Dendritic Structures Functionalized with Boron Clusters, in Particular Carboranes, and Their Biological Properties †
Abstract
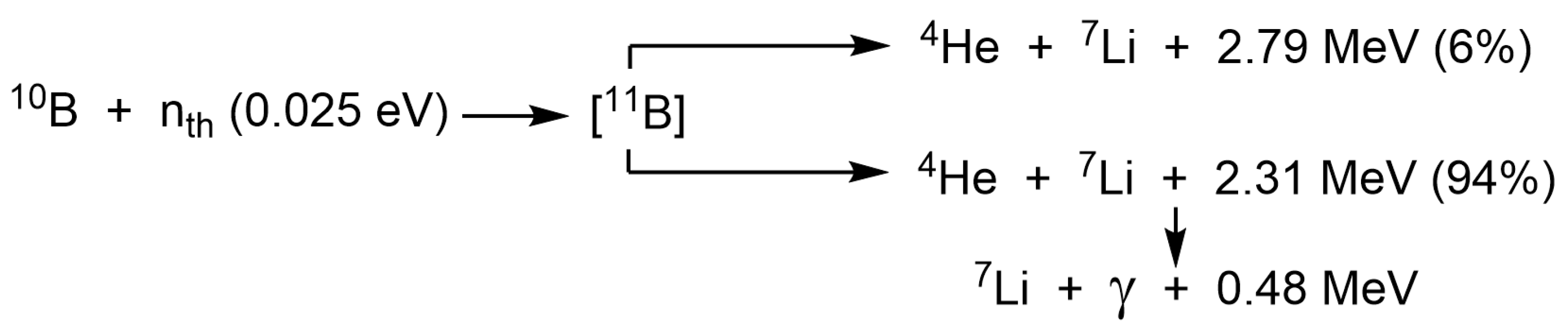
1. Introduction
2. Boron Clusters on the Surface of Dendrimers
2.1. Boronated PAMAM Dendrimers
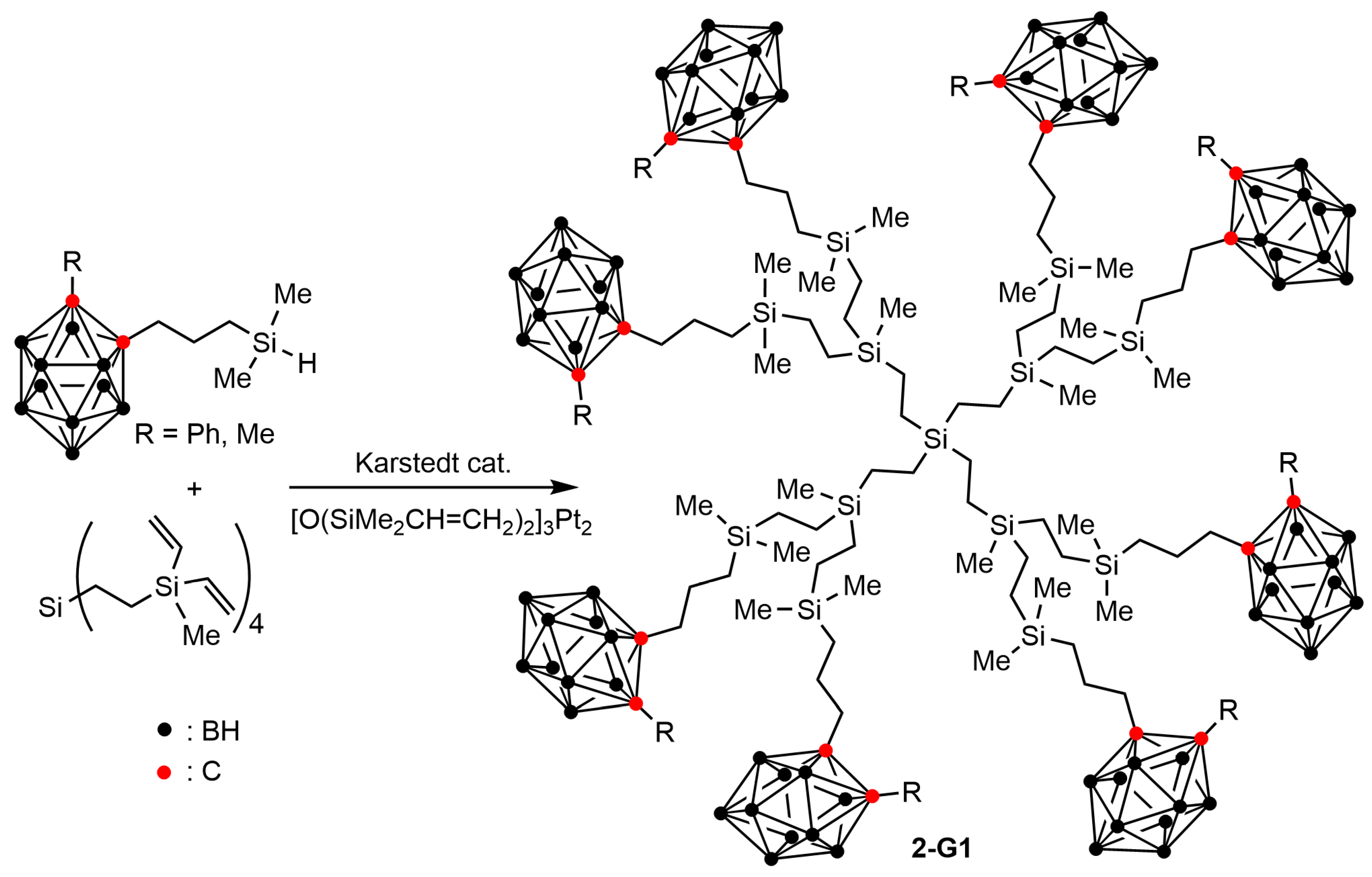
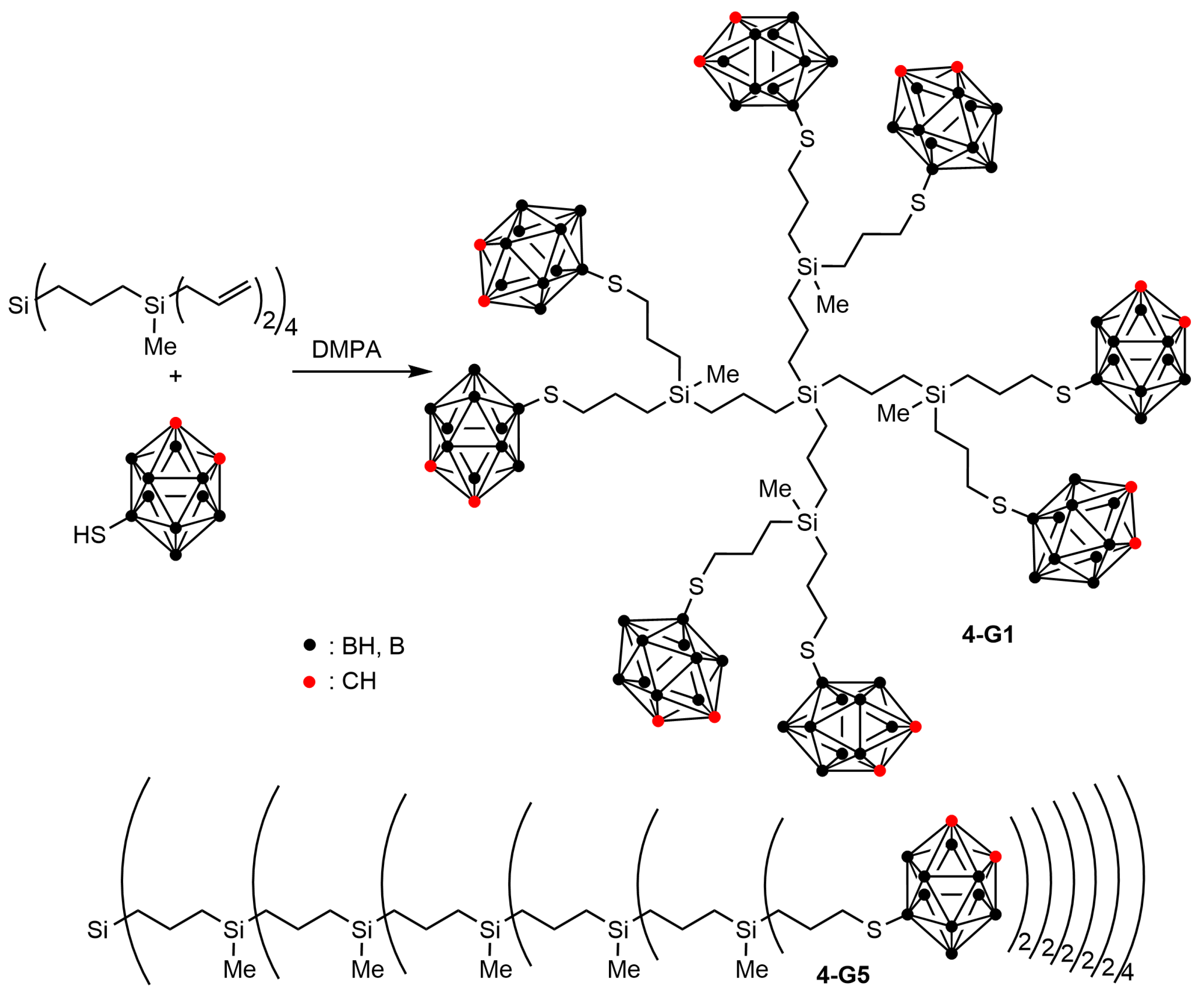
2.2. Boronated Carbosilane Dendrimers
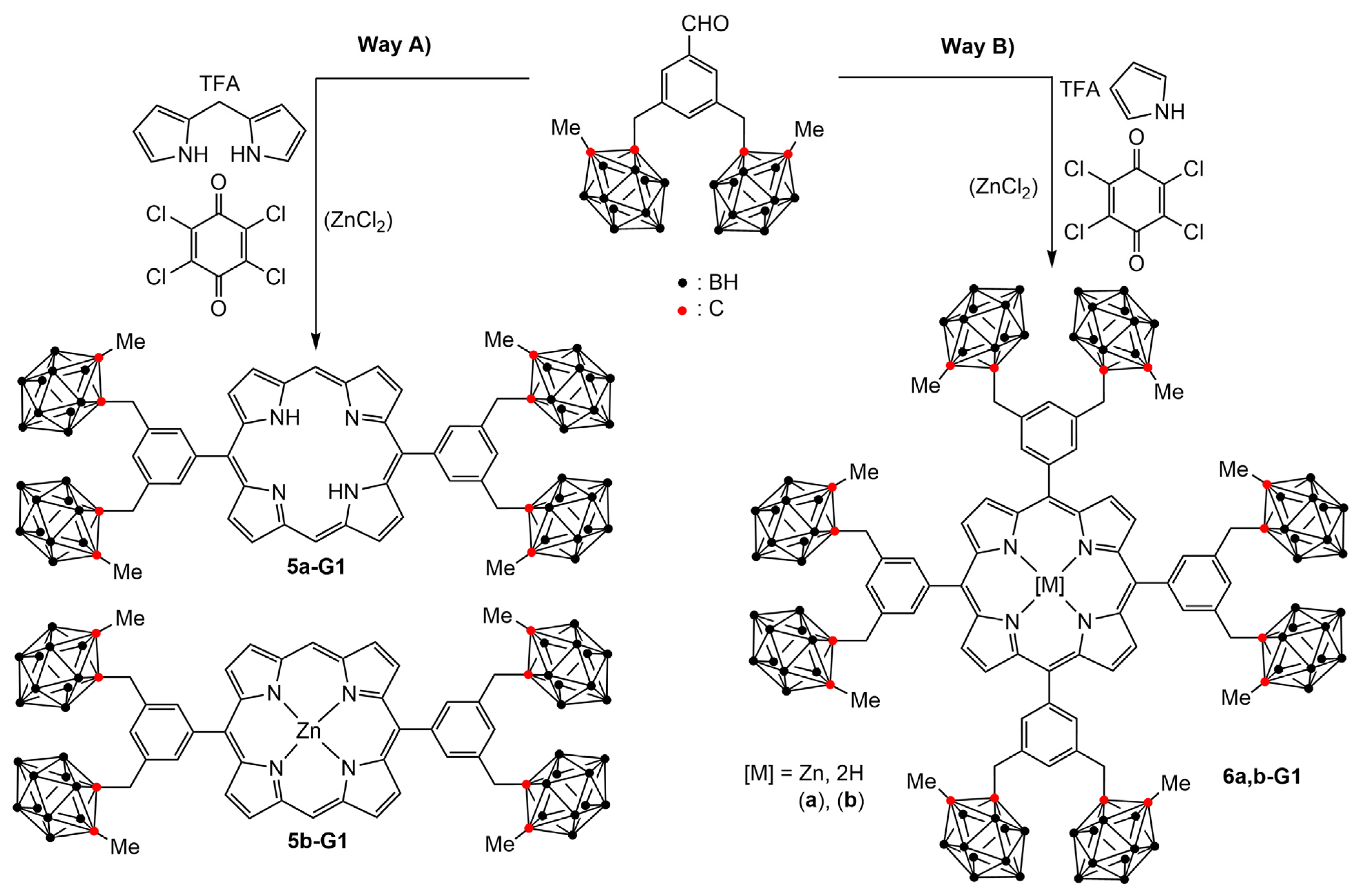
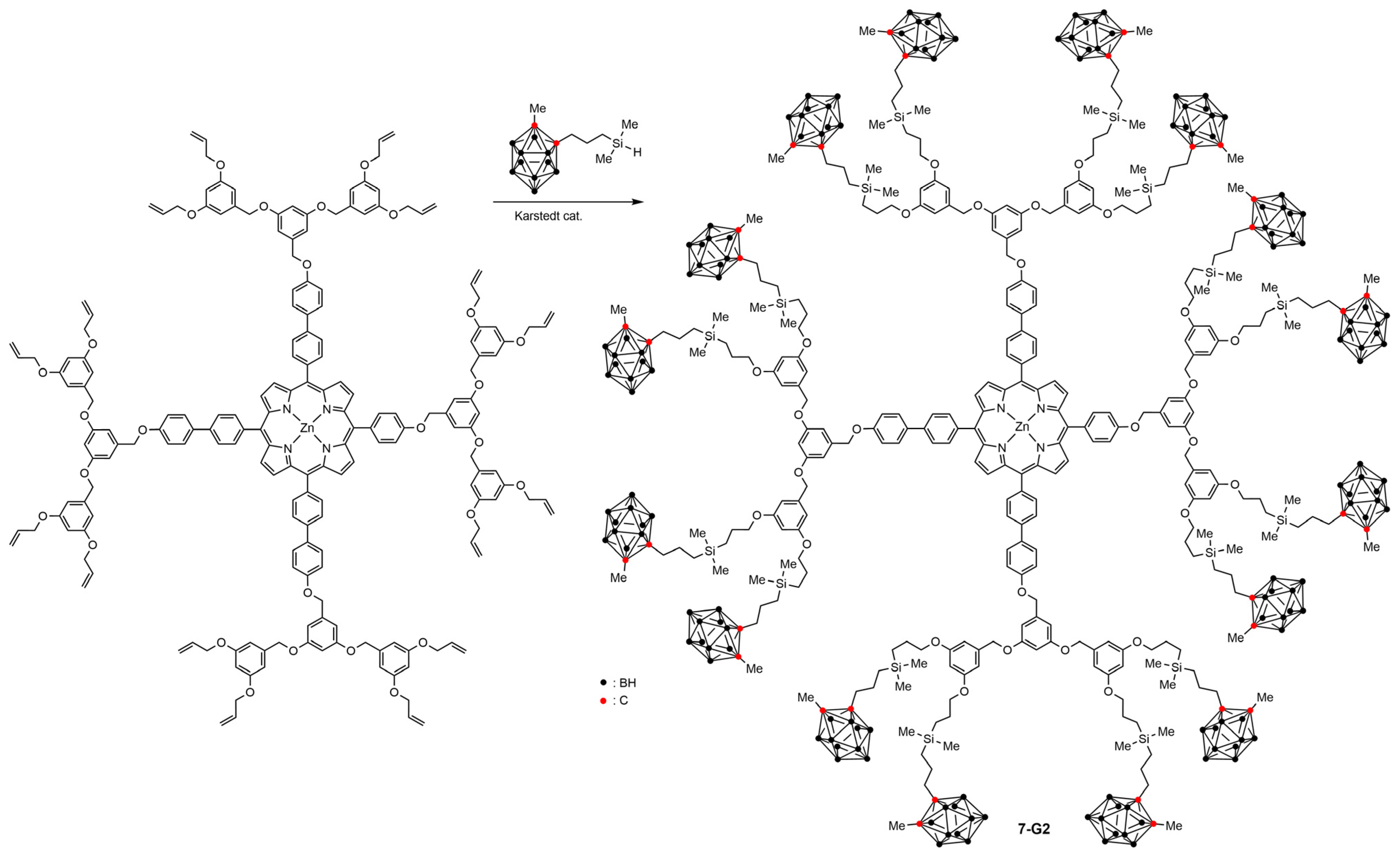
2.3. Boronated Dendrimers Having a Porphyrin Core
2.4. Boronated Dendrimers Having a Phenylene Core
2.5. Miscellaneous Boronated Dendrimers
3. Boron Clusters inside Dendrimers
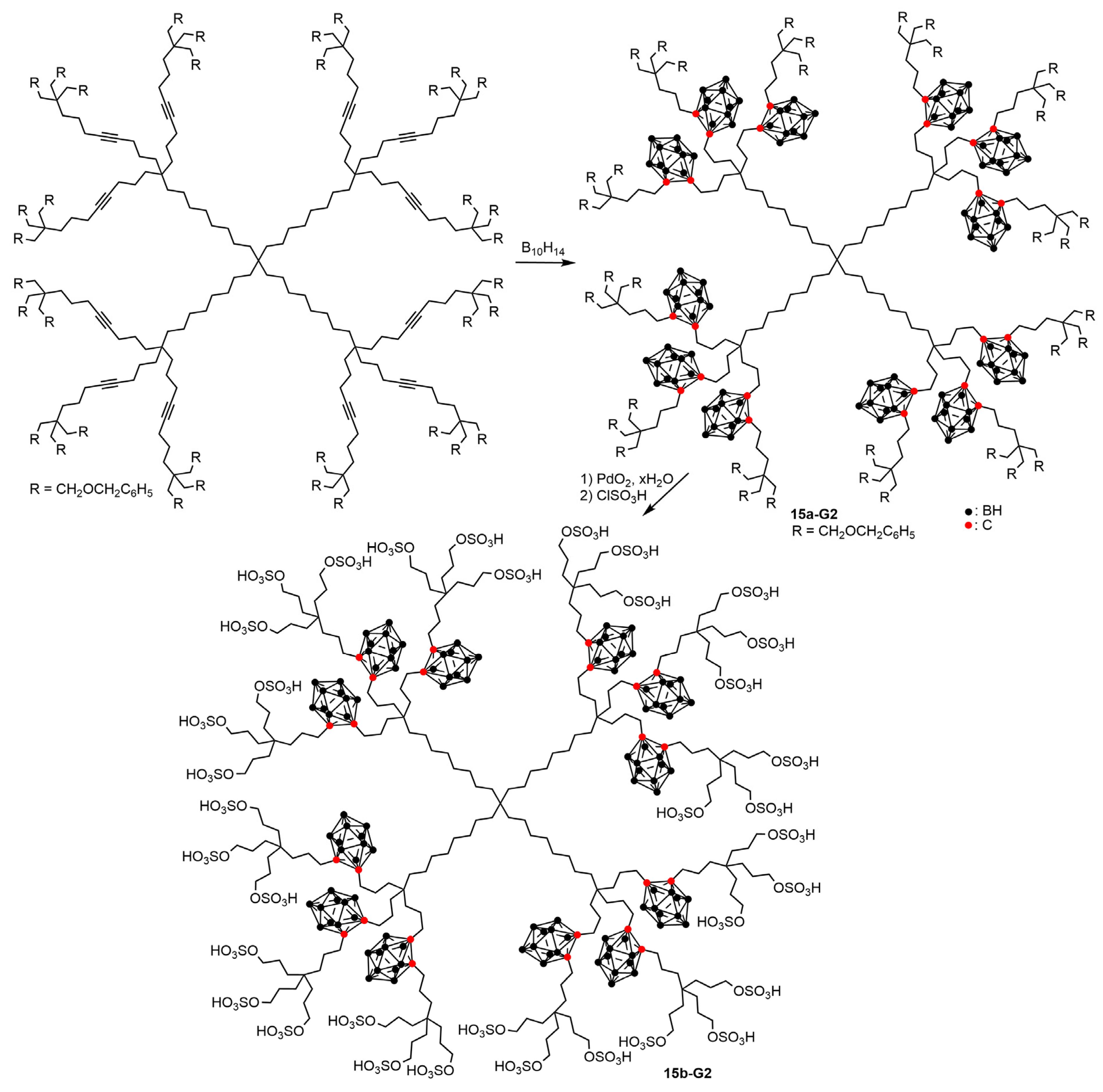
3.1. Carboranes inside Hydrocarbon-Based Dendrimers
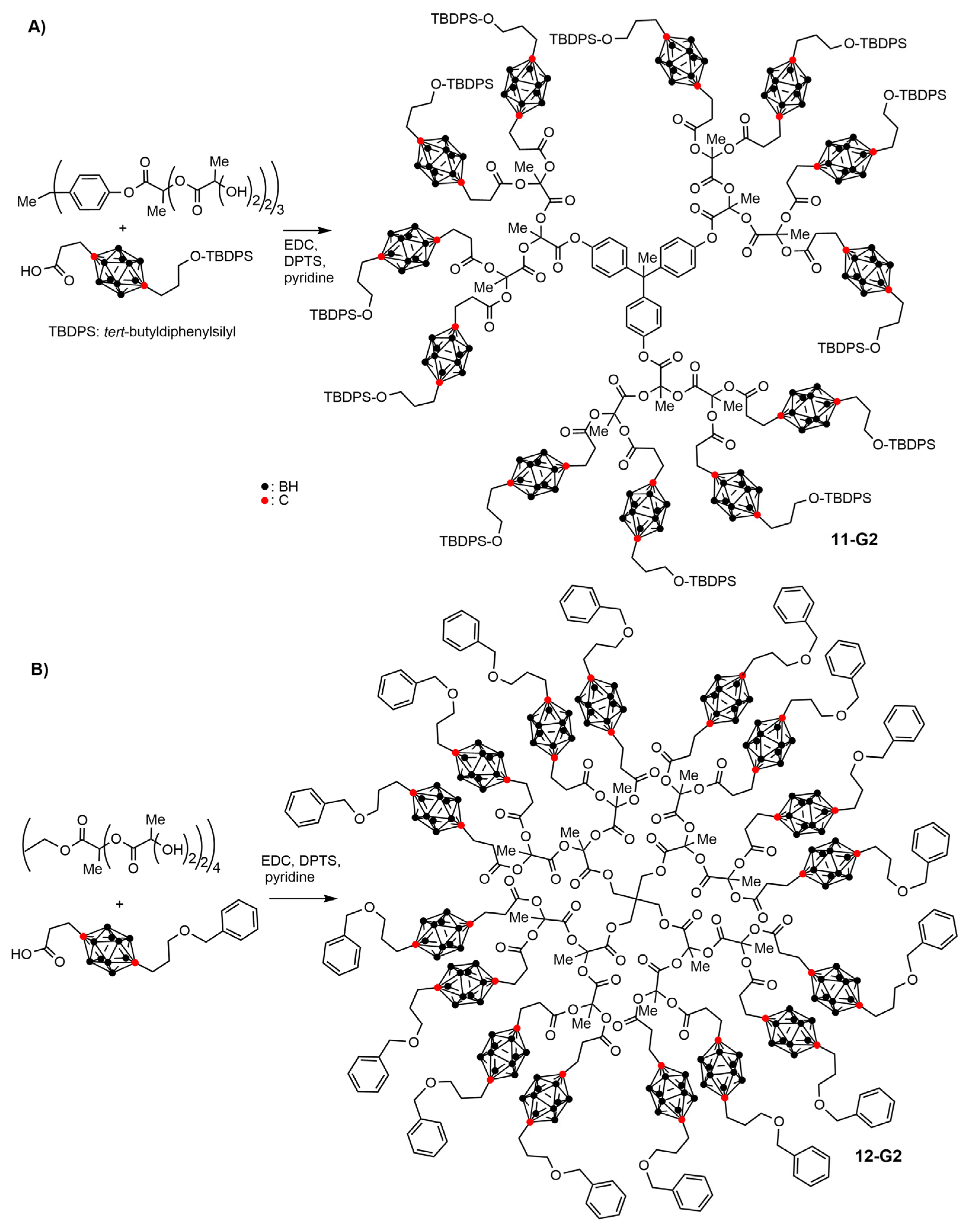
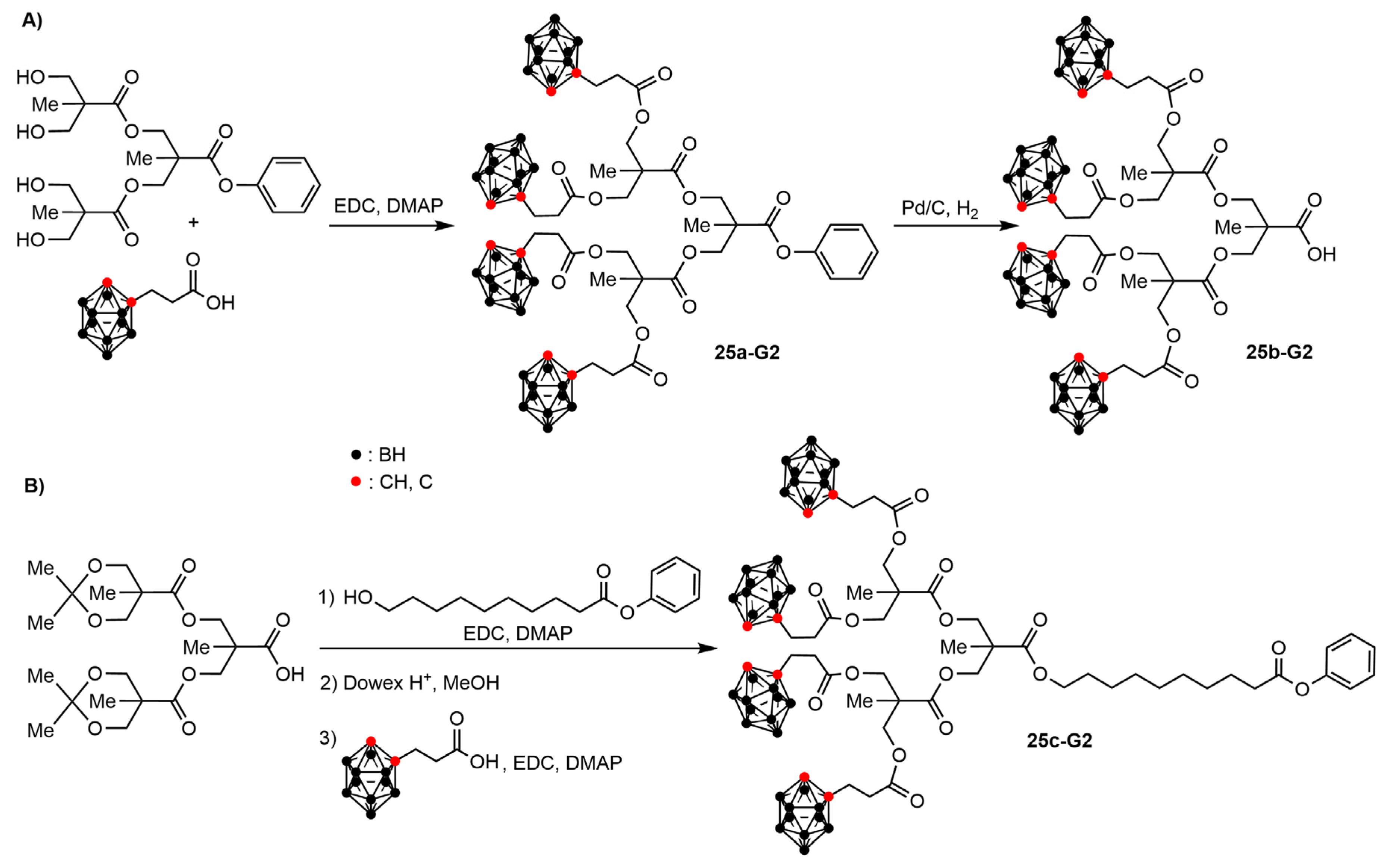
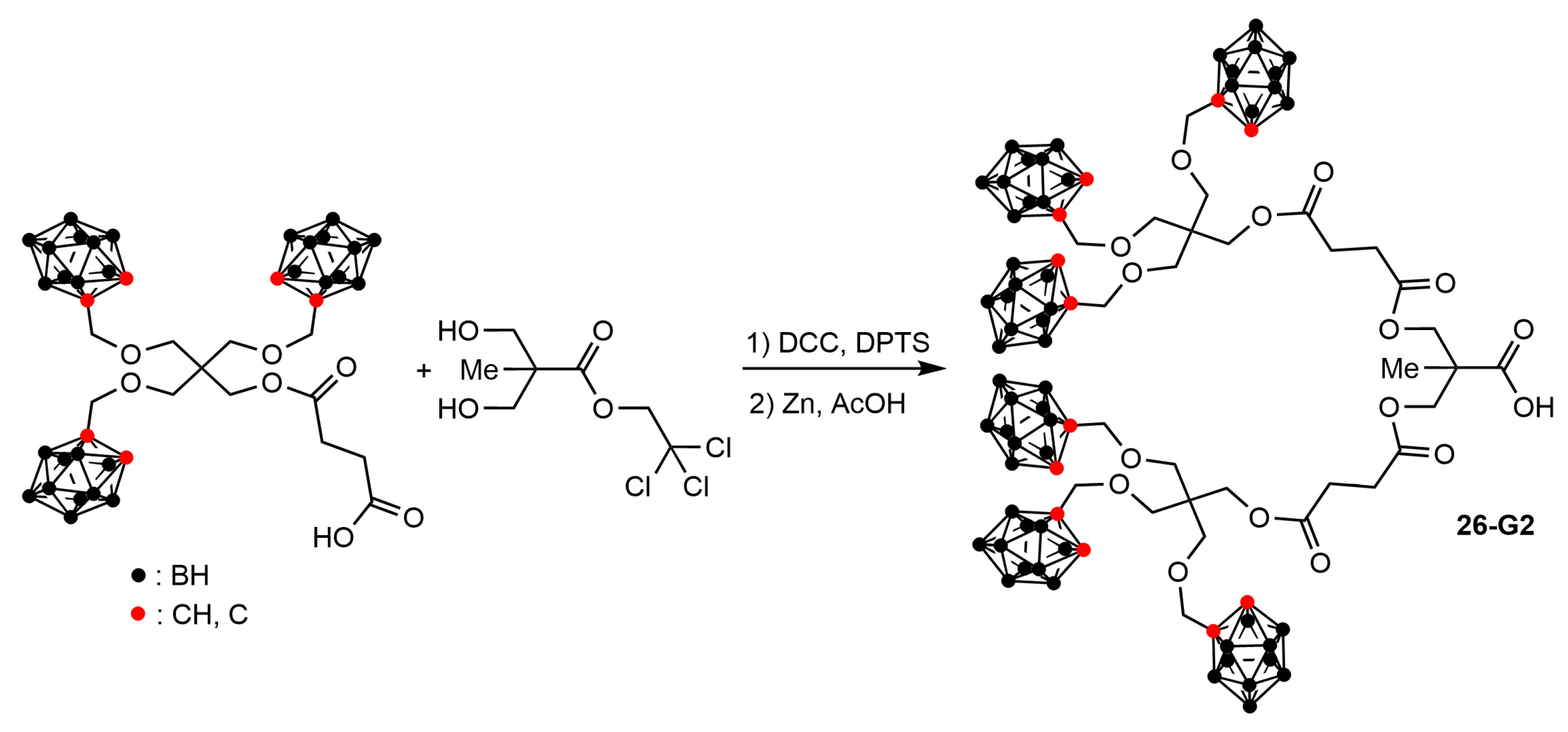
3.2. Carboranes inside Polyester-Based Dendrimers
3.3. Carboranes inside Various Small Dendrimers
4. Dendrons and Dendritic Structures Functionalized with Boron Clusters
4.1. Boron Clusters at the Core of Dendrons
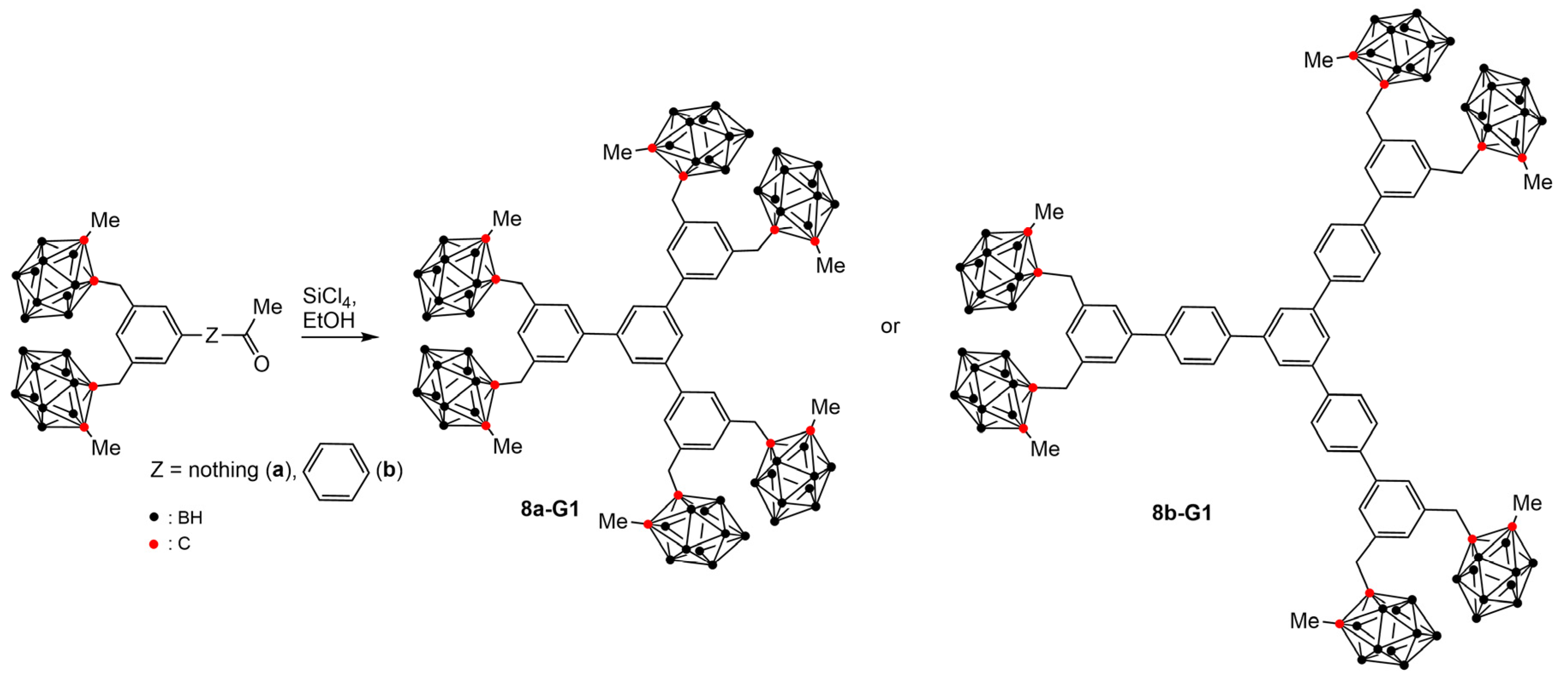
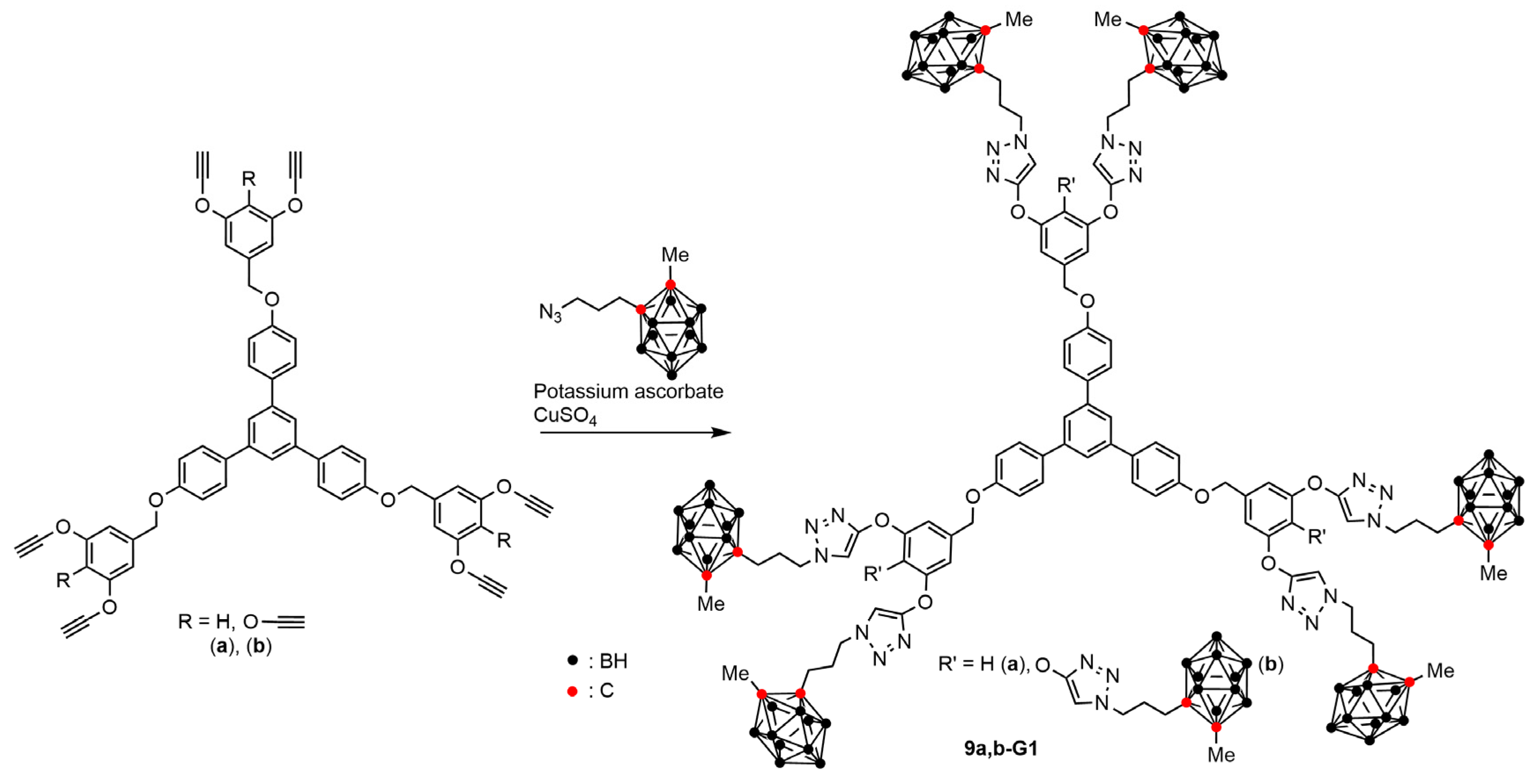
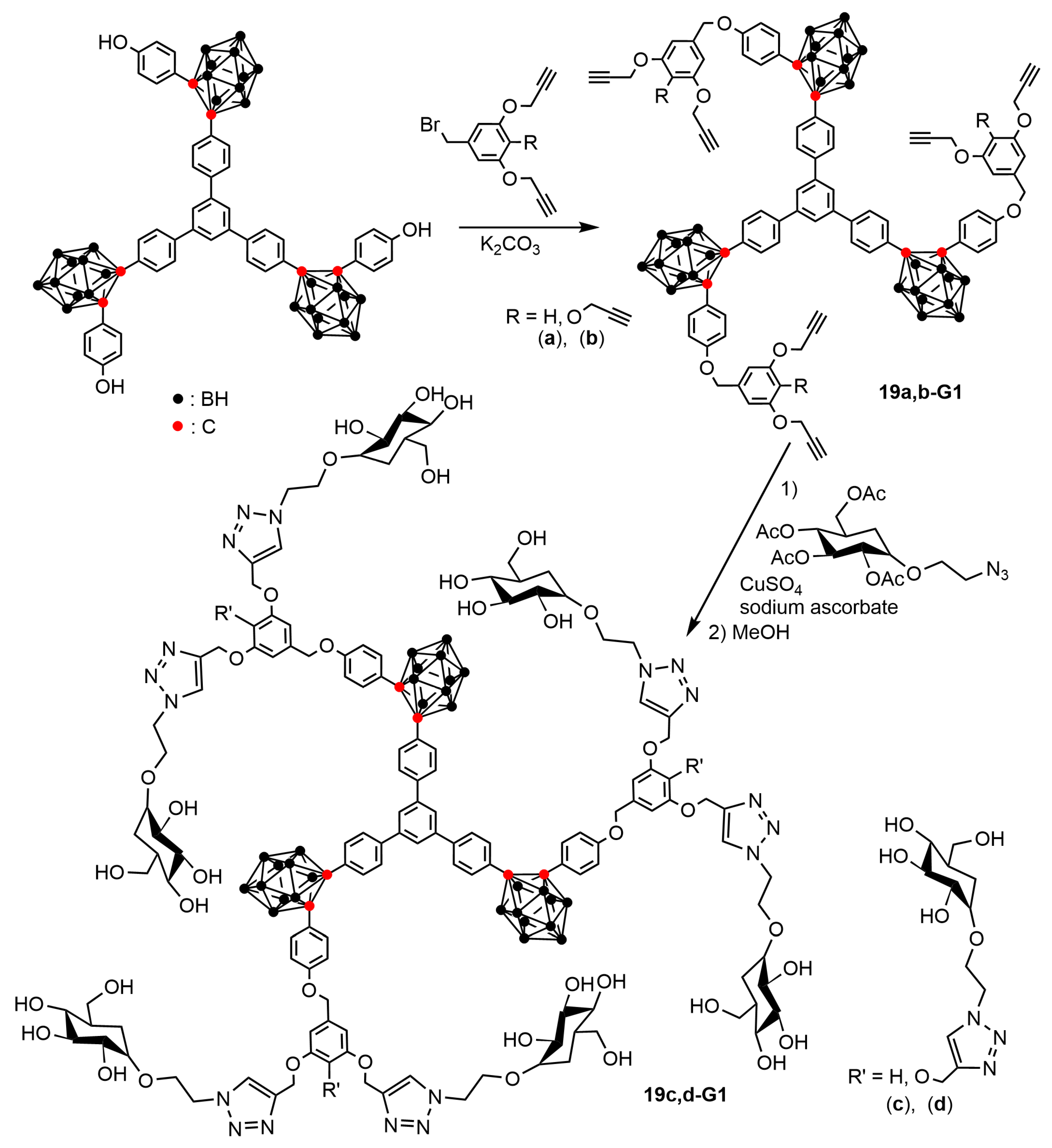
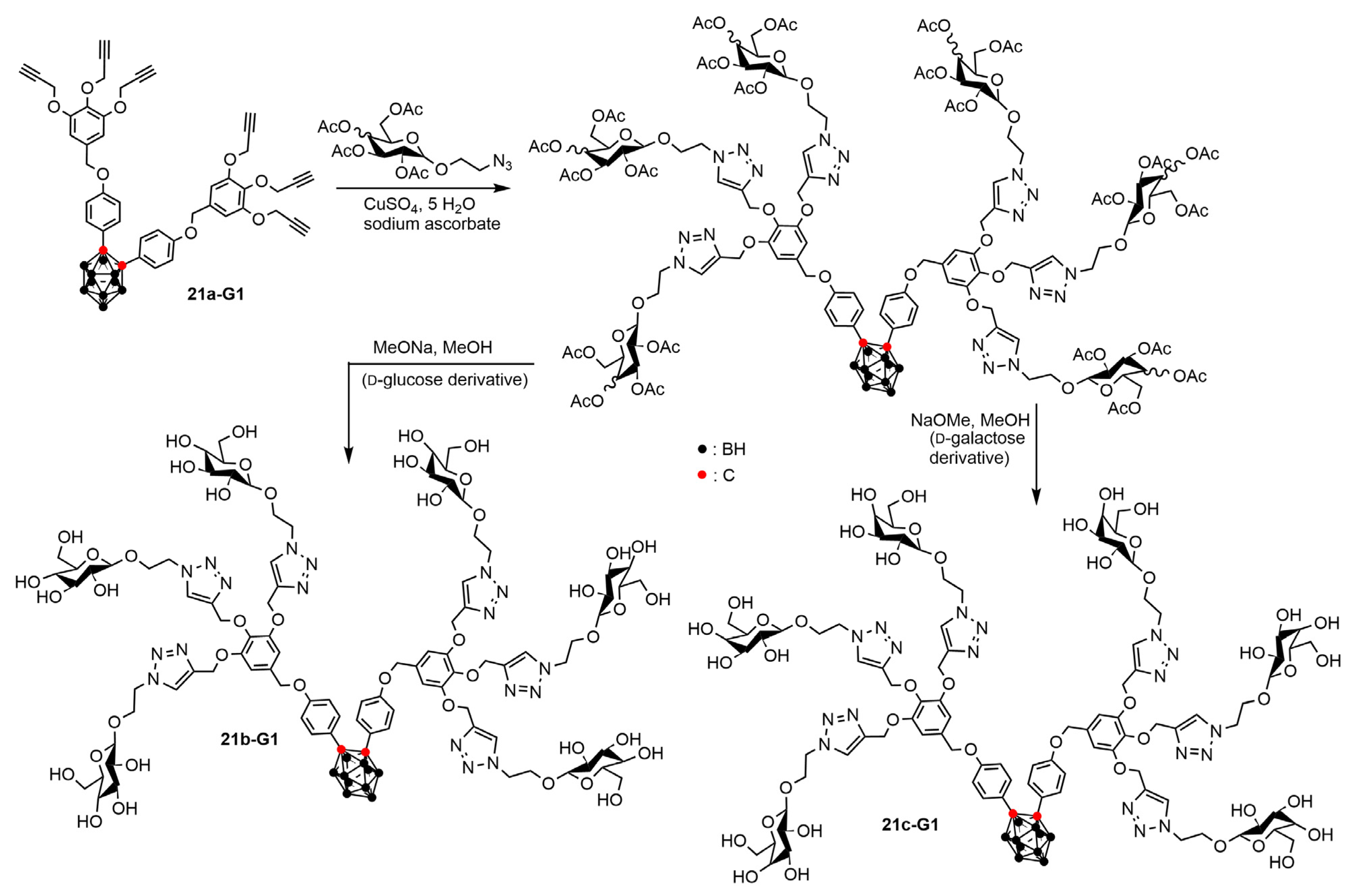
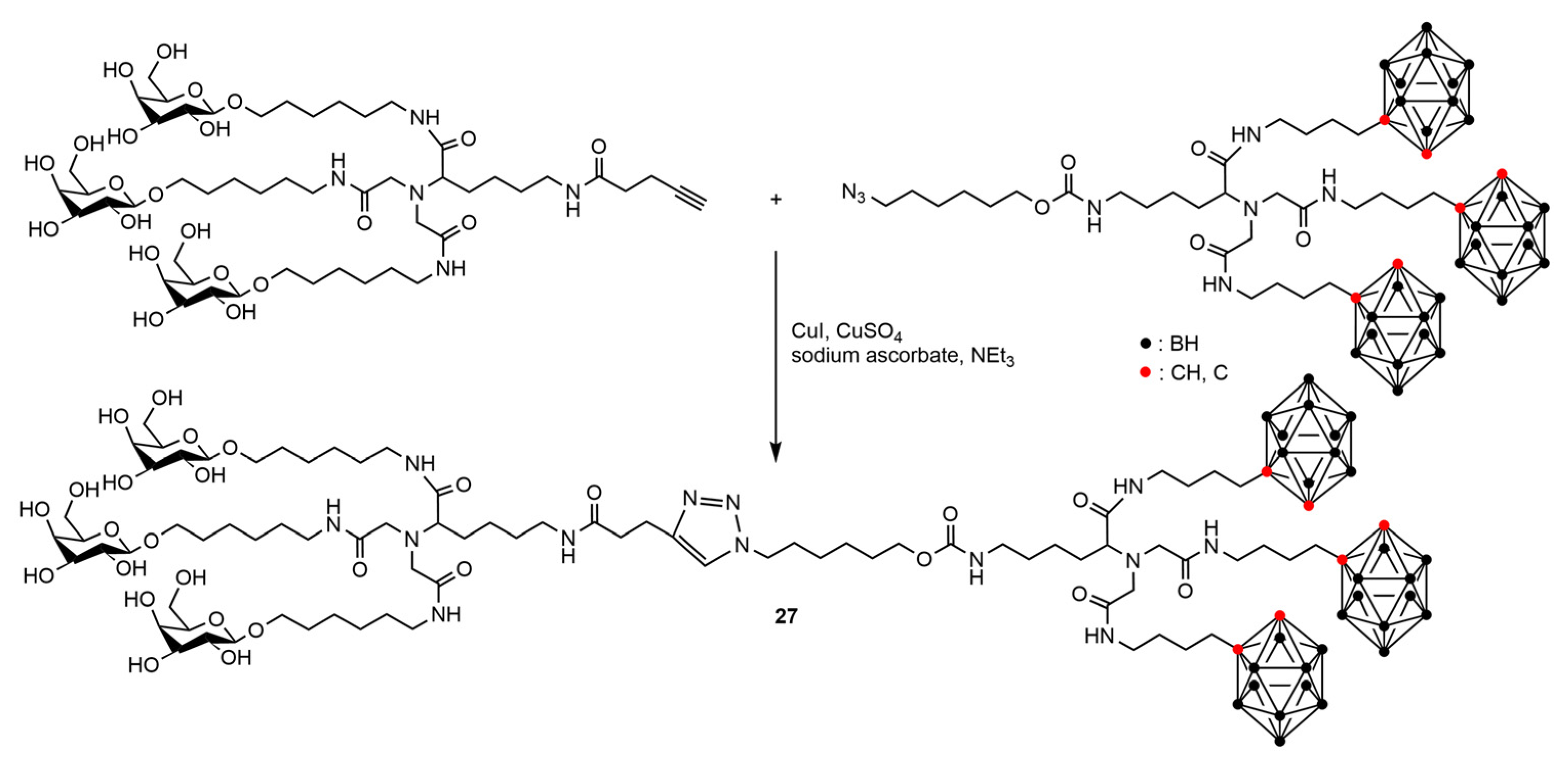
4.2. Boron Clusters on the Surface of Dendrons
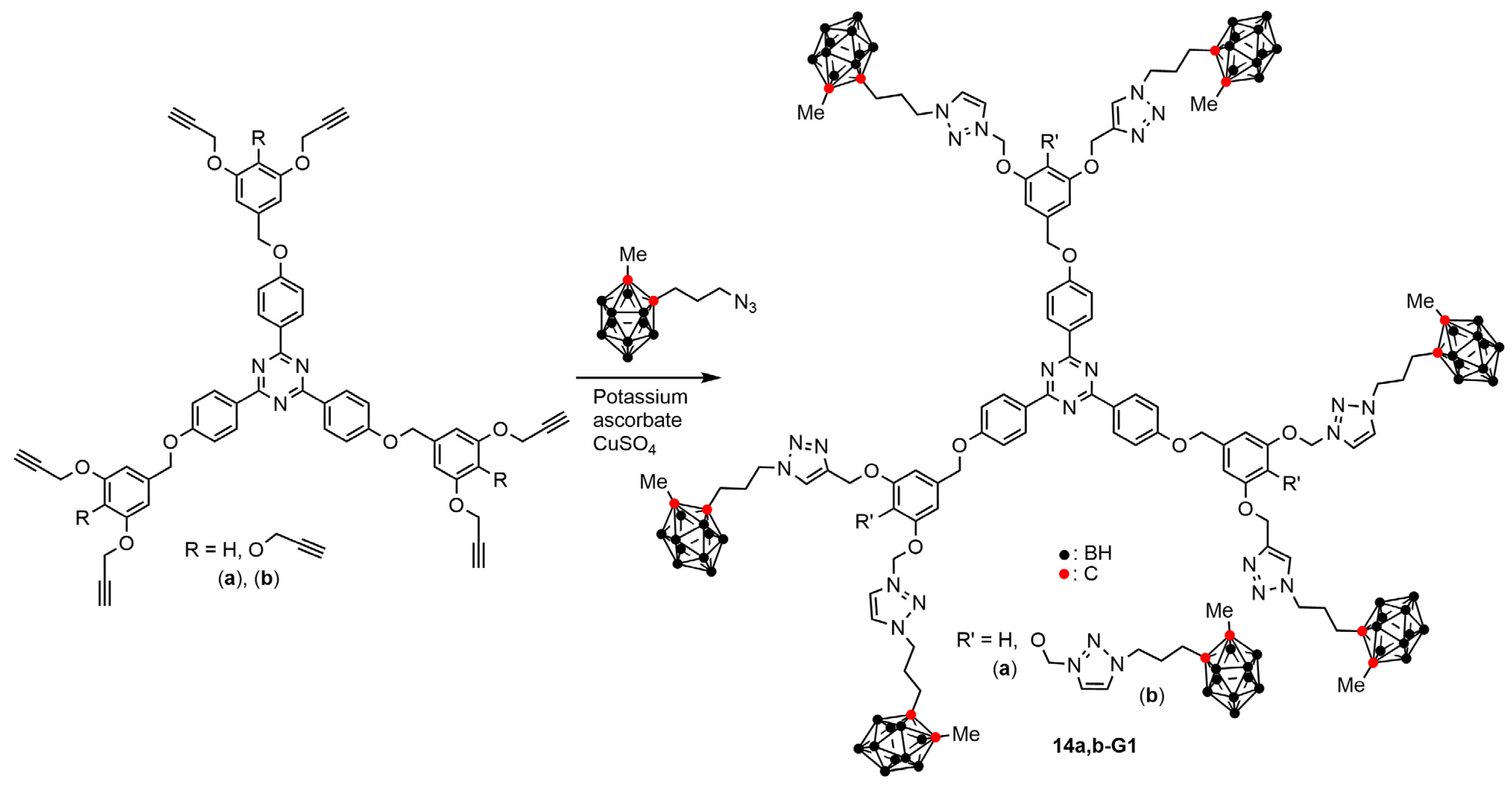
4.3. Carboranes on a Part of the Surface of Janus Dendrimers
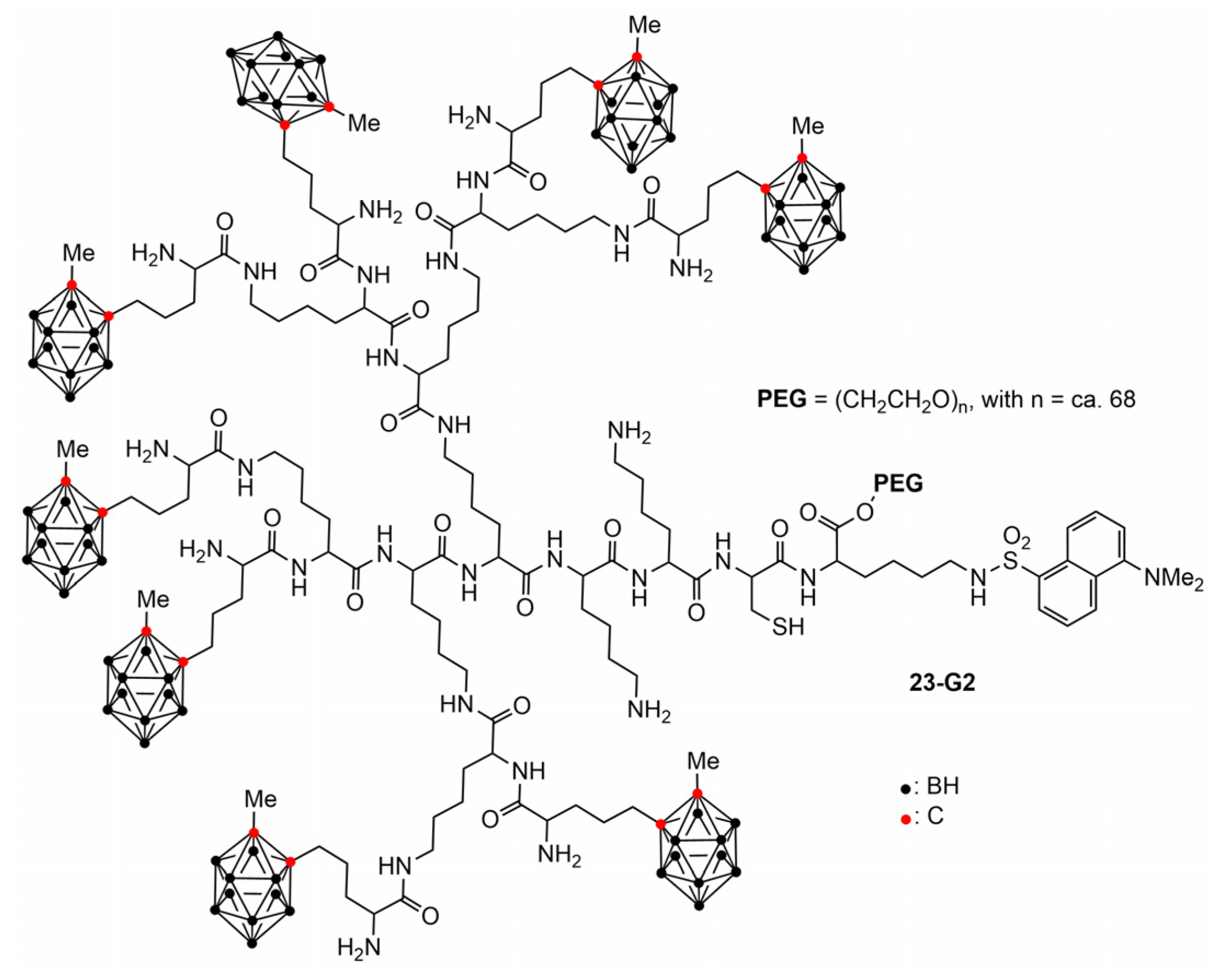
5. Biological Properties of Dendritic Compounds Having Boron Clusters Somewhere in Their Structure
5.1. Anti-Cancer Properties of Dendritic Structures Functionalized with Boron Clusters
5.1.1. Anti-Cancer Properties and In Vivo Localization of Boronated PAMAM Dendrimers
5.1.2. Anti-Cancer Properties and In Vivo Localization of Other Boronated Dendrimers
5.2. BNCT Properties of Dendritic Structures Functionalized with Boron Clusters
5.2.1. BNCT Properties of Boronated PAMAM Dendrimers
5.2.2. BNCT Properties of Diverse Boronated Dendritic Structures
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sergeeva, A.P.; Popov, I.A.; Piazza, Z.A.; Li, W.L.; Romanescu, C.; Wang, L.S.; Boldyrev, A.I. Understanding Boron through Size-Selected Clusters: Structure, Chemical Bonding, and Fluxionality. Acc. Chem. Res. 2014, 47, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Grimes, R.N. Carboranes, 3rd ed.; Elsevier Academic Press: London, UK, 2016; pp. 1–1041. [Google Scholar]
