Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Design
2.2.1. Induction of Type 2 Diabetes
2.2.2. Rationale of Selection of Salbutamol Dose
2.3. Estimation of Body Weight, Gastrocnemius (GN) Muscle Weight, and Blood Glucose Levels
2.4. Estimation of Body Composition
2.5. Behavioral Parameters
2.5.1. Assessment of Forelimb Grip Strength by Grip Strength Meter
2.5.2. Assessment of Locomotor Activity by Actophotometer Test
2.5.3. Assessment of Muscle Strength by Wire-Hanging Test
2.5.4. Assessment of Muscle Coordination by Rotarod Test
2.5.5. Assessment of Gait Speed by Footprint Test
2.6. Estimation of Total and Myofibrillar Protein Concentration
2.7. Assessment of Oxidative Stress Markers and Antioxidative Status
2.7.1. Estimation of Lipid Peroxidation by Malondialdehyde
2.7.2. Estimation of Protein Carbonyl Content
2.7.3. Estimation of Catalase Activity by H2O2 Decomposition
2.7.4. Estimation of Reduced Glutathione Activity by Ellman’s Reagent
2.7.5. Estimation of Superoxide Dismutase Activity by Pyrogallol Activity
2.8. Estimation of Cellular Toxicity by Histological Analysis
2.9. Estimation of Serum Testosterone, GDF-8, Inflammatory Markers, and Lipid Markers
2.10. 1H NMR-Based GN Muscle Metabolomics Profiling
2.10.1. Sample Preparation
2.10.2. NMR Measurements
2.10.3. Spectral Assignment and Concentration Profiling
2.10.4. Multivariate Data Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of Salbutamol on Blood Glucose Levels, Body Weight, and GN Muscle Weight in HFD/STZ-Induced Diabetic Rats
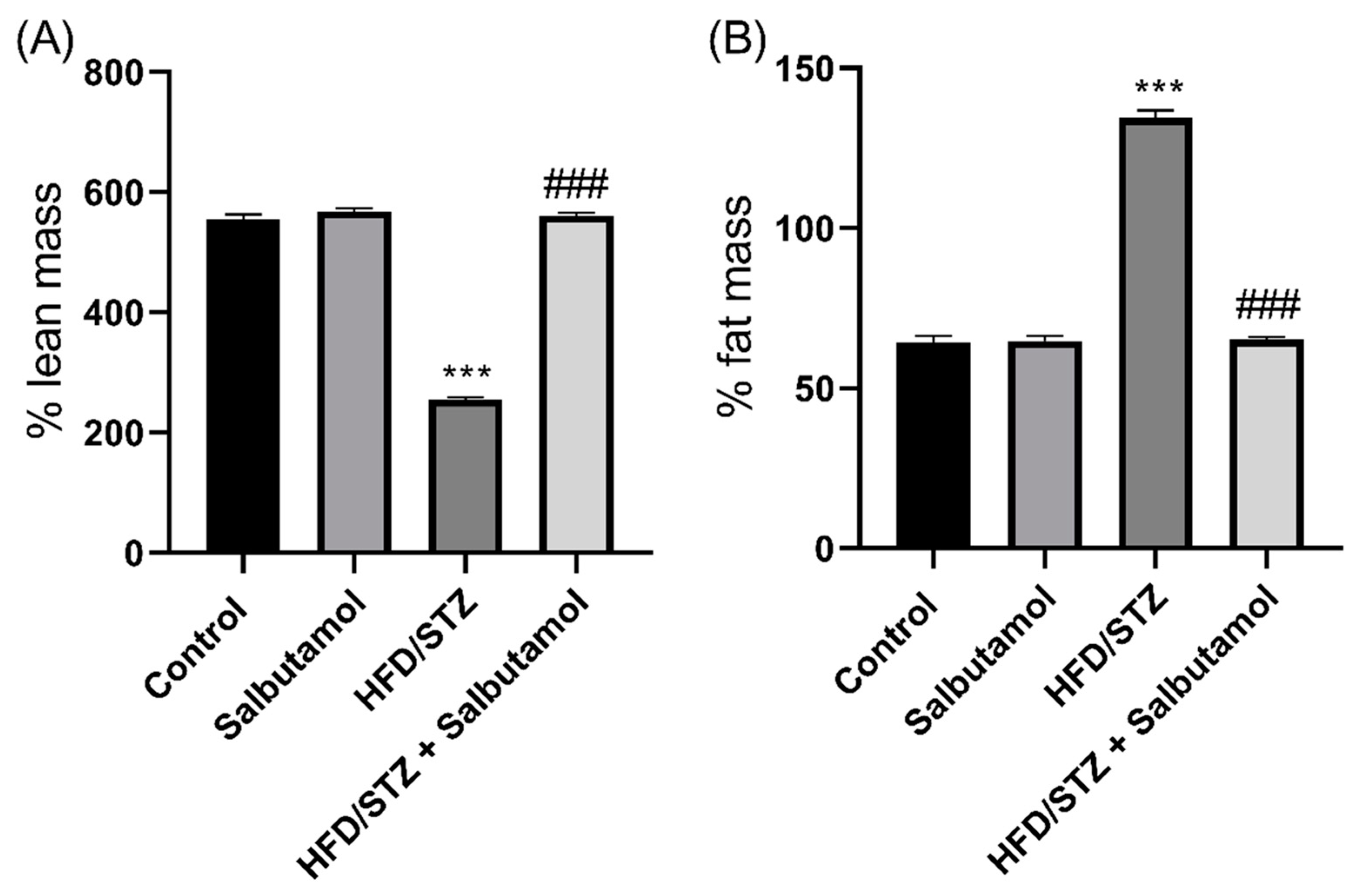
3.2. Effect of Salbutamol on Body Composition in HFD/STZ-Induced Diabetic Rats
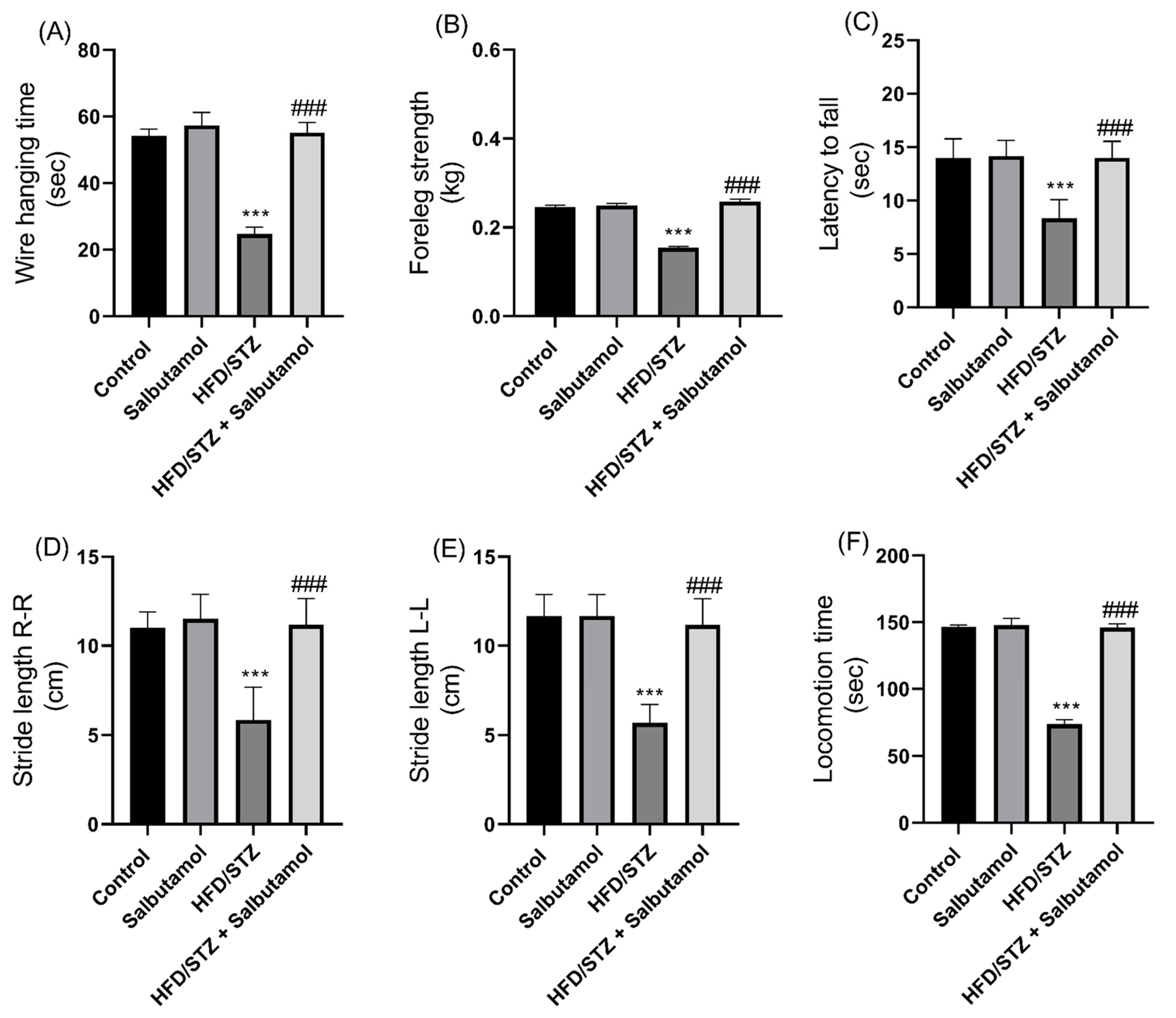
3.3. Effect of Salbutamol on Muscle Strength and Motor Coordination in HFD/STZ-Induced Diabetic Rats
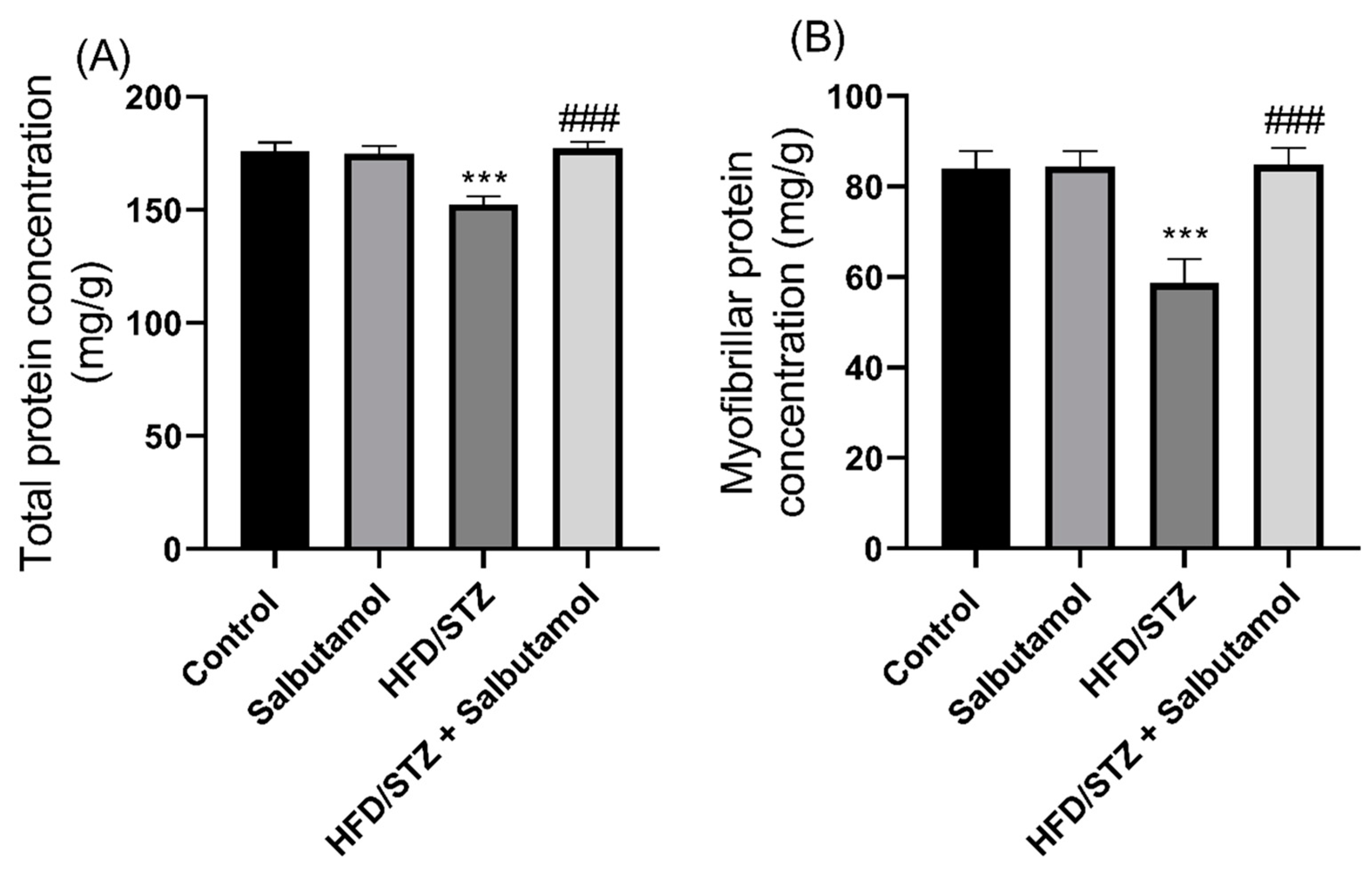
3.4. Effect of Salbutamol on Total and Myofibrillar Protein Concentration in HFD/STZ-Induced Diabetic Rats
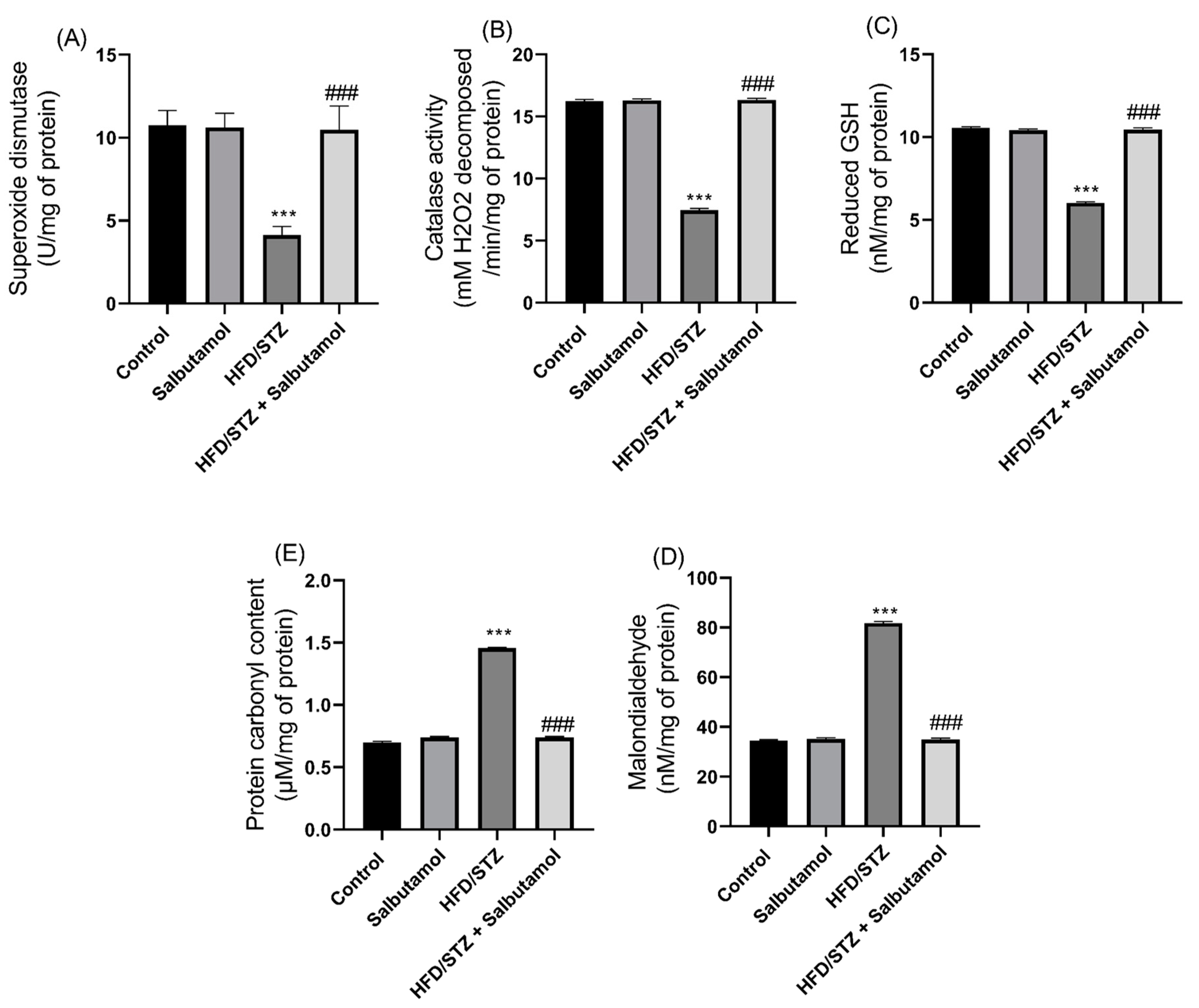
3.5. Effect of Salbutamol on Oxidative Stress and Antioxidant Status in HFD/STZ-Induced Diabetic Rats
3.6. Effect of Salbutamol on the Cellular Architecture of GN Muscle in HFD/STZ-Induced Diabetic Rats
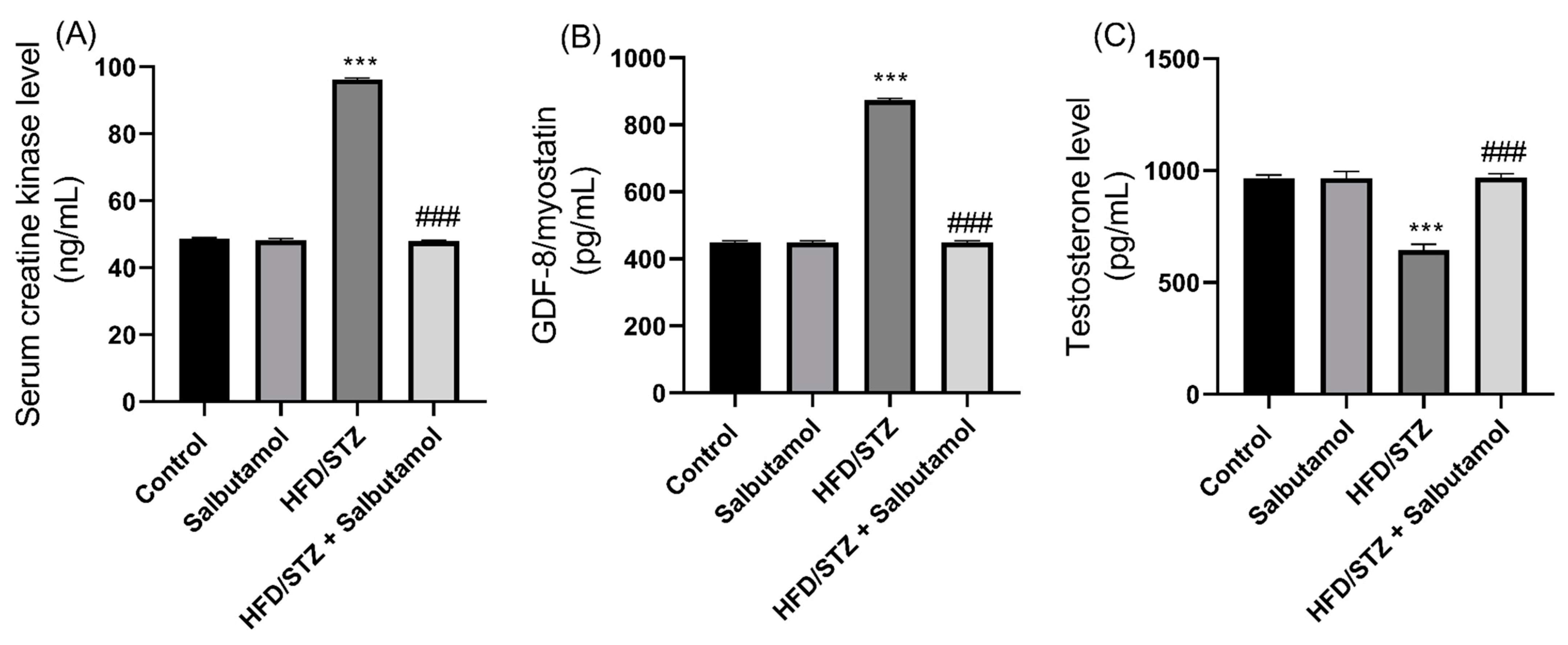
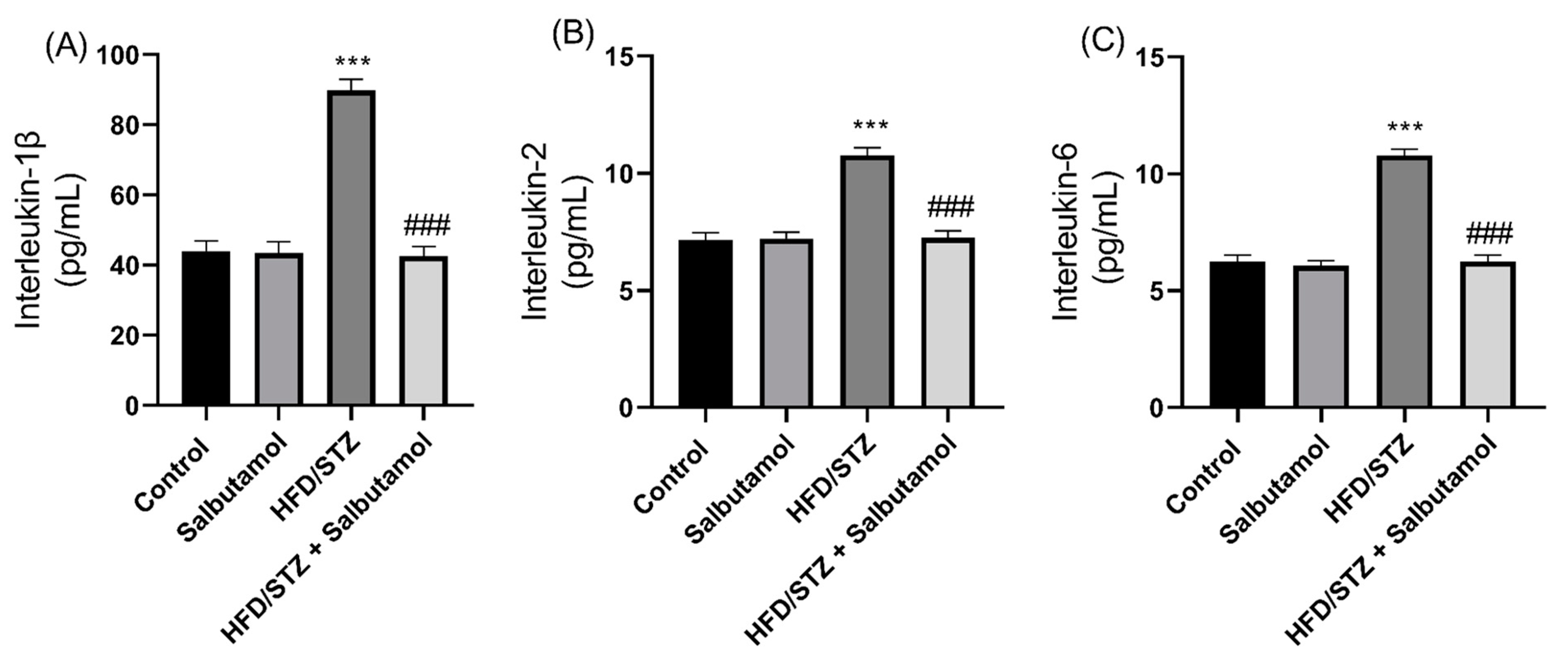
3.7. Effect of Salbutamol on Serum Level of Creatine Kinase, GDF-8, Testosterone, and Pro-Inflammatory Markers in HFD/STZ-Induced Diabetic Rats
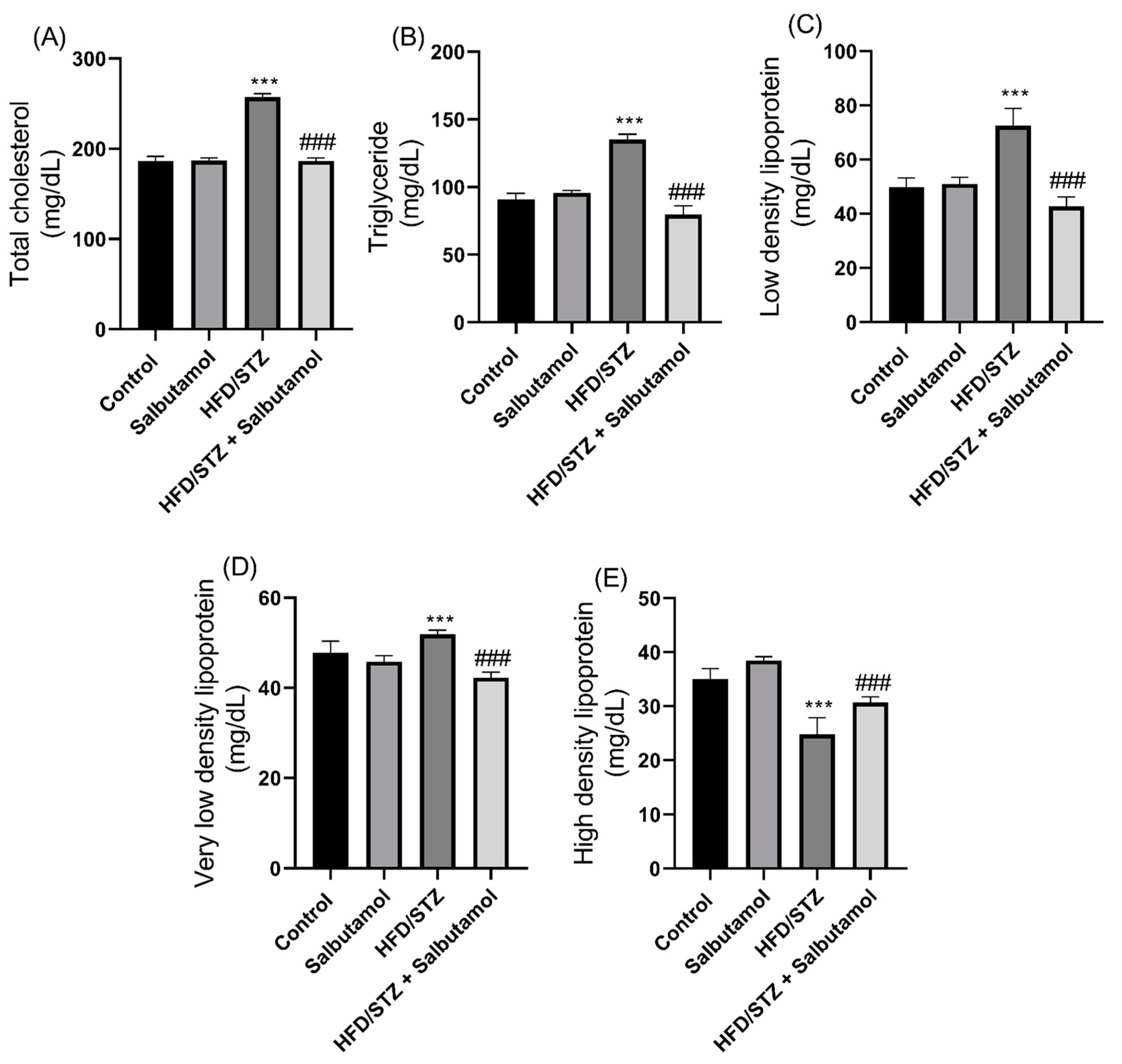
3.8. Effect of Salbutamol on Serum Lipid Profile in HFD/STZ-Induced Diabetic Rats
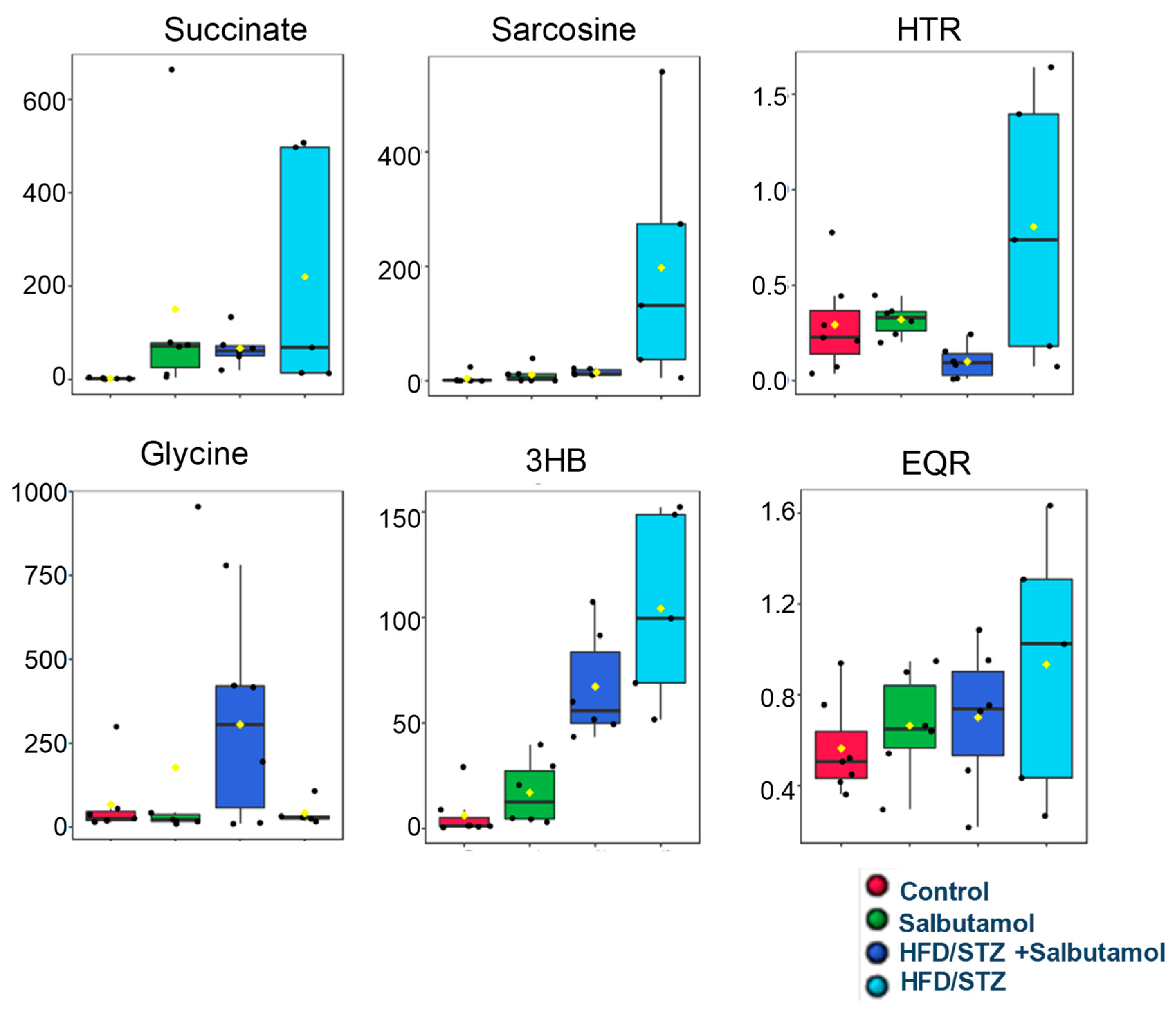
