Factors That Influence Sustained Release from Hot-Melt Extrudates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Hot-Melt Extrusion
2.2.2. Homogeneity of Flurbiprofen Content in the Extrudates
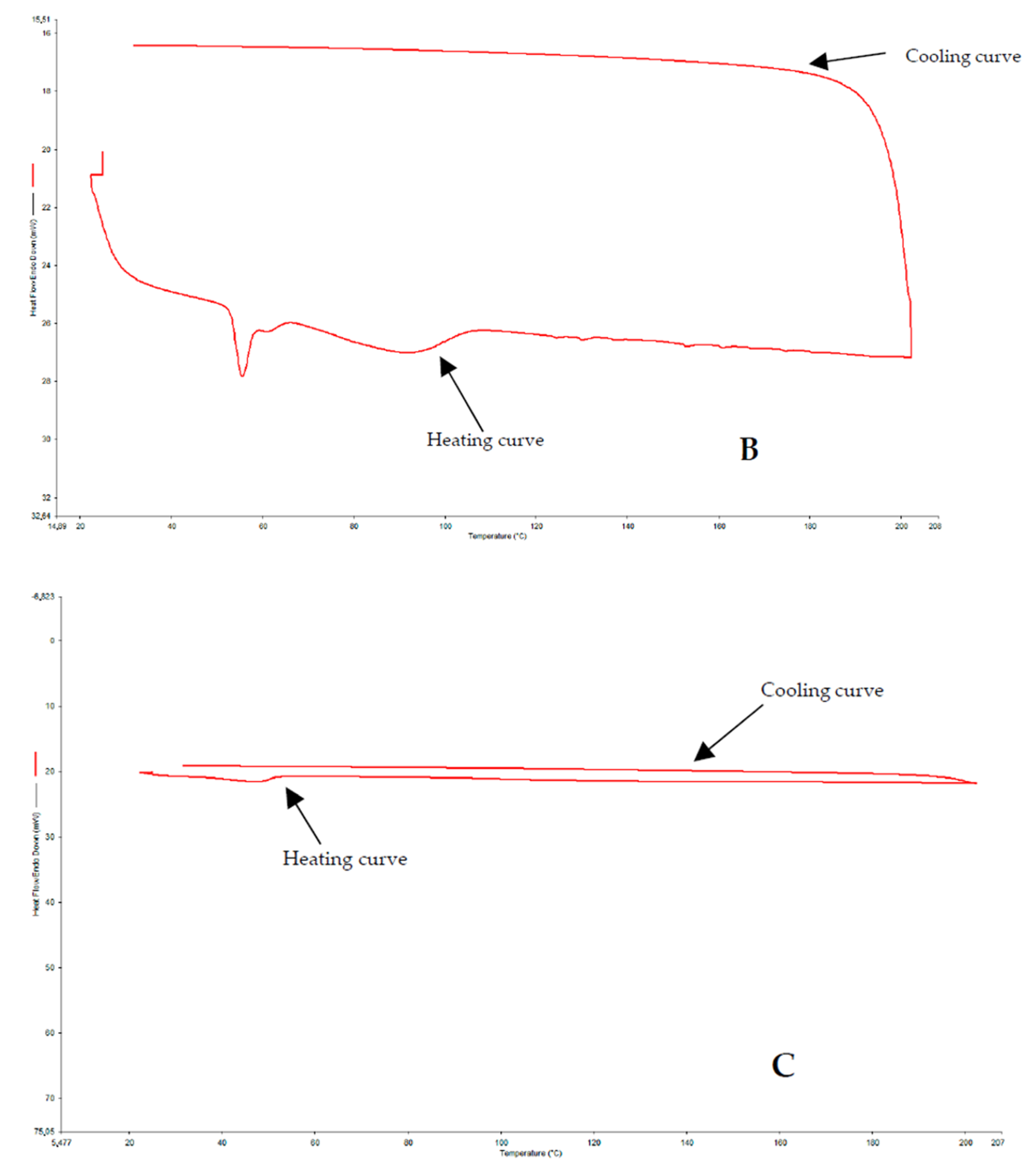
2.2.3. Differential Scanning Calorimetry (DSC)
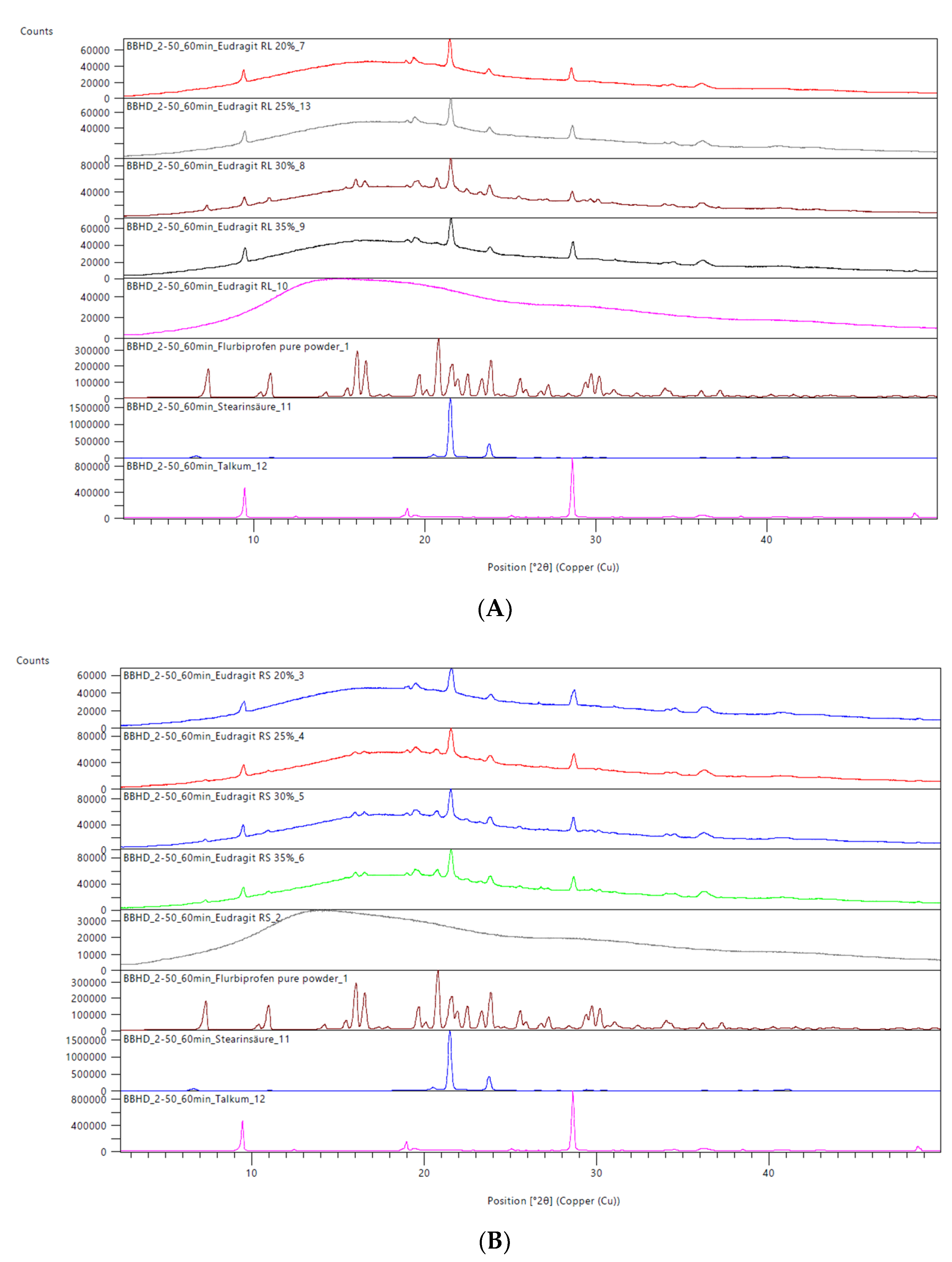
2.2.4. X-ray Powder Diffraction Analysis (XRPD)
2.2.5. Milling of the Extrudates into Various Particle Size Ranges
2.2.6. Dissolution Testing
2.2.7. HPLC Analysis
2.2.8. Data Presentation and Statistics
3. Results
3.1. Hot-Melt Extrusion
3.2. Content Uniformity
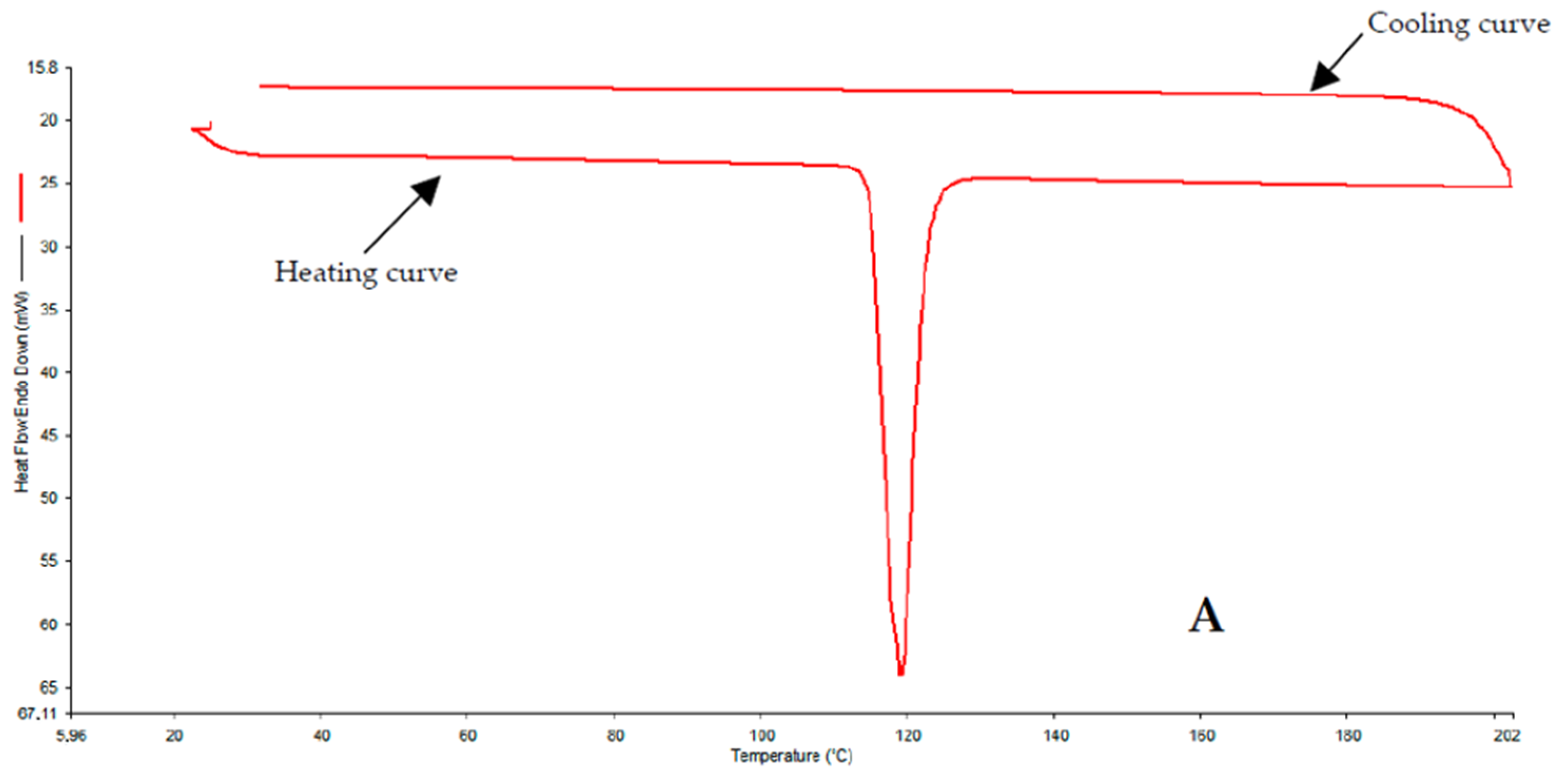
3.3. DSC
3.4. X-ray Powder Diffraction Analysis
3.5. Dissolution Testing
3.5.1. Dissolution Testing of the Eudragit RL Extrudates: Drug Loading Effect