- Heying, T.L.; Ager, J.W., Jr.; Clark, S.L.; Mangold, D.J.; Goldstein, H.L.; Hillman, M.; Polak, R.J.; Szymanski, J.W. A New Series of Organoboranes. I. Carboranes from the Reaction of Decaborane with Acetylenic Compounds. Inorg. Chem. 1963, 2, 1089–1092. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Stanko, V.I.; Brattsev, V.A.; Chapovskii, Y.A.; Struchkov, Y.T. The structure of B10C2H12 (Barene) and its derivatives. Bull. Acad. Sci. USSR Div. Chem. Sci. 1963, 12, 1911. [Google Scholar] [CrossRef]
- Grafstein, D.; Dvorak, J. Neocarboranes, a New Family of Stable Organoboranes Isomeric with the Carboranes. Inorg. Chem. 1963, 2, 1128–1133. [Google Scholar] [CrossRef]
- Edvenson, G.M.; Gaines, D.F. Thermal Isomerization of Regiospecifically 10B-Labeled Icosahedral Carboranes. Inorg. Chem. 1990, 29, 1210–1216. [Google Scholar] [CrossRef]
- Bregadze, V.I. Fifty years of carborane chemistry: The history of discovery and the first results. Russ. Chem. Bull. 2014, 63, 1021–1026. [Google Scholar] [CrossRef]
- Zhu, Y.; Siwei, X.; Maguire, J.A.; Hosmane, N.S. Application of Cycloaddition Reactions to the Syntheses of Novel Boron Compounds. Molecules 2010, 15, 9437–9449. [Google Scholar] [CrossRef]
- Scholz, M.; Hey-Hawkins, E. Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. [Google Scholar] [CrossRef]
- Barth, R.F.; Soloway, A.H. Boron Neutron-Capture Therapy of primary and metastatic brain-tumors. Mol. Chem. Neuropathol. 1994, 21, 139–154. [Google Scholar] [CrossRef]
- Barth, R.F.; Coderre, J.A.; Vicente, M.G.H.; Blue, T.E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Ouali, A.; DelavauxNicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; Wiley: Chichester, UK, 2011; pp. 1–538. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers—Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst dendrimers—Molecular level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Zhu, Y.H.; Maguire, J.A.; Kaim, W.; Takagaki, M. Nano and dendritic structured carboranes and metallacarboranes: From materials to cancer therapy. J. Organomet. Chem. 2009, 694, 1690–1697. [Google Scholar] [CrossRef]
- Gao, S.M.; Hosmane, N.S. Dendrimer- and nanostructure-supported carboranes and metallacarboranes: An account. Russ. Chem. Bull. 2014, 63, 788–810. [Google Scholar] [CrossRef]
- Vinas, C.; Nunez, R.; Bennour, I.; Teixidor, F. Periphery Decorated and Core Initiated Neutral and Polyanionic Borane Large Molecules: Forthcoming and Promising Properties for Medicinal Applications. Curr. Med. Chem. 2019, 26, 5036–5076. [Google Scholar] [CrossRef]
- Wu, G.; Barth, R.F.; Yang, W.; Lee, R.J.; Tjark, W.; Backer, M.V.; Backer, J.M. Boron containing macromolecules and nanovehicles as delivery agents for neutron capture therapy. Anti-Cancer Agents Med. Chem. 2006, 6, 167–184. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Zhu, Y.; Maguire, J.A.; Hosmane, S.N.; Chakrabarti, A. Boron nanostructures—From materials to cancer therapy: An account. Main Group Chem. 2010, 9, 153–166. [Google Scholar] [CrossRef]
- Pitto-Barry, A. Polymers and boron neutron capture therapy (BNCT): A potent combination. Polym. Chem. 2021, 12, 2035–2044. [Google Scholar] [CrossRef]
- Liko, F.; Hindre, F.; Fernandez-Megia, E. Dendrimers as Innovative Radiopharmaceuticals in Cancer Radionanotherapy. Biomacromolecules 2016, 17, 3103–3114. [Google Scholar] [CrossRef]
- Barth, R.F.; Adams, D.M.; Soloway, A.H.; Alam, F.; Darby, M.V. Boronated starburst dendrimer monoclonal-antibody immunoconjugates—Evaluations as a potential delivery system for Neutron-Capture Therapy. Bioconjug. Chem. 1994, 5, 58–66. [Google Scholar] [CrossRef]
- Capala, J.; Barth, R.F.; Bendayan, M.; Lauzon, M.; Adams, D.M.; Soloway, A.H.; Fenstermaker, R.A.; Carlsson, J. Boronated epidermal growth factor as a potential targeting agent for boron neutron capture therapy of brain tumors. Bioconjug. Chem. 1996, 7, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Wu, G.; Chatterjee, M.; Yang, W.L.; Sekido, M.; Diop, L.A.; Muller, R.; Sudimack, J.J.; Lee, R.J.; Barth, R.F.; et al. Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug. Chem. 2003, 14, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Backer, M.V.; Gaynutdinov, T.I.; Patel, V.; Bandyopadhyaya, A.K.; Thirumamagal, B.T.S.; Tjarks, W.; Barth, R.F.; Claffey, K.; Backer, J.M. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol. Cancer Ther. 2005, 4, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Campo, A.; Vinas, C.; Teixidor, F.; Nunez, R.; Sillanpaa, R.; Kivekas, R. Modular construction of neutral and anionic carboranyl-containing carbosilane-based dendrimers. Macromolecules 2007, 40, 5644–5652. [Google Scholar] [CrossRef]
- Djeda, R.; Ruiz, J.; Astruc, D.; Satapathy, R.; Dash, B.P.; Hosmane, N.S. “Click” Synthesis and Properties of Carborane-Appended Large Dendrimers. Inorg. Chem. 2010, 49, 10702–10709. [Google Scholar] [CrossRef]