3.9. Effect of Salbutamol on GN Muscle Metabolomics Using 1H NMR-Based Technique in HFD/STZ-Induced Diabetic Rats
3.10. Disturbed Interlinking Metabolic Pathways in Diabetes-Induced Skeletal Muscle Atrophy
4. Discussion
5. Conclusions
6. Limitation of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Souza, D.M.; Al-Sajee, D.; Hawke, T.J. Diabetic myopathy: Impact of diabetes mellitus on skeletal muscle progenitor cells. Front. Physiol. 2013, 4, 379. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ochoa, E.O.; Llanos, P.; Lanner, J.T. The Underlying Mechanisms of Diabetic Myopathy. J. Diabetes Res. 2017, 2017, 7485738. [Google Scholar] [CrossRef] [PubMed]
- Rusbana, T.B.; Agista, A.Z.; Saputra, W.D.; Ohsaki, Y.; Watanabe, K.; Ardiansyah, A.; Budijanto, S.; Koseki, T.; Aso, H.; Komai, M.; et al. Supplementation with Fermented Rice Bran Attenuates Muscle Atrophy in a Diabetic Rat Model. Nutrients 2020, 12, 2409. [Google Scholar] [CrossRef] [PubMed]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know about Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Yang, J. Enhanced skeletal muscle for effective glucose homeostasis. Prog. Mol. Biol. Transl. Sci. 2014, 121, 133–163. [Google Scholar]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R.; Catalano, M.G.; Brignardello, E.; Danni, O.; Boccuzzi, G. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes 2004, 53, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Okun, J.G.; Rusu, P.M.; Chan, A.Y.; Wu, Y.; Yap, Y.W.; Sharkie, T.; Schumacher, J.; Schmidt, K.V.; Roberts-Thomson, K.M.; Russell, R.D.; et al. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat. Metab. 2021, 3, 394–409. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef] [PubMed]
- Price, S.R.; Bailey, J.L.; Wang, X.; Jurkovitz, C.; England, B.K.; Ding, X.; Phillips, L.S.; Mitch, W.E. Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin-proteasome proteolytic pathway by a mechanism including gene transcription. J. Clin. Investig. 1996, 98, 1703–1708. [Google Scholar] [CrossRef]
- Gonçalves, D.A.P.; Lira, E.C.; Baviera, A.M.; Cao, P.; Zanon, N.M.; Arany, Z.; Bedard, N.; Tanksale, P.; Wing, S.S.; Lecker, S.H.; et al. Mechanisms Involved in 3′,5′-Cyclic Adenosine Monophosphate-Mediated Inhibition of the Ubiquitin-Proteasome System in Skeletal Muscle. Endocrinology 2009, 150, 5395–5404. [Google Scholar]
- Gonçalves, D.A.; Silveira, W.A.; Lira, E.C.; Graca, F.A.; Paula-Gomes, S.; Zanon, N.M.; Kettelhut, I.C.; Navegantes, L.C.C. Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. Am. J. Physiol. Endocrinol. Metab. 2012, 302, 123–133. [Google Scholar] [CrossRef]
