Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Solution Preparation
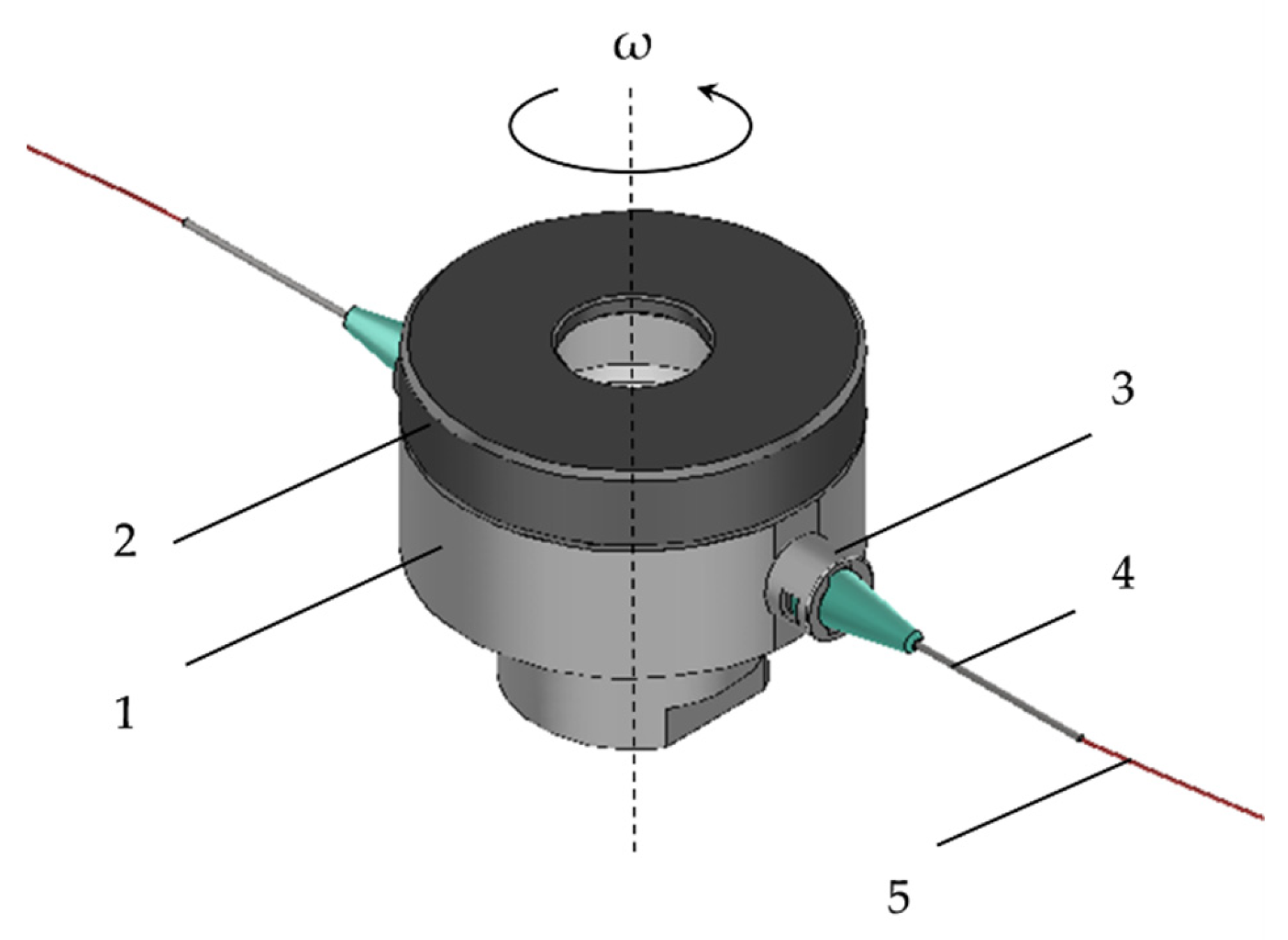
2.3. Centrigual Spinning
2.4. Scanning Electron Microscopy (SEM)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. Determination of Aceclofenac Content by HPLC
2.8. In Vitro Dissolution Studies
2.9. Residual Ethanol Content Determination of the Microfibrous Samples Using Gas Chromatography (GC)
2.10. Viscosity Measurement
3. Results and Discussions
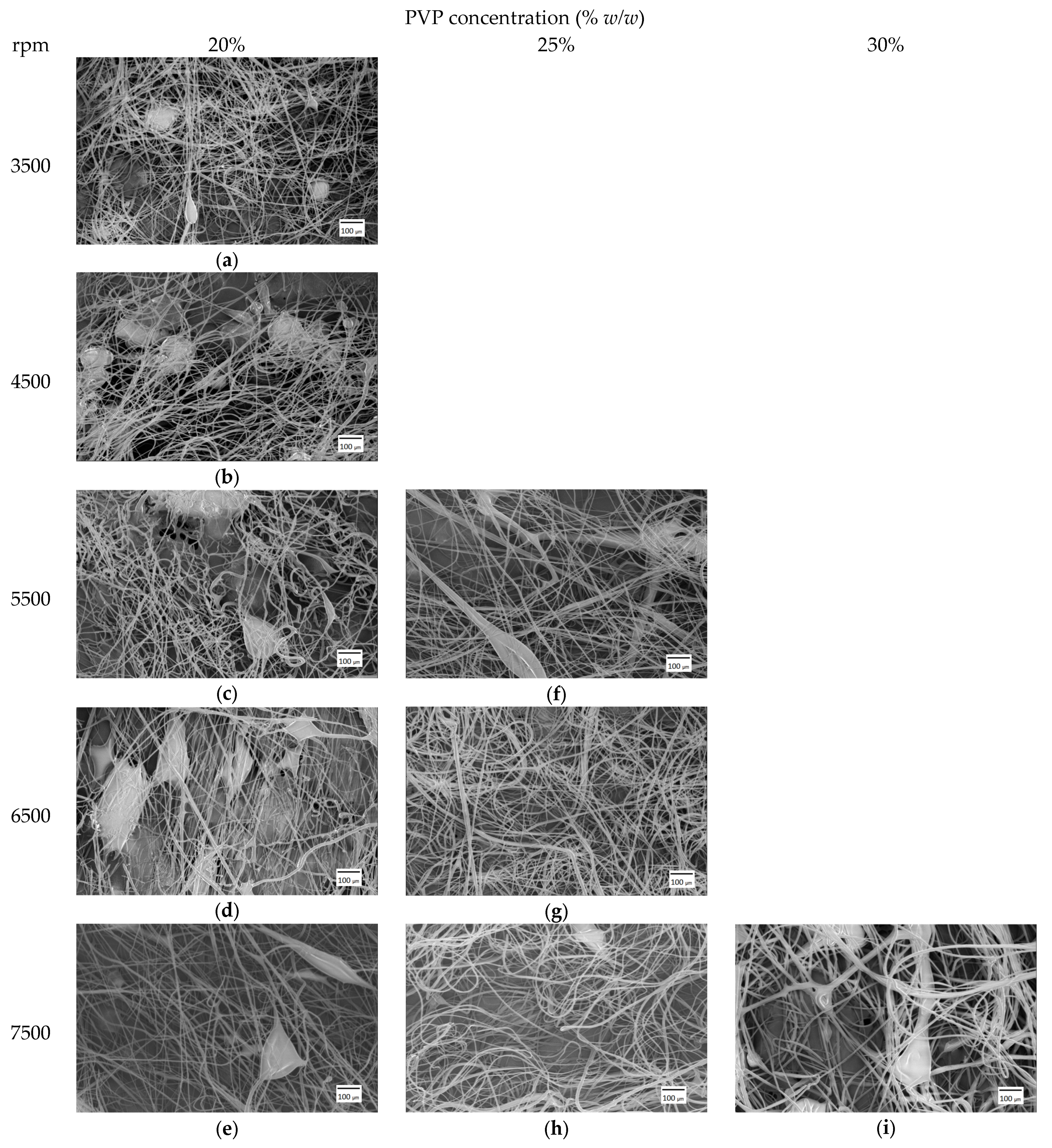
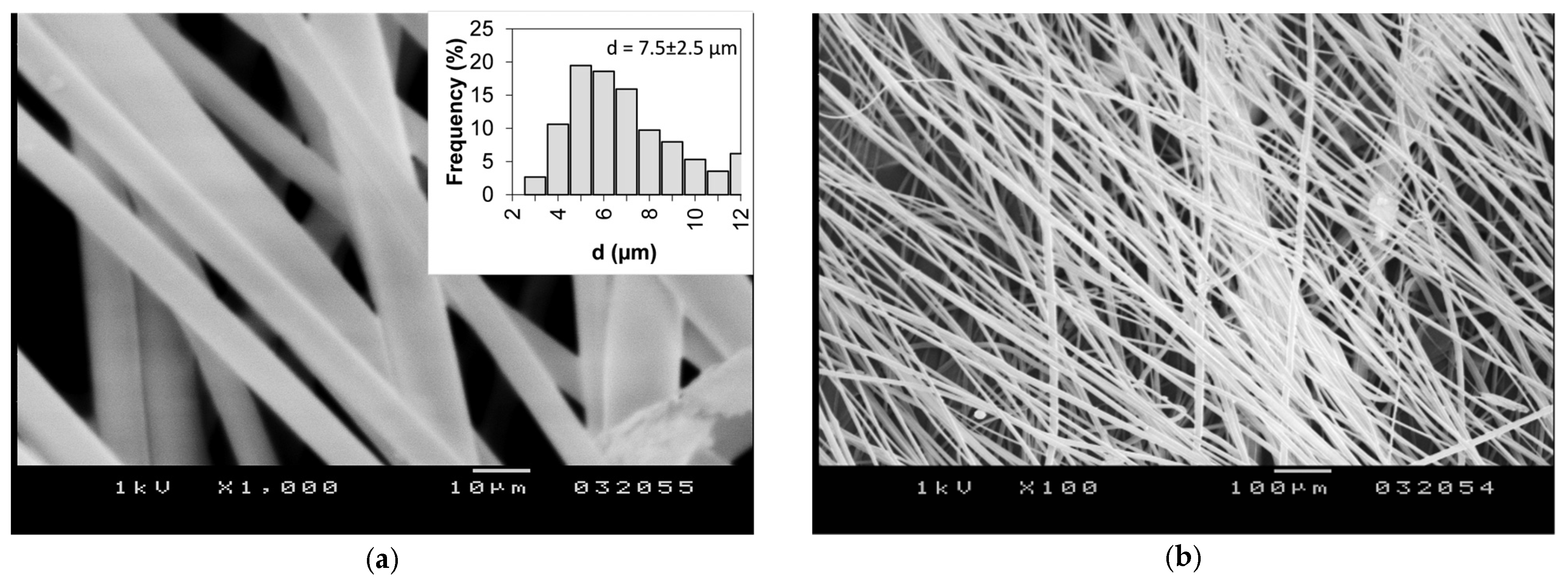
3.1. Fiber Mat Characterization
3.2. Determination of Aceclofenac and Ethanol Content of the Microfibrous Webs