- Minyaylo, E.O.; Anisimov, A.A.; Zaitsev, A.V.; Milenin, S.A.; Tikhonov, P.A.; Vyshivannaya, O.V.; Ol’shevskaya, V.A.; Nikiforova, G.G.; Buzin, M.I.; Peregudov, A.S.; et al. Boron-substituted carborane-carbosilane dendrimers: Synthesis and properties. React. Funct. Polym. 2020, 157, 104746. [Google Scholar] [CrossRef]
- Minyaylo, E.O.; Zubova, V.Y.; Zaitsev, A.V.; Ol’shevskaya, V.A.; Nikiforova, G.G.; Buzin, M.I.; Anisimov, A.A.; Muzafarov, A.M. Studies on the effect of polyhedral carboranes on the physicochemical properties of polycarboranosiloxanes. Polym. Chem. 2023, 14, 1514–1525. [Google Scholar] [CrossRef]
- Vicente, M.G.H.; Wickramasinghe, A.; Nurco, D.J.; Wang, H.J.H.; Nawrocky, M.M.; Makar, M.S.; Miura, M. Synthesis, toxicity and biodistribution of two 5,15-di 3,5-(nido-carboranylmethyl)phenyl porphyrins in EMT-6 tumor bearing mice. Bioorg. Med. Chem. 2003, 11, 3101–3108. [Google Scholar] [CrossRef]
- Gottumukkala, V.; Luguya, R.; Fronczek, F.R.; Vicente, M.G.H. Synthesis and cellular studies of an octa-anionic 5,10,15,20-tetra 3,5-(nido-carboranylmethyl)phenyl porphyrin (H2OCP) for application in BNCT. Bioorg. Med. Chem. 2005, 13, 1633–1640. [Google Scholar] [CrossRef]
- Cabrera-Gonzalez, J.; Xochitiotzi-Flores, E.; Vinas, C.; Teixidor, F.; Garcia-Ortega, H.; Farfan, N.; Santillan, R.; Parella, T.; Nunez, R. High-Boron-Content Porphyrin-Cored Aryl Ether Dendrimers: Controlled Synthesis, Characterization, and Photophysical Properties. Inorg. Chem. 2015, 54, 5021–5031. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Gaillard, E.R.; Maguire, J.A.; Hosmane, N.S. Synthesis and Properties of Carborane-Appended C-3-Symmetrical Extended pi Systems. J. Am. Chem. Soc. 2010, 132, 6578–6587. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Bode, B.P.; Reidl, C.T.; Sawicki, J.W.; Mason, A.J.; Maguire, J.A.; Hosmane, N.S. “Click” Chemistry-Mediated Phenylene-Cored Carborane Dendrimers. Organometallics 2012, 31, 2931–2935. [Google Scholar] [CrossRef]
- Gonzalez-Campo, A.; Ferrer-Ugalde, A.; Vinas, C.; Teixidor, F.; Sillanpaa, R.; Rodriguez-Romero, J.; Santillan, R.; Farfan, N.; Nunez, R. A Versatile Methodology for the Controlled Synthesis of Photoluminescent High-Boron-Content Dendrimers. Chem.-Eur. J. 2013, 19, 6299–6312. [Google Scholar] [CrossRef]
- Parrott, M.C.; Marchington, E.B.; Valliant, J.F.; Adronov, A. Synthesis and properties of carborane-functionalized aliphatic polyester dendrimers. J. Am. Chem. Soc. 2005, 127, 12081–12089. [Google Scholar] [CrossRef]