- Gonçalves, D.A.; Silveira, W.A.; Manfredi, L.H.; Graca, F.A.; Amrani, A.; Bertaggia, E.; Neill, B.T.O.; Lautherbach, N.; Machado, J.; Nogara, J.; et al. Insulin/IGF1 signalling mediates the effects of β(2) -adrenergic agonist on muscle proteostasis and growth. J. Cachexia Sarcopenia Muscle 2019, 10, 455–475. [Google Scholar] [CrossRef]
- Hostrup, M.; Kalsen, A.; Auchenberg, M.; Bangsbo, J.; Backer, V. Effects of acute and 2-week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scand J. Med. Sci. Sports 2016, 26, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.J.; Lynch, M.E. Comparison of the effects of salbutamol and clenbuterol on skeletal muscle mass and carcass composition in senescent rats. Metabolism 1994, 43, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Hostrup, M.; Reitelseder, S.; Jessen, S.; Kalsen, A.; Nyberg, M.; Egelund, J.; Kreiberg, M.; Kristensen, C.M.; Thomassen, M.; Pilegaard, H.; et al. Beta2-adrenoceptor agonist salbutamol increases protein turnover rates and alters signalling in skeletal muscle after resistance exercise in young men. J. Physiol. 2018, 596, 4121–4139. [Google Scholar] [CrossRef] [PubMed]
- Jessen, S.; Reitelseder, S.; Kalsen, A.; Kreiberg, M.; Onslev, J.; Gad, A.; Ørtenblad, N.; Backer, V.; Holm, L.; Bangsbo, J.; et al. β2-Adrenergic agonist salbutamol augments hypertrophy in MHCIIa fibers and sprint mean power output but not muscle force during 11 weeks of resistance training in young men. J. Appl. Physiol. 2021, 130, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Koeberl, D.D.; Case, L.E.; Desai, A.; Smith, E.C.; Walters, C.; Han, S.-O.; Thurberg, B.L.; Young, S.P.; Bali, D.; Kishnani, P.S. Improved muscle function in a phase I/II clinical trial of albuterol in Pompe disease. Mol. Genet. Metab. 2020, 129, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Fauman, E.; Erte, I.; Perry, J.R.; Kastenmüller, G.; Shin, S.Y.; Petersen, A.K.; Hyde, C.; Psatha, M.; Ward, K.J.; et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013, 62, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ortiz, M.; Nuñez Ramos, P.; Oregioni, A.; Claus, S.P. NMR metabolomics identifies over 60 biomarkers associated with Type II Diabetes impairment in db/db mice. Metabolomics 2019, 15, 89. [Google Scholar] [CrossRef]