3.3. Solid-State Characterization of the Obtained Microfibrous Webs
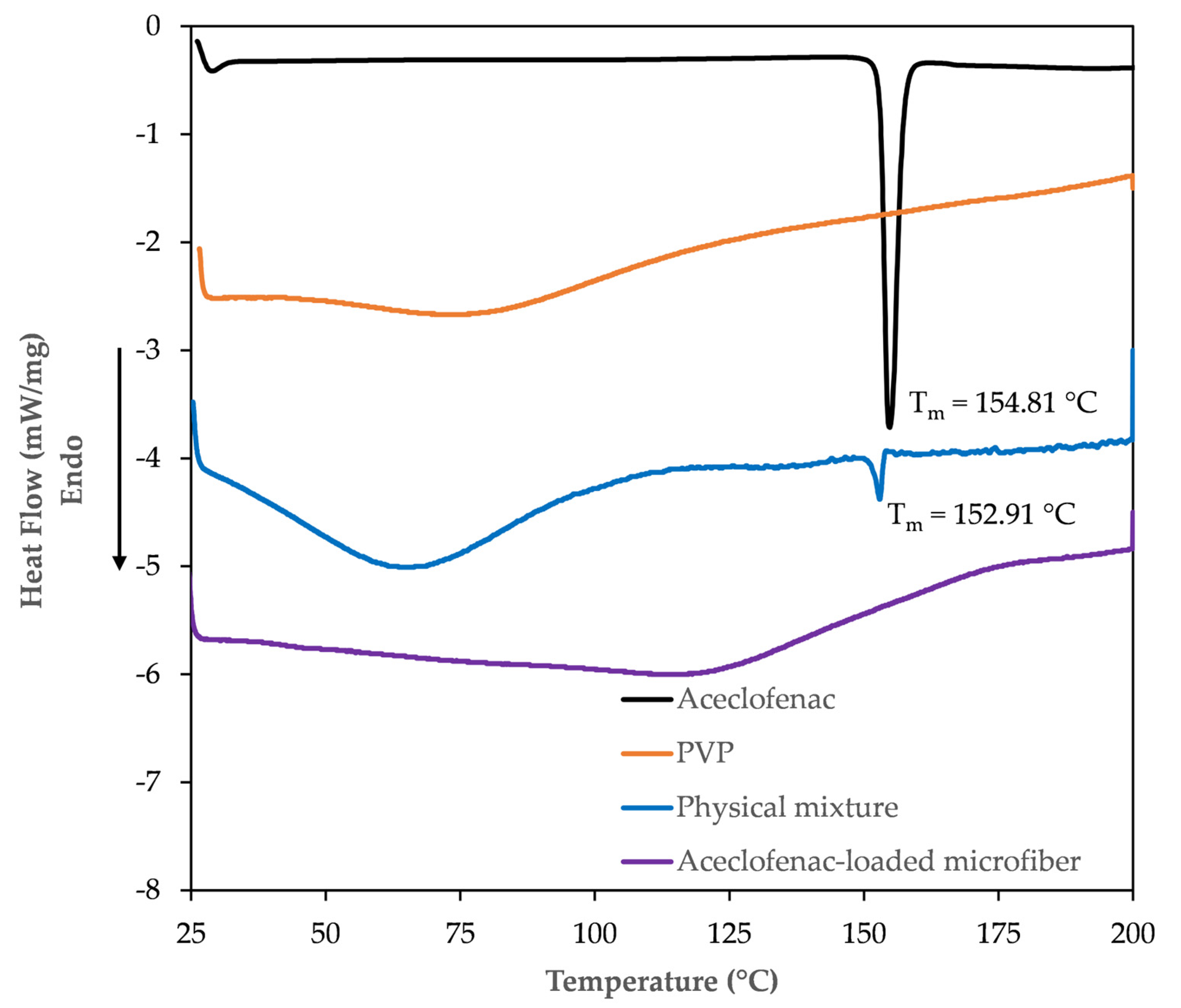
3.3.1. DSC Study
3.3.2. FT-IR Study
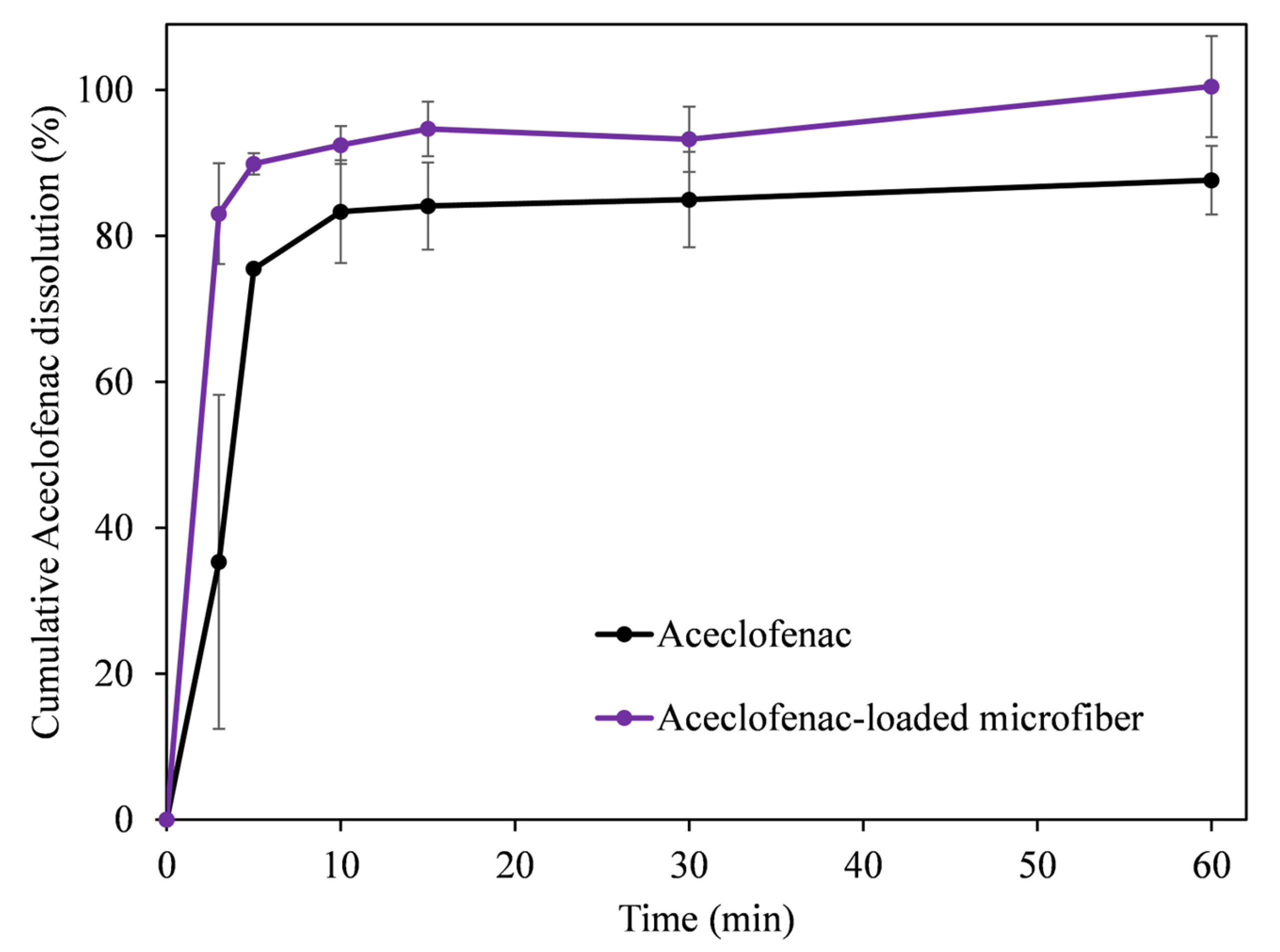
3.4. In Vitro Dissolution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A Review on Recent Advances in Application of Electrospun Nanofiber Materials as Biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Kesici Güler, H.; Cengiz Callioglu, F. A New Composite Nanofibrous Biomaterial Development for Drug Delivery Applications. Express Polym. Lett. 2023, 17, 487–501. [Google Scholar] [CrossRef]
- Gergely, A.; Kántor, J.; Bitay, E.; Biró, D. Electrospinning of Polymer Fibres Using Recycled PET. Acta Mater. Transylvanica 2019, 2, 19–26. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology 1996, 7, 216. [Google Scholar] [CrossRef]
- Taylor, G.I. Electrically Driven Jets. Proc. R. Soc. Lond. A Math. Phys. Sci. 1969, 313, 453–475. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R.; Ceylan, M.; Jabbarnia, A. Recent Progress on Conventional and Non-Conventional Electrospinning Processes. Fibers Polym. 2013, 14, 1235–1247. [Google Scholar] [CrossRef]
- Wu, C.M.; Chou, M.H. Acoustic–Electric Conversion and Piezoelectric Properties of Electrospun Polyvinylidene Fluoride/Silver Nanofibrous Membranes. Express Polym. Lett. 2020, 14, 103–114. [Google Scholar] [CrossRef]
- Guarino, V.; Iannotti, V.; Ausanio, G.; Ambrosio, L.; Lanotte, L. Elastomagnetic Nanofiber Wires by Magnetic Field Assisted Electrospinning. Express Polym. Lett. 2019, 13, 419–428. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of Electrospinning Technology: Applications in the Pharmaceutical Industry. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 12, e1611. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Cho, Y.-S.; Kim, W.D. Stability Analysis for Multi-Jets Electrospinning Process Modified with a Cylindrical Electrode. Eur. Polym. J. 2006, 42, 2031–2038. [Google Scholar] [CrossRef]
- Theron, S.A.; Yarin, A.L.; Zussman, E.; Kroll, E. Multiple Jets in Electrospinning: Experiment and Modeling. Polymer 2005, 46, 2889–2899. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Zhou, L.; Xu, Z.; Xing, J.; Feng, Q. Recent Progress of the Needleless Electrospinning for High Throughput of Nanofibers. Recent Pat. Nanotechnol. 2019, 13, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, X.; Lin, T. Upward Needleless Electrospinning of Nanofibers. J. Eng. