Abstract
Chronic inflammatory respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis, present ongoing challenges in terms of effective treatment and management. These diseases are characterized by persistent inflammation in the airways, leading to structural changes and compromised lung function. There are several treatments available for them, such as bronchodilators, immunomodulators, and oxygen therapy. However, there are still some shortcomings in the effectiveness and side effects of drugs. To achieve optimal therapeutic outcomes while minimizing systemic side effects, targeted therapies and precise drug delivery systems are crucial to the management of these diseases. This comprehensive review focuses on the role of drug delivery systems in chronic inflammatory respiratory diseases, particularly nanoparticle-based drug delivery systems, inhaled corticosteroids (ICSs), novel biologicals, gene therapy, and personalized medicine. By examining the latest advancements and strategies in these areas, we aim to provide a thorough understanding of the current landscape and future prospects for improving treatment outcomes in these challenging conditions.
1. Introduction
Chronic inflammatory respiratory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), affect millions of people worldwide and are a leading cause for the increase in lung disease morbidity and mortality [1]. Asthma, as a heterogeneous clinical syndrome, affects over 300 million people worldwide [2]. COPD, a disease mainly associated with long-term smoking, became the third leading cause of death globally in 2020 [3]. Although there are several existing treatments, limited efficacy, side effects, and individual variability still cannot be ignored [4,5,6]. In recent years, there has been a growing interest in the development of targeted drug delivery systems for the treatment of these diseases [7,8,9]. Nanoparticle-based drug delivery systems, inhaled corticosteroids (ICSs), novel biologicals, gene therapy, and personalized medicine have emerged as promising approaches to deliver drugs more effectively and with fewer side effects.
Currently, the development of new nanoparticle-based drug delivery systems that can target specific cells such as lung epithelial cells and macrophages, while minimizing systemic side effects, have received significant attention [10]. These systems utilize nanoparticles, which are tiny particles ranging from 1 to 100 nanometers in size, to encapsulate and deliver drugs directly to the affected areas of the lungs [11]. By modifying the surface properties of nanoparticles, researchers can enhance their ability to selectively bind to specific cell types in the lungs, thereby improving drug delivery efficiency and reducing off-target effects [12]. Furthermore, nanoparticle-based drug delivery systems can protect the drugs from degradation and enhance their stability, ensuring sustained release and prolonged therapeutic effects [13].
In addition to nanoparticle-based systems, inhaled corticosteroids (ICSs) have long been used as a standard treatment for chronic inflammatory respiratory diseases [14,15]. ICSs work by reducing inflammation in the airways, thus alleviating symptoms and preventing exacerbation. Researchers are also exploring novel biological targets and innovative methods for delivering biologicals to the lungs. Gene therapy approaches, including viral-vector-based delivery systems and CRISPR–Cas9 technology, represent another exciting frontier in the treatment of chronic inflammatory respiratory diseases [16,17]. Moreover, personalized medicine approaches take into account an individual’s unique characteristics, such as genetics, biomarkers, and lifestyle factors, to tailor treatments to their specific needs [8,18]. By utilizing advanced diagnostic tools like genomic sequencing and biomarker analysis, healthcare providers can identify patient subgroups who are more likely to respond to a particular therapy, thus optimizing treatment outcomes [19,20]. However, several challenges remain, including optimizing delivery efficiency, ensuring safety, and addressing ethical considerations.
The purpose of this review is to provide an overview of the current research progress in nanoparticle-based drug delivery systems, ICS, novel biologicals, gene therapy, and personalized medicine for the treatment of chronic inflammatory respiratory diseases. In this review, we examine recent advancements, discuss limitations, and explore future directions for each of these therapeutic approaches.
2. Nanoparticle-Based Drug Delivery Systems
The application of nanotechnology continues to provide effective strategies in treating various chronic diseases and improving treatment outcomes. Using nanocarrier systems such as liposomes, micelles, and nanoparticles for pulmonary drug delivery has been proven to be a promising noninvasive treatment strategy for achieving drug deposition and controlled release in the lungs [10] (Figure 1). These systems involve the use of engineered particles with dimensions on the nanometer scale to deliver drugs directly to target cells in the lungs [21]. Nanoparticles have several advantages over conventional drug delivery methods, including improved bioavailability, enhanced targeting, and reduced toxicity [22,23].
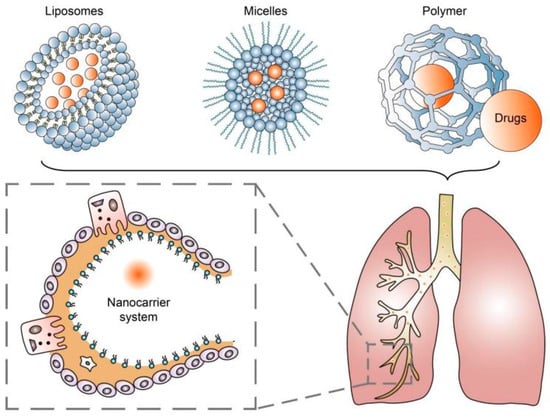
Figure 1.
Nanocarrier systems can achieve drug deposition and controlled release in the lungs.
Liposomes are spherical vesicles composed of lipid bilayers that can encapsulate both hydrophilic and hydrophobic drugs [24]. The size, surface charge, and lipid composition of lipid nanoparticles (LNPs) can be tailored to enhance drug stability, prolong circulation time, and improve biocompatibility [25]. Furthermore, conjugating small-molecule ligands, peptides [26], or monoclonal antibodies [27] to the surface of an LNP can endow it with targeting ability. For example, folate receptors are often found to be overexpressed on macrophages, which makes folate-coupled LNP a great option for delivering anti-inflammatory drugs [28]. There are many factors that can affect the release of cargo carried by LNPs, including temperature, changes in pH values, enzymes, light, etc. Among them, the mechanism of pH change is the most studied, and can cause LNPs to undergo phase transition and achieve higher membrane permeability [29].
In addition to LNPs, there are also some other nanoparticles that have their own characteristics (Table 1). Micelles are another kind of nanoparticle consisting of amphiphilic molecules that form a core-shell structure [30]. Their great solubility allows them to easily penetrate the increased alveolar fluid barrier present in chronic inflammatory lung diseases. A new kind of stabilized phospholipid nanomicelles (SSMs) can reach deep lung tissue and successfully extend the half-life of budesonide in the lung to 18–20 h [31]. Magnetic nanoparticles (MNPs) developed using the magnetofection technique have wide-ranging applications in the fields of biological research and medicine, including drug and gene therapy, magnetic targeting (such as in cancer therapies), and diagnostic imaging as contrast enhancers [32,33]. A representative example is the superparamagnetic iron oxide nanoparticle (SPION), a type of nanoparticle with special magnetism that can be guided through an external magnetic field to locations within the body [34]. They can accurately transport the drugs coated on their surface, mainly some inflammation-related molecular antibodies like IL4Rα and ST2, to the site of the inflammatory lesion [35,36]. A kind of selective organ targeting (SORT) nanoparticle was designed to release its cargo in a controlled manner; it can target the site of inflammation in the lungs and elsewhere while minimizing exposure of healthy tissue in other parts of the body [37]. This targeted drug delivery approach has the potential to reduce drug toxicity and improve patient outcomes [38]. Recently, a growing number of hybrid nanoparticles (HNPs) have emerged that can simultaneously possess the characteristics of different nanoparticles [39]. This has sparked a trend of exploring different combinations of nanoparticles.