- Del Coco, L.; Vergara, D.; De Matteis, S.; Mensà, E.; Sabbatinelli, J.; Prattichizzo, F.; Bonfigli, A.R.; Storci, G.; Bravaccini, S.; Pirini, F.; et al. NMR-Based Metabolomic Approach Tracks Potential Serum Biomarkers of Disease Progression in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2019, 8, 720. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.S.; Jensen, J.M.; Jakobsen, J.; Ulhøj, B.P.; Andersen, H. Striated muscle fiber size, composition, and capillary density in diabetes in relation to neuropathy and muscle strength. J. Diabetes 2014, 6, 462–471. [Google Scholar] [CrossRef]
- Ato, S.; Kido, K.; Sato, K.; Fujita, S. Type 2 diabetes causes skeletal muscle atrophy but does not impair resistance training-mediated myonuclear accretion and muscle mass gain in rats. Exp. Physiol. 2019, 104, 1518–1531. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, B.; Halici, Z.; Cadirci, E.; Karagoz, E.; Bayraktutan, Z.; Yayla, M.; Akpinar, E.; Atamanalp, S.S.; Unal, D.; Karamese, M. Comparative study on effects of nebulized and oral salbutamol on a cecal ligation and puncture-induced sepsis model in rats. Drug Res. 2015, 65, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Caccia, S.; Fong, M.H. Kinetics and distribution of the β-adrenergic agonist salbutamol in rat brain. J. Pharm. Pharmacol. 1984, 36, 200–202. [Google Scholar] [CrossRef]
- Nixon, J.P.; Zhang, M.; Wang, C.; Kuskowski, M.A.; Novak, C.M.; Levine, J.A.; Billington, C.J.; Kotz, C.M. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity 2010, 18, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- China, S.P.; Pal, S.; Chattopadhyay, S.; Porwal, K.; Kushwaha, S.; Bhattacharyya, S.; Mittal, M.; Gurjar, A.A.; Barbhuyan, T.; Singh, A.K.; et al. Globular adiponectin reverses osteo-sarcopenia and altered body composition in ovariectomized rats. Bone 2017, 105, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O.A.; Tilson, H.A.; Byrd, W.C.; Riley, M.T. A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav. Toxicol. 1979, 1, 233–236. [Google Scholar]
- Cabe, P.A.; Tilson, H.A.; Mitchell, C.L.; Dennis, R. A simple recording grip strength device. Pharmacol. Biochem. Behav. 1978, 8, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.; Saraswat, N.; Chandra, P.; Khalid, M.; Kabra, A. Isolation of Thymol from Trachyspermum ammi Fruits for Treatment of Diabetes and Diabetic Neuropathy in STZ-Induced Rats. Biomed. Res. Int. 2022, 2022, 8263999. [Google Scholar] [CrossRef]
