Cationic Calix[4]arene Vectors to Efficiently Deliver AntimiRNA Peptide Nucleic Acids (PNAs) and miRNA Mimics
Abstract
1. Introduction
2. Materials and Methods
2.1. Calix[4]arene Vectors Synthesis and Resuspension
2.2. Cell Lines and Culture Conditions
2.3. Transfection Procedures
2.4. FACS Analysis
2.5. RNA Extraction
2.6. Quantification of miRNAs
2.7. MTT Assay
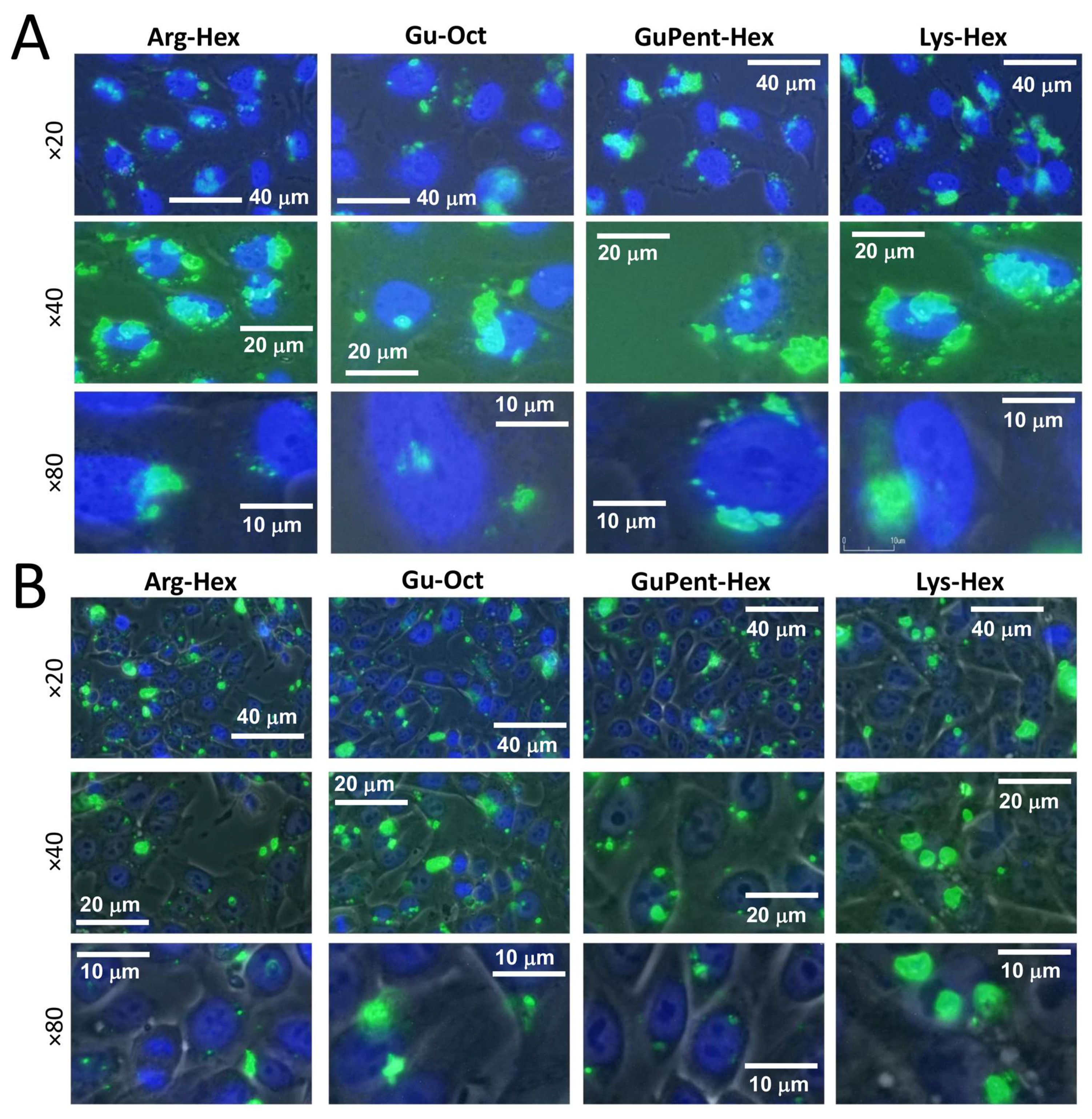
2.8. Cell Imaging
2.9. Statistical Analysis
3. Results
3.1. Design of the Calix[4]arene Vectors
3.2. Employment of Calix[4]arene Vectors for the Delivery of antimiRNA Peptide Nucleic Acids (PNAs)
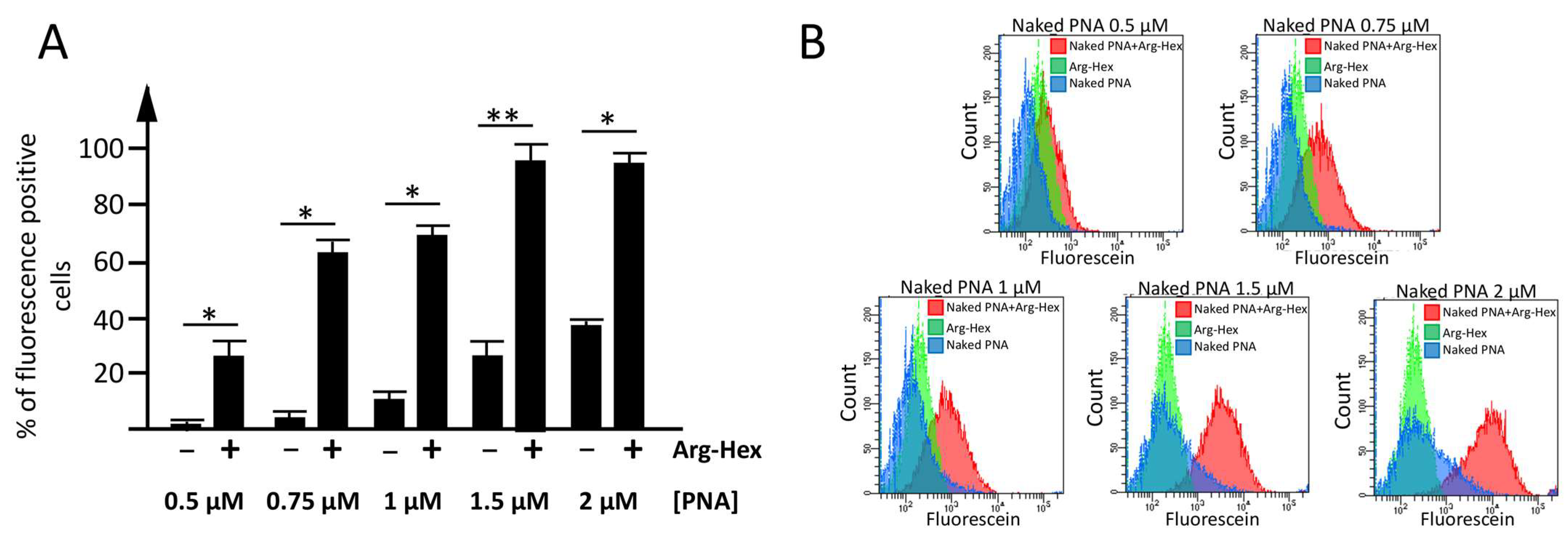
3.2.1. Assessment of the Optimal Transfection Conditions
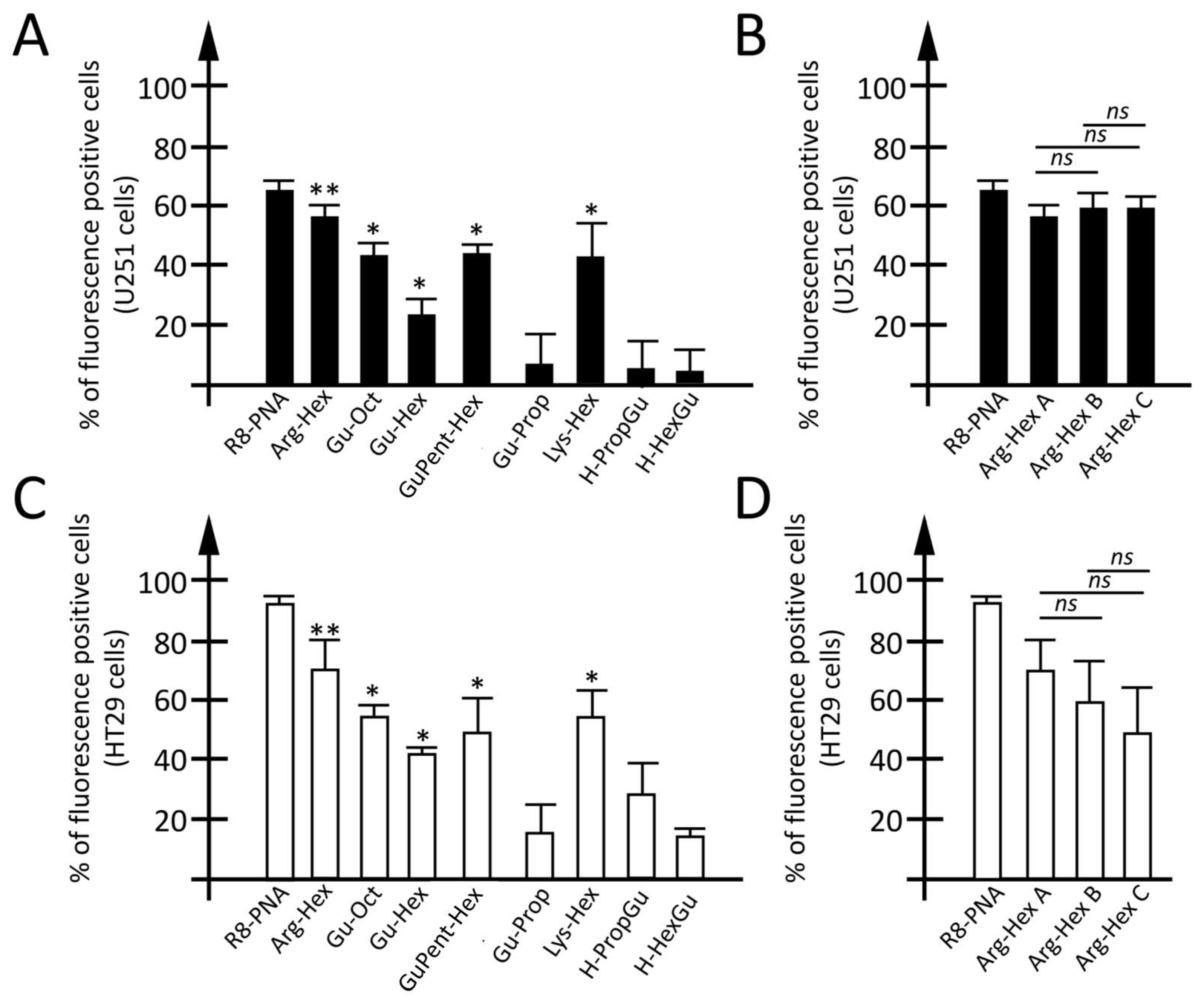
3.2.2. Employment of Calix[4]arene Vectors for the Delivery of PNA
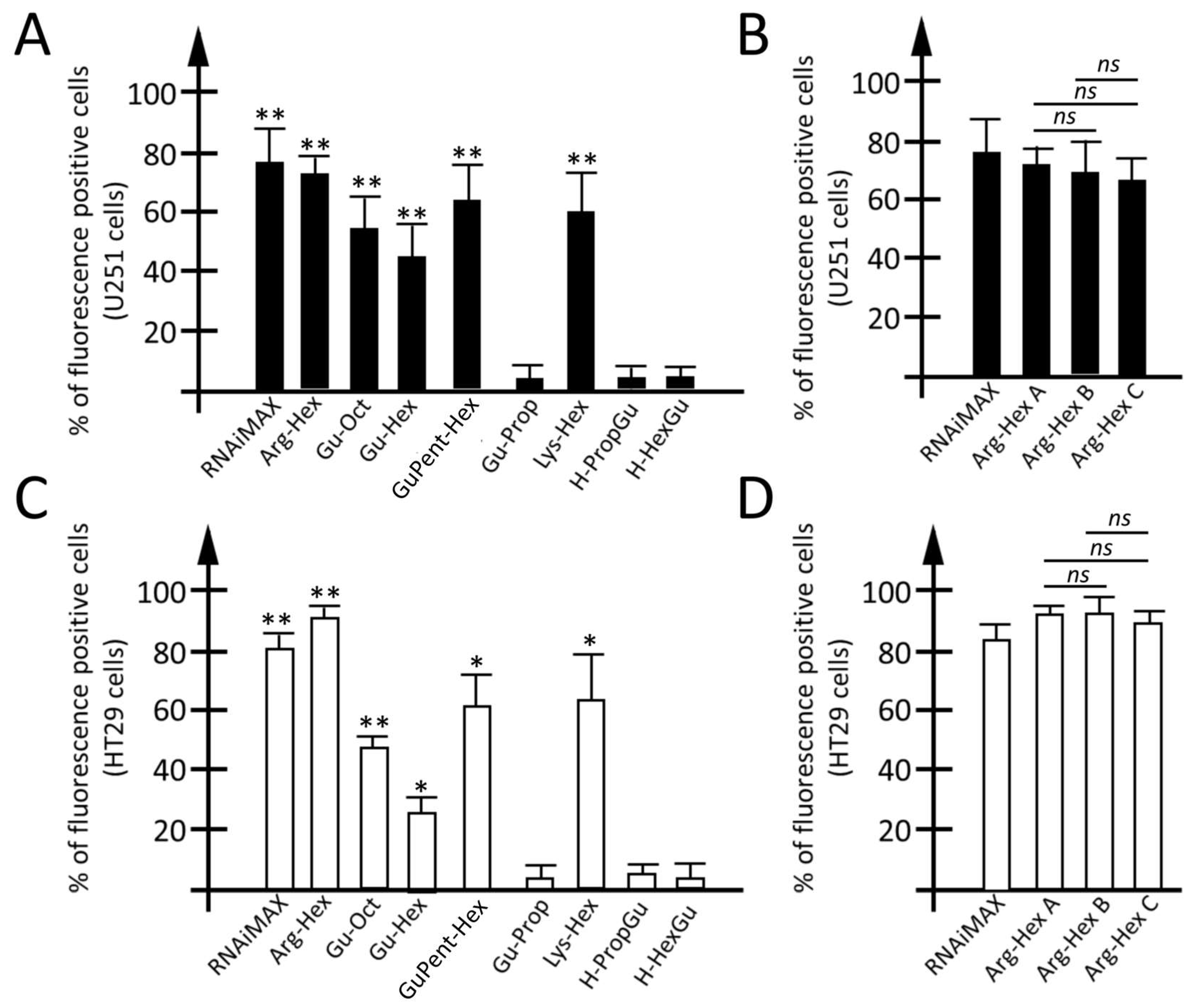
3.3. Employment of Calix[4]arene Vectors for the Delivery of miRNA Molecules
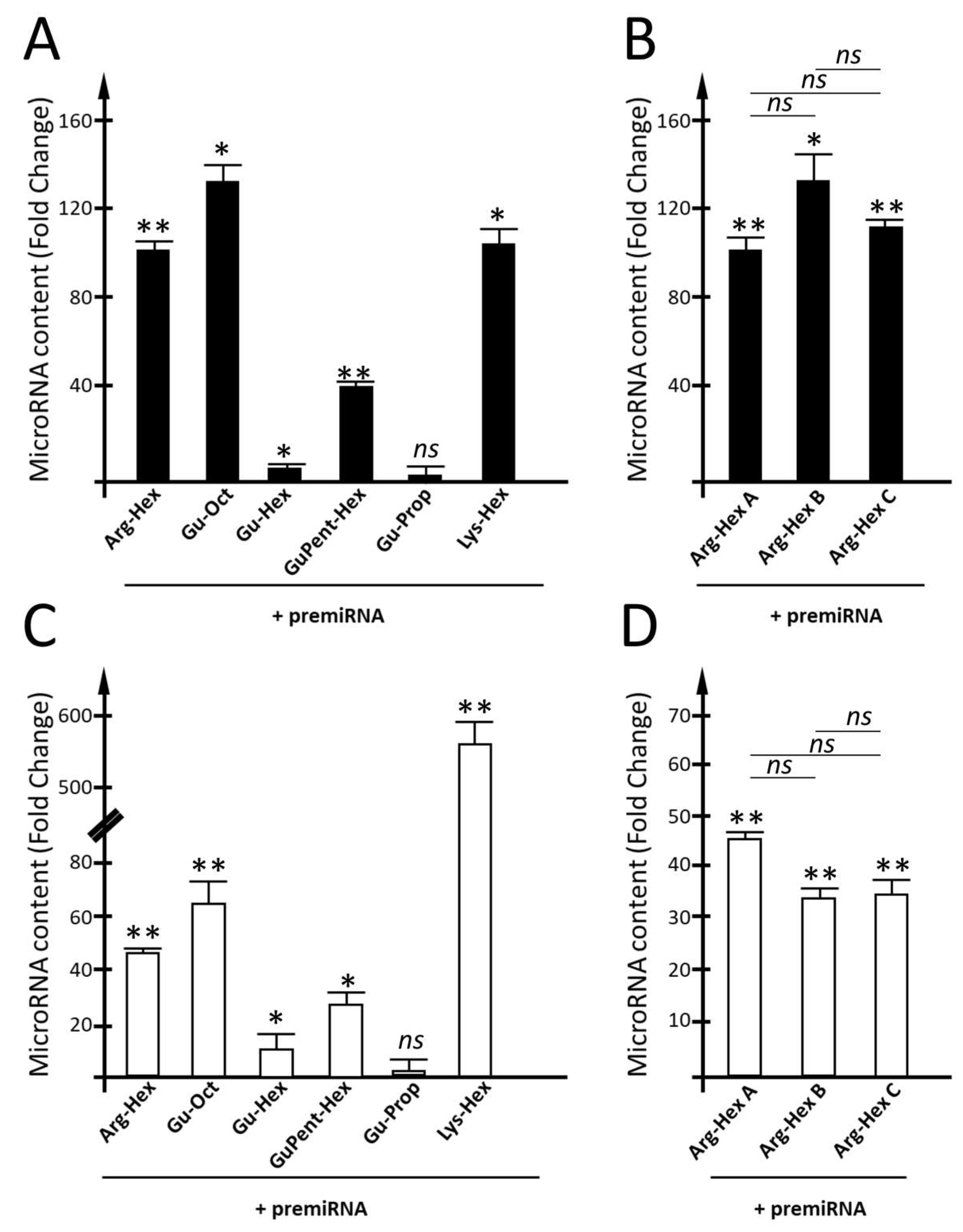
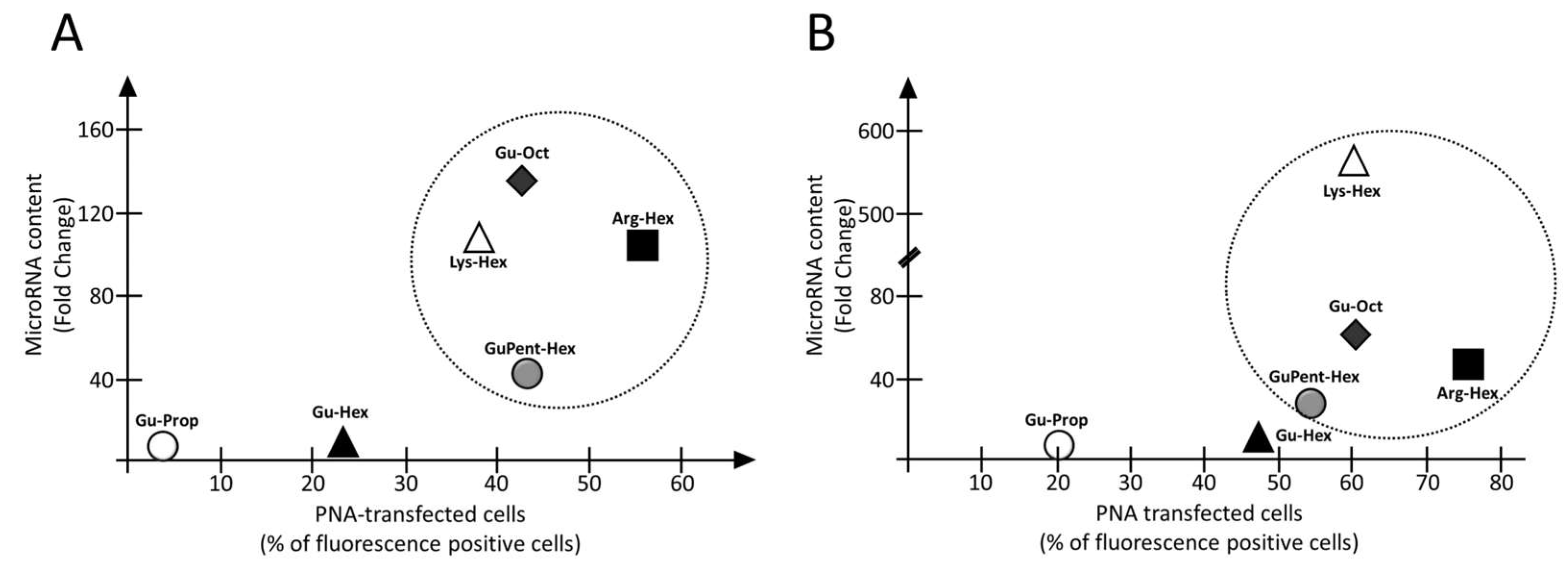
3.4. Employment of Calix[4]arene Vectors for the Delivery of premiRNA Molecules
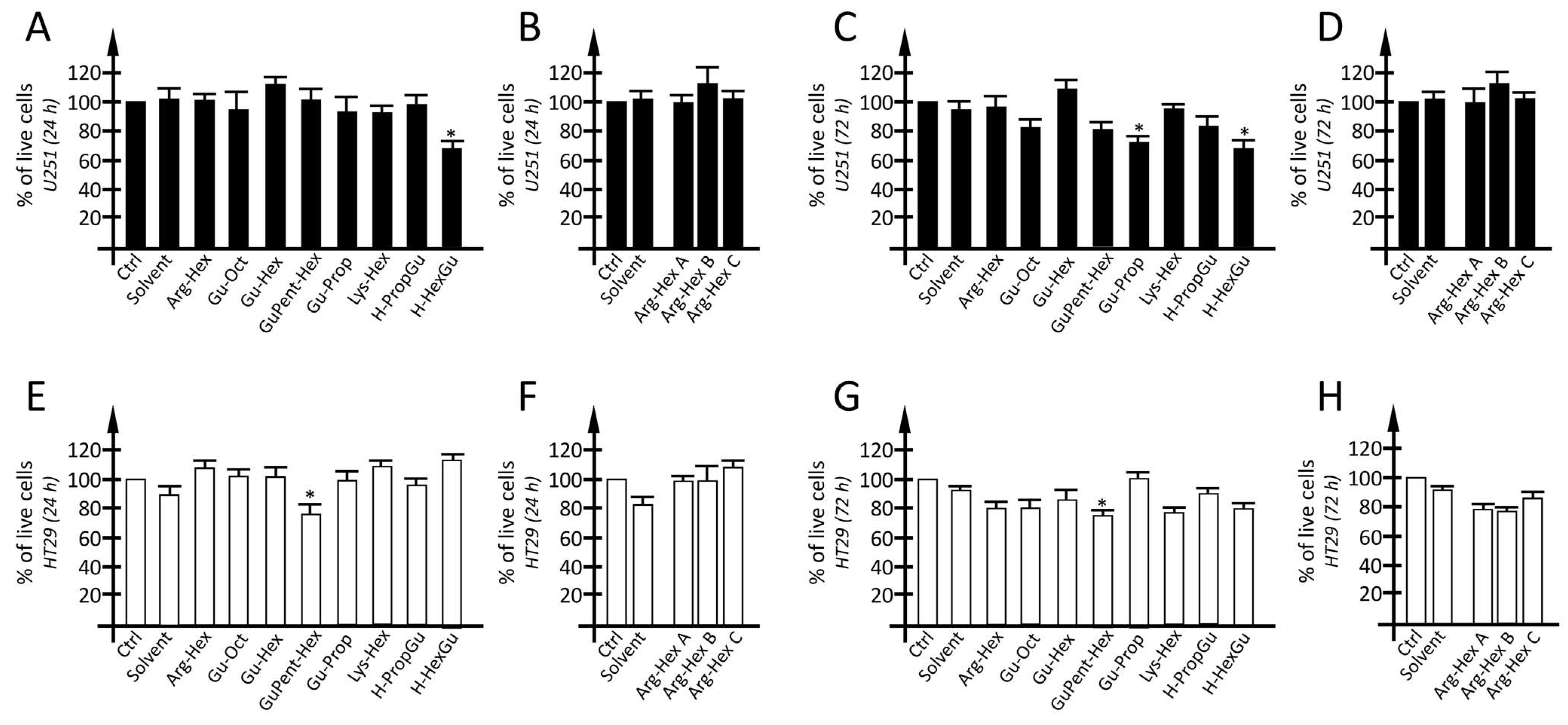
3.5. Evaluation of Calix[4]arene Vectors Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, J.A.; Ventura, A. The biological functions of miRNAs: Lessons from in vivo studies. Trends Cell Biol. 2015, 25, 137–147. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; Macrae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Iorio, M.V.; Croce, C.M. Causes and Consequences of MicroRNA Dysregulation. Cancer J. 2012, 18, 215–222. [Google Scholar] [CrossRef]
- Melo, S.A.; Esteller, M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett. 2011, 585, 2087–2099. [Google Scholar] [CrossRef]
- He, L.; Zhang, F.; Zhu, Y.; Lu, M. A crosstalk between circular RNA, microRNA, and messenger RNA in the development of various brain cognitive disorders. Front. Mol. Neurosci. 2022, 15, 960657. [Google Scholar] [CrossRef]
- Sugai, T.; Sugimoto, R.; Eizuka, M.; Osakabe, M.; Yamada, S.; Yanagawa, N.; Matsumoto, T.; Suzuki, H. Comprehensive Analysis of microRNA Expression During the Progression of Colorectal Tumors. Dig. Dis. Sci. 2022, 68, 813–823. [Google Scholar] [CrossRef]
- Reid, G.; Kao, S.C.; Pavlakis, N.; Brahmbhatt, H.; MacDiarmid, J.; Clarke, S.; Boyer, M.; van Zandwijk, N. Clinical development of TargomiRs, a miRNA mimic-based treatment for patients with recurrent thoracic cancer. Epigenomics 2016, 8, 1079–1085. [Google Scholar] [CrossRef]
- Reda El Sayed, S.; Cristante, J.; Guyon, L.; Denis, J.; Chabre, O.; Cherradi, N. MicroRNA Therapeutics in Cancer: Current Advances and Challenges. Cancers 2021, 13, 2680. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, L.; Li, S.; Chen, F.; Au-Yeung, K.K.-W.; Shi, C. MicroRNA as an Important Target for Anticancer Drug Development. Front. Pharmacol. 2021, 12, 736323. [Google Scholar] [CrossRef]
- Lima, J.F.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Anti-miRNA oligonucleotides: A comprehensive guide for design. RNA Biol. 2018, 15, 338–352. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Jopling, C.L. Targeting microRNA-122 to Treat Hepatitis C Virus Infection. Viruses 2010, 2, 1382–1393. [Google Scholar] [CrossRef]
- Kunden, R.D.; Khan, J.Q.; Ghezelbash, S.; Wilson, J.A. The Role of the Liver-Specific microRNA, miRNA-122 in the HCV Replication Cycle. Int. J. Mol. Sci. 2020, 21, 5677. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Catalano, D.; Talis, A.; Szabo, G.; Bala, S. Protective effect of LNA-anti-miR-132 therapy on liver fibrosis in mice. Mol. Ther. Nucleic Acids 2021, 25, 155–167. [Google Scholar] [CrossRef]
- Smith, C.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; Rebhan, M.A.E.; Crivelli, S.E.M.; Denzler, R.; Stoffel, M.; Hall, J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014, 42, 609–621. [Google Scholar] [CrossRef]
- Stelma, F.; van der Ree, M.H.; Sinnige, M.J.; Brown, A.; Swadling, L.; de Vree, J.M.L.; Willemse, S.B.; van der Valk, M.; Grint, P.; Neben, S.; et al. Immune phenotype and function of natural killer and T cells in chronic hepatitis C patients who received a single dose of anti-MicroRNA-122, RG-101. Hepatology 2017, 66, 57–68. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef]
- Saarbach, J.; Sabale, P.M.; Winssinger, N. Peptide nucleic acid (PNA) and its applications in chemical biology, diagnostics, and therapeutics. Curr. Opin. Chem. Biol. 2019, 52, 112–124. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, A.; Puri, N. Peptide nucleic acids: Advanced tools for biomedical applications. J. Biotechnol. 2017, 259, 148–159. [Google Scholar] [CrossRef]
- Pierce, J.B.; Zhou, H.; Simion, V.; Feinberg, M.W. Long Noncoding RNAs as Therapeutic Targets. Adv. Exp. Med. Biol. 2022, 1363, 161–175. [Google Scholar] [CrossRef]
- Wang, Y.; Malik, S.; Suh, H.-W.; Xiao, Y.; Deng, Y.; Fan, R.; Huttner, A.; Bindra, R.S.; Singh, V.; Saltzman, W.M.; et al. Anti-seed PNAs targeting multiple oncomiRs for brain tumor therapy. Sci. Adv. 2023, 9, eabq7459. [Google Scholar] [CrossRef]
- Proshkina, G.; Shramova, E.; Ryabova, A.; Katrivas, L.; Giannini, C.; Malpicci, D.; Levi-Kalisman, Y.; Deyev, S.; Kotlyar, A. Novel Small Multilamellar Liposomes Containing Large Quantities of Peptide Nucleic Acid Selectively Kill Breast Cancer Cells. Cancers 2022, 14, 4806. [Google Scholar] [CrossRef]
- Cesaro, E.; Falanga, A.P.; Catapano, R.; Greco, F.; Romano, S.; Borbone, N.; Pastore, A.; Marzano, M.; Chiurazzi, F.; D’Errico, S.; et al. Exploring a peptide nucleic acid-based antisense approach for CD5 targeting in chronic lymphocytic leukemia. PLoS ONE 2022, 17, e0266090. [Google Scholar] [CrossRef]
- Gasparello, J.; Gambari, L.; Papi, C.; Rozzi, A.; Manicardi, A.; Corradini, R.; Gambari, R.; Finotti, A. High Levels of Apoptosis Are Induced in the Human Colon Cancer HT-29 Cell Line by Co-Administration of Sulforaphane and a Peptide Nucleic Acid Targeting miR-15b-5p. Nucleic Acid Ther. 2020, 30, 164–174. [Google Scholar] [CrossRef]
- Brognara, E.; Fabbri, E.; Montagner, G.; Gasparello, J.; Manicardi, A.; Corradini, R.; Bianchi, N.; Finotti, A.; Breveglieri, G.; Borgatti, M.; et al. High levels of apoptosis are induced in human glioma cell lines by co-administration of peptide nucleic acids targeting miR-221 and miR-222. Int. J. Oncol. 2016, 48, 1029–1038. [Google Scholar] [CrossRef]
- Fabbri, E.; Tamanini, A.; Jakova, T.; Gasparello, J.; Manicardi, A.; Corradini, R.; Sabbioni, G.; Finotti, A.; Borgatti, M.; Lampronti, I.; et al. A Peptide Nucleic Acid against MicroRNA miR-145-5p Enhances the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Calu-3 Cells. Molecules 2017, 23, 71. [Google Scholar] [CrossRef]
- Gupta, A.; Quijano, E.; Liu, Y.; Bahal, R.; Scanlon, S.E.; Song, E.; Hsieh, W.-C.; Braddock, D.E.; Ly, D.H.; Saltzman, W.M.; et al. Anti-tumor Activity of miniPEG-γ-Modified PNAs to Inhibit MicroRNA-210 for Cancer Therapy. Mol. Ther. Nucleic Acids 2017, 9, 111–119. [Google Scholar] [CrossRef]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhaas, J.B.; Saltzman, W.M.; Slack, F.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, E1695–E1704. [Google Scholar] [CrossRef]
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018, 183, 428–444. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Kao, S.C.; Fulham, M.; Wong, K.; Cooper, W.; Brahmbhatt, H.; MacDiarmid, J.; Pattison, S.; Sagong, J.O.; Huynh, Y.; Leslie, F.; et al. A Significant Metabolic and Radiological Response after a Novel Targeted MicroRNA-based Treatment Approach in Malignant Pleural Mesothelioma. Am. J. Respir. Crit. Care Med. 2015, 191, 1467–1469. [Google Scholar] [CrossRef]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Javed, N.; Rahman, J.U.; Abu-Izneid, T.; Alam Khan, F. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin. Cancer Biol. 2021, 69, 391–398. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Simonson, B.; Das, S. MicroRNA Therapeutics: The Next Magic Bullet? Mini-Rev. Med. Chem. 2015, 15, 467–474. [Google Scholar] [CrossRef]
- Abd-Aziz, N.; Kamaruzman, N.I.; Poh, C.L. Development of MicroRNAs as Potential Therapeutics against Cancer. J. Oncol. 2020, 2020, 8029721. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam, R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kelin, A.V.; Milstein, S.; et al. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef]
- Matsuda, S.; Keiser, K.; Nair, J.K.; Charisse, K.; Manoharan, R.M.; Kretschmer, P.; Peng, C.G.; Kelin, A.V.; Kandasamy, P.; Willoughby, J.L.; et al. siRNA Conjugates Carrying Sequentially Assembled Trivalent N-Acetylgalactosamine Linked Through Nucleosides Elicit Robust Gene Silencing In Vivo in Hepatocytes. ACS Chem. Biol. 2015, 10, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Samarsky, D.; Liu, L.; Xu, Q.; Zhang, W.; Zhu, G.; Wu, P.; Zuo, X.; Deng, H.; et al. Tumor-targeted in vivo gene silencing via systemic delivery of cRGD-conjugated siRNA. Nucleic Acids Res. 2014, 42, 11805–11817. [Google Scholar] [CrossRef]
- Ämmälä, C.; Drury, W.J.; Knerr, L.; Ahlstedt, I.; Stillemark-Billton, P.; Wennberg-Huldt, C.; Andersson, E.-M.; Valeur, E.; Jansson-Löfmark, R.; Janzén, D.; et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 2018, 4, eaat3386. [Google Scholar] [CrossRef]
- Alam, M.R.; Ming, X.; Fisher, M.; Lackey, J.G.; Rajeev, K.G.; Manoharan, M.; Juliano, R.L. Multivalent Cyclic RGD Conjugates for Targeted Delivery of Small Interfering RNA. Bioconjugate Chem. 2011, 22, 1673–1681. [Google Scholar] [CrossRef]
- Klein, A.F.; Varela, M.A.; Arandel, L.; Holland, A.; Naouar, N.; Arzumanov, A.; Seoane, D.; Revillod, L.; Bassez, G.; Ferry, A.; et al. Peptide-conjugated oligonucleotides evoke long-lasting myotonic dystrophy correction in patient-derived cells and mice. J. Clin. Investig. 2019, 129, 4739–4744. [Google Scholar] [CrossRef]
- Tushir-Singh, J. Antibody-siRNA conjugates: Drugging the undruggable for anti-leukemic therapy. Expert Opin. Biol. Ther. 2017, 17, 325–338. [Google Scholar] [CrossRef]
- Song, E.; Zhu, P.; Lee, S.-K.; Chowdhury, D.; Kussman, S.; Dykxhoorn, D.M.; Feng, Y.; Palliser, D.; Weiner, D.B.; Shankar, P.; et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005, 23, 709–717. [Google Scholar] [CrossRef]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004, 432, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Hadwiger, P.; John, M.; Vornlocher, H.-P.; Unverzagt, C. Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells. Bioorganic Med. Chem. Lett. 2004, 14, 4975–4977. [Google Scholar] [CrossRef] [PubMed]
- Bost, J.P.; Barriga, H.; Holme, M.N.; Gallud, A.; Maugeri, M.; Gupta, D.; Lehto, T.; Valadi, H.; Esbjörner, E.K.; Stevens, M.M.; et al. Delivery of Oligonucleotide Therapeutics: Chemical Modifications, Lipid Nanoparticles, and Extracellular Vesicles. ACS Nano 2021, 15, 13993–14021. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.J.; Cheng, C.J.; Quijano, E.; Weller, C.; Kristofik, N.; Duong, N.; Hoimes, C.; Egan, M.E.; Saltzman, W.M. Surface modified poly(β amino ester)-containing nanoparticles for plasmid DNA delivery. J. Control Release 2012, 164, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef]
- Volpi, S.; Cancelli, U.; Neri, M.; Corradini, R. Multifunctional Delivery Systems for Peptide Nucleic Acids. Pharmaceuticals 2020, 14, 14. [Google Scholar] [CrossRef]
- Gupta, A.; Bahal, R.; Gupta, M.; Glazer, P.M.; Saltzman, W.M. Nanotechnology for delivery of peptide nucleic acids (PNAs). J. Control Release 2016, 240, 302–311. [Google Scholar] [CrossRef]
- Malik, S.; Asmara, B.; Moscato, Z.; Mukker, J.K.; Bahal, R. Advances in Nanoparticle-based Delivery of Next Generation Peptide Nucleic Acids. Curr. Pharm. Des. 2018, 24, 5164–5174. [Google Scholar] [CrossRef]
- Pooga, M.; Soomets, U.; Hällbrink, M.; Valkna, A.; Saar, K.; Rezaei, K.; Kahl, U.; Hao, J.-X.; Xu, X.-J.; Wiesenfeld-Hallin, Z.; et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 1998, 16, 857–861. [Google Scholar] [CrossRef]
- Rogers, F.A.; Lin, S.S.; Hegan, D.C.; Krause, D.S.; Glazer, P.M. Targeted Gene Modification of Hematopoietic Progenitor Cells in Mice Following Systemic Administration of a PNA-peptide Conjugate. Mol. Ther. 2012, 20, 109–118. [Google Scholar] [CrossRef]
- Tan, X.; Bruchez, M.P.; Armitage, B.A. Closing the Loop: Constraining TAT Peptide by γPNA Hairpin for Enhanced Cellular Delivery of Biomolecules. Bioconjugate Chem. 2018, 29, 2892–2898. [Google Scholar] [CrossRef]
- Kauffman, W.B.; Guha, S.; Wimley, W.C. Synthetic molecular evolution of hybrid cell penetrating peptides. Nat. Commun. 2018, 9, 2568. [Google Scholar] [CrossRef] [PubMed]
- Ndeboko, B.; Ramamurthy, N.; Lemamy, G.J.; Jamard, C.; Nielsen, P.E.; Cova, L. Role of Cell-Penetrating Peptides in Intracellular Delivery of Peptide Nucleic Acids Targeting Hepadnaviral Replication. Mol. Ther. Nucleic Acids 2017, 9, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.M.; Kim, M.H.; Yu, G.S.; Um, B.H.; Park, H.K.; Lee, H.-I.; Lee, K.T.; Suh, Y.D.; Choi, J.S. Enhanced splicing correction effect by an oligo-aspartic acid–PNA conjugate and cationic carrier complexes. J. Control Release 2014, 175, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ljungstrøm, T.; Knudsen, H.; Nielsen, P.E. Cellular Uptake of Adamantyl Conjugated Peptide Nucleic Acids. Bioconjugate Chem. 1999, 10, 965–972. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, K.; Shen, G.; Wooley, K.L.; Taylor, J.-S.A. Cationic Shell-Cross-Linked Knedel-like (cSCK) Nanoparticles for Highly Efficient PNA Delivery. Mol. Pharm. 2009, 6, 615–626. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, K.; Wooley, K.L.; Taylor, J.-S. Imaging mRNA Expression in Live Cells via PNA·DNA Strand Displacement-Activated Probes. J. Nucleic Acids 2012, 2012, 962652. [Google Scholar] [CrossRef]
- Shen, Y.; Shrestha, R.; Ibricevic, A.; Gunsten, S.P.; Welch, M.J.; Wooley, K.L.; Brody, S.L.; Taylor, J.-S.A.; Liu, Y. Antisense peptide nucleic acid-functionalized cationic nanocomplex for in vivo mRNA detection. Interface Focus 2013, 3, 20120059. [Google Scholar] [CrossRef]
- Cheng, C.J.; Saltzman, W.M. Polymer Nanoparticle-Mediated Delivery of MicroRNA Inhibition and Alternative Splicing. Mol. Pharm. 2012, 9, 1481–1488. [Google Scholar] [CrossRef]
- Bahal, R.; Quijano, E.; McNeer, N.A.; Liu, Y.; Bhunia, D.C.; Lopez-Giraldez, F.; Fields, R.J.; Saltzman, W.M.; Ly, D.H.; Glazer, P.M. Single-Stranded γPNAs for In Vivo Site-Specific Genome Editing via Watson-Crick Recognition. Curr. Gene Ther. 2014, 14, 331–342. [Google Scholar] [CrossRef]
- McNeer, N.A.; Schleifman, E.B.; Cuthbert, A.; Brehm, M.; Jackson, A.; Cheng, C.; Anandalingam, K.; Kumar, P.; Shultz, L.D.; Greiner, D.L.; et al. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene Ther. 2013, 20, 658–669. [Google Scholar] [CrossRef]
- Ricciardi, A.S.; Bahal, R.; Farrelly, J.S.; Quijano, E.; Bianchi, A.H.; Luks, V.L.; Putman, R.; López-Giráldez, F.; Coşkun, S.; Song, E.; et al. In utero nanoparticle delivery for site-specific genome editing. Nat. Commun. 2018, 9, 2481. [Google Scholar] [CrossRef] [PubMed]
- Schleifman, E.B.; McNeer, N.A.; Jackson, A.; Yamtich, J.; Brehm, M.A.; Shultz, L.D.; Greiner, D.L.; Kumar, P.; Saltzman, W.M.; Glazer, P.M. Site-specific Genome Editing in PBMCs with PLGA Nanoparticle-delivered PNAs Confers HIV-1 Resistance in Humanized Mice. Mol. Ther. Nucleic Acids 2013, 2, e135. [Google Scholar] [CrossRef] [PubMed]
- McNeer, N.A.; Anandalingam, K.; Fields, R.J.; Caputo, C.; Kopic, S.; Gupta, A.; Quijano, E.; Polikoff, L.; Kong, Y.; Bahal, R.; et al. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat. Commun. 2015, 6, 6952. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Prasetyanto, E.A.; Septiadi, D.; Manicardi, A.; Brognara, E.; Gambari, R.; Corradini, R.; De Cola, L. Combined Delivery of Temozolomide and Anti-miR221 PNA Using Mesoporous Silica Nanoparticles Induces Apoptosis in Resistant Glioma Cells. Small 2015, 11, 5687–5695. [Google Scholar] [CrossRef]
- Ma, X.; Devi, G.; Qu, Q.; Toh, D.-F.K.; Chen, G.; Zhao, Y. Intracellular Delivery of Antisense Peptide Nucleic Acid by Fluorescent Mesoporous Silica Nanoparticles. Bioconjugate Chem. 2014, 25, 1412–1420. [Google Scholar] [CrossRef]
- Beavers, K.R.; Mares, J.W.; Swartz, C.M.; Zhao, Y.; Weiss, S.M.; Duvall, C.L. In Situ Synthesis of Peptide Nucleic Acids in Porous Silicon for Drug Delivery and Biosensing. Bioconjugate Chem. 2014, 25, 1192–1197. [Google Scholar] [CrossRef]
- Beavers, K.R.; Werfel, T.A.; Shen, T.; Kavanaugh, T.E.; Kilchrist, K.V.; Mares, J.W.; Fain, J.S.; Wiese, C.B.; Vickers, K.C.; Weiss, S.M.; et al. Porous Silicon and Polymer Nanocomposites for Delivery of Peptide Nucleic Acids as Anti-MicroRNA Therapies. Adv. Mater. 2016, 28, 7984–7992. [Google Scholar] [CrossRef]
- Kelly, I.B., 3rd; Fletcher, R.B.; McBride, J.R.; Weiss, S.M.; Duvall, C.L. Tuning Composition of Polymer and Porous Silicon Composite Nanoparticles for Early Endosome Escape of Anti-microRNA Peptide Nucleic Acids. ACS Appl. Mater. Interfaces 2020, 12, 39602–39611. [Google Scholar] [CrossRef]
- Neri, M.; Kang, J.; Zuidema, J.M.; Gasparello, J.; Finotti, A.; Gambari, R.; Sailor, M.J.; Bertucci, A.; Corradini, R. Tuning the Loading and Release Properties of MicroRNA-Silencing Porous Silicon Nanoparticles by Using Chemically Diverse Peptide Nucleic Acid Payloads. ACS Biomater. Sci. Eng. 2022, 8, 4123–4131. [Google Scholar] [CrossRef]
- Bagnacani, V.; Franceschi, V.; Bassi, M.; Lomazzi, M.; Donofrio, G.; Sansone, F.; Casnati, A.; Ungaro, R. Arginine clustering on calix[4]arene macrocycles for improved cell penetration and DNA delivery. Nat. Commun. 2013, 4, 1721–1727. [Google Scholar] [CrossRef]
- Gasparello, J.; Lomazzi, M.; Papi, C.; D’aversa, E.; Sansone, F.; Casnati, A.; Donofrio, G.; Gambari, R.; Finotti, A. Efficient Delivery of MicroRNA and AntimiRNA Molecules Using an Argininocalix[4]arene Macrocycle. Mol. Ther. Nucleic Acids 2019, 18, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Gasparello, J.; Manicardi, A.; Casnati, A.; Corradini, R.; Gambari, R.; Finotti, A.; Sansone, F. Efficient cell penetration and delivery of peptide nucleic acids by an argininocalix[4]arene. Sci. Rep. 2019, 9, 3036. [Google Scholar] [CrossRef] [PubMed]
- Finotti, A.; Gasparello, J.; Casnati, A.; Corradini, R.; Gambari, R.; Sansone, F. Delivery of Peptide Nucleic Acids Using an Argininocalix[4]arene as Vector. Methods Mol. Biol. 2021, 2211, 123–143. [Google Scholar] [CrossRef]
- Wang, T.; Larcher, L.M.; Ma, L.; Veedu, R.N. Systematic Screening of Commonly Used Commercial Transfection Reagents towards Efficient Transfection of Single-Stranded Oligonucleotides. Molecules 2018, 23, 2564. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Dudič, M.; Donofrio, G.; Rivetti, C.; Baldini, L.; Casnati, A.; Cellai, S.; Ungaro, R. DNA Condensation and Cell Transfection Properties of Guanidinium Calixarenes: Dependence on Macrocycle Lipophilicity, Size, and Conformation. J. Am. Chem. Soc. 2006, 128, 14528–14536. [Google Scholar] [CrossRef] [PubMed]
- Bagnacani, V.; Sansone, F.; Donofrio, G.; Baldini, L.; Casnati, A.; Ungaro, R. Macrocyclic Nonviral Vectors: High Cell Transfection Efficiency and Low Toxicity in a Lower Rim Guanidinium Calix[4]arene. Org. Lett. 2008, 10, 3953–3956. [Google Scholar] [CrossRef]
- Bagnacani, V.; Franceschi, V.; Fantuzzi, L.; Casnati, A.; Donofrio, G.; Sansone, F.; Ungaro, R. Lower Rim Guanidinocalix[4]arenes: Macrocyclic Nonviral Vectors for Cell Transfection. Bioconjugate Chem. 2012, 23, 993–1002. [Google Scholar] [CrossRef]
- Lomazzi, M.; Franceschi, V.; Bagnacani, V.; Vezzoni, C.A.; Donofrio, G.; Casnati, A.; Sansone, F. A Structure-Activity Investigation on Modified Analogues of an Argininocalixarene Based Non-viral Gene Vector. Eur. J. Org. Chem. 2021, 2021, 4076–4087. [Google Scholar] [CrossRef]
- Morbioli, I.; Casnati, A.; Esko, J.D.; Tor, Y.; Sansone, F. Calixarene-decorated liposomes for intracellular cargo delivery. Org. Biomol. Chem. 2021, 19, 6598–6602. [Google Scholar] [CrossRef]
- Gasparello, J.; Papi, C.; Zurlo, M.; Gambari, L.; Rozzi, A.; Manicardi, A.; Corradini, R.; Gambari, R.; Finotti, A. Treatment of Human Glioblastoma U251 Cells with Sulforaphane and a Peptide Nucleic Acid (PNA) Targeting miR-15b-5p: Synergistic Effects on Induction of Apoptosis. Molecules 2022, 27, 1299. [Google Scholar] [CrossRef]
- Brognara, E.; Fabbri, E.; Bazzoli, E.; Montagner, G.; Ghimenton, C.; Eccher, A.; Cantù, C.; Manicardi, A.; Bianchi, N.; Finotti, A.; et al. Uptake by human glioma cell lines and biological effects of a peptide nucleic acids targeting miR-221. J. Neurooncol. 2014, 118, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lülf, H.; Bertucci, A.; Septiadi, D.; Corradini, R.; De Cola, L. Multifunctional Inorganic Nanocontainers for DNA and Drug Delivery into Living Cells. Chem. Eur. J. 2014, 20, 10900–10904. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, M.; Shiraishi, T.; Nielsen, P.E. Cooperative Cellular Uptake and Activity of Octaarginine Antisense Peptide Nucleic acid (PNA) Conjugates. Biomolecules 2019, 9, 554. [Google Scholar] [CrossRef]
- Fuchs, S.M.; Raines, R.T. Pathway for Polyarginine Entry into Mammalian Cells. Biochemistry 2004, 43, 2438–2444. [Google Scholar] [CrossRef]
- Yi, T. Identifying RISC Components Using Ago2 Immunoprecipitation and Mass Spectrometry. Methods Mol. Biol. 2018, 1720, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Ghavami, M.; Nielsen, P.E. In Vitro Cellular Delivery of Peptide Nucleic Acid (PNA). Methods Mol. Biol. 2020, 2105, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Chen, B.-C.; Han, J.-C.; Wei, L.; Pan, X.-B. Delivery of cell-penetrating peptide-peptide nucleic acid conjugates by assembly on an oligonucleotide scaffold. Sci. Rep. 2015, 5, 17640. [Google Scholar] [CrossRef]
- Schlosser, K.; Taha, M.; Deng, Y.; Stewart, D.J. Systemic delivery of MicroRNA mimics with polyethylenimine elevates pulmonary microRNA levels, but lacks pulmonary selectivity. Pulm. Circ. 2017, 8, 2045893217750613. [Google Scholar] [CrossRef]
- Kapadia, C.H.; Luo, B.; Dang, M.N.; Irvin-Choy, N.; Valcourt, D.M.; Day, E.S. Polymer nanocarriers for MicroRNA delivery. J. Appl. Polym. Sci. 2020, 137, 48651. [Google Scholar] [CrossRef]
- Papi, C.; Gasparello, J.; Zurlo, M.; Manicardi, A.; Corradini, R.; Cabrini, G.; Gambari, R.; Finotti, A. Combined Treatment of Bronchial Epithelial Calu-3 Cells with Peptide Nucleic Acids Targeting miR-145-5p and miR-101-3p: Synergistic Enhancement of the Expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Gene. Int. J. Mol. Sci. 2022, 23, 9348. [Google Scholar] [CrossRef]
- Zurlo, M.; Romagnoli, R.; Oliva, P.; Gasparello, J.; Finotti, A.; Gambari, R. Synergistic effects of the combined treatment of U251 and T98G glioma cells with an anti-tubulin tetrahydrothieno[2,3-c]pyridine derivative and a peptide nucleic acid targeting miR-221-3p. Int. J. Oncol. 2021, 59, 61. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Yari, N.; Jamnani, F.R.; Mahdian, R.; Karimi, M.; Zeinali, S.; Rafiee, M.H.; Azizi, M. The role of miRNA-377 as a tumor suppressor in lung cancer by negative regulation of genes belonging to ErbB signaling pathway. Mol. Biol. Rep. 2022, 49, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Fabbri, E.; Santangelo, A.; Bezzerri, V.; Cantù, C.; Di Gennaro, G.; Finotti, A.; Ghimenton, C.; Eccher, A.; Dechecchi, M.; et al. miRNA array screening reveals cooperative MGMT-regulation between miR-181d-5p and miR-409-3p in glioblastoma. Oncotarget 2016, 7, 28195–28206. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Borgatti, M.; Montagner, G.; Bianchi, N.; Finotti, A.; Lampronti, I.; Bezzerri, V.; Dechecchi, M.C.; Cabrini, G.; Gambari, R. Expression of microRNA-93 and Interleukin-8 during Pseudomonas aeruginosa–Mediated Induction of Proinflammatory Responses. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1144–1155. [Google Scholar] [CrossRef]
- Gasparello, J.; D’Aversa, E.; Breveglieri, G.; Borgatti, M.; Finotti, A.; Gambari, R. In vitro induction of interleukin-8 by SARS-CoV-2 Spike protein is inhibited in bronchial epithelial IB3-1 cells by a miR-93-5p agomiR. Int. Immunopharmacol. 2021, 101, 108201. [Google Scholar] [CrossRef] [PubMed]
- Movahed, M.G.; Ali, A.A.; Ghazanfari, T.; Modaresi, M. Cytokine patterns in cystic fibrosis patients with different microbial infections in oropharyngeal samples. Cytokine 2022, 160, 156038. [Google Scholar] [CrossRef]
- Williamson, M.; Casey, M.; Gabillard-Lefort, C.; Alharbi, A.; Teo, Y.Q.J.; McElvaney, N.G.; Reeves, E.P. Current evidence on the effect of highly effective CFTR modulation on interleukin-8 in cystic fibrosis. Expert Rev. Respir. Med. 2022, 16, 43–56. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The Role of Interleukin-8 in Lung Inflammation and Injury: Implications for the Management of COVID-19 and Hyperinflammatory Acute Respiratory Distress Syndrome. Front. Pharmacol. 2022, 12, 808797. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparello, J.; Papi, C.; Zurlo, M.; Volpi, S.; Gambari, R.; Corradini, R.; Casnati, A.; Sansone, F.; Finotti, A. Cationic Calix[4]arene Vectors to Efficiently Deliver AntimiRNA Peptide Nucleic Acids (PNAs) and miRNA Mimics. Pharmaceutics 2023, 15, 2121. https://doi.org/10.3390/pharmaceutics15082121
Gasparello J, Papi C, Zurlo M, Volpi S, Gambari R, Corradini R, Casnati A, Sansone F, Finotti A. Cationic Calix[4]arene Vectors to Efficiently Deliver AntimiRNA Peptide Nucleic Acids (PNAs) and miRNA Mimics. Pharmaceutics. 2023; 15(8):2121. https://doi.org/10.3390/pharmaceutics15082121
Chicago/Turabian StyleGasparello, Jessica, Chiara Papi, Matteo Zurlo, Stefano Volpi, Roberto Gambari, Roberto Corradini, Alessandro Casnati, Francesco Sansone, and Alessia Finotti. 2023. "Cationic Calix[4]arene Vectors to Efficiently Deliver AntimiRNA Peptide Nucleic Acids (PNAs) and miRNA Mimics" Pharmaceutics 15, no. 8: 2121. https://doi.org/10.3390/pharmaceutics15082121
APA StyleGasparello, J., Papi, C., Zurlo, M., Volpi, S., Gambari, R., Corradini, R., Casnati, A., Sansone, F., & Finotti, A. (2023). Cationic Calix[4]arene Vectors to Efficiently Deliver AntimiRNA Peptide Nucleic Acids (PNAs) and miRNA Mimics. Pharmaceutics, 15(8), 2121. https://doi.org/10.3390/pharmaceutics15082121