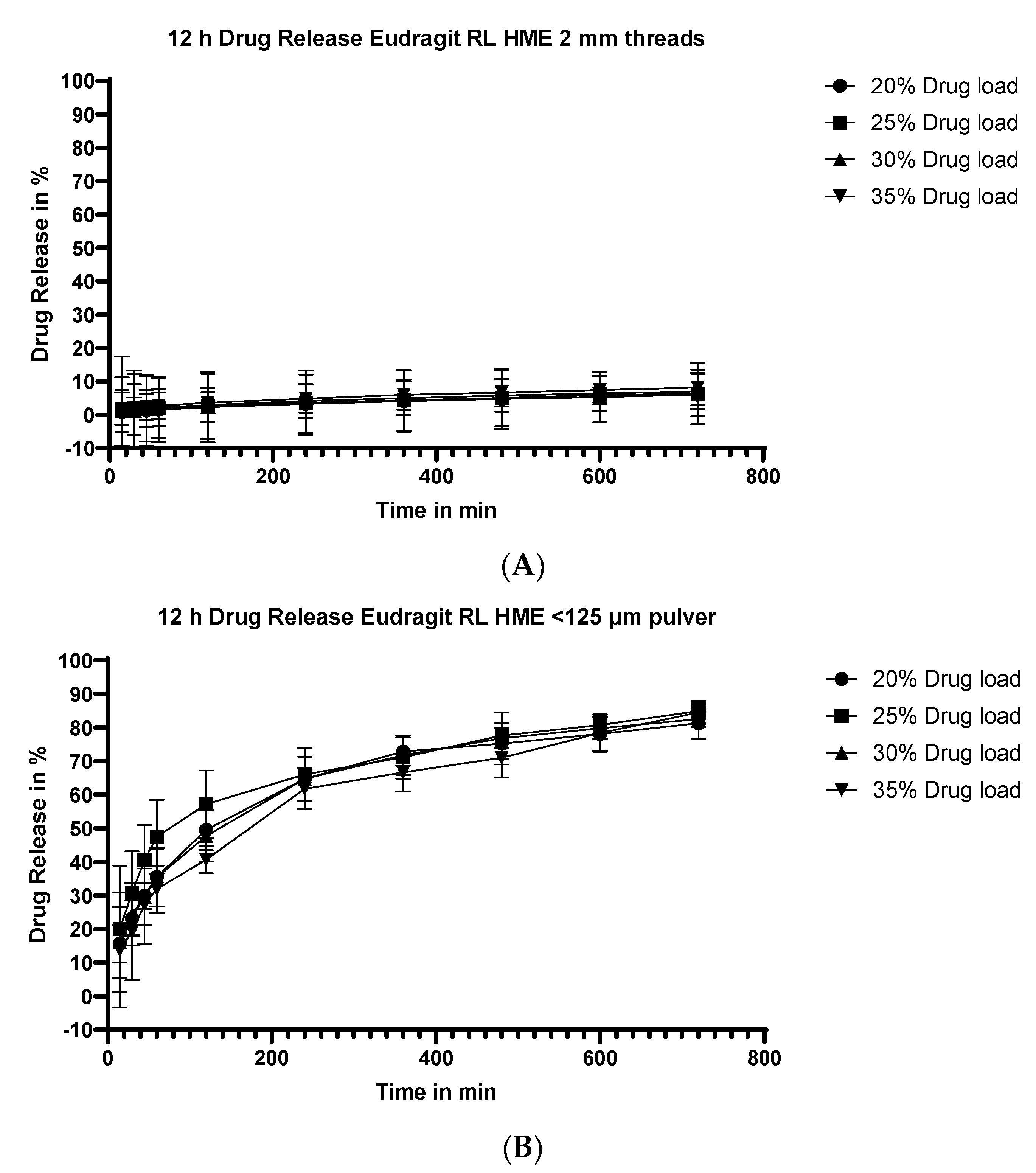
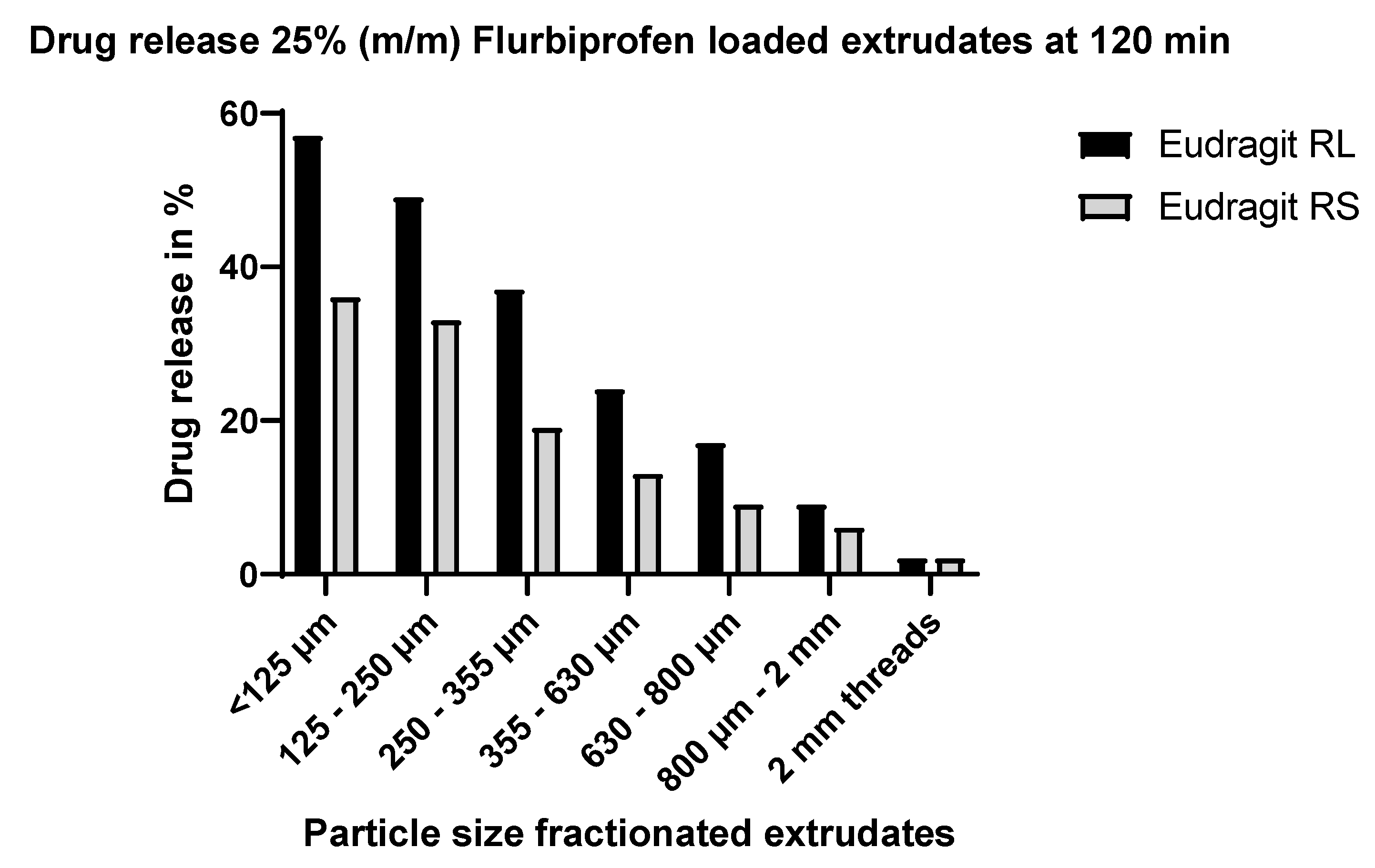
3.5.2. Dissolution Testing of the Eudragit RL Extrudates: Effect of Particle Size

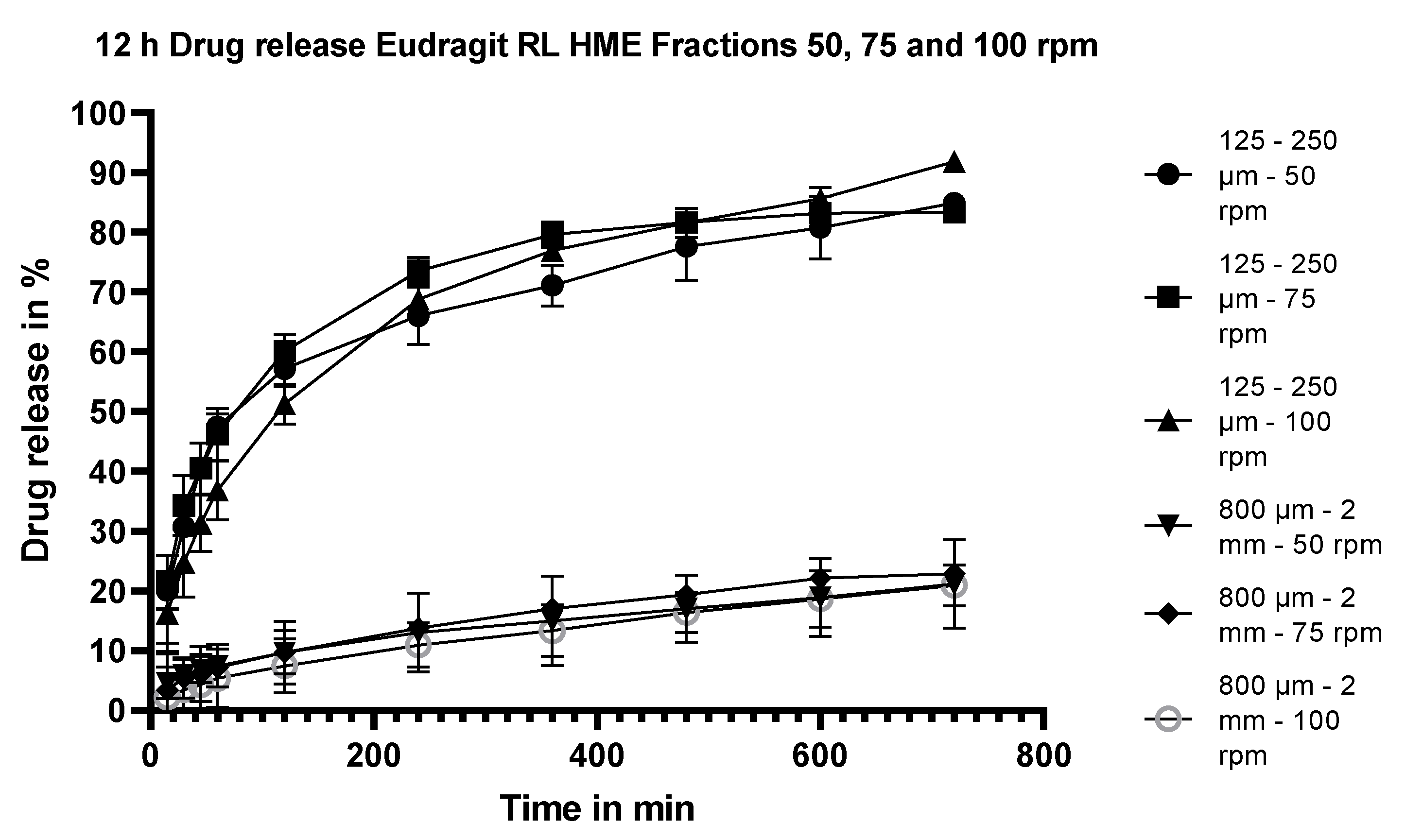
3.5.3. Dissolution Testing of the Eudragit RL Extrudates: Effect of Stirring Rate
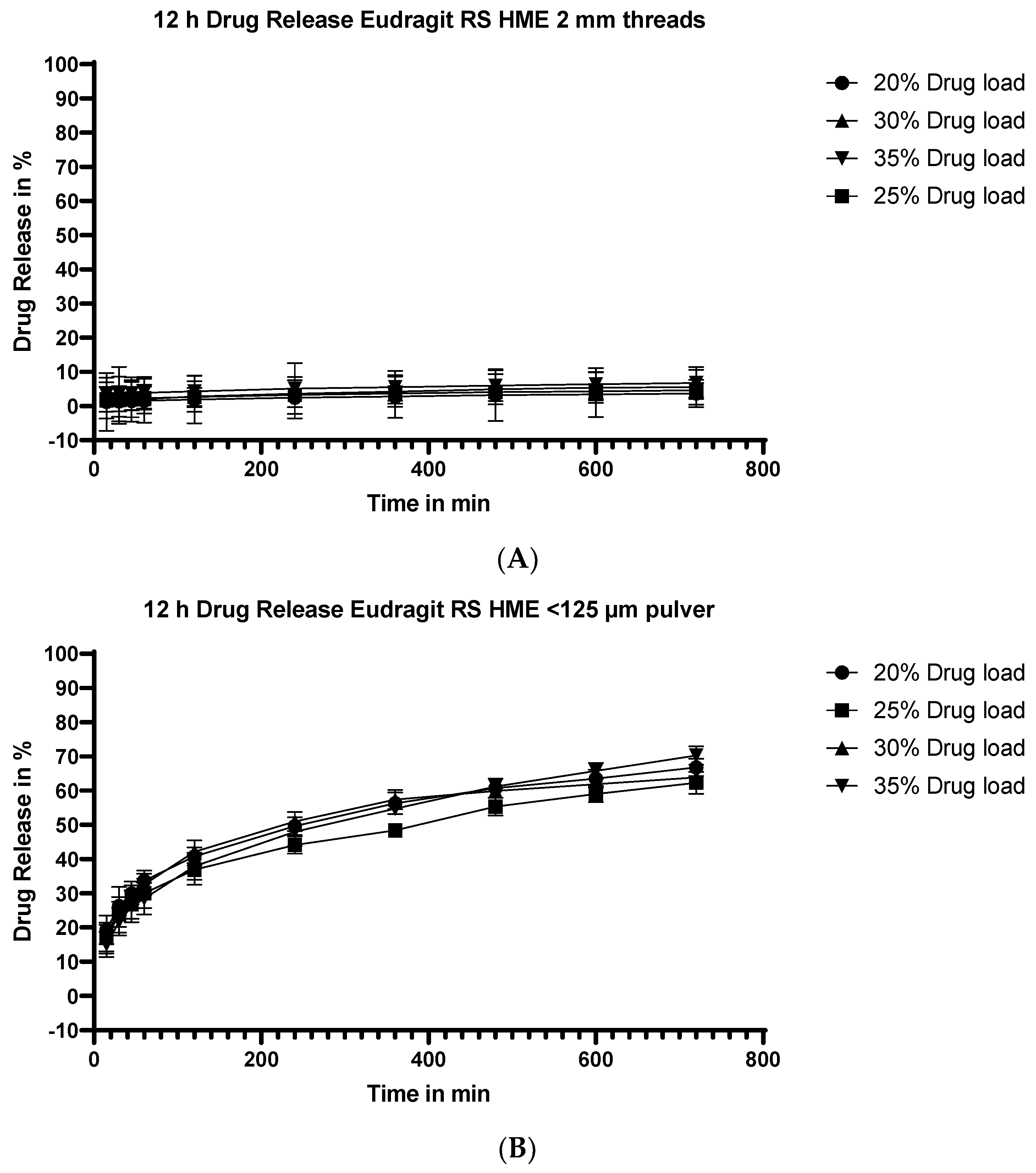
3.5.4. Dissolution Testing of the Eudragit RS Extrudates: Effect of Drug Load
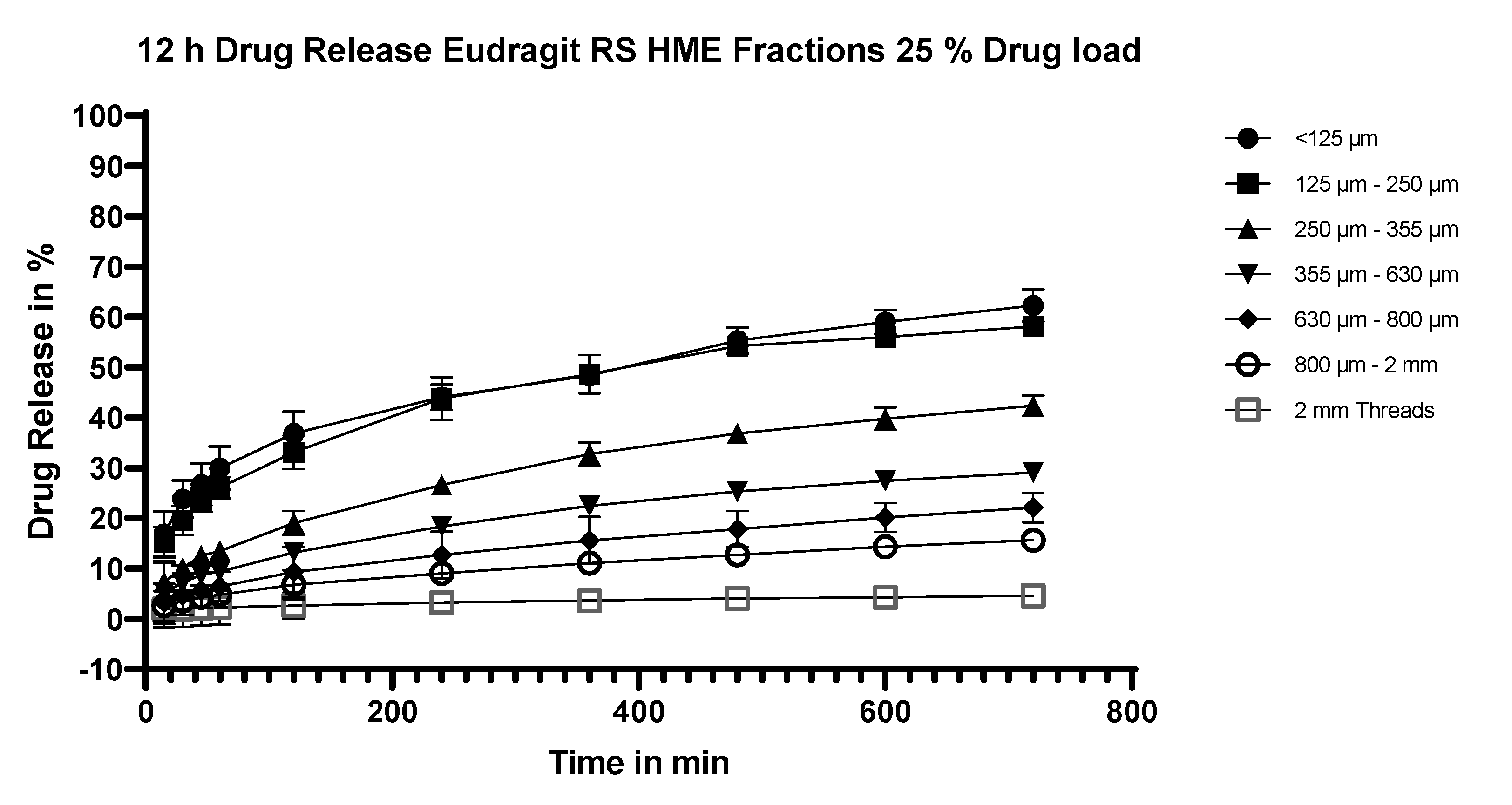
3.5.5. Dissolution Testing of the Eudragit RS Extrudates: Effect of Particle Size
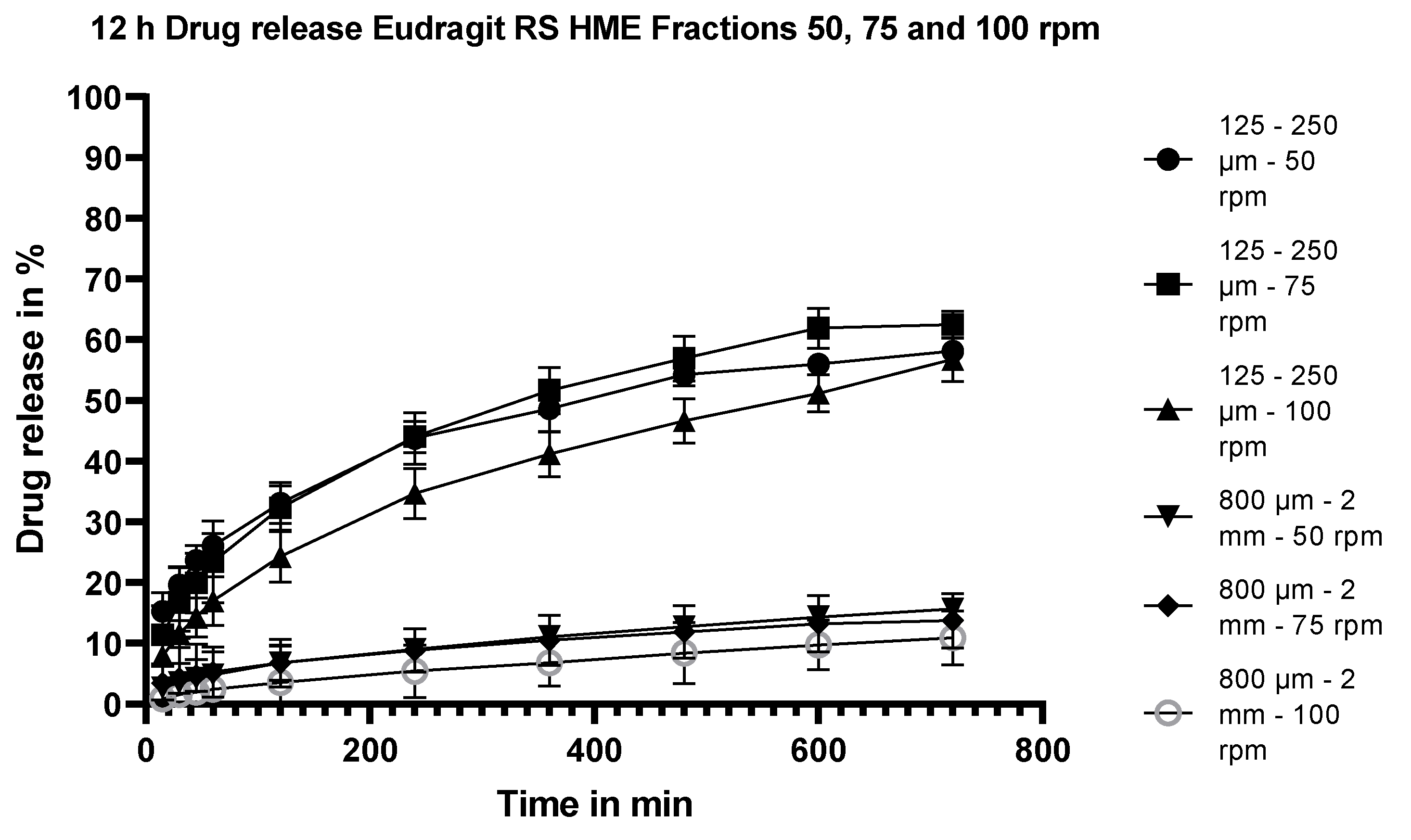
3.5.6. Dissolution Testing of the Eudragit RS Extrudates: Effect of Stirring Rate
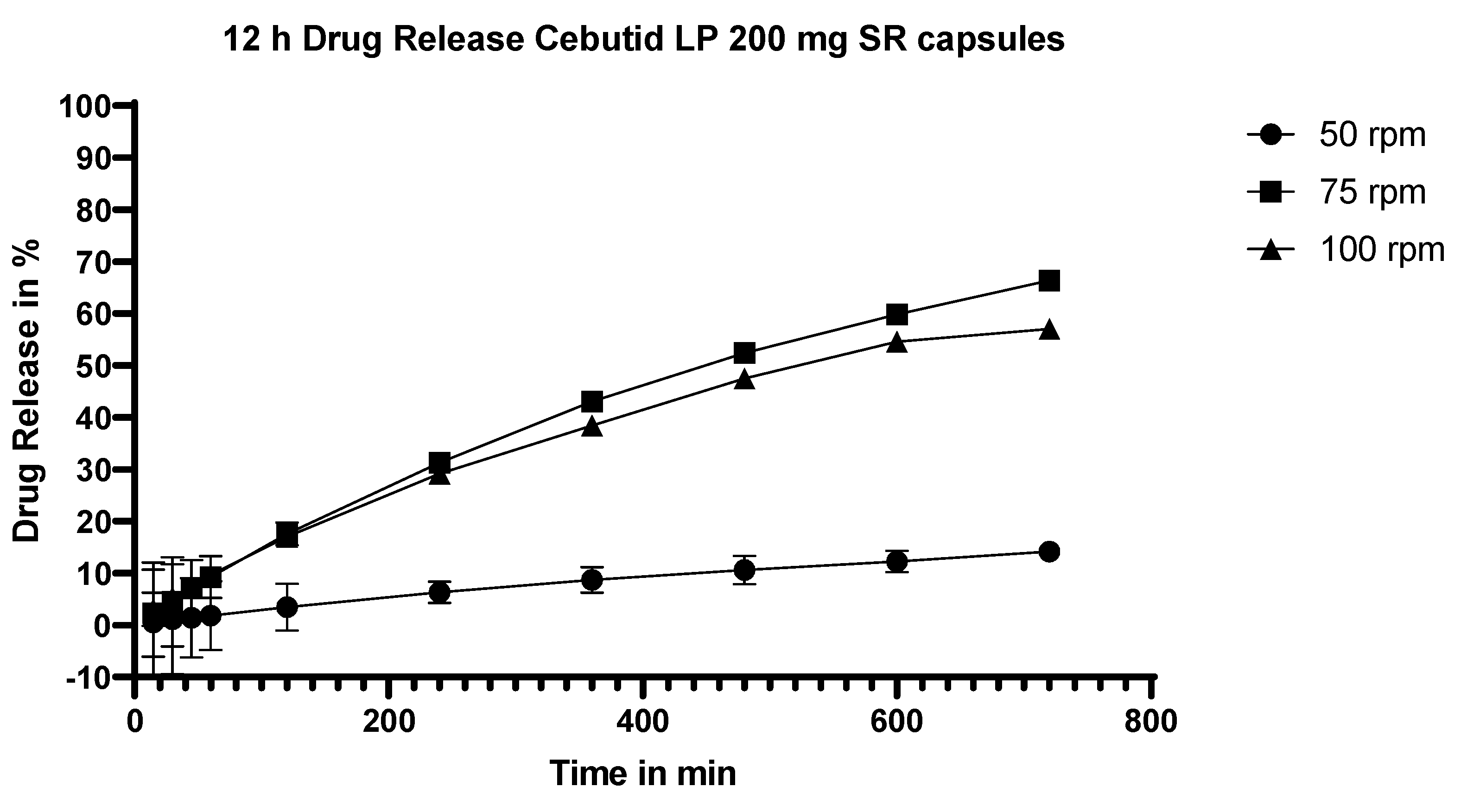
3.5.7. Dissolution Testing of the Commercial Product, Cebutid LP