- Mu, S.; OuYang, L.; Liu, B.; Zhu, Y.; Li, K.; Zhan, M.; Liu, Z.; Jia, Y.; Lei, W.; Reiner, A. Preferential interneuron survival in the transition zone of 3-NP-induced striatal injury in rats. J. Neurosci. Res. 2011, 89, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.S.; Bartos, I.; Márka, Z.; Akay, T.; Márka, S.; Mann, R.S. Quantification of gait parameters in freely walking rodents. BMC Biol. 2015, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Gehrig, S.M.; Léger, B.; Trieu, J.; Walrand, S.; Murphy, K.T.; Lynch, G.S. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice. J. Physiol. 2010, 588, 4811–4823. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, T.A., Jr.; Farrar, R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Wehr, N.B.; Levine, R.L. Quantification of protein carbonylation. Methods Mol. Biol. 2013, 965, 265–281. [Google Scholar] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Kinugawa, S.; Hirabayashi, K.; Suga, T.; Yokota, T.; Takahashi, M.; Fukushima, A.; Homma, T.; Ono, T.; Sobirin, M.A.; et al. Angiotensin II receptor blocker improves the lowered exercise capacity and impaired mitochondrial function of the skeletal muscle in type 2 diabetic mice. J. Appl. Physiol. 2013, 114, 844–857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Guleria, A.; Misra, D.P.; Rawat, A.; Dubey, D.; Khetrapal, C.L.; Bacon, P.; Misra, R.; Kumar, D. NMR-Based Serum Metabolomics Discriminates Takayasu Arteritis from Healthy Individuals: A Proof-of-Principle Study. J. Proteome Res. 2015, 14, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0--The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Guleria, A.; Bajpai, N.K.; Rawat, A.; Khetrapal, C.L.; Prasad, N.; Kumar, D. Metabolite characterisation in peritoneal dialysis effluent using high-resolution (1) H and (1) H-(13) C NMR spectroscopy. Magn. Reson. Chem. 2014, 52, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Guleria, A.; Pratap, A.; Dubey, D.; Rawat, A.; Chaurasia, S.; Sukesh, E.; Phatak, S.; Ajmani, S.; Kumar, U.; Khetrapal, C.L.; et al. NMR based serum metabolomics reveals a distinctive signature in patients with Lupus Nephritis. Sci. Rep. 2016, 6, 35309. [Google Scholar] [CrossRef] [PubMed]
- Walejko, J.M.; Chelliah, A.; Keller-Wood, M.; Gregg, A.; Edison, A.S. Global Metabolomics of the Placenta Reveals Distinct Metabolic Profiles between Maternal and Fetal Placental Tissues Following Delivery in Non-Labored Women. Metabolites 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Prakash, V.; Gupta, N.; Kumar, A.; Kant, R.; Kumar, D. Serum Metabolic Disturbances in Lung Cancer Investigated through an Elaborative NMR-Based Serum Metabolomics Approach. ACS Omega 2022, 7, 5510–5520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Kumar, A.; Singh, S.; Arya, P.; Singh, S.K.; Chaurasia, R.N.; Singh, A.; Kumar, D. An elaborative NMR based plasma metabolomics study revealed metabolic derangements in patients with mild cognitive