302. [Google Scholar] [CrossRef]


| Code | PC:S | Chol (mM) | PG/Et (%) | R123 (%) | Size (nm) | η (cP) | %EE | E | pH |
|---|---|---|---|---|---|---|---|---|---|
| ELP3-R | 85:15 | - | - | 0.1 | 217 ± 15 | 4382 ± 105 | 85.2 | 46.8 | 7.0 |
| ETHO2-R | 60:0 | 25 | 0.5 | 0.1 | 128 ± 19 | 4818 ± 117 | 98.1 | 63.2 | 7.1 |
| ELP3-gel-R | 85:15 | - | - | 0.1 | 231 ± 13 | 5983 ± 132 | - | - | 6.8 |
| ETHO2-gel-R | 60:0 | 12.5 | 0.25 | 0.1 | 252 ± 32 | 5872 ± 152 | - | - | 6.9 |
| Code | Flux (µg/cm2/h) | * D (cm2/h) | * p (cm/h) | Diffusion Rate (mg/cm2/h1/2) | r2 |
|---|---|---|---|---|---|
| Synthetic Membrane | |||||
| ELP3R | 37.42 | 7.98 × 10−6 | 0.32 × 10−3 | 0.0648 | 0.761 |
| ETHO2R | 234.94 | 7.57 × 10−5 | 0.45 × 10−3 | 0.406 | 0.810 |
| ELP3-GR | 43.44 | 2.52 × 10−5 | 0.42 × 10−3 | 0.0752 | 0.845 |
| ETHO2-GR | 78.42 | 8.24 × 10−5 | 0.67 × 10−3 | 0.135 | 0.836 |
| Human cultured skin EpiDerm | |||||
| ELP3R | 28.61 | 4.73 × 10−6 | 0.25 × 10−3 | 0.049 | 0.722 |
| ETHO2R | 169.58 | 3.51 × 10−5 | 0.63 × 10−3 | 0.293 | 0.865 |
| ELP3-GR | 88.55 | 1.05 × 10−4 | 0.36 × 10−3 | 0.153 | 0.87 |
| ETHO2-GR | 136.45 | 2.4 × 10−4 | 0.80 × 10−3 | 0.236 | 0.844 |
| Rat abdominal skin | |||||
| ELP3R | 70.03 | 3.54 × 10−5 | 0.126 × 10−2 | 0.121 | 0.763 |
| ETHO2R | 182.42 | 1.05 × 10−4 | 0.3 × 10−2 | 0.315 | 0.744 |
| ELP3-GR | 65.55 | 5.75 × 10−5 | 0.11 × 10−3 | 0.113 | 0.869 |
| ETHO2-GR | 88.64 | 1.64 × 10−4 | 0.24 × 10−3 | 0.153 | 0.874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, A.; Altamimi, M.A.; Afzal, O.; Altamimi, A.S.A.; Ramzan, M.; Khuroo, T. Mechanistic of Vesicular Ethosomes and Elastic Liposomes on Permeation Profiles of Acyclovir across Artificial Membrane, Human Cultured EpiDerm, and Rat Skin: In Vitro-Ex Vivo Study. Pharmaceutics 2023, 15, 2189. https://doi.org/10.3390/pharmaceutics15092189
Hussain A, Altamimi MA, Afzal O, Altamimi ASA, Ramzan M, Khuroo T. Mechanistic of Vesicular Ethosomes and Elastic Liposomes on Permeation Profiles of Acyclovir across Artificial Membrane, Human Cultured EpiDerm, and Rat Skin: In Vitro-Ex Vivo Study. Pharmaceutics. 2023; 15(9):2189. https://doi.org/10.3390/pharmaceutics15092189
Chicago/Turabian StyleHussain, Afzal, Mohammad A. Altamimi, Obaid Afzal, Abdulmalik S. A. Altamimi, Mohhammad Ramzan, and Tahir Khuroo. 2023. "Mechanistic of Vesicular Ethosomes and Elastic Liposomes on Permeation Profiles of Acyclovir across Artificial Membrane, Human Cultured EpiDerm, and Rat Skin: In Vitro-Ex Vivo Study" Pharmaceutics 15, no. 9: 2189. https://doi.org/10.3390/pharmaceutics15092189
APA StyleHussain, A., Altamimi, M. A., Afzal, O., Altamimi, A. S. A., Ramzan, M., & Khuroo, T. (2023). Mechanistic of Vesicular Ethosomes and Elastic Liposomes on Permeation Profiles of Acyclovir across Artificial Membrane, Human Cultured EpiDerm, and Rat Skin: In Vitro-Ex Vivo Study. Pharmaceutics, 15(9), 2189. https://doi.org/10.3390/pharmaceutics15092189