Table 1.
Therapeutic applications of nanoparticles in chronic inflammatory respiratory diseases.
Despite the promise of nanoparticle-based drug delivery, there are still several research challenges that need to be addressed. For example, there is a need to develop nanoparticles with optimal physicochemical properties, such as particle size, surface charge, and stability, to ensure effective drug delivery [46]. Recent research has reported that the structure of mesoporous silica nanoparticles (MSNs) can be well controlled with several parameters such as pH, surfactant, silica precursor, and temperature. For instance, Pan et al. prepared a series of size-controlled MSNs with a range of 25–105 nm by simply changing the amount of the basic catalyst triethanolamine (TEA) added [47]. So, it is believed that MSNs have significant potential to serve as nanocarriers for pulmonary drug delivery [48]. Additionally, researchers need to carefully evaluate the safety and toxicity of nanoparticle-based drug delivery systems. While some studies have shown promising results, others have raised concerns about the potential for long-term toxicity and negative environmental impacts of nanoparticle-based drug delivery [49,50]. Currently, it is widely believed that the cytotoxicity of nanoparticles is mainly related to their large surface area and small size [51]. Yuan et al. concluded through their study on the effects of 20, 30, and 40 nm zinc oxide nanoparticles on human embryonic lung fibroblasts that cytotoxicity is concentration-dependent, therefore calling for the minimum therapeutic concentration [52]. Other researchers found that the surface charge and solubility are also associated with the cytotoxicity of nanoparticles [53,54].
Moving forward, researchers are exploring several future directions for nanoparticle-based drug delivery systems. For example, considering that there is a large amount of mucus oozing out of the lungs during chronic inflammatory diseases, researchers are developing new mucus-penetrating nanoparticles (MPPs). Uptake mechanism studies revealed that caveolae-mediated endocytosis and macropinocytosis contributed to the absorption of MPPs [55]. In vivo research results showed a more than five-fold increase in drug bioavailability [56]. Others are investigating new methods for optimizing nanoparticle design and surface modification to improve targeting and drug release [40,57]. Additionally, some researchers are investigating the potential of combining nanoparticles with other treatment modalities such as gene therapy or immunotherapy [46,58]. Finally, there is growing interest in developing personalized nanoparticle-based drug delivery approaches that can be tailored to individual patients based on their unique disease characteristics and genetic profiles [59].
Through targeted drug delivery, nanoparticles have the potential to improve therapeutic efficacy and reduce systemic side effects. Overall, nanoparticle-based drug delivery systems hold great promise for the treatment of chronic inflammatory respiratory diseases.
3. Inhaled Corticosteroids (ICSs)
Inhaled corticosteroids (ICSs) are widely used as a treatment option for chronic respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). These medications work by reducing the production of inflammatory mediators in the airways, which helps prevent or reduce inflammation, bronchoconstriction, and mucus production. According to the Global Initiative for Asthma (GINA) report [1], ICSs have been shown to improve lung function, reduce exacerbation, and improve quality of life in patients with chronic respiratory diseases.
However, there are some current challenges with ICS delivery that limit their efficacy. One major challenge is achieving the optimal distribution of the medication throughout the lungs. ICS particles can become trapped in the mouth or throat, reducing their effectiveness in the lower airways [60]. Patients may also have difficulty using their inhaler correctly, leading to reduced medication delivery and efficacy [61]. Moreover, selecting the appropriate ICS dose for each patient can be challenging, as individual needs can vary significantly [62].
To optimize ICS delivery and improve its efficacy, several methods have been developed. One approach involves the use of spacer devices, which help to slow down the speed of medication delivery and improve medication deposition in the lungs [63]. Another approach is the development of more efficient ICS formulations, such as fine-particle ICSs, which have shown improved efficacy compared with conventional ICS formulations [64]. Fine-particle ICSs have greater deposition in the small airways compared with conventional ICSs [65]. According to a meta-analysis, fine-particle ICSs have significantly higher odds of achieving asthma control [66]. The combination of ICSs and other drugs is also worth further optimization (Figure 2). Additionally, research advancements have explored smart inhalers that can monitor medication adherence and provide feedback to patients [67]. Nowadays, four kinds of inhalers (nebulizers, dry powder inhalers (DPIs), pressurized metered-dose inhalers (pMDIs), and soft mist inhalers (SMIs)) are widely used (Table 2). Recently, artificial intelligence (AI)-based intelligent inhalers have attracted much attention, as they can enable real-time regulation of inhalation plans. For example, intelligent dry powder inhalers (DPIs) constructed based on artificial neural networks (ANNs) have effectively improved the bioavailability of drugs [68], but additional data are still needed to train more advanced models to output better drug delivery plans [69].
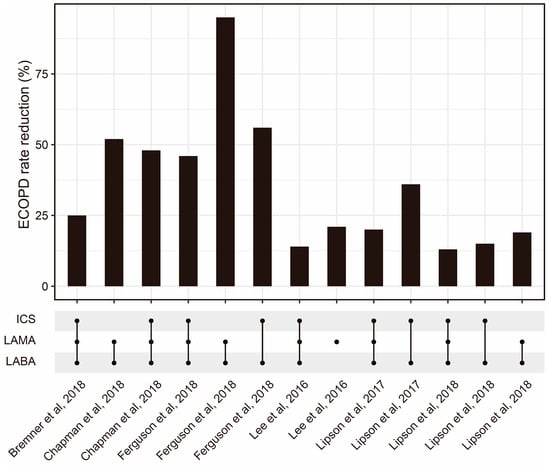
Figure 2.
The ECOPD rate reduction from ICSs combined with other drug regimens reported by some published studies [70,71,72,73,74,75]. Abbreviations: ECOPD: Exacerbation of chronic obstructive pulmonary disease; ICS: Inhaled corticosteroid; LAMA: Long-acting muscarinic antagonist; LABA: Long-acting beta2-adrenergic agonist.

Table 2.
Different kinds of ICS inhalers.
While there have been notable advancements, it is important to acknowledge that there are still existing limitations concerning the use of ICSs that necessitate careful consideration and remediation. For example, some studies have suggested that long-term use of ICSs may increase the risk of pneumonia and cataracts [82,83]. Moreover, further research is needed to determine the optimal ICS dose and duration of treatment for individual patients [84].
Future directions for research in ICS delivery are focused on several areas. Personalized ICS dosing strategies based on individual patient characteristics and disease severity are being explored [85]. Investigations are currently underway to explore new ICS formulations that utilize innovative drug delivery technologies, including nanotechnology and microencapsulation [86].
Thus, ICSs remain an effective treatment option for chronic respiratory diseases, but proper delivery optimization is crucial to their efficacy and safety.
4. Novel Biologicals
Biologicals are a class of drugs that are produced using living cells or organisms and have revolutionized the treatment of many respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF). Biologicals target specific proteins and immune cells involved in the inflammation and damage of the airways and lungs, offering a more precise and effective treatment option compared with traditional medications [87].