impairment: A study on north Indian population. Metab. Brain Dis. 2021, 36, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Sharma, S.; Durgappa, M.; Gupta, N.; Raj, R.; Kumar, A.; Sharma, P.N.; Krishna, V.P.; Kumar, R.V.; Guleria, A.; et al. Serum Metabolic Disturbances Associated with Acute-on-chronic Liver Failure in Patients with Underlying Alcoholic Liver Diseases: An Elaborative NMR-based Metabolomics Study. J. Pharm. Bioallied Sci. 2021, 13, 276–282. [Google Scholar] [PubMed]
- Cassiède, M.; Nair, S.; Dueck, M.; Mino, J.; McKay, R.; Mercier, P.; Quémerais, B.; Lacy, P. Assessment of (1)H NMR-based metabolomics analysis for normalization of urinary metals against creatinine. Clin. Chim. Acta 2017, 464, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Costa Rosa, L.F. The effect of BCAA supplementation upon the immune response of triathletes. Med. Sci. Sports Exerc. 2000, 32, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Mamyrova, G.; Targoff, I.N.; Huber, A.M.; Malley, J.D.; Rice, M.M.; Miller, F.W.; Rider, L.G. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine 2013, 92, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Cao, Y.; Zhang, Y.; Liu, J.; Bao, Y.; Wang, C.; Jia, W.; Zhao, A. Random forest in clinical metabolomics for phenotypic discrimination and biomarker selection. Evid. Based Complement. Altern. Med. 2013, 2013, 298183. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.W.; Rothwell, N.J.; Stock, M.J.; Winter, P.D. Chronic effects of beta 2-adrenergic agonists on body composition and protein synthesis in the rat. Biosci. Rep. 1984, 4, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Collomp, K.; Candau, R.; Lasne, F.; Labsy, Z.; Préfaut, C.; De Ceaurriz, J. Effects of short-term oral salbutamol administration on exercise endurance and metabolism. J. Appl. Physiol. 2000, 89, 430–436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Nielsen, A.R.; Fischer, C.P.; Hansen, J.; Pedersen, B.K.; Plomgaard, P. Plasma and muscle myostatin in relation to type 2 diabetes. PLoS ONE 2012, 7, e37236. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Singh, A.; Phogat, J.; Dahuja, A.; Dabur, R. Magnoflorine prevent the skeletal muscle atrophy via Akt/mTOR/FoxO signal pathway and increase slow-MyHC production in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2021, 267, 113510. [Google Scholar] [CrossRef] [PubMed]
- Uzkeser, H.; Cadirci, E.; Halici, Z.; Odabasoglu, F.; Polat, B.; Yuksel, T.N.; Ozaltin, S.; Atalay, F. Anti-inflammatory and antinociceptive effects of salbutamol on acute and chronic models of inflammation in rats: Involvement of an antioxidant mechanism. Mediat. Inflamm. 2012, 2012, 438912. [Google Scholar] [CrossRef]