- Lerouge, F.; Vinas, C.; Teixidor, F.; Nunez, R.; Abreu, A.; Xochitiotzi, E.; Santillan, R.; Farfan, N. High boron content carboranyl-functionalized aryl ether derivatives displaying photoluminescent properties. Dalton Trans. 2007, 1898–1903. [Google Scholar] [CrossRef]
- Jena, B.B.; Jena, S.R.; Swain, B.R.; Mahanta, C.S.; Samanta, L.; Dash, B.P.; Satapathy, R. Triazine-cored dendritic molecules containing multiple o-carborane clusters. Appl. Organomet. Chem. 2020, 34, e5754. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Keith, J.M.; Baker, G.R.; Escamilla, G.H. Chemistry within a unimolecular micelle precursor—Boron superclusters by site-specific and depth-specific transformation of dendrimers. 37. Chemistry of micelles. Angew. Chem. Int. Ed. Engl. 1994, 33, 666–668. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N. Metallo- and metalloido-micellane™ derivatives—Incorporation of metals and nonmetals within unimolecular superstructures. Macromol. Symp. 1994, 77, 63–71. [Google Scholar] [CrossRef]
- Parrott, M.C.; Valliant, J.F.; Adronov, A. Thermally induced phase transition of carborane-functionalized aliphatic polyester dendrimers in aqueous media. Langmuir 2006, 22, 5251–5255. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Synthesis of a new class of carborane-containing star-shaped molecules via silicon tetrachloride promoted cyclotrimerization reactions. Org. Lett. 2008, 10, 2247–2250. [Google Scholar] [CrossRef]
- Minyaylo, E.O.; Zaitsev, A.V.; Ol’shevskaya, V.A.; Peregudov, A.S.; Anisimov, A.A. The CH-functionalization of B-substituted organosilicon derivatives of polyhedral carboranes as a way to obtain new materials. Mendeleev Commun. 2023, 33, 47–49. [Google Scholar] [CrossRef]
- Swain, B.R.; Jena, S.R.; Beriha, S.K.; Mahanta, C.S.; Jena, B.B.; Prasanth, T.; Samanta, L.; Satapathy, R.; Dash, B.P. Synthesis and anticancer properties of dendritic glycoconjugates containing multiple o-carborane clusters. New J. Chem. 2023, 47, 10296–10308. [Google Scholar] [CrossRef]
- Nemoto, H.; Wilson, J.G.; Nakamura, H.; Yamamoto, Y. Polyols of a cascade type as a water-solubilizing element of carborane derivatives for Boron Neutron-Capture Therapy. J. Org. Chem. 1992, 57, 435. [Google Scholar] [CrossRef]