4. Discussion
4.1. DSC and XRPD
4.2. Loading Capacity
4.3. Dissolution Testing
4.3.1. Effect of Particle Size
4.3.2. Effect of API Load
4.3.3. Effect of Polymer Type
4.3.4. Effect of Stirring Speed on Release from Extrudates
4.3.5. Commercial Product vs. Extrudates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Aita, I.; Ponsar, H.; Quodbach, J. A Critical Review on 3D-printed Dosage Forms. Curr. Pharm. Des. 2018, 24, 4957–4978. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.; Lee, B.-J.; Tran, T.T. Recent studies on the processes and formulation impacts in the development of solid dispersions by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2021, 164, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S.; Jain, D.; Agarwal, Y.; Amin, P. Hot-melt extrusion: Highlighting recent advances in pharmaceutical applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102452. [Google Scholar] [CrossRef]
- Cantin, O.; Siepmann, F.; Willart, J.; Danede, F.; Karrout, Y. PEO hot melt extrudates for controlled drug delivery: Importance of the type of drug and loading. J. Drug Deliv. Sci. Technol. 2021, 61, 102238. [Google Scholar] [CrossRef]
- Patki, M.; Palekar, S.; Nukala, P.K.; Vartak, R.; Patel, K. Overdose and Alcohol Sensitive Immediate Release System (OASIS) for Deterring Accidental Overdose or Abuse of Drugs. AAPS PharmSciTech 2021, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Emam, M.F.; Taha, N.F.; Mursi, N.M.; Emara, L.H. Preparation, characterization and in-Vitro/in-Vivo evaluation of meloxicam extruded pellets with enhanced bioavailability and stability. Drug Dev. Ind. Pharm. 