Research is ongoing to identify new biological targets for the treatment of respiratory diseases. For example, interleukin-33 (IL-33) is a protein that has been shown to promote allergic inflammation in asthma and may be a potential target for biologicals [88]. Other targets include prostaglandin D2 (PGD2) and its receptor, chemoattractant receptor-homologous molecule expressed on T-helper type-2 cells (CRTH2), which are involved in airway smooth muscle contraction and inflammation [89], and the protein periostin, which plays a role in lung tissue remodeling in asthma and IPF [90]. These novel targets offer the potential for more personalized and targeted therapies for respiratory diseases (Table 3).

Table 3.
Novel biological targets of chronic inflammatory respiratory diseases.
Effective delivery of biologicals to the lungs is critical for their efficacy. Various innovative methods have been developed to improve drug delivery, including nebulizers, dry powder inhalers, and intravenous infusions [104]. Additionally, recent advancements in nanotechnology have opened up new possibilities for targeted drug delivery to specific areas of the lungs [105]. For example, a new exosome membrane–modified M2 macrophages targeted nanomedicine has been proved to be effective for allergic asthma in vivo [40]. The progress of these delivery methods provides the potential for achieving the specific action of biopharmaceuticals at the organ level.
Research on biologicals for respiratory diseases has made significant advancements in recent years. For example, studies have shown the efficacy of biologicals targeting interleukin-5 (IL-5) and interleukin-4/13 (IL-4/13) in asthma [106] and the effectiveness of nintedanib, a tyrosine kinase inhibitor, in slowing the progression of IPF [107]. However, there are also limitations to biological therapy, including high costs and the risk of adverse effects such as allergic reactions and infections [108].
Developing personalized biological therapies and improving drug delivery methods will undoubtedly be the main trends in the future. For example, studies have explored the use of biomarkers to identify patients who may benefit from specific biologicals and the development of smart inhalers that can monitor adherence and provide feedback to patients [109]. Additionally, research is ongoing to develop new biologicals that target novel pathways and cells involved in respiratory diseases [110].
So far, biologicals have transformed the treatment of respiratory diseases, offering more precise and targeted therapies. Three anti-IL-5 biologicals and one anti-IL-4R biological have recently emerged as promising treatments for type 2 (T2) asthma [111]. There is also evidence that itepekimab could reduce the annualized exacerbation rate and improve lung function in former smokers with COPD [112]. Further research is needed to optimize the efficacy, safety, and cost-effectiveness of these treatments.
5. Gene Therapy
Gene therapy is a promising approach for the treatment of respiratory diseases, including asthma, cystic fibrosis, alpha-1 antitrypsin deficiency, and pulmonary hypertension. This therapeutic approach involves the delivery of genetic material to replace or supplement faulty genes, prevent the expression of harmful genes, or introduce new genes to cells [113]. Gene therapy offers the potential for long-lasting effects compared with traditional pharmacological treatments.
Several gene therapy approaches have been developed for respiratory diseases, including viral-vector-based delivery systems and clustered regularly interspaced short palindromic repeats–CRISPR-associated protein 9 (CRISPR–Cas9) technology. Viral vectors, such as adeno-associated viruses (AAVs) and lentiviruses, are commonly used to deliver the therapeutic gene to target cells. AAVs have shown promise in clinical trials for cystic fibrosis and other genetic lung diseases [114]. CRISPR–Cas9 technology allows precise editing of defective genes in living cells and has been used to correct mutations in animal models of cystic fibrosis and alpha-1 antitrypsin deficiency [115] (Table 4). However, there are still limitations to these approaches, such as immune responses to viral vectors and potential off-target effects of genome editing.

Table 4.
Treatments based on gene therapy for chronic inflammatory respiratory diseases.
In recent years, research on gene therapy for respiratory diseases has achieved remarkable advancements. For example, clinical trials of AAV gene therapy targeting CFTR for cystic fibrosis have shown significant improvements in the lung function and quality of life in patients [124]. Additionally, promising results have been seen in preclinical studies using CRISPR–Cas9 gene editing for cystic fibrosis and other respiratory diseases [125]. However, it is important to acknowledge that there are still problems in this field, including the necessity for enhanced delivery techniques and thorough investigation of the potential risks linked to genome editing. Further research and development are imperative to answer these questions [126].
Gene therapy approaches for respiratory diseases remain to be optimized. This includes the development of more efficient and targeted delivery methods such as aerosolized nanoparticles for lung-specific delivery [127]. The natural wrapping property of exosomes can protect genetic material from degradation and attack by the immune system, making it an excellent carrier [128]. Additionally, research is exploring the use of gene therapy in combination with other therapies, such as stem cell therapy, to enhance therapeutic efficacy [129]. Furthermore, ethical considerations surrounding genome editing, including potential unintended effects and the need for informed consent, require continued discussion and investigation.
Gene therapy offers the potential for long-lasting effects compared with traditional pharmacological treatments. Ongoing research is needed to optimize the safety and efficacy of gene therapy approaches and to address the limitations and ethical concerns associated with this promising therapeutic approach.
6. Personalized Medicine
Personalized medicine is an approach to healthcare that considers individual variability in genes, environment, and lifestyle for the prevention, diagnosis, and treatment of diseases. In the context of respiratory diseases, personalized medicine aims to tailor treatment strategies to the unique needs of patients based on their genetic and molecular characteristics as well as other clinical and environmental factors [8]. Implementing this approach has the power not only to enhance patient outcomes but also to alleviate healthcare costs.
Personalized medicine has several advantages for patients with chronic inflammatory respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD). By identifying biomarkers and other factors (like serum immunoglobulins, sputum microbiome, and prognostic imaging biomarkers) that contribute to disease progression and exacerbation, physicians can develop more targeted treatment plans that minimize side effects and maximize efficacy [130]. For example, some patients with severe asthma may benefit from biological therapies targeting specific cytokines or immune cells. Additionally, personalized medicine may enable early identification of patients at risk for disease progression or exacerbation, allowing proactive interventions to prevent severe symptoms and hospitalizations.
Current research in personalized medicine for respiratory diseases is focused on identifying biomarkers and developing diagnostic tools to better classify patients based on their underlying disease mechanisms. For example, studies have identified gene expression profiles associated with different subtypes of asthma and COPD [131,132]. Additionally, researchers are exploring the use of wearable sensors and other technologies to monitor patient symptoms and disease activity in real time, enabling more timely interventions and adjustments to treatment plans.
The latest advancements in personalized medicine for respiratory diseases have exhibited promising outcomes, showcasing improved patient well-being and the capacity for cost savings within the healthcare system. For example, a study of biomarker-guided asthma management found significant reductions in asthma exacerbation and healthcare utilization compared with standard care [133]. However, there are still limitations to the implementation of personalized medicine in clinical practice, such as the cost and availability of diagnostic tests and therapies, as well as ethical considerations surrounding the use of genetic information in treatment decisions [134].
Improving the accuracy and accessibility of diagnostic tests and expanding the range of targeted therapies available to patients are key points of personalized medicine for respiratory diseases. For example, researchers are exploring the use of artificial intelligence and machine learning algorithms to better predict patient outcomes and identify optimal treatment strategies [135]. Additionally, studies are investigating the potential benefits of combining multiple targeted therapies for patients with complex disease mechanisms. Furthermore, ongoing discussions around ethical and regulatory issues will continue to shape the development and implementation of personalized medicine in clinical practice.