- Nemoto, H.; Cai, J.P.; Yamamoto, Y. Synthesis of a water-soluble o-carborane bearing a uracil moiety via a palladium-catalyzed reaction under essential neutral conditions. J. Chem. Soc.-Chem. Commun. 1994, 577–578. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Cai, J.P.; Nakamura, H.; Sadayori, N.; Asao, N.; Nemoto, H. Synthesis of Netropsin and Distamycin analogs bearing O-carborane and their DNA recognition. J. Org. Chem. 1995, 60, 3352–3357. [Google Scholar] [CrossRef]
- Swain, B.R.; Mahanta, C.S.; Jena, B.B.; Beriha, S.K.; Nayak, B.; Satapathy, R.; Dash, B.P. Preparation of dendritic carboranyl glycoconjugates as potential anticancer therapeutics. RSC Adv. 2020, 10, 34764–34774. [Google Scholar] [CrossRef] [PubMed]
- Stocker, W.; Karakaya, B.; Schurmann, B.L.; Rabe, J.P.; Schluter, A.D. Ordered dendritic nanorods with a poly(p-phenylene) backbone. J. Am. Chem. Soc. 1998, 120, 7691–7695. [Google Scholar] [CrossRef]
- Benhabbour, S.R.; Parrott, M.C.; Gratton, S.E.A.; Adronov, A. Synthesis and properties of carborane-containing dendronized polymers. Macromolecules 2007, 40, 5678–5688. [Google Scholar] [CrossRef]
- Qualmann, B.; Kessels, M.M.; Musiol, H.J.; Sierralta, W.D.; Jungblut, P.W.; Moroder, L. Synthesis of boron-rich lysine dendrimers as protein labels in electron microscopy. Angew. Chem. Int. Ed. Engl. 1996, 35, 909–911. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.-I.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef]
- Galie, K.M.; Mollard, A.; Zharov, I. Polyester-based carborane-containing dendrons. Inorg. Chem. 2006, 45, 7815–7820. [Google Scholar] [CrossRef]
- Mollard, A.; Zharov, I. Tricarboranyl pentaerythritol-based building block. Inorg. Chem. 2006, 45, 10172–10179. [Google Scholar] [CrossRef]
- Caminade, A.M.; Laurent, R.; Delavaux-Nicot, B.; Majoral, J.P. “Janus” dendrimers: Syntheses and properties. New J. Chem. 2012, 36, 217–226. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, Y.C.; Chou, F.I.; Liang, C.F.; Lin, E.W.; Chuang, Y.J.; Lin, C.C. Design of multivalent galactosyl carborane as a targeting specific agent for potential application to boron neutron capture therapy. Chem. Commun. 2012, 48, 612–614. [Google Scholar] [CrossRef]
- Dubey, R.; Kushal, S.; Mollard, A.; Vojtovich, L.; Oh, P.; Levin, M.D.; Schnitzer, J.E.; Zharov, I.; Olenyuk, B.Z. Tumor Targeting, Trifunctional Dendritic Wedge. Bioconjug. Chem. 2015, 26, 78–89. [Google Scholar] [CrossRef]