- Arneson-Wissink, P.C.; Hogan, K.A.; Ducharme, A.M.; Samani, A.; Jatoi, A.; Doles, J.D. The wasting-associated metabolite succinate disrupts myogenesis and impairs skeletal muscle regeneration. JCSM Rapid Commun. 2020, 3, 56–69. [Google Scholar] [CrossRef]
- Jessen, S.; Baasch-Skytte, T.; Onslev, J.; Eibye, K.; Backer, V.; Bangsbo, J.; Hostrup, M. Muscle hypertrophic effect of inhaled beta2-agonist is associated with augmented insulin-stimulated whole-body glucose disposal in young men. J. Physiol. 2022, 600, 2345–2357. [Google Scholar] [CrossRef]
- Lee, S.; Xu, H.; Van Vleck, A.; Mawla, A.M.; Li, A.M.; Ye, J.; Huising, M.O.; Annes, J.P. β-Cell Succinate Dehydrogenase Deficiency Triggers Metabolic Dysfunction and Insulinopenic Diabetes. Diabetes 2022, 71, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stancáková, A.; Pihlajamäki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, H.; Kumar, D.; Dubey, D.; Kumar, S.; Chaurasia, S.; Guleria, A.; Majumder, S.; Singh, R.; Agarwal, V.; Misra, R. Metabolomics analysis revealed significantly higher synovial Phe/Tyr ratio in reactive arthritis and undifferentiated spondyloarthropathy. Rheumatology 2020, 59, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Wannemacher, R.W., Jr.; Klainer, A.S.; Dinterman, R.E.; Beisel, W.R. The significance and mechanism of an increased serum phenylalanine-tyrosine ratio during infection. Am. J. Clin. Nutr. 1976, 29, 997–1006. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Ianuzzo, C.D. Decay of succinate dehydrogenase activity in rat skeletal muscle following streptozotocin injection. Horm. Metab. Res. 1976, 8, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Dollet, L.; Kuefner, M.; Caria, E.; Rizo-Roca, D.; Pendergrast, L.; Abdelmoez, A.M.; Karlsson, H.K.R.; Björnholm, M.; Dalbram, E.; Treebak, J.T.; et al. Glutamine Regulates Skeletal Muscle Immunometabolism in Type 2 Diabetes. Diabetes 2022, 71, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Benarrosh, A.; Garnotel, R.; Henry, A.; Arndt, C.; Gillery, P.; Motte, J.; Bakchine, S. A young adult with sarcosinemia. No benefit from long duration treatment with memantine. JIMD Rep. 2013, 9, 93–96. [Google Scholar] [PubMed]
- Huang, C.F.; Chen, A.; Lin, S.Y.; Cheng, M.L.; Shiao, M.S.; Mao, T.Y. A metabolomics approach to investigate the proceedings of mitochondrial dysfunction in rats from prediabetes to diabetes. Saudi J. Biol. Sci. 2021, 28, 4762–4769. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, B.; Macchione, B.; Monteleone, M.; Naccarato, A.; Sindona, G.; Tagarelli, A. Sarcosine as a marker in prostate cancer progression: A rapid and simple method for its quantification in human urine by solid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2011, 400, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.O.; Arias, E.; Diaz, A.; Burgos, E.S.; Guan, F.; Tiano, S.; Mao, K.; Green, C.L.; Qiu, Y.; Shah, H.; et al. Sarcosine Is Uniquely Modulated by Aging and Dietary Restriction in Rodents and Humans. Cell Rep. 2018, 25, 663–676.e666. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Blondin, D.P.; Hooks, B.G.; Parmar, G.; Alecu, I.; Patten, D.A.; Cuillerier, A.; O’Dwyer, C.; Thrush, A.B.; Fullerton, M.D.; et al. Exercise training enhances muscle mitochondrial metabolism in diet-resistant obesity. eBioMedicine 2022, 83, 104192. [Google Scholar] [CrossRef]


| Parameters | Time Points | Control | Salbutamol | HFD/STZ | HFD/STZ + Salbutamol |
|---|---|---|---|---|---|
| Blood Glucose level (mg/dL) | 0 week | 124.83 ± 1.47 | 124.33 ± 1.21 | 124.66 ± 3.26 | 124.66 ± 2.94 |
| 4 weeks | 124.83 ± 1.94 | 125.16 ± 1.72 | 371.16 ± 14.90 *** | 370.83 ± 15.43 ns | |
| Body weight (g) | 0 week | 185.16 ± 2.31 | 194.50 ± 3.08 | 184.50 ± 2.07 | 175.00 ± 3.03 |
| 4 weeks | 206.66 ± 2.80 | 226.66 ± 1.96 | 306.33 ± 3.01 *** | 203.50 ± 1.51 ### | |
| GN muscle weight (mg) | 4 weeks | 783.50 ± 2.88 | 804.66 ± 2.73 | 338.66 ± 2.50 *** | 776.16 ± 6.76 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Prajapati, P.; Singh, G.; Kumar, D.; Mishra, V.; Kim, S.-C.; Raorane, C.J.; Raj, V.; Kushwaha, S. Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats. Pharmaceutics 2023, 15, 2101. https://doi.org/10.3390/pharmaceutics15082101
Kumar A, Prajapati P, Singh G, Kumar D, Mishra V, Kim S-C, Raorane CJ, Raj V, Kushwaha S. Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats. Pharmaceutics. 2023; 15(8):2101. https://doi.org/10.3390/pharmaceutics15082101
Chicago/Turabian StyleKumar, Anand, Priyanka Prajapati, Gurvinder Singh, Dinesh Kumar, Vikas Mishra, Seong-Cheol Kim, Chaitany Jayprakash Raorane, Vinit Raj, and Sapana Kushwaha. 2023. "Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats" Pharmaceutics 15, no. 8: 2101. https://doi.org/10.3390/pharmaceutics15082101
APA StyleKumar, A., Prajapati, P., Singh, G., Kumar, D., Mishra, V., Kim, S.-C., Raorane, C. J., Raj, V., & Kushwaha, S. (2023). Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats. Pharmaceutics, 15(8), 2101. https://doi.org/10.3390/pharmaceutics15082101