In conclusion, personalized medicine allows treatment plans to be more targeted, effective, and tailored to individual patient needs. Ongoing research is needed to address the limitations and ethical considerations associated with this approach and to optimize the accuracy and accessibility of diagnostic tests and therapies. Due to its high heterogeneity, personalized healthcare needs to be organically combined with various other therapies to revitalize the lungs of patients with chronic inflammatory diseases (Figure 3).
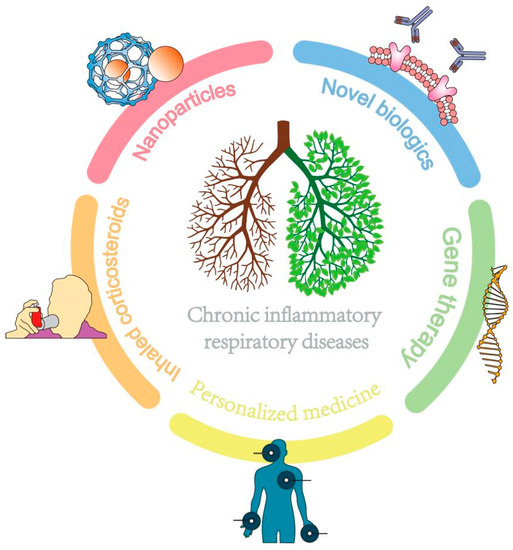
Figure 3.
The combination of multiple therapies benefits patients with chronic inflammatory lung diseases.
7. Conclusions
Targeted drug delivery systems, including nanoparticle-based systems, ICSs, novel biologicals, gene therapy, and personalized medicine, hold great promise for the treatment of chronic inflammatory respiratory diseases. Ongoing research focuses on developing new delivery systems that can specifically target lung cells while minimizing systemic side effects. Furthermore, novel biological targets and innovative methods for delivering biologicals to the lungs are also being explored. Gene therapy approaches, including viral-vector-based delivery systems and CRISPR–Cas9 technology, show potential for treating respiratory diseases. Personalized medicine approaches could improve treatment outcomes by tailoring therapies to individuals based on their unique characteristics. Finally, combining different drug delivery systems, such as using organ-specific nanoparticles to deliver gene-targeting drugs according to disease subtypes, can further enhance drug efficacy. The utilization of an exosome-based vector system, which efficiently and specifically delivers mRNA or CRISPR–Cas9 plasmids to target cells, also holds promise for targeted gene therapy both in vitro and in vivo.
The clinical implications of these advancements are significant, as targeted drug delivery systems have the potential to improve patient outcomes and reduce healthcare costs. Healthcare professionals should consider integrating these approaches into their practice as they become more widely available. However, there is still a considerable journey from the laboratory bench to clinical application. Hence, additional research is needed to refine and optimize these approaches for maximum effectiveness. It is important to address safety concerns related to nanoparticle-based delivery systems and gene therapy as well as to develop improved methods for delivering biologicals to the lungs. Moreover, identifying optimal personalized medicine approaches is of paramount importance to ensure that treatments align with the specific demands and characteristics of individual patients.
To sum up, the use of targeted drug delivery systems represents a promising approach to the treatment of chronic inflammatory respiratory diseases. Further research is required to fine-tune and optimize these approaches as well as to identify the most effective personalized medicine strategies. For instance, utilizing biological models that closely resemble the human lung environment, such as lung organoids, can better reflect the effect of new drug delivery systems. Given the clinical heterogeneity of chronic inflammatory pulmonary disease, machine learning methods offer distinct advantages in calculating personalized treatment plans and predicting treatment outcomes in advance, leveraging the patient’s phenotype, subphenotype, and internal characteristics. Furthermore, defining refined subtypes of chronic inflammatory lung diseases based on multiple omics features can better capture the unique characteristics of each patient. Ultimately, the goal is to improve patient outcomes and reduce healthcare costs by delivering treatments that are tailored to individual patient needs.
Author Contributions
J.W.: Responsible for data collection and analysis and writing the initial draft of the manuscript. P.W.: Involved in the revision and supplementation of the manuscript. Y.S.: Involved in data interpretation; contributed to the revision and editing of the manuscript. D.H.: Responsible for guiding and organizing the study. Involved in data interpretation and made important revisions and edits to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Shanghai Municipal Health Commission (Grant No. 202040174); Science and Technology Commission of Shanghai Municipality (Grant No. 22Y11900800); Core Discipline Improvement Project, 3-Year (2020–2022) Action Plan of Shanghai Public Health System Development (GWV-10.1-XK26); Class A, Core Medicine Discipline Improvement Project of Jinshan District (JSZK2019A01); and Discipline Platform Improvement Project, 3-Year Talents Echelon Action Plan of Jinshan Hospital, Fudan University (XPT-2020-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support from the Shanghai Municipal Health Commission; the Science and Technology Commission of Shanghai Municipality; the Center of Emergency and Critical Medicine, Jinshan Hospital of Fudan University; the Key Laboratory of Chemical Injury, Emergency and Critical Medicine of Shanghai Municipal Health Commission; and the Research Center for Chemical Injury, Emergency and Critical Medicine of Fudan University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2021, 59, 2102730. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Raherison, C.; Girodet, P.-O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in asthma: New understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Christenson, S.A.; Smith, S.A.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17074. [Google Scholar] [CrossRef]
- Porsbjerg, C.M.; Sverrild, A.; Lloyd, C.M.; Menzies-Gow, A.N.; Bel, E.H. Anti-alarmins in asthma: Targeting the airway epithelium with next-generation biologics. Eur. Respir. J. 2020, 56, 2000260. [Google Scholar] [CrossRef]
- Brightling, C.; Greening, N. Airway inflammation in COPD: Progress to precision medicine. Eur. Respir. J. 2019, 54, 1900651. [Google Scholar] [CrossRef]