2021, 47, 163–175. [Google Scholar] [CrossRef]
- Dumpa, N.R.; Sarabu, S.; Bandari, S.; Zhang, F.; Repka, M.A. Chronotherapeutic Drug Delivery of Ketoprofen and Ibuprofen for Improved Treatment of Early Morning Stiffness in Arthritis Using Hot-Melt Extrusion Technology. AAPS PharmSciTech 2018, 19, 2700–2709. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dumpa, N.; Bandari, S.; Durig, T.; Repka, M.A. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech 2020, 21, 243. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/psusa/flurbiprofen-list-nationally-authorised-medicinal-products-psusa/00001450/201911_en.pdf (accessed on 14 May 2023).
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Flurbiprofen: A review of its pharmacological properties and therapeutic use in rheumatic diseases. Drugs 1979, 18, 417–438. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.C.; Schuna, A.A.; Sundstrom, W.R. A Review of Selected Investigational Nonsteroidal Antiinflammatory Drugs of the 1980s. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1986, 6, 10–25. [Google Scholar] [CrossRef]
- Benvenuti, C.; Guidoni, G.; Longoni, A.; Mordini, M. Controlled study on flurbiprofen and diclofenac in the treatment of rheu-matic disorders. Int. J. Tissue React. 1983, 5, 61–65. [Google Scholar]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3394#section=Metabolite-Description (accessed on 14 May 2023).
