Biopolymer-Based Nanosystems: Potential Novel Carriers for Kidney Drug Delivery
Abstract
:1. Introduction
2. Targeted Kidney Strategies
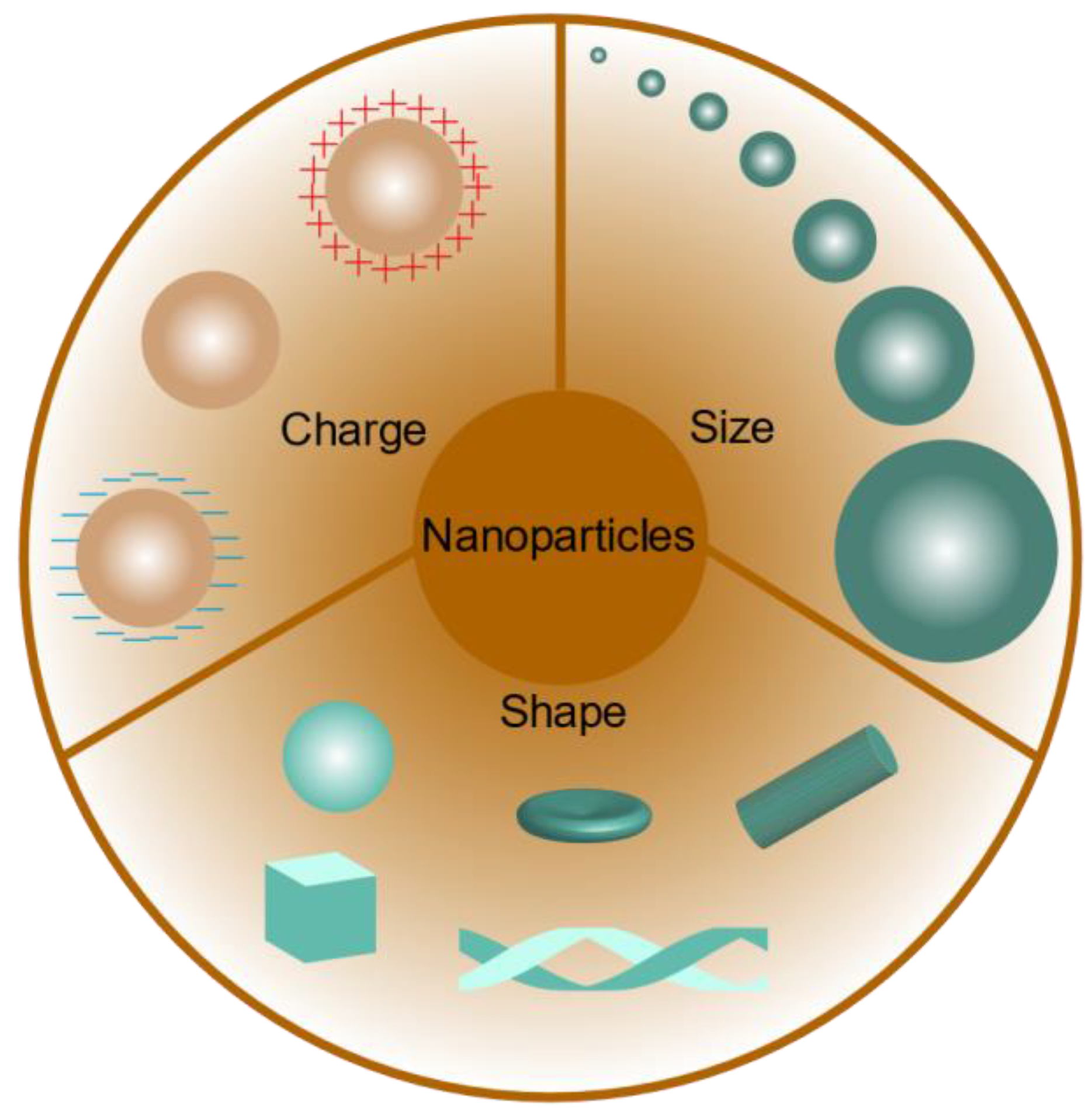
3. Influence of the Physicochemical Properties of Nanoparticles on Kidney Targeting
3.1. Nanoparticle Size
3.2. Nanoparticle Charge
3.3. Nanoparticle Shape
4. Biopolymers and Their Application in Renal Diseases
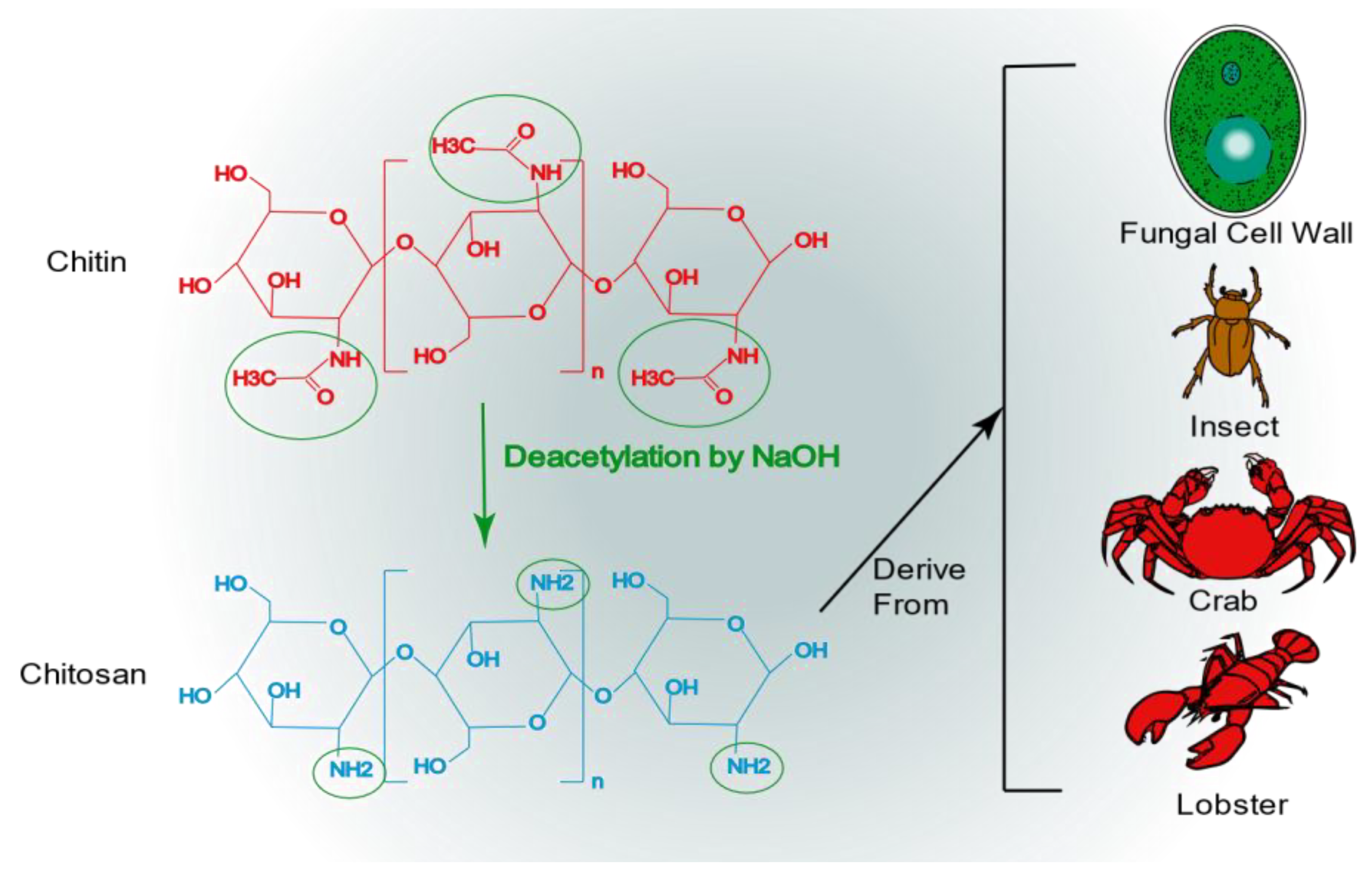
4.1. Chitosan
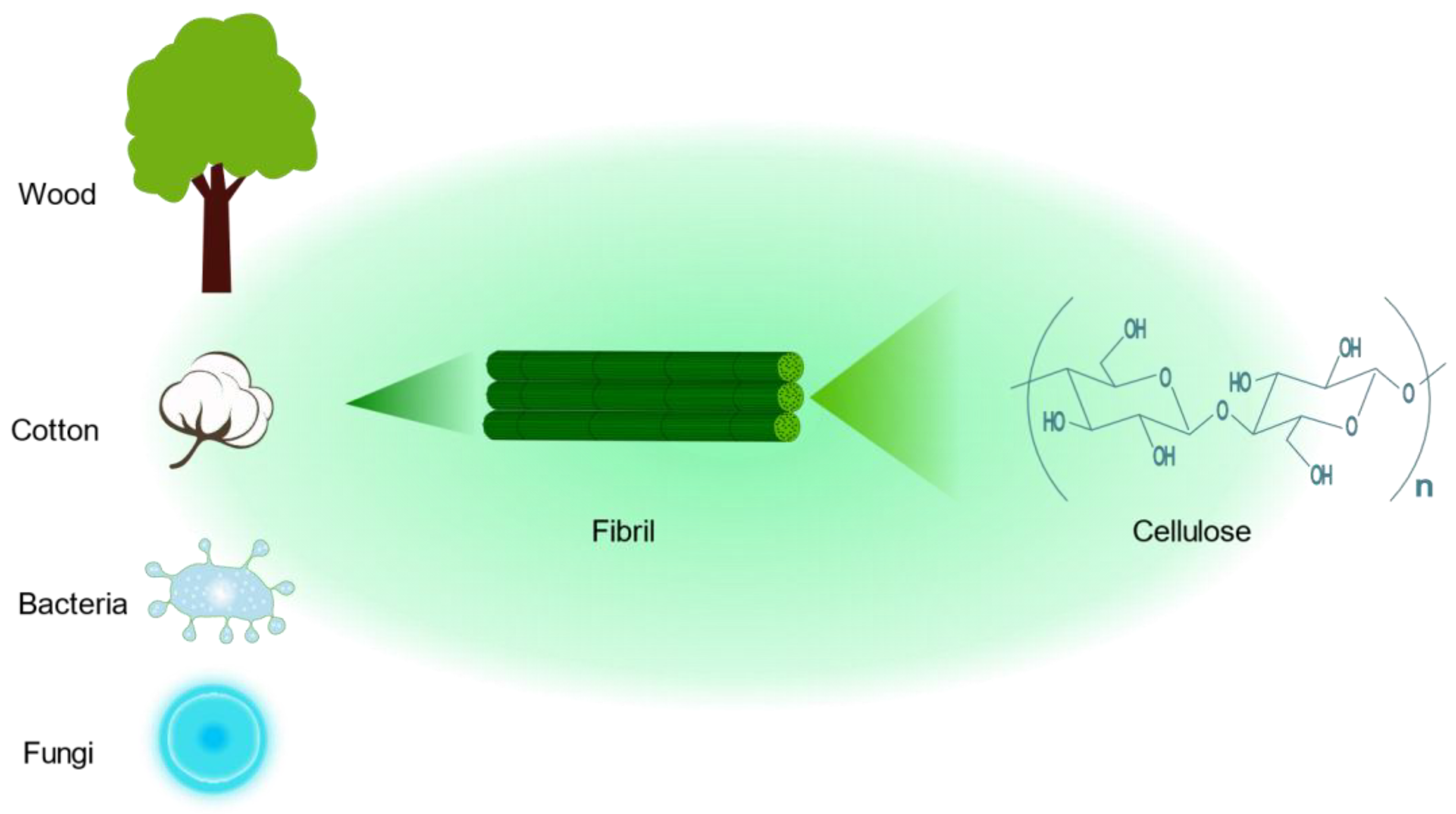
4.2. Cellulose
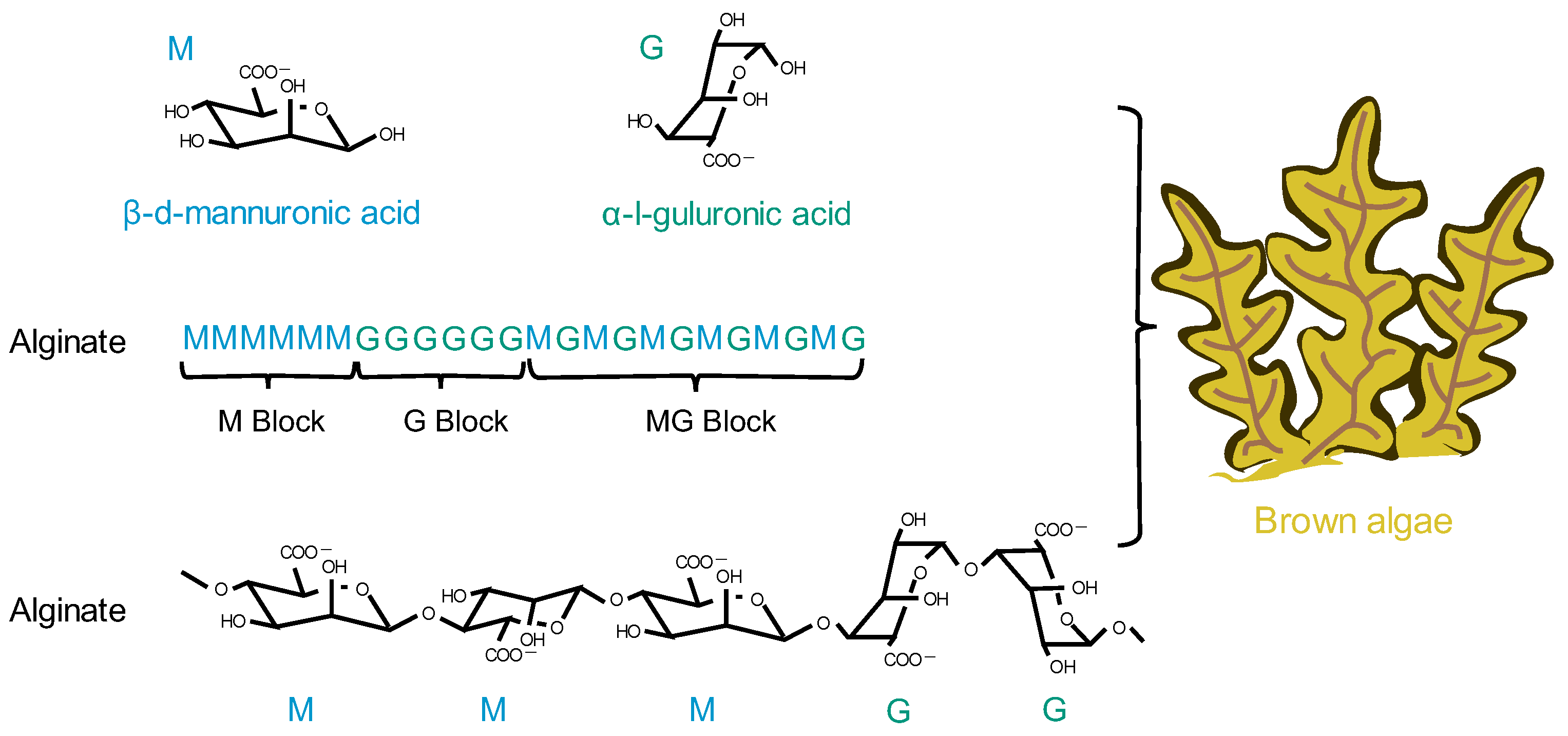
4.3. Alginate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef]
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xiang, Y.; Li, H.; Chen, A.; Dong, Z. Inflammation in kidney repair: Mechanism and therapeutic potential. Pharmacol. Ther. 2022, 237, 108240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, C. From AKI to CKD: Maladaptive Repair and the Underlying Mechanisms. Int. J. Mol. Sci. 2022, 23, 10880. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.-M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Zhang, M.; Hu, C.; Zhang, X.; Li, C.; Nie, S.; Huang, Z.; Zhao, Z.; Hou, F.F.; et al. Prevalence of Chronic Kidney Disease in China: Results from the Sixth China Chronic Disease and Risk Factor Surveillance. JAMA Intern. Med. 2023, 183, 298–310. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- August, P. Chronic Kidney Disease—Another Step Forward. N. Engl. J. Med. 2023, 388, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422D. [Google Scholar] [CrossRef]
- Turner, J.M.; Bauer, C.; Abramowitz, M.K.; Melamed, M.L.; Hostetter, T.H. Treatment of chronic kidney disease. Kidney Int. 2012, 81, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, Y.-S. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target Ther. 2022, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Chandra Hembram, K.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314. [Google Scholar] [CrossRef]
- De Leo, V.; Maurelli, A.M.; Giotta, L.; Catucci, L. Liposomes containing nanoparticles: Preparation and applications. Colloids Surf. B Biointerfaces 2022, 218, 112737. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liu, X.-R.; Chen, Q.-B.; Li, Y.; Zhou, J.-L.; Zhou, L.-Y.; Zou, T. Hyaluronic acid and albumin based nanoparticles for drug delivery. J. Control. Release 2021, 331, 416–433. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Q.; Zhang, L.; Sun, H.; Ding, F.; Li, Y.; Chen, P. Fe3O4 magnetic nanoparticles ameliorate albumin-induced tubulointerstitial fibrosis by autophagy related to Rab7. Colloids Surf. B Biointerfaces 2021, 198, 111470. [Google Scholar] [CrossRef]
- Jeon, M.; Halbert, M.V.; Stephen, Z.R.; Zhang, M. Iron Oxide Nanoparticles as T1 Contrast Agents for Magnetic Resonance Imaging: Fundamentals, Challenges, Applications, and Prospectives. Adv. Mater. 2021, 33, e1906539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Z.; Wu, Y.; Zhang, W.; Zhang, T.; Yu, B.-Y.; Zheng, X.; Tian, J. Renal-clearable and biodegradable black phosphorus quantum dots for photoacoustic imaging of kidney dysfunction. Anal. Chim. Acta 2022, 1204, 339737. [Google Scholar] [CrossRef] [PubMed]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xue, L.-F.; Hu, B.; Liu, H.-H.; Huang, S.-B.; Khan, S.; Meng, Y. Calycosin-loaded nanoliposomes as potential nanoplatforms for treatment of diabetic nephropathy through regulation of mitochondrial respiratory function. J. Nanobiotechnology 2021, 19, 178. [Google Scholar] [CrossRef]
- Bowey, K.; Tanguay, J.-F.; Tabrizian, M. Liposome technology for cardiovascular disease treatment and diagnosis. Expert Opin. Drug Deliv. 2012, 9, 249–265. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Hyperglycemia-induced oxidative stress in isolated proximal tubules of mouse: The in vitro effects of myricitrin and its solid lipid nanoparticle. Arch. Physiol. Biochem. 2021, 127, 422–428. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Elfaky, M.A.; Thabit, A.K.; Sirwi, A.; Fahmy, U.A.; Bahabri, R.M.; Al-Awad, E.A.; Basaeed, L.F. Development of a Novel Pharmaceutical Formula of Nanoparticle Lipid Carriers of Gentamicin/α-Tocopherol and In Vivo Assessment of the Antioxidant Protective Effect of α-Tocopherol in Gentamicin-Induced Nephrotoxicity. Antibiotics 2019, 8, 234. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Zoheir, K.M.A.; Mohamed, N.A.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Abdelhameed, M.F.; Farrag, A.R.H.; Elshamy, A.I. Cytoprotective potentialities of carvacrol and its nanoemulsion against cisplatin-induced nephrotoxicity in rats: Development of nano-encasulation form. Heliyon 2022, 8, e09198. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, T.; Wang, L.; Hu, B.; Darabi, A.; Lin, J.; Xing, M.M.Q.; Qiu, X. Studying Different Binding and Intracellular Delivery Efficiency of ssDNA Single-Walled Carbon Nanotubes and Their Effects on LC3-Related Autophagy in Renal Mesangial Cells via miRNA-382. ACS Appl. Mater. Interfaces 2015, 7, 25733–25740. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Jiang, Z.; Jin, H.; Sun, S.; Chen, C.; Zhang, J.; Guo, Z.; Liu, X. Effects of gallic acid biofabricated rGO nanosheets combined with radiofrequency radiation for the treatment of renal cell carcinoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 846–852. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Wang, J.; Poon, C.; Chin, D.; Milkowski, S.; Lu, V.; Hallows, K.R.; Chung, E.J. Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery. Nano Res. 2018, 11, 5584–5595. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Kan, M.; Yang, J.; Gong, Q.; Jin, R.; Dai, Y.; Jin, J.; Zang, H. Gypenoside XLIX loaded nanoparticles targeting therapy for renal fibrosis and its mechanism. Eur. J. Pharmacol. 2021, 910, 174501. [Google Scholar] [CrossRef]
- Matsuura, S.; Katsumi, H.; Suzuki, H.; Hirai, N.; Hayashi, H.; Koshino, K.; Higuchi, T.; Yagi, Y.; Kimura, H.; Sakane, T.; et al. l-Serine-modified polyamidoamine dendrimer as a highly potent renal targeting drug carrier. Proc. Natl. Acad. Sci. USA 2018, 115, 10511–10516. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, D.; Wang, H.; Zhang, P. Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases. Pharmaceutics 2023, 15, 1899. [Google Scholar] [CrossRef]
- Guo, L.; Luo, S.; Du, Z.; Zhou, M.; Li, P.; Fu, Y.; Sun, X.; Huang, Y.; Zhang, Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017, 8, 878. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Pan, S.; Rodrigues, J.; Elkodous, M.A.; Danquah, M.K. Medical applications of biopolymer nanofibers. Biomater. Sci. 2022, 10, 4107–4118. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, Y. Recent advances of chitosan-based nanoparticles for biomedical and biotechnological applications. Int. J. Biol. Macromol. 2022, 203, 379–388. [Google Scholar] [CrossRef]
- Liu, K.; Zhuang, Y.; Chen, J.; Yang, G.; Dai, L. Research Progress on the Preparation and High-Value Utilization of Lignin Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7254. [Google Scholar] [CrossRef] [PubMed]
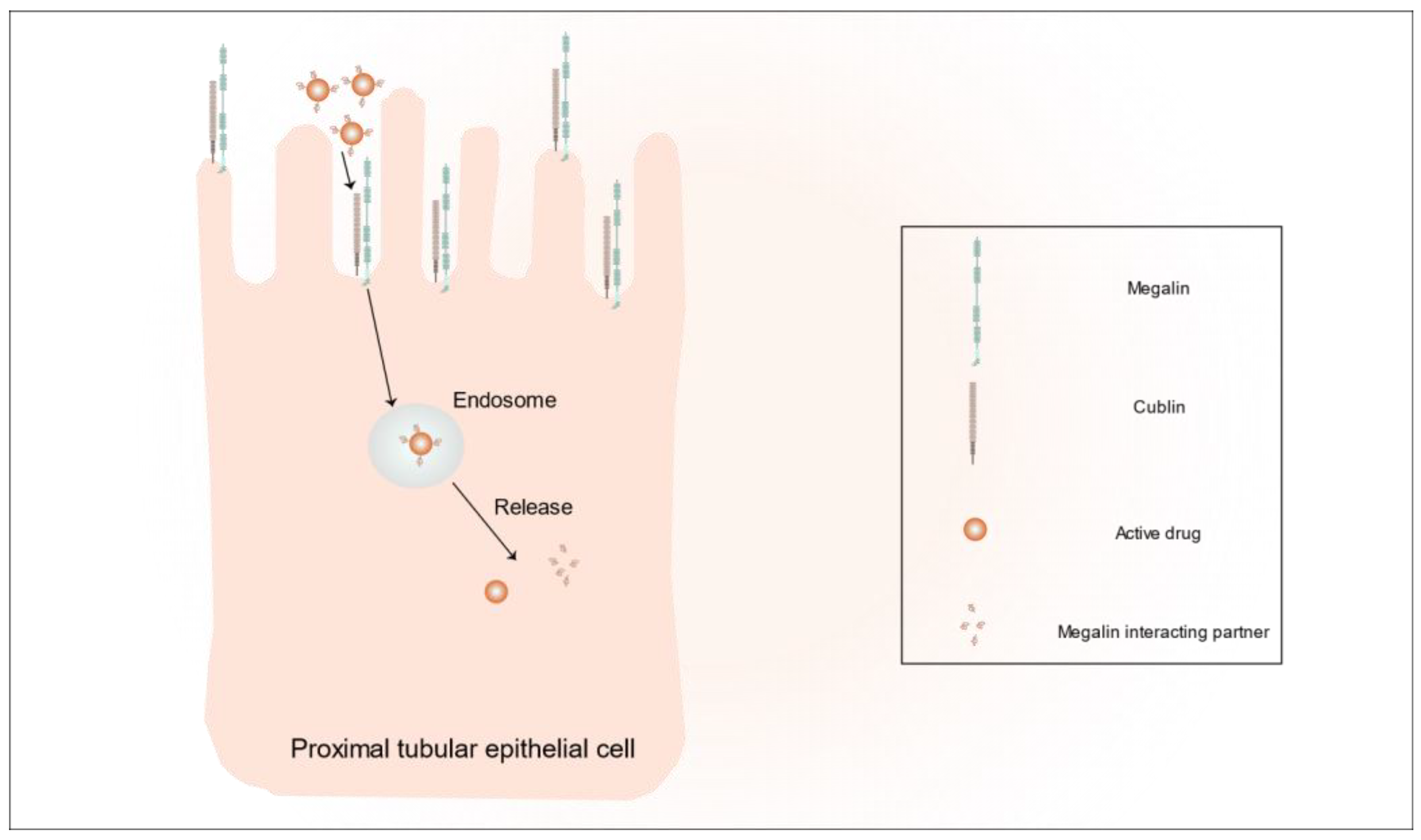
- Perez Bay, A.E.; Schreiner, R.; Benedicto, I.; Paz Marzolo, M.; Banfelder, J.; Weinstein, A.M.; Rodriguez-Boulan, E.J. The fast-recycling receptor Megalin defines the apical recycling pathway of epithelial cells. Nat. Commun. 2016, 7, 11550. [Google Scholar] [CrossRef] [PubMed]
- Elsakka, E.G.E.; Mokhtar, M.M.; Hegazy, M.; Ismail, A.; Doghish, A.S. Megalin, a multi-ligand endocytic receptor, and its participation in renal function and diseases: A review. Life Sci. 2022, 308, 120923. [Google Scholar] [CrossRef]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 2012, 27, 223–236. [Google Scholar] [CrossRef]
- Leheste, J.R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Aucouturier, P.; Moskaug, J.O.; Otto, A.; Christensen, E.I.; et al. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 1999, 155, 1361–1370. [Google Scholar] [CrossRef]
- Nastase, M.V.; Zeng-Brouwers, J.; Wygrecka, M.; Schaefer, L. Targeting renal fibrosis: Mechanisms and drug delivery systems. Adv. Drug Deliv. Rev. 2018, 129, 295–307. [Google Scholar] [CrossRef]
- Prakash, J.; de Borst, M.H.; Lacombe, M.; Opdam, F.; Klok, P.A.; van Goor, H.; Meijer, D.K.F.; Moolenaar, F.; Poelstra, K.; Kok, R.J. Inhibition of renal rho kinase attenuates ischemia/reperfusion-induced injury. J. Am. Soc. Nephrol. 2008, 19, 2086–2097. [Google Scholar] [CrossRef]
- Prakash, J.; de Borst, M.H.; van Loenen-Weemaes, A.M.; Lacombe, M.; Opdam, F.; van Goor, H.; Meijer, D.K.F.; Moolenaar, F.; Poelstra, K.; Kok, R.J. Cell-specific delivery of a transforming growth factor-beta type I receptor kinase inhibitor to proximal tubular cells for the treatment of renal fibrosis. Pharm. Res. 2008, 25, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.M.; van Dorenmalen, K.M.A.; Pieters, E.H.E.; Lacombe, M.; Pato, J.; Storm, G.; Hennink, W.E.; Kok, R.J. Imatinib-ULS-lysozyme: A proximal tubular cell-targeted conjugate of imatinib for the treatment of renal diseases. J. Control. Release 2012, 157, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.M.; Harmsen, S.; Pieters, E.H.E.; Sparidans, R.W.; Lacombe, M.; Szokol, B.; Orfi, L.; Kéri, G.; Storm, G.; Hennink, W.E.; et al. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int. J. Nanomed. 2012, 7, 417–433. [Google Scholar] [CrossRef]
- Zhou, P.; Sun, X.; Zhang, Z. Kidney-targeted drug delivery systems. Acta Pharm. Sin. B 2014, 4, 37–42. [Google Scholar] [CrossRef]
- Qiao, H.; Sun, M.; Su, Z.; Xie, Y.; Chen, M.; Zong, L.; Gao, Y.; Li, H.; Qi, J.; Zhao, Q.; et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials 2014, 35, 7157–7171. [Google Scholar] [CrossRef]
- Gao, S.; Hein, S.; Dagnæs-Hansen, F.; Weyer, K.; Yang, C.; Nielsen, R.; Christensen, E.I.; Fenton, R.A.; Kjems, J. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics 2014, 4, 1039–1051. [Google Scholar] [CrossRef]
- Oroojalian, F.; Rezayan, A.H.; Mehrnejad, F.; Nia, A.H.; Shier, W.T.; Abnous, K.; Ramezani, M. Efficient megalin targeted delivery to renal proximal tubular cells mediated by modified-polymyxin B-polyethylenimine based nano-gene-carriers. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 770–782. [Google Scholar] [CrossRef]
- Wischnjow, A.; Sarko, D.; Janzer, M.; Kaufman, C.; Beijer, B.; Brings, S.; Haberkorn, U.; Larbig, G.; Kübelbeck, A.; Mier, W. Renal Targeting: Peptide-Based Drug Delivery to Proximal Tubule Cells. Bioconjug. Chem. 2016, 27, 1050–1057. [Google Scholar] [CrossRef]
- Janzer, M.; Larbig, G.; Kübelbeck, A.; Wischnjow, A.; Haberkorn, U.; Mier, W. Drug Conjugation Affects Pharmacokinetics and Specificity of Kidney-Targeted Peptide Carriers. Bioconjug. Chem. 2016, 27, 2441–2449. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, K.; Zhang, X.; Chung, E.J. The effect of size, charge, and peptide ligand length on kidney targeting by small, organic nanoparticles. Bioeng. Transl. Med. 2020, 5, e10173. [Google Scholar] [CrossRef]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, F.; Chade, A.R.; Bidwell, G.L. Utilizing a Kidney-Targeting Peptide to Improve Renal Deposition of a Pro-Angiogenic Protein Biopolymer. Pharmaceutics 2019, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dai, W.; Liu, Z.; He, L. Renal Proximal Tubular Cells: A New Site for Targeted Delivery Therapy of Diabetic Kidney Disease. Pharmaceuticals 2022, 15, 1494. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Liu, C.; Chen, Y.; Yao, M.; Liao, S.; Xin, W.; Gong, S.; Guan, X.; Li, Y.; Xiong, J.; et al. Cobaltosic oxide-polyethylene glycol-triphenylphosphine nanoparticles ameliorate the acute-to-chronic kidney disease transition by inducing BNIP3-mediated mitophagy. Kidney Int. 2023, 103, 903–916. [Google Scholar] [CrossRef]
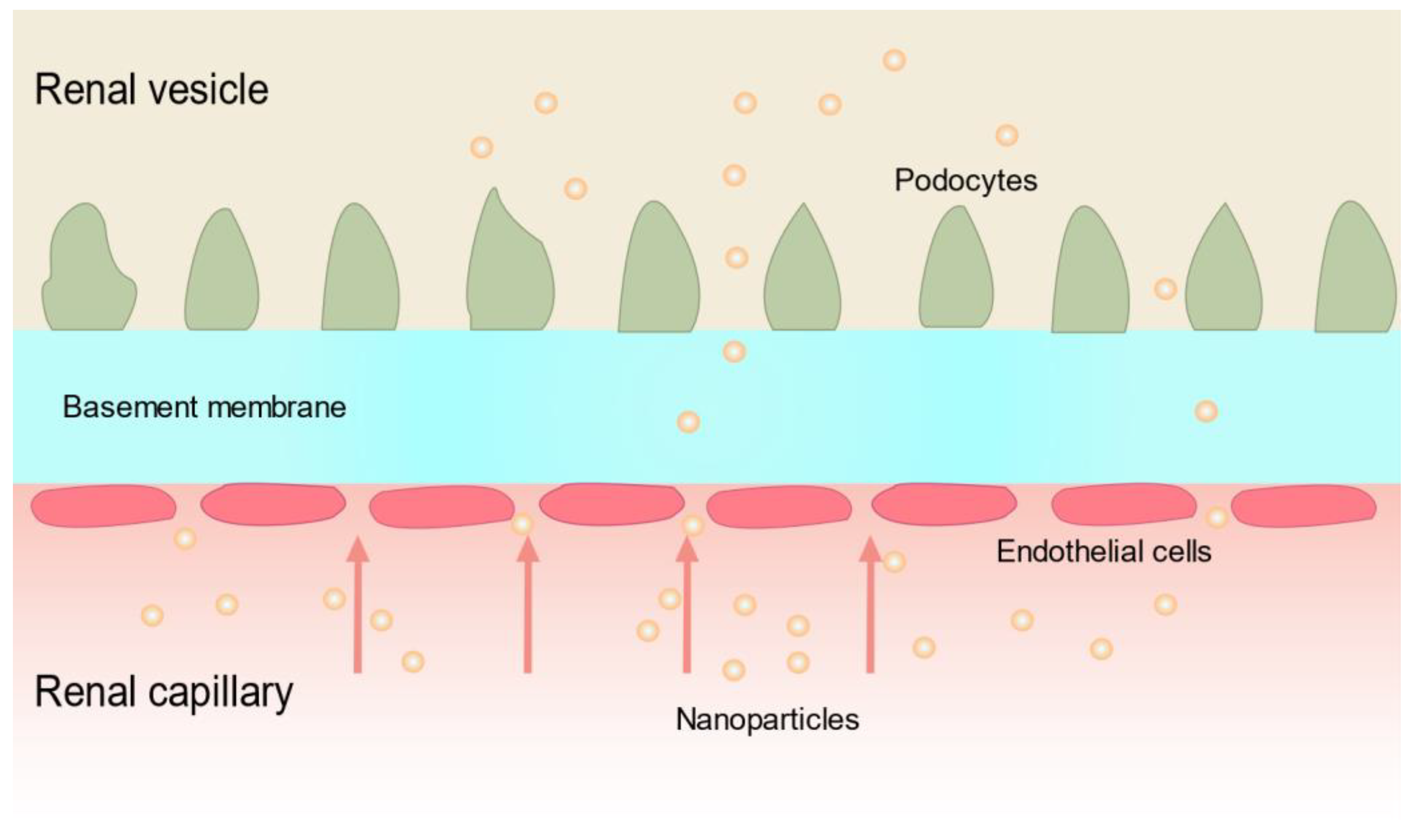
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef]
- Akilesh, S.; Huber, T.B.; Wu, H.; Wang, G.; Hartleben, B.; Kopp, J.B.; Miner, J.H.; Roopenian, D.C.; Unanue, E.R.; Shaw, A.S. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 967–972. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Ng, B.D.; Minton, D.R.; Gudas, L.J.; Park, C.Y.; Heller, D.A. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015, 15, 2358–2364. [Google Scholar] [CrossRef]
- Williams, R.M.; Shah, J.; Tian, H.S.; Chen, X.; Geissmann, F.; Jaimes, E.A.; Heller, D.A. Selective Nanoparticle Targeting of the Renal Tubules. Hypertension 2018, 71, 87–94. [Google Scholar] [CrossRef]
- Han, S.J.; Williams, R.M.; D’Agati, V.; Jaimes, E.A.; Heller, D.A.; Lee, H.T. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 2020, 98, 76–87. [Google Scholar] [CrossRef]
- Finch, N.C.; Fawaz, S.S.; Neal, C.R.; Butler, M.J.; Lee, V.K.; Salmon, A.J.; Lay, A.C.; Stevens, M.; Dayalan, L.; Band, H.; et al. Reduced Glomerular Filtration in Diabetes Is Attributable to Loss of Density and Increased Resistance of Glomerular Endothelial Cell Fenestrations. J. Am. Soc. Nephrol. 2022, 33, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Weil, E.J.; Lemley, K.V.; Mason, C.C.; Yee, B.; Jones, L.I.; Blouch, K.; Lovato, T.; Richardson, M.; Myers, B.D.; Nelson, R.G. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int. 2012, 82, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C. Nanomedicines for renal disease: Current status and future applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef]
- Liu, J.B.; Yu, M.X.; Zhou, C.; Zheng, J. Renal clearable inorganic nanoparticles: A new frontier of bionanotechnology. Mater. Today 2013, 16, 477–486. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Rampersaud, S.; Fang, J.; Wei, Z.; Fabijanic, K.; Silver, S.; Jaikaran, T.; Ruiz, Y.; Houssou, M.; Yin, Z.; Zheng, S.; et al. The Effect of Cage Shape on Nanoparticle-Based Drug Carriers: Anticancer Drug Release and Efficacy via Receptor Blockade Using Dextran-Coated Iron Oxide Nanocages. Nano Lett. 2016, 16, 7357–7363. [Google Scholar] [CrossRef]
- Calderon, A.J.; Bhowmick, T.; Leferovich, J.; Burman, B.; Pichette, B.; Muzykantov, V.; Eckmann, D.M.; Muro, S. Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers. J. Control. Release 2011, 150, 37–44. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Aghbashlo, M.; Amiri, H.; Moosavi Basri, S.M.; Rastegari, H.; Lam, S.S.; Pan, J.; Gupta, V.K.; Tabatabaei, M. Tuning chitosan’s chemical structure for enhanced biological functions. Trends Biotechnol. 2023, 41, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, J.; Mi, Y.; Miao, Q.; Tan, W.; Guo, Z. Preparation of imidazole acids grafted chitosan with enhanced antioxidant, antibacterial and antitumor activities. Carbohydr. Polym. 2023, 315, 120978. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, R.; Zhang, X.; Pang, G.; Li, L.; Han, C.; Liu, B.; Xue, X.; Liu, J.; Sun, T.; et al. A programmable oral bacterial hydrogel for controllable production and release of nanovaccine for tumor immunotherapy. Biomaterials 2023, 299, 122147. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.-L.; Ng, I.S.; Lin, G.-Y.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Hosseini, M.; Amiri, M.; Ghanbari, M.; Mahdi, M.A.; Abdulsahib, W.K.; Salavati-Niasari, M. Drug delivery based on chitosan, β-cyclodextrin and sodium carboxymethyl cellulose as well as nanocarriers for advanced leukemia treatment. Biomed. Pharmacother. 2022, 153, 113369. [Google Scholar] [CrossRef]
- Alhodieb, F.S.; Barkat, M.A.; Barkat, H.A.; Hadi, H.A.; Khan, M.I.; Ashfaq, F.; Rahman, M.A.; Hassan, M.Z.; Alanezi, A.A. Chitosan-modified nanocarriers as carriers for anticancer drug delivery: Promises and hurdles. Int. J. Biol. Macromol. 2022, 217, 457–469. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Prajapati, C.; Agrawal, Y.O.; Agnihotri, V.V.; Mahajan, U.B.; Patil, K.R.; Patil, D.D.; Patil, C.R. Development and biological evaluation of protective effect of kidney targeted N-acetylated chitosan nanoparticles containing thymoquinone for the treatment of DNA damage in cyclophosphamide-induced haemorrhagic cystitis. Int. J. Biol. Macromol. 2022, 214, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-X.; Sun, X.; Gong, T.; Ding, H.; Fu, Y.; Zhang, Z.-R. Randomly 50% N-acetylated low molecular weight chitosan as a novel renal targeting carrier. J. Drug Target. 2007, 15, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Taweel, G.M.A.; Hidayathulla, S. Nano-composites chitosan-curcumin synergistically inhibits the oxidative stress induced by toxic metal cadmium. Int. J. Biol. Macromol. 2018, 108, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Duan, S.; Zhao, M.; Jiao, Q.; Bai, Y.; Yu, L.; Du, B.; Cheng, G. Co-delivery of celastrol and lutein with pH sensitive nano micelles for treating acute kidney injury. Toxicol. Appl. Pharmacol. 2022, 450, 116155. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shu, G.; Jin, F.; Qi, J.; Xu, X.; Du, Y.; Yu, H.; Wang, J.; Sun, M.; You, Y.; et al. ROS-responsive chitosan-SS31 prodrug for AKI therapy via rapid distribution in the kidney and long-term retention in the renal tubule. Sci. Adv. 2020, 6, eabb7422. [Google Scholar] [CrossRef]
- Robert, A.; Meunier, B. How to Define a Nanozyme. ACS Nano 2022, 16, 6956–6959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, L.; Qiu, K.; Liao, X.; Rees, T.W.; Zhao, Z.; Ji, L.; Chao, H. An Ultrasmall RuO2 Nanozyme Exhibiting Multienzyme-like Activity for the Prevention of Acute Kidney Injury. ACS Appl. Mater. Interfaces 2020, 12, 31205–31216. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Tang, Q.; Chen, X.; Li, Z. Nanocrystalline cellulose extracted from bast fibers: Preparation, characterization, and application. Carbohydr. Polym. 2022, 290, 119462. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, R.; Sun, J.; Liu, J.; Zhu, Q. Naturally Derived Janus Cellulose Nanomaterials: Anisotropic Cellulose Nanomaterial Building Blocks and Their Assembly into Asymmetric Structures. ACS Nano 2022, 16, 13468–13491. [Google Scholar] [CrossRef]
- Keller, M.B.; Sørensen, T.H.; Krogh, K.B.R.M.; Wogulis, M.; Borch, K.; Westh, P. Activity of fungal β-glucosidases on cellulose. Biotechnol. Biofuels 2020, 13, 121. [Google Scholar] [CrossRef]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Iglesias, M.; Lizundia, E.; Lanceros-Méndez, S. Water-Soluble Cellulose Derivatives as Suitable Matrices for Multifunctional Materials. Biomacromolecules 2019, 20, 2786–2795. [Google Scholar] [CrossRef]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Ghilan, A.; Nicu, R.; Ciolacu, D.E.; Ciolacu, F. Insight into the Latest Medical Applications of Nanocellulose. Materials 2023, 16, 4447. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jawaid, M.; Kian, L.K.; Khan, A.A.P.; Asiri, A.M. Isolation and Production of Nanocrystalline Cellulose from Conocarpus Fiber. Polymers 2021, 13, 1835. [Google Scholar] [CrossRef]
- Baseer, R.A.; Dacrory, S.; El Gendy, M.A.M.; Ewies, E.F.; Kamel, S. A biodegradable film based on cellulose and thiazolidine bearing UV shielding property. Sci. Rep. 2022, 12, 7887. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Bangar, S.P.; Harussani, M.M.; Ilyas, R.A.; Ashogbon, A.O.; Singh, A.; Trif, M.; Jafari, S.M. Surface modifications of cellulose nanocrystals: Processes, properties, and applications. Food Hydrocoll. 2022, 130, 107689. [Google Scholar] [CrossRef]
- Chen, R.; Ma, Z.H.; Sun, D.Y.; Wang, X.; Han, Y. Cellulose I nanocrystals (CNCs I) prepared in mildly acidic lithium bromide trihydrate (MALBTH) and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2022, 201, 59–66. [Google Scholar] [CrossRef]
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2022, 62, 989–1002. [Google Scholar] [CrossRef]
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Gong, J.; Wan, Z.; Zhou, J.; Chang, C.; Zhang, D. Micropatterned Hydrogels with Highly Ordered Cellulose Nanocrystals for Visually Monitoring Cardiomyocytes. Small 2022, 18, e2202235. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, F.; Zhang, L. Copolymer-Functionalized Cellulose Nanocrystals as a pH- and NIR-Triggered Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. Biomacromolecules 2022, 23, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, M.; Mu, G.; Ren, H.; He, C.; Xie, Q.; Liu, Q.; Wang, J.; Cha, R. Adsorptivity of cationic cellulose nanocrystals for phosphate and its application in hyperphosphatemia therapy. Carbohydr. Polym. 2021, 255, 117335. [Google Scholar] [CrossRef]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef]
- Gutekunst, L. An Update on Phosphate Binders: A Dietitian’s Perspective. J. Ren. Nutr. 2016, 26, 209–218. [Google Scholar] [CrossRef]
- Wong, S.; Alidori, S.; Mello, B.P.; Almeida, B.A.; Ulmert, D.; Brendel, M.B.; Scheinberg, D.A.; McDevitt, M.R. Fibrillar pharmacology of functionalized nanocellulose. Sci. Rep. 2021, 11, 157. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Q.; Zhu, G.; Ma, J.; Lin, N. Size effect of cellulose nanocrystals in cellular internalization and exosome-packaging exocytosis. Carbohydr. Polym. 2022, 298, 120131. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Ismadji, S.; Gunawan, S. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, X.; Du, C.; Su, Y.; Yin, L.; Tan, X.; Liu, H.; Wang, Y.; Xu, L.; Xu, X. An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy. Biomed. Pharmacother. 2022, 153, 113438. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Jiang, D.; Yu, B.; Ni, D.; Li, M.; Long, Y.; Ellison, P.A.; Siamof, C.M.; Cheng, L.; Barnhart, T.E.; et al. Nanostructured polyvinylpyrrolidone-curcumin conjugates allowed for kidney-targeted treatment of cisplatin induced acute kidney injury. Bioact. Mater. 2023, 19, 282–291. [Google Scholar] [CrossRef]
- Mrudulakumari Vasudevan, U.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohydr. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and functional assessment of alginate fibers prepared by metal-calcium ion complex coagulation bath. Carbohydr. Polym. 2020, 232, 115693. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2021, 170, 424–436. [Google Scholar] [CrossRef]
- He, Q.; Tong, T.; Yu, C.; Wang, Q. Advances in Algin and Alginate-Hybrid Materials for Drug Delivery and Tissue Engineering. Mar. Drugs 2022, 21, 14. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Sosnowska, K.; Zakrzeska, A.; Kasacka, I.; Lewandowska, A.; Winnicka, K. The Influence of Chitosan Cross-linking on the Properties of Alginate Microparticles with Metformin Hydrochloride-In Vitro and In Vivo Evaluation. Molecules 2017, 22, 182. [Google Scholar] [CrossRef]
- Heidarisasan, S.; Ziamajidi, N.; Karimi, J.; Abbasalipourkabir, R. Effects of insulin-loaded chitosan-alginate nanoparticles on RAGE expression and oxidative stress status in the kidney tissue of rats with type 1 diabetes. Iran. J. Basic. Med. Sci. 2018, 21, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Man, J.; Li, J.; Cui, X.; Zhang, C.; Shi, W.; Li, D.; Zhang, S.; Li, J. Histone Deacetylase Inhibitor-loaded Calcium Alginate Microspheres for Acute Kidney Injury Treatment. ACS Appl. Bio. Mater. 2020, 3, 6457–6465. [Google Scholar] [CrossRef] [PubMed]
| Types of Nanoparticles | Subclasses | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|
| Inorganic nanoparticles | Fe3O4 magnetic nanoparticles | Superparamagnetic, anti-inflammatory, and antioxidative stress effects. | Toxicity, complex preparation process. | [20,21] |
| Gold nanoparticles | Easy to fabricate, highly stable surface chemistry, and multi-functionality. | Toxicity | [1] | |
| Quantum dots | Excellent photo-stability, high quantum yield, mainly used for renal photoacoustic imaging. | In vivo toxicity, distribution, metabolism, and excretion issues | [22,23] | |
| Lipid nanoparticles | Liposome | Hydrophilic and lipophilic, surface modifiable with targeted ligands, biocompatible. | Costly and quick to remove. | [24,25] |
| Solid lipid nanoparticles (SLNs) | Biocompatible and biodegradable, high surface area. | Low drug loading, excretion of drugs under storage conditions. | [26,27] | |
| Nanostructured lipid carriers (NLCs) | Compared to SLNs, they have a better drug encapsulation rate, higher drug loading capacity, and lower drug spillage during storage. | Stability and storage issues. | [27,28] | |
| Nanoemulsion | High bioavailability, good stability, and long shelf life. | Toxicity | [29,30] | |
| Carbon-based nanoparticles | Carbon nanotubes(CNTs) | Excellent adsorption capacity and high surface area. | Poor solubility, low biodegradability, and toxicity issues. | [31,32] |
| Graphene | Excellent optical properties, electrical conductivity, and high mechanical strength. | Toxicity and cell viability issues. | [33,34] | |
| Polymer nanoparticles | Micelles | Stable, customizable drug release characteristics on demand. | Some polymers may be limited by the immune response | [35,36] |
| Synthetic polymers | Easy chemical coupling, good drug loading or encapsulation, increased drug cycle time, uniform particle size distribution, and modifiable physicochemical properties. | Limited process technology and immunogenicity. | [1,37] | |
| Biopolymers | Biodegradable, eliminated from the body via normal metabolic pathways, non-toxic, biocompatible, and poorly immunogenic | [1] | ||
| Dendrimers | Surface attachable specific targeting ligands, high permeability, and enhanced solubility. | Toxicity and untimely release of drugs. | [38] | |
| Biomimetic nanoparticles | Cell-membrane coated nanoparticles | Extended blood circulation, high biocompatibility, and low side effects. | Some cell membranes have the potential to promote tumor growth or disease progression in their own right. | [39] |
| Natural protein-based nanoparticles | Biocompatibility, biodegradability, and easy size control. | High cost and rapid degradation. | [40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Dai, W.; Xiao, L.; Sun, L.; He, L. Biopolymer-Based Nanosystems: Potential Novel Carriers for Kidney Drug Delivery. Pharmaceutics 2023, 15, 2150. https://doi.org/10.3390/pharmaceutics15082150
Li H, Dai W, Xiao L, Sun L, He L. Biopolymer-Based Nanosystems: Potential Novel Carriers for Kidney Drug Delivery. Pharmaceutics. 2023; 15(8):2150. https://doi.org/10.3390/pharmaceutics15082150
Chicago/Turabian StyleLi, Hao, Wenni Dai, Li Xiao, Lin Sun, and Liyu He. 2023. "Biopolymer-Based Nanosystems: Potential Novel Carriers for Kidney Drug Delivery" Pharmaceutics 15, no. 8: 2150. https://doi.org/10.3390/pharmaceutics15082150
APA StyleLi, H., Dai, W., Xiao, L., Sun, L., & He, L. (2023). Biopolymer-Based Nanosystems: Potential Novel Carriers for Kidney Drug Delivery. Pharmaceutics, 15(8), 2150. https://doi.org/10.3390/pharmaceutics15082150