- Wang, J.; Hu, K.; Cai, X.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin. B 2022, 12, 18–32. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Nano-delivery to the lung—By inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, J.; Wu, J.; Suk, J.S. Enhancing nanoparticle penetration through airway mucus to improve drug delivery efficacy in the lung. Expert Opin. Drug Deliv. 2021, 18, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Yang, G.; Choi, E.; Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 2022, 10, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.-A. Budesonide/Glycopyrronium/Formoterol: A Review in COPD. Drugs 2021, 81, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.M.; Dixon, A.E.; Krishnan, J.A.; Lemanske, R.F.; Pace, W.; Schatz, M. Managing Asthma in Adolescents and Adults: 2020 Asthma Guideline Update from the National Asthma Education and Prevention Program. JAMA 2020, 324, 2301–2317. [Google Scholar] [CrossRef]
- Werder, R.B.; Liu, T.; Abo, K.M.; Lindstrom-Vautrin, J.; Villacorta-Martin, C.; Huang, J.; Hinds, A.; Boyer, N.; Bullitt, E.; Liesa, M.; et al. CRISPR interference interrogation of COPD GWAS genes reveals the functional significance of desmoplakin in iPSC-derived alveolar epithelial cells. Sci. Adv. 2022, 8, eabo6566. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Wouters, E.F.; Wouters, B.B.; Augustin, I.M.; Houben-Wilke, S.; Vanfleteren, L.E.; Franssen, F.M. Personalised pulmonary rehabilitation in COPD. Eur. Respir. Rev. 2018, 27, 170125. [Google Scholar] [CrossRef]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef]
- Castaldi, P.J.; Yun, J.; Estepar, R.S.J.; Ross, J.C.; Cho, M.H.; Hersh, C.P.; Kinney, G.L.; Young, K.A.; Regan, E.A.; Lynch, D.A.; et al. Machine Learning Characterization of COPD Subtypes. Chest 2020, 157, 1147–1157. [Google Scholar] [CrossRef]
- Passi, M.; Shahid, S.; Chockalingam, S.; Sundar, I.K.; Packirisamy, G. Conventional and Nanotechnology Based Approaches to Combat Chronic Obstructive Pulmonary Disease: Implications for Chronic Airway Diseases. Int. J. Nanomed. 2020, 15, 3803–3826. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Im, J.; Nho, R.S. Advanced Therapeutic Strategies for Chronic Lung Disease Using Nanoparticle-Based Drug Delivery. J. Clin. Med. 2016, 5, 82. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Thorley, A.J.; Tetley, T.D. New perspectives in nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef]
- Pohlit, H.; Bellinghausen, I.; Frey, H.; Saloga, J. Recent advances in the use of nanoparticles for allergen-specific immunotherapy. Allergy 2017, 72, 1461–1474. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Low, P.S. Targeting of Nanoparticles: Folate Receptor. Cancer Nanotechnol. 2010, 624, 249–265. [Google Scholar] [CrossRef]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Xu, H.; Yao, Q.; Cai, C.; Gou, J.; Zhang, Y.; Zhong, H.; Tang, X. Amphiphilic poly(amino acid) based micelles applied to drug delivery: The in vitro and in vivo challenges and the corresponding potential strategies. J. Control. Release 2015, 199, 84–97. [Google Scholar] [CrossRef]
- Sahib, M.; Darwis, Y.; Peh, K.K.; Abdulameer, S.; Tan, Y.T.F. Rehydrated sterically stabilized phospholipid nanomicelles of budesonide for nebulization: Physicochemical characterization and in vitro, in vivo evaluations. Int. J. Nanomed. 2011, 6, 2351–2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Q.; Huang, T.; Ling, D.; Gao, J. New Insights into Biocompatible Iron Oxide Nanoparticles: A Potential Booster of Gene Delivery to Stem Cells. Small 2020, 16, e2001588. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 1–29. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Halwani, R.; Shaik, A.S.; Ratemi, E.; Afzal, S.; Kenana, R.; Al-Muhsen, S.; Al Faraj, A. A novel anti-IL4Rα nanoparticle efficiently controls lung inflammation during asthma. Exp. Mol. Med. 2016, 48, e262. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, W.; Wang, H.; Yue, J.; Mao, Y.; Zhou, W.; Kong, X.; Guo, Q.; Zhang, L.; Xu, P.; et al. Anti-ST2 Nanoparticle Alleviates Lung Inflammation by Targeting ILC2s-CD4+T Response. Int. J. Nanomed. 2020, 15, 9745–9758. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Zuo, X.; Guo, X.; Gu, Y.; Zheng, H.; Zhou, Z.; Wang, X.; Jiang, S.; Wang, G.; Xu, C.; Wang, F. Recent Advances in Nanomaterials for Asthma Treatment. Int. J. Mol. Sci. 2022, 23, 14427. [Google Scholar] [CrossRef]
- Soares, D.C.F.; Domingues, S.C.; Viana, D.B.; Tebaldi, M.L. Polymer-hybrid nanoparticles: Current advances in biomedical applications. Biomed. Pharmacother. 2020, 131, 110695. [Google Scholar] [CrossRef]
- Pei, W.; Li, X.; Bi, R.; Zhang, X.; Zhong, M.; Yang, H.; Zhang, Y.; Lv, K. Exosome membrane-modified M2 macrophages targeted nanomedicine: Treatment for allergic asthma. J. Control. Release 2021, 338, 253–267. [Google Scholar] [CrossRef]
- Dauletbaev, N.; Cammisano, M.; Herscovitch, K.; Lands, L.C. Stimulation of the RIG-I/MAVS Pathway by Polyinosinic:Polycytidylic Acid Upregulates IFN-β in Airway Epithelial Cells with Minimal Costimulation of IL-8. J. Immunol. 2015, 195, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Costabile, G.; Durantie, E.; Brocca, P.; Rondelli, V.; Russo, A.; Russo, G.; Miro, A.; Quaglia, F.; Petri-Fink, A.; et al. Hybrid Lipid/Polymer Nanoparticles for Pulmonary Delivery of siRNA: Development and Fate Upon In Vitro Deposition on the Human Epithelial Airway Barrier. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 170–181. [Google Scholar] [CrossRef]
- Dormenval, C.; Lokras, A.; Cano-Garcia, G.; Wadhwa, A.; Thanki, K.; Rose, F.; Thakur, A.; Franzyk, H.; Foged, C. Identification of Factors of Importance for Spray Drying of Small Interfering RNA-Loaded Lipidoid-Polymer Hybrid Nanoparticles for Inhalation. Pharm. Res. 2019, 36, 142. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.-Q. Inhaled siRNA nanoparticles targeting IL11 inhibit lung fibrosis and improve pulmonary function post-bleomycin challenge. Sci. Adv. 2022, 8, eabn7162. [Google Scholar] [CrossRef]
- Tagalakis, A.D.; McAnulty, R.J.; Devaney, J.; Bottoms, S.E.; Wong, J.B.; Elbs, M.; Writer, M.J.; Hailes, H.C.; Tabor, A.B.; O’Callaghan, C.; et al. A Receptor-targeted Nanocomplex Vector System Optimized for Respiratory Gene Transfer. Mol. Ther. 2008, 16, 907–915. [Google Scholar] [CrossRef]
- Blank, F.; Fytianos, K.; Seydoux, E.; Rodriguez-Lorenzo, L.; Petri-Fink, A.; von Garnier, C.; Rothen-Rutishauser, B. Interaction of biomedical nanoparticles with the pulmonary immune system. J. Nanobiotechnol. 2017, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Lee, Y.; Kim, M.; Lee, S.; Jon, S.; Lee, S.-H. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials 2017, 140, 37–44. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, V.; Kim, K.-H.; Yip, A.C.; Sohn, J. Environmental impacts of nanomaterials. J. Environ. Manag. 2018, 225, 261–271. [Google Scholar] [CrossRef]
- Ali, M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv. Colloid Interface Sci. 2023, 314, 102881. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-H.; Chen, Y.; Zha, H.-X.; Song, L.-J.; Li, C.-Y.; Li, J.-Q.; Xia, X.-H. Determination, characterization and cytotoxicity on HELF cells of ZnO nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, A.; Mudunkotuwa, I.A.; Geary, S.M.; Morris, A.S.; Mapuskar, K.A.; Spitz, D.R.; Grassian, V.H.; Salem, A.K. Size-dependent cytotoxicity of copper oxide nanoparticles in lung epithelial cells. Environ. Sci. Nano 2016, 3, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Cheng, H.; Cui, Z.; Guo, S.; Zhang, X.; Huo, Y.; Mao, S. Mucoadhesive versus mucopenetrating nanoparticles for oral delivery of insulin. Acta Biomater. 2021, 135, 506–519. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- da Silva, A.L.; de Oliveira, G.P.; Kim, N.; Cruz, F.F.; Kitoko, J.Z.; Blanco, N.G.; Martini, S.V.; Hanes, J.; Rocco, P.R.M.; Suk, J.S.; et al. Nanoparticle-based thymulin gene therapy therapeutically reverses key pathology of experimental allergic asthma. Sci. Adv. 2020, 6, eaay7973. [Google Scholar] [CrossRef]
- Hou, C.; Bai, H.; Wang, Z.; Qiu, Y.; Kong, L.-L.; Sun, F.; Wang, D.; Yin, H.; Zhang, X.; Mu, H.; et al. A hyaluronan-based nanosystem enables combined anti-inflammation of mTOR gene silencing and pharmacotherapy. Carbohydr. Polym. 2018, 195, 339–348. [Google Scholar] [CrossRef]
- Kubczak, M.; Michlewska, S.; Bryszewska, M.; Aigner, A.; Ionov, M. Nanoparticles for local delivery of siRNA in lung therapy. Adv. Drug Deliv. Rev. 2021, 179, 114038. [Google Scholar] [CrossRef]
- Latorre, M.; Bacci, E.; Seccia, V.; Bartoli, M.L.; Cardini, C.; Cianchetti, S.; Cristofani, L.; Di Franco, A.; Miccoli, M.; Puxeddu, I.; et al. Upper and lower airway inflammation in severe asthmatics: A guide for a precision biologic treatment. Ther. Adv. Respir. Dis. 2020, 14, 1753466620965151. [Google Scholar] [CrossRef]
- Aalbers, R.; Vogelmeier, C.; Kuna, P. Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respir. Med. 2016, 111, 1–7. [Google Scholar] [CrossRef]
- Evans, D.J.; Kew, K.M.; Anderson, D.E.; Boyter, A.C. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database Syst. Rev. 2015, 2015, CD011437. [Google Scholar] [CrossRef] [PubMed]
- Dorinsky, P.; DePetrillo, P.; DeAngelis, K.; Trivedi, R.; Darken, P.; Gillen, M. Relative Bioavailability of Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Administered with and without a Spacer: Results of a Phase I, Randomized, Crossover Trial in Healthy Adults. Clin. Ther. 2020, 42, 634–648. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.W.; Colice, G.; Grigg, J.; van Aalderen, W.; Martin, R.J.; Israel, E.; Postma, D.S.; Roche, N.; Phipatanakul, W.; Hillyer, E.V.; et al. Real-Life Outcomes for Patients with Asthma Prescribed Spacers for Use with Either Extrafine- or Fine-Particle Inhaled Corticosteroids. J. Allergy Clin. Immunol. Pract. 2017, 5, 1040–1049.e4. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Dekhuijzen, R.; van der Molen, T.; Martin, R.J.; van Aalderen, W.; Roche, N.; Guilbert, T.W.; Israel, E.; van Eickels, D.; Khalid, J.M.; et al. Asthma-Related Outcomes in Patients Initiating Extrafine Ciclesonide or Fine-Particle Inhaled Corticosteroids. Allergy Asthma Immunol. Res. 2017, 9, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sonnappa, S.; McQueen, B.; Postma, D.S.; Martin, R.J.; Roche, N.; Grigg, J.; Guilbert, T.; Gouder, C.; Pizzichini, E.; Niimi, A.; et al. Extrafine Versus Fine Inhaled Corticosteroids in Relation to Asthma Control: A Systematic Review and Meta-Analysis of Observational Real-Life Studies. J. Allergy Clin. Immunol. Pract. 2018, 6, 907–915.e7. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; De Simoni, A.; Wileman, V.; Holliday, L.; Newby, C.J.; Chisari, C.; Ali, S.; Zhu, N.; Padakanti, P.; Pinprachanan, V.; et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Syst. Rev. 2022, 6, CD013030. [Google Scholar] [CrossRef]
- de Boer, A.H.; Hagedoorn, P.; Hoppentocht, M.; Buttini, F.; Grasmeijer, F.; Frijlink, H.W. Dry powder inhalation: Past, present and future. Expert Opin. Drug Deliv. 2016, 14, 499–512. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Pathare, S.R.; More, M.A.; Waykhinde, N.A.; Laddha, U.D.; Salunkhe, K.S.; Kshirsagar, S.J.; Patil, S.S.; Ramteke, K.H. Dry Powder Inhaler with the technical and practical obstacles, and forthcoming platform strategies. J. Control. Release 2023, 355, 292–311. [Google Scholar] [CrossRef]
- Bremner, P.R.; Birk, R.; Brealey, N.; Ismaila, A.S.; Zhu, C.Q.; Lipson, D.A. Single-inhaler fluticasone furoate/umeclidinium/vilanterol versus fluticasone furoate/vilanterol plus umeclidinium using two inhalers for chronic obstructive pulmonary disease: A randomized non-inferiority study. Respir. Res. 2018, 19, 19. [Google Scholar] [CrossRef]
- Chapman, K.R.; Hurst, J.R.; Frent, S.-M.; Larbig, M.; Fogel, R.; Guerin, T.; Banerji, D.; Patalano, F.; Goyal, P.; Pfister, P.; et al. Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.T.; Rabe, K.F.; Martinez, F.J.; Fabbri, L.M.; Wang, C.; Ichinose, M.; Bourne, E.; Ballal, S.; Darken, P.; DeAngelis, K.; et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir. Med. 2018, 6, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-D.; Xie, C.-M.; Yunus, F.; Itoh, Y.; Ling, X.; Yu, W.-C.; Kiatboonsri, S. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: A randomized, multicentre study in East Asia. Respirology 2016, 21, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Barnacle, H.; Birk, R.; Brealey, N.; Locantore, N.; Lomas, D.A.; Ludwig-Sengpiel, A.; Mohindra, R.; Tabberer, M.; Zhu, C.-Q.; et al. FULFIL Trial: Once-Daily Triple Therapy for Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 196, 438–446. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Martin, A.R.; Finlay, W.H. Nebulizers for drug delivery to the lungs. Expert Opin. Drug Deliv. 2015, 12, 889–900. [Google Scholar] [CrossRef]
- Khan, I.; Hussein, S.; Houacine, C.; Sadozai, S.K.; Islam, Y.; Bnyan, R.; Elhissi, A.; Yousaf, S. Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. Int. J. Pharm. 2021, 598, 120376. [Google Scholar] [CrossRef]
- Dhayanandamoorthy, Y.; Antoniraj, M.G.; Kandregula, C.A.B.; Kandasamy, R. Aerosolized hyaluronic acid decorated, ferulic acid loaded chitosan nanoparticle: A promising asthma control strategy. Int. J. Pharm. 2020, 591, 119958. [Google Scholar] [CrossRef]
- Montoro, J.; Antolín-Amérigo, D.; Izquierdo-Domínguez, A.; Zapata, J.; González, G.; Valero, A. Impact of Asthma Inhalers on Global Climate: A Systematic Review of Their Carbon Footprint and Clinical Outcomes in Spain. J. Investig. Allergol. Clin. Immunol. 2023, 33, 250–262. [Google Scholar] [CrossRef]
- Kaur, I.; Aggarwal, B.; Gogtay, J. Integration of dose counters in pressurized metered-dose inhalers for patients with asthma and chronic obstructive pulmonary disease: Review of evidence. Expert Opin. Drug Deliv. 2015, 12, 1301–1310. [Google Scholar] [CrossRef]
- Komalla, V.; Wong, C.Y.J.; Sibum, I.; Muellinger, B.; Nijdam, W.; Chaugule, V.; Soria, J.; Ong, H.X.; Buchmann, N.A.; Traini, D. Advances in soft mist inhalers. Expert Opin. Drug Deliv. 2023. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219. [Google Scholar] [CrossRef] [PubMed]
- Mattishent, K.; Thavarajah, M.; Blanco, P.; Gilbert, D.; Wilson, A.M.; Loke, Y.K. Meta-Review: Adverse Effects of Inhaled Corticosteroids Relevant to Older Patients. Drugs 2014, 74, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Cazzola, M.; Matera, M.G.; Rogliani, P. Adding a LAMA to ICS/LABA Therapy: A Meta-analysis of Triple Combination Therapy in COPD. Chest 2019, 155, 758–770. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Jackson, D.J.; Mauger, D.T.; Boehmer, S.J.; Phipatanakul, W.; Sheehan, W.J.; Moy, J.N.; Paul, I.M.; Bacharier, L.B.; Cabana, M.D.; et al. Individualized therapy for persistent asthma in young children. J. Allergy Clin. Immunol. 2016, 138, 1608–1618.e12. [Google Scholar] [CrossRef]
- Casula, L.; Sinico, C.; Valenti, D.; Pini, E.; Pireddu, R.; Schlich, M.; Lai, F.; Fadda, A.M. Delivery of beclomethasone dipropionate nanosuspensions with an electronic cigarette. Int. J. Pharm. 2021, 596, 120293. [Google Scholar] [CrossRef]
- Brandsma, C.; Berge, M.V.D.; Hackett, T.; Brusselle, G.; Timens, W. Recent advances in chronic obstructive pulmonary disease pathogenesis: From disease mechanisms to precision medicine. J. Pathol. 2020, 250, 624–635. [Google Scholar] [CrossRef]
- Werder, R.B.; Ullah, A.; Rahman, M.M.; Simpson, J.; Lynch, J.P.; Collinson, N.; Rittchen, S.; Rashid, R.B.; Sikder, A.A.; Handoko, H.Y.; et al. Targeting the P2Y13 Receptor Suppresses IL-33 and HMGB1 Release and Ameliorates Experimental Asthma. Am. J. Respir. Crit. Care Med. 2022, 205, 300–312. [Google Scholar] [CrossRef]
- Chantveerawong, T.; Sangkangjanavanich, S.; Chiewchalermsri, C.; Pradubpongsa, P.; Mitthamsiri, W.; Jindarat, S.; Wang, M.; Akdis, M.; Sokolowska, M.; Akdis, C.A.; et al. Increased circulating CRTH2+Tregs are associated with asthma control and exacerbation. Allergy 2022, 77, 681–685. [Google Scholar] [CrossRef]
- Burgess, J.K.; Jonker, M.R.; Berg, M.; Hacken, N.T.H.T.; Meyer, K.B.; Berge, M.v.D.; Nawijn, M.C.; Heijink, I.H. Periostin: Contributor to abnormal airway epithelial function in asthma? Eur. Respir. J. 2021, 57, 2001286. [Google Scholar] [CrossRef]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, M.; Kuna, P. Targeting the PGD2/CRTH2/DP1 Signaling Pathway in Asthma and Allergic Disease: Current Status and Future Perspectives. Drugs 2017, 77, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xiong, Y.; Li, W.; Cui, X.; Cheng, X.; Leng, Q.; He, R. IL-37 inhibits IL-4/IL-13-induced CCL11 production and lung eosinophilia in murine allergic asthma. Allergy 2018, 73, 1642–1652. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Tsai, Y.-G.; Lin, Y.-C.; Hsu, Y.-L.; Chen, Y.-T.; Tsai, M.-K.; Liao, W.-T.; Lin, Y.-C.; Hung, C.-H. IL-25 Induced ROS-Mediated M2 Macrophage Polarization via AMPK-Associated Mitophagy. Int. J. Mol. Sci. 2021, 23, 3. [Google Scholar] [CrossRef]
- Jember, A.G.; Zuberi, R.; Liu, F.T.; Croft, M. Development of Allergic Inflammation in a Murine Model of Asthma Is Dependent on the Costimulatory Receptor Ox40. J. Exp. Med. 2001, 193, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Riemma, M.A.; Cerqua, I.; Romano, B.; Irollo, E.; Bertolino, A.; Camerlingo, R.; Granato, E.; Rea, G.; Scala, S.; Terlizzi, M.; et al. Sphingosine-1-phosphate/TGF-β axis drives epithelial mesenchymal transition in asthma-like disease. Br. J. Pharmacol. 2022, 179, 1753–1768. [Google Scholar] [CrossRef]
- Wang, J.; Lai, X.; Yao, S.; Chen, H.; Cai, J.; Luo, Y.; Wang, Y.; Qiu, Y.; Huang, Y.; Wei, X.; et al. Nestin promotes pulmonary fibrosis via facilitating recycling of TGF-β receptor I. Eur. Respir. J. 2022, 59, 2003721. [Google Scholar] [CrossRef]
- Wollin, L.; Wex, E.; Pautsch, A.; Schnapp, G.; Hostettler, K.E.; Stowasser, S.; Kolb, M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 2015, 45, 1434–1445. [Google Scholar] [CrossRef]
- Aumiller, V.; Balsara, N.; Wilhelm, J.; Günther, A.; Königshoff, M. WNT/β-Catenin Signaling Induces IL-1β Expression by Alveolar Epithelial Cells in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2013, 49, 96–104. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix Metalloproteinases as Therapeutic Targets for Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef]
- Davies, J.C.; Wainwright, C.E.; Canny, G.J.; Chilvers, M.A.; Howenstine, M.S.; Munck, A.; Mainz, J.G.; Rodriguez, S.; Li, H.; Yen, K.; et al. Efficacy and Safety of Ivacaftor in Patients Aged 6 to 11 Years with Cystic Fibrosis with aG551DMutation. Am. J. Respir. Crit. Care Med. 2013, 187, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, X.; Zeng, Y.; Lin, D.; Wu, J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021, 14, 2067–2089. [Google Scholar] [CrossRef]
- Tay, H.L.; Foster, P.S. Biologics or immunotherapeutics for asthma? Pharmacol. Res. 2020, 158, 104782. [Google Scholar] [CrossRef]
- Wollin, L.; Distler, J.H.; Redente, E.F.; Riches, D.W.H.; Stowasser, S.; Schlenker-Herceg, R.; Maher, T.M.; Kolb, M. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur. Respir. J. 2019, 54, 1900161. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Adcock, I.M.; Benito-Villalvilla, C.; Bianchini, R.; Bjermer, L.; Caramori, G.; Cari, L.; Chung, K.F.; Diamant, Z.; Eguiluz-Gracia, I.; et al. Comparing biologicals and small molecule drug therapies for chronic respiratory diseases: An EAACI Taskforce on Immunopharmacology position paper. Allergy 2019, 74, 432–448. [Google Scholar] [CrossRef]