- Carli, F.; Capone, G.; Colombo, I.; Magarotto, L.; Motta, A. Surface and transport properties of acrylic polymers influencing drug release from porous matrices. Int. J. Pharm. 1984, 21, 317–329. [Google Scholar] [CrossRef]
- Alshetaili, A.; Almutairy, B.K.; Alshehri, S.M.; Repka, M.A. Development and Characterization of Sustained-Released Donepezil Hydrochloride Solid Dispersions Using Hot Melt Extrusion Technology. Pharmaceutics 2021, 13, 213. [Google Scholar] [CrossRef]
- Park, J.B.; Lee, B.J.; Kang, C.Y.; Tiwari, R.V.; Repka, M.A. Process analytical quality control of tailored drug release formulation prepared via hot-melt extrusion technology. J. Drug Deliv. Sci. Technol. 2017, 38, 51–58. [Google Scholar] [CrossRef]
- Bounartzi, M.; Panagopoulou, A.; Kantiranis, N.; Malamataris, S.; Nikolakakis, I. Effect of plasticiser type on the hot melt extrusion of venlafaxine hydrochloride. J. Pharm. Pharmacol. 2013, 66, 297–308. [Google Scholar] [CrossRef]
- Tiwari, R.; Agarwal, S.K.; Murthy, R.S.R.; Tiwari, S. Formulation and Evaluation of Sustained Release Extrudes Prepared via Novel Hot Melt Extrusion Technique. J. Pharm. Innov. 2014, 9, 246–258. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. 3D-Printed Network Structures as Controlled-Release Drug Delivery Systems: Dose Adjustment, API Release Analysis and Prediction. AAPS PharmSciTech 2018, 19, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.; Patel, N.; Patel, K.; Nottingham, E.; Singh, M. Sustained release dosage form of noscapine HCl using hot melt extrusion (HME) technique: Formulation and pharmacokinetics. Drug Deliv. Transl. Res. 2020, 11, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef]
- Kempin, W.; Franz, C.; Koster, L.C.; Schneider, F.; Bogdahn, M.; Weitschies, W.; Seidlitz, A. Assessment of different polymers and drug loads for fused deposition modeling of drug loaded implants. Eur. J. Pharm. Biopharm. 2017, 115, 84–93. [Google Scholar] [CrossRef]
- Blom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech 2019, 20, 52. [Google Scholar]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Viciosa, M.T.; Danède, F.; Affouard, F.; Correia, N.T. Molecular Mobility of Amorphous S-Flurbiprofen: A Dielectric Relaxation Spectroscopy Approach. Mol. Pharm. 2014, 11, 112–130. [Google Scholar] [CrossRef]
- Baek, H.H.; Kwon, S.Y.; Rho, S.-J.; Lee, W.S.; Yang, H.-J.; Hah, J.-M.; Choi, H.-G.; Kim, Y.-R.; Yong, C.S. Enhanced solubility and bioavailability of flurbiprofen by cycloamylose. Arch. Pharmacal Res. 2011, 34, 391–397. [Google Scholar] [CrossRef]
- Merck & Co. The Merck Index 2001; Merck & Co.: Whitehouse Station, NJ, USA, 2021. [Google Scholar]
- Paradkar, A.; Maheshwari, M.; Tyagi, A.K.; Chauhan, B.; Kadam, S.S. Preparation and characterization of flurbiprofen beads by melt solidification technique. AAPS PharmSciTech 2003, 4, 514–522. [Google Scholar] [CrossRef]
- Pajula, K.; Taskinen, M.; Lehto, V.P.; Ketolainen, J.; Korhonen, O. Predicting the formation and stability of amorphous small molecule binary mixtures from computationally determined Flory—Huggins interaction parameter and phase diagram. Mol. Pharm. 2010, 7, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Wzorek, A.; Kolbus, A.; Urbaniak, M.; Han, J.; Soloshonok, V.A.; Klika, K.D. Flurbiprofen: A Study of the Behavior of the Scalemate by Chromatography, Sublimation, and NMR. Symmetry 2021, 13, 543. [Google Scholar] [CrossRef]
- Desai, D.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H.; Phuapradit, W.; Vaka, S.R.K. Selection of Solid-State Plasticizers as Processing Aids for Hot-Melt Extrusion. J. Pharm. Sci. 2018, 107, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Flurbiprofen Tablets Monograph. In The United States Pharmacopeia and National Formulary USP 41–NF 36; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2018.
- Dissolution Test of Solid Dosage Forms. In The European Pharmacopeia, 10th ed.; European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2019; Chapter 2.9.3; pp. 326–333.
- Klumpp, L.; Leigh, M.; Dressman, J. Dissolution behavior of various drugs in different FaSSIF versions. Eur. J. Pharm. Sci. 2020, 142, 105138. [Google Scholar] [CrossRef]
- Jung, F.; Nothnagel, L.; Gao, F.; Thurn, M.; Vogel, V.; Wacker, M.G. A comparison of two biorelevant in vitro drug release methods for nanotherapeutics based on advanced physiologically-based pharmacokinetic modelling. Eur. J. Pharm. Biopharm. 2018, 127, 462–470. [Google Scholar] [CrossRef]
- ICH. ICH M9 on Biopharmaceutics Classification System Based Biowaivers. In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; ICH Harmonized Tripartite Guideline: Geneva, Switzerland, 2020. [Google Scholar]
- <711>Dissolution. In The United States Pharmacopeia and National Formulary USP 41–NF 36; The United States Pharma-Copeial Convention, Inc.: Rockville, MD, USA, 2018; p. 6461.
- Duan, J.Z.; Riviere, K.; Marroum, P. In Vivo Bioequivalence and In Vitro Similarity Factor (f2) for Dissolution Profile Comparisons of Extended Release Formulations: How and When Do They Match? Pharm. Res. 2011, 28, 1144–1156. [Google Scholar] [CrossRef]
- Hofsäss, M.A.; Dressman, J. Evaluation of Differences in Dosage Form Performance of Generics Using BCS-Based Biowaiver Specifications and Biopharmaceutical Modeling–Case Examples Amoxicillin and Doxycycline. J. Pharm. Sci. 2020, 109, 2437–2453. [Google Scholar] [CrossRef]
- Hopfenberg, H.; Apicella, A.; Saleeby, D. Factors affecting water sorption in and solute release from glassy ethylene—Vinyl alcohol copolymers. J. Membr. Sci. 1981, 8, 273–282. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Shah, S.; Maddineni, S.; Lu, J.; Repka, M.A. Melt extrusion with poorly soluble drugs. Int. J. Pharm. 2013, 453, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.M.; McGinity, J.W.; Iii, R.O.W. Enhancing bioavailability through thermal processing. Int. J. Pharm. 2013, 450, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-Melt Extrusion: A Roadmap for Product Development. AAPS PharmSciTech 2021, 22, 184. [Google Scholar] [CrossRef] [PubMed]
- Benival, D.; Salave, S.; Prayag, K.; Rana, D.; Amate, P.; Pardhe, R.; Jadhav, A.; Jindal, A.B. Recent Progress in Hot Melt Extrusion Technology in Pharmaceutical Dosage Form Design. Recent Patents Drug Deliv. Formul. 2022, 16, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Palazi, E.; Karavas, E.; Barmpalexis, P.; Kostoglou, M.; Nanaki, S.; Christodoulou, E.; Bikiaris, D.N. Melt extrusion process for adjusting drug release of poorly water soluble drug felodipine using different polymer matrices. Eur. J. Pharm. Sci. 2018, 114, 332–345. [Google Scholar] [CrossRef]
- Laukamp, E.J.; Vynckier, A.-K.; Voorspoels, J.; Thommes, M.; Breitkreutz, J. Development of sustained and dual drug release co-extrusion formulations for individual dosing. Eur. J. Pharm. Biopharm. 2015, 89, 357–364. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Moseson, D.E.; Richard, C.A.; Swinney, M.R.; Horava, S.D.; Oucherif, K.A.; Cox, A.L.; Hawkins, E.D.; Li, Y.; DeNeve, D.F.; et al. Development of hot-melt extruded drug/polymer matrices for sustained delivery of meloxicam. J. Control. Release 2022, 342, 189–200. [Google Scholar] [CrossRef]
- Loreti, G.; Maroni, A.; Del Curto, M.D.; Melocchi, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. Eur. J. Pharm. Sci. 2014, 52, 77–85. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Prolonged drug release properties for orodispersible films by combining hot-melt extrusion and solvent casting methods. Eur. J. Pharm. Biopharm. 2018, 129, 66–73. [Google Scholar] [CrossRef]
- Bezerra, G.S.N.; Lima, T.A.d.M.d.; Colbert, D.M.; Geever, J.; Geever, L. Formulation and Evaluation of Fenbendazole Extended-Release Extrudes Processed by Hot-Melt Extrusion. Polymers 2022, 14, 4188. [Google Scholar] [CrossRef]
- Yang, F.; Su, Y.; Small, J.; Huang, C.; Martin, G.E.; Farrington, A.M.; DiNunzio, J.; Brown, C.D. Probing the Molecular-Level Interactions in an Active Pharmaceutical Ingredient (API)—Polymer Dispersion and the Resulting Impact on Drug Product Formulation. Pharm. Res. 2020, 37, 94. [Google Scholar] [CrossRef] [PubMed]
- Glaessl, B.; Siepmann, F.; Tucker, I.; Rades, T.; Siepmann, J. Mathematical modeling of drug release from Eudragit RS-based delivery systems. J. Drug Deliv. Sci. Technol. 2010, 20, 127–133. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef] [PubMed]
- Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006, 321, 1–11. [Google Scholar] [CrossRef]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical Aspects and Pathophysiology of Inflammatory Bowel Disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018766s020lbl.pdf (accessed on 14 May 2023).