- Hu, N.; Tanaka, H.; Kakino, R.; Yoshikawa, S.; Miyao, M.; Akita, K.; Isohashi, K.; Aihara, T.; Nihei, K.; Ono, K. Evaluation of a treatment planning system developed for clinical boron neutron capture therapy and validation against an independent Monte Carlo dose calculation system. Radiat. Oncol. 2021, 16, 243. [Google Scholar] [CrossRef]
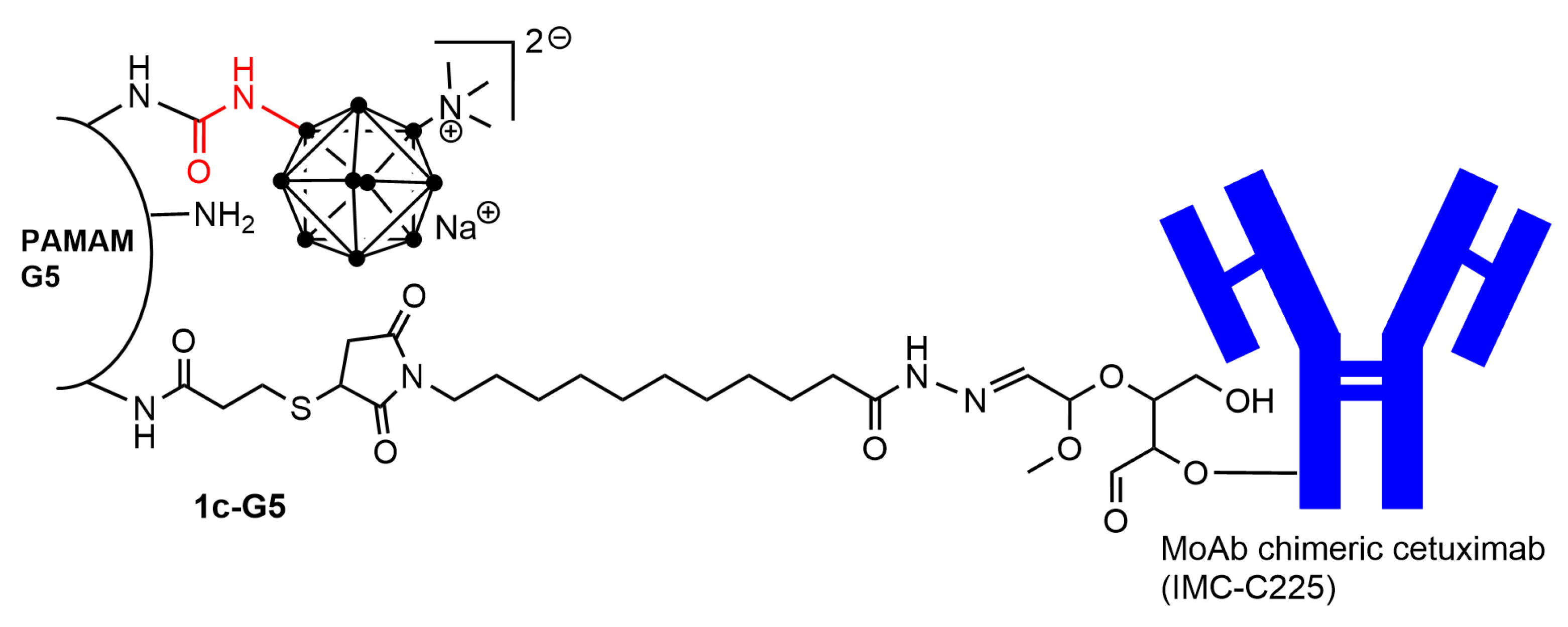
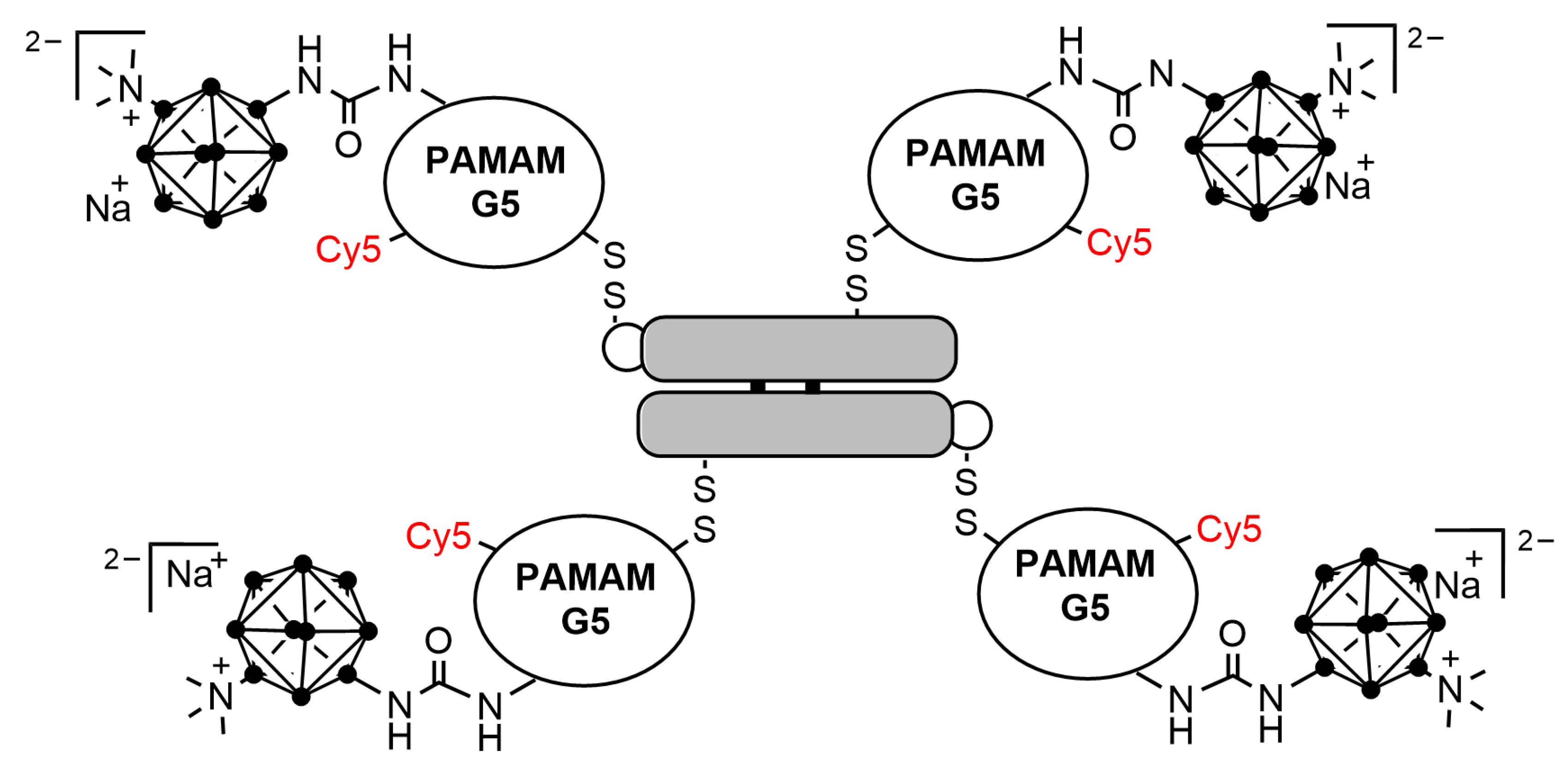
- Wu, G.; Barth, R.F.; Yang, W.L.; Chatterjee, M.; Tjarks, W.; Ciesielski, M.J.; Fenstermaker, R.A. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug. Chem. 2004, 15, 185–194. [Google Scholar] [CrossRef]
- Luderer, M.J.; de la Puente, P.; Azab, A.K. Advancements in Tumor Targeting Strategies for Boron Neutron Capture Therapy. Pharm. Res. 2015, 32, 2824–2836. [Google Scholar] [CrossRef]
- Barth, R.F.; Yang, W.L.; Adams, D.M.; Rotaru, J.H.; Shukla, S.; Sekido, M.; Tjarks, W.; Fenstermaker, R.A.; Ciesielski, M.; Nawrocky, M.M.; et al. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer Res. 2002, 62, 3159–3166. [Google Scholar]
- Yang, W.L.; Barth, R.F.; Wu, G.; Bandyopadhyaya, A.K.; Thirumamagal, B.T.S.; Tjarks, W.; Binns, P.J.; Riley, K.; Patel, H.; Coderre, J.A.; et al. Boronated epidermal growth factor as a delivery agent for neutron capture therapy of EGF receptor positive gliomas. Appl. Radiat. Isot. 2004, 61, 981–985. [Google Scholar] [CrossRef]
- Barth, R.F.; Wu, G.; Yang, W.L.; Binns, P.J.; Riley, K.J.; Patel, H.; Coderre, J.A.; Tjarks, W.; Bandyopadhyaya, A.K.; Thirumamagal, B.T.S.; et al. Neutron capture therapy of epidermal growth factor (plus) gliomas using boronated cetuximab (IMC-C225) as a delivery agent. Appl. Radiat. Isot. 2004, 61, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yang, W.L.; Barth, R.F.; Kawabata, S.; Swindall, M.; Bandyopadhyaya, A.K.; Tjarks, W.; Khorsandi, B.; Blue, T.E.; Ferketich, A.K.; et al. Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin. Cancer Res. 2007, 13, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Barth, R.F.; Wu, G.; Huo, T.Y.; Tjarks, W.; Ciesielski, M.; Fenstermaker, R.A.; Ross, B.D.; Wikstrand, C.J.; Riley, K.J.; et al. Convection enhanced delivery of boronated EGF as a molecular targeting agent for neutron capture therapy of brain tumors. J. Neuro-Oncol. 2009, 95, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Barth, R.F.; Wu, G.; Kawabata, S.; Sferra, T.J.; Bandyopadhyaya, A.K.; Tjarks, W.; Ferketich, A.K.; Moeschberger, M.L.; Binns, P.J.; et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin. Cancer Res. 2006, 12, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Wu, G.; Barth, R.F.; Swindall, M.R.; Bandyopadhyaya, A.K.; Tjarks, W.; Tordoff, K.; Moeschberger, M.; Sferra, T.J.; Binns, P.J.; et al. Molecular targeting and treatment of composite EGFR and EGFRvIII-positive gliomas using boronated monoclonal antibodies. Clin. Cancer Res. 2008, 14, 883–891. [Google Scholar] [CrossRef]
- Yang, W.; Barth, R.F.; Wu, G.; Tjarks, W.; Binns, P.; Riley, K. Boron neutron capture therapy of EGFR or EGFRvIII positive gliomas using either boronated monoclonal antibodies or epidermal growth factor as molecular targeting agents. Appl. Radiat. Isot. 2009, 67, S328–S331. [Google Scholar] [CrossRef]
- Available online: https://isnct.net/bnct-boron-neutron-capture-therapy/accelerator-based-bnct-projects-2021/ (accessed on 10 July 2023).
- Nunez, R.; Romero, I.; Teixidor, F.; Vinas, C. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef]
- Sumitani, S.; Nagasaki, Y. Boron neutron capture therapy assisted by boron-conjugated nanoparticles. Polym. J. 2012, 44, 522–530. [Google Scholar] [CrossRef]
- Carlsson, J.; Kullberg, E.B.; Capala, J.; Sjoberg, S.; Edwards, K.; Gedda, L. Ligand liposomes and boron neutron capture therapy. J. Neuro-Oncol. 2003, 62, 47–59. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.L.; Zhang, J.C. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334. [Google Scholar] [CrossRef] [PubMed]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminade, A.-M.; Milewski, M.; Hey-Hawkins, E. Dendritic Structures Functionalized with Boron Clusters, in Particular Carboranes, and Their Biological Properties. Pharmaceutics 2023, 15, 2117. https://doi.org/10.3390/pharmaceutics15082117
Caminade A-M, Milewski M, Hey-Hawkins E. Dendritic Structures Functionalized with Boron Clusters, in Particular Carboranes, and Their Biological Properties. Pharmaceutics. 2023; 15(8):2117. https://doi.org/10.3390/pharmaceutics15082117
Chicago/Turabian StyleCaminade, Anne-Marie, Max Milewski, and Evamarie Hey-Hawkins. 2023. "Dendritic Structures Functionalized with Boron Clusters, in Particular Carboranes, and Their Biological Properties" Pharmaceutics 15, no. 8: 2117. https://doi.org/10.3390/pharmaceutics15082117
APA StyleCaminade, A.-M., Milewski, M., & Hey-Hawkins, E. (2023). Dendritic Structures Functionalized with Boron Clusters, in Particular Carboranes, and Their Biological Properties. Pharmaceutics, 15(8), 2117. https://doi.org/10.3390/pharmaceutics15082117