- Häußermann, S.; Arendsen, L.J.; Pritchard, J.N. Smart dry powder inhalers and intelligent adherence management. Adv. Drug Deliv. Rev. 2022, 191, 114580. [Google Scholar] [CrossRef] [PubMed]
- Drazen, J.M.; Harrington, D. New Biologics for Asthma. N. Engl. J. Med. 2018, 378, 2533–2534. [Google Scholar] [CrossRef]
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Celli, B.R.; Wechsler, M.E.; Abdulai, R.M.; Luo, X.; Boomsma, M.M.; Staudinger, H.; Horowitz, J.E.; Baras, A.; Ferreira, M.A.; et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: A genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir. Med. 2021, 9, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Ghivizzani, S.C. Viral vectors for gene therapy. Pharmacol. Ther. 1998, 80, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Ping, Y. Delivery of genome-editing biomacromolecules for treatment of lung genetic disorders. Adv. Drug Deliv. Rev. 2021, 168, 196–216. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Chen, J.-C.; Chen, L.-C.; Kuo, M.-L. Adeno-Associated Virus-Mediated Interleukin-12 Gene Expression Alleviates Lung Inflammation and Type 2 T-Helper-Responses in Ovalbumin-Sensitized Asthmatic Mice. Hum. Gene Ther. 2022, 33, 1052–1061. [Google Scholar] [CrossRef]
- Do, D.C.; Mu, J.; Ke, X.; Sachdeva, K.; Qin, Z.; Wan, M.; Ishmael, F.T.; Gao, P. miR-511-3p protects against cockroach allergen–induced lung inflammation by antagonizing CCL2. J. Clin. Investig. 2019, 4, 126832. [Google Scholar] [CrossRef]
- Li, R.; Wang, F.; Wei, J.; Lin, Y.; Tang, G.; Rao, L.; Ma, L.; Xu, Q.; Wu, J.; Lv, Q.; et al. The Role of Macrophage Migration Inhibitory Factor (MIF) in Asthmatic Airway Remodeling. Allergy Asthma Immunol. Res. 2021, 13, 88–105. [Google Scholar] [CrossRef]
- Ustiugova, A.S.; Dvorianinova, E.M.; Melnikova, N.V.; Dmitriev, A.A.; Kuprash, D.V.; Afanasyeva, M.A. CRISPR/Cas9 genome editing demonstrates functionality of the autoimmunity-associated SNP rs12946510. Biochim. Biophys. Acta 2023, 1869, 166599. [Google Scholar] [CrossRef]
- Shen, S.; Sanchez, M.E.; Blomenkamp, K.; Corcoran, E.M.; Marco, E.; Yudkoff, C.J.; Jiang, H.; Teckman, J.H.; Bumcrot, D.; Albright, C.F. Amelioration of Alpha-1 Antitrypsin Deficiency Diseases with Genome Editing in Transgenic Mice. Hum. Gene Ther. 2018, 29, 861–873. [Google Scholar] [CrossRef]
- Zieger, M.; Borel, F.; Greer, C.; Gernoux, G.; Blackwood, M.; Flotte, T.R.; Mueller, C. Liver-directed SERPINA1 gene therapy attenuates progression of spontaneous and tobacco smoke-induced emphysema in α1-antitrypsin null mice. Mol. Ther. Methods Clin. Dev. 2022, 25, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Porritt, M.J.; Ericson, E.; Taheri-Ghahfarokhi, A.; Clausen, M.; Magnusson, L.; Admyre, T.; Nitsch, R.; Mayr, L.; Aasehaug, L.; et al. Therapeutic Genome Editing with CRISPR/Cas9 in a Humanized Mouse Model Ameliorates α1-antitrypsin Deficiency Phenotype. Ebiomedicine 2018, 29, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Thornell, I.M.; Singh, B.K.; Shah, V.S.; Stoltz, D.A.; McCray, P.B.; Zabner, J.; Sinn, P.L. A Novel AAV-mediated Gene Delivery System Corrects CFTR Function in Pigs. Am. J. Respir. Cell Mol. Biol. 2019, 61, 747–754. [Google Scholar] [CrossRef]
- Moss, R.B.; Milla, C.; Colombo, J.; Accurso, F.; Zeitlin, P.L.; Clancy, J.P.; Spencer, L.T.; Pilewski, J.; Waltz, D.A.; Dorkin, H.L.; et al. Repeated Aerosolized AAV-CFTR for Treatment of Cystic Fibrosis: A Randomized Placebo-Controlled Phase 2B Trial. Hum. Gene Ther. 2007, 18, 726–732. [Google Scholar] [CrossRef]
- Geurts, M.H.; de Poel, E.; Amatngalim, G.D.; Oka, R.; Meijers, F.M.; Kruisselbrink, E.; van Mourik, P.; Berkers, G.; Groot, K.M.d.W.-D.; Michel, S.; et al. CRISPR-Based Adenine Editors Correct Nonsense Mutations in a Cystic Fibrosis Organoid Biobank. Cell Stem Cell 2020, 26, 503–510.e7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S.; Chakraborty, C. CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol. Ther. 2021, 29, 571–586. [Google Scholar] [CrossRef]
- Zimmermann, C.M.; Baldassi, D.; Chan, K.; Adams, N.B.; Neumann, A.; Porras-Gonzalez, D.L.; Wei, X.; Kneidinger, N.; Stoleriu, M.G.; Burgstaller, G.; et al. Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J. Control. Release 2022, 351, 137–150. [Google Scholar] [CrossRef]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.C.; Amatullah, H.; Vaswani, C.M.; Maron-Gutierrez, T.; Kim, M.; Mei, S.H.; Szaszi, K.; Monteiro, A.P.T.; Varkouhi, A.K.; Herreroz, R.; et al. Mesenchymal Stromal (stem) Cell (MSC) therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur. Respir. J. 2022, 59, 2004216. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Obeidat, M.; Sadatsafavi, M.; Sin, D.D. Introduction to precision medicine in COPD. Eur. Respir. J. 2019, 53, 1802460. [Google Scholar] [CrossRef]
- Ramsheh, M.Y.; Haldar, K.; Esteve-Codina, A.; Purser, L.F.; Richardson, M.; Müller-Quernheim, J.; Greulich, T.; Nowinski, A.; Barta, I.; Stendardo, M.; et al. Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: A bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe 2021, 2, e300–e310. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.-J.; Cant, E.; Keir, H.R.; Barton, A.K.; Kuzmanova, E.; Shuttleworth, M.; Pollock, J.; Finch, S.; Polverino, E.; Bottier, M.; et al. Endotyping Chronic Obstructive Pulmonary Disease, Bronchiectasis, and the “Chronic Obstructive Pulmonary Disease–Bronchiectasis Association”. Am. J. Respir. Crit. Care Med. 2022, 206, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Porsbjerg, C.M.; Robijn, A.L.; Gibson, P.G. Biomarker-guided management reduces exacerbations in non-eosinophilic asthma in pregnancy: A secondary analysis of a randomized controlled trial. Respirology 2020, 25, 719–725. [Google Scholar] [CrossRef]
- Malinovschi, A. Limited use of biomarker-guided therapy in mild asthma. Lancet Respir. Med. 2020, 8, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Cao, H.; FitzGerald, J.M.; Iannotti, N.; Yang, E.; Kocks, J.W.H.; Kostikas, K.; Price, D.; Reddel, H.K.; Tsiligianni, I.; et al. Artificial Intelligence/Machine Learning in Respiratory Medicine and Potential Role in Asthma and COPD Diagnosis. J. Allergy Clin. Immunol. Pract. 2021, 9, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).