| Flurbiprofen | Eudragit RL | Eudragit RS | |
|---|---|---|---|
| Melting temperature | 110–111 °C a 114–118 °C b | - | - |
| Glass transition temperature | −8.15 °C | 63 °C | 64 °C |
| Degradation temperature | 184 °C | 166 °C | 170 °C |
| Components | 20% Flurbiprofen | 25% Flurbiprofen | 33% Flurbiprofen | 25% Flurbiprofen | Function |
|---|---|---|---|---|---|
| Flurbiprofen | 20% | 25% | 33% | 25% | API |
| Talcum | 2.5% | 2.5% | 2.5% | 4% | Homogenization |
| Eudragit RS PO | 75% | 70% | 62% | - | Polymer |
| Eudragit RL PO | - | - | - | 67% | Polymer |
| Mg Stearate | 2.5% | 2.5% | 2.5% | - | Plasticizer |
| Stearic acid | - | - | - | 4% | Plasticizer |
| Components | 20% Flurbiprofen | 25% Flurbiprofen | 30% Flurbiprofen | 35% Flurbiprofen | Function |
|---|---|---|---|---|---|
| Flurbiprofen | 20% | 25% | 30% | 35% | API |
| Talcum | 4% | 4% | 4% | 4% | Homogenization |
| Eudragit RS PO | 72% | 67% | 62% | 57% | Polymer |
| Stearic acid | 4% | 4% | 4% | 4% | Plasticizer |
| Fraction | Particle Size Fraction |
|---|---|
| 1 | 2 mm threads |
| 2 | 800 µm–2 mm |
| 3 | 630 µm–800 µm |
| 4 | 355 µm–630 µm |
| 5 | 250 µm–355 µm |
| 6 | 125 µm–250 µm |
| 7 | <125 µm |
| Parameter | Value |
|---|---|
| Mobile phase and pH | ACN: H2O: TFA 58:42:0.1 pH 6.5 |
| Flow rate | 1.0 mL/min |
| Absorption wavelength | 248 nm |
| Retention time | 8.8 min |
| Correlation coefficient | 0.999 |
| LOQ | 2.26 µg/mL |
| Method reference | Nothnagel and Jung [40] |
| Flurbiprofen % Loading (m/m) | Start | Middle | End | Mean | Rel.SD |
|---|---|---|---|---|---|
| E-RL extrudates | |||||
| 20% | |||||
| 1. Batch | 99.19% | 100.89% | 99.56% | 99.88% | 0.89% |
| 2. Batch | 101.63% | 99.74% | 100.24% | 100.54% | 0.97% |
| 25% | |||||
| 1. Batch | 101.53% | 100.65% | 100.22% | 100.80% | 0.67% |
| 2. Batch | 99.67% | 100.61% | 99.27% | 100.15% | 0.66% |
| 30% | |||||
| 1. Batch | 100.71% | 100.67% | 99.55% | 100.31% | 0.66% |
| 2. Batch | 100.25% | 99.29% | 99.47% | 99.67% | 0.68% |
| 35% | |||||
| 1. Batch | 100.41% | 99.81% | 99.19% | 99.81% | 0.61% |
| 2. Batch | 100.21% | 101.47% | 100.04% | 100.58% | 0.89% |
| E-RS extrudates | |||||
| 20% | |||||
| 1. Batch | 101.92% | 99.51% | 99.18% | 100.21% | 1.49% |
| 2. Batch | 101.47% | 99.35% | 98.95% | 99.93% | 1.50% |
| 25% | |||||
| 1. Batch | 99.93% | 100.21% | 101.73% | 100.63% | 0.97% |
| 2. Batch | 100.17% | 101.09% | 98.63% | 100.64% | 0.65% |
| 30% | |||||
| 1. Batch | 99.43% | 99.21% | 97.97% | 98.87% | 0.80% |
| 2. Batch | 99.88% | 100.98% | 99.89% | 100.44% | 0.77% |
| 35% | |||||
| 1. Batch | 101.60% | 99.76% | 98.84% | 100.07% | 1.41% |
| 2. Batch | 99.39% | 101.74% | 99.53% | 100.57% | 1.65% |
| Burst Release from the Fractionated Eudragit RL Extrudates at 25% (/m/m) Drug Load | |||||||
|---|---|---|---|---|---|---|---|
| Fractions | <125 µm | 125–250 µm | 250–355 µm | 355–630 µm | 630–800 µm | 800 µm–2 mm | 2 mm Threads |
| Drug release (%) at 15 min | 20.10 | 20.04 | 11.46 | 8.28 | 5.58 | 4.64 | 0.96 |
| Drug release (%) at 720 min | 84.90 | 83.14 | 81.01 | 58.57 | 43.87 | 21.18 | 6.30 |
| Burst Release in % | 23.6 | 24.1 | 14.1 | 14.1 | 12.7 | 21.9 | 15.2 |
| Burst release from the fractionated Eudragit RS extrudates at 25% (/m/m) drug load | |||||||
| Drug release (%) at 15 min | 16.92 | 15.27 | 7.34 | 5.26 | 3.26 | 2.66 | 1.98 |
| Drug release (%) at 720 min | 62.26 | 58.09 | 42.38 | 29.06 | 22.13 | 15.67 | 4.59 |
| Burst Release in % | 26.8 | 26.2 | 17.3 | 18.1 | 14.7 | 16.9 | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansuroglu, Y.; Dressman, J. Factors That Influence Sustained Release from Hot-Melt Extrudates. Pharmaceutics 2023, 15, 1996. https://doi.org/10.3390/pharmaceutics15071996
Mansuroglu Y, Dressman J. Factors That Influence Sustained Release from Hot-Melt Extrudates. Pharmaceutics. 2023; 15(7):1996. https://doi.org/10.3390/pharmaceutics15071996
Chicago/Turabian StyleMansuroglu, Yaser, and Jennifer Dressman. 2023. "Factors That Influence Sustained Release from Hot-Melt Extrudates" Pharmaceutics 15, no. 7: 1996. https://doi.org/10.3390/pharmaceutics15071996
APA StyleMansuroglu, Y., & Dressman, J. (2023). Factors That Influence Sustained Release from Hot-Melt Extrudates. Pharmaceutics, 15(7), 1996. https://doi.org/10.3390/pharmaceutics15071996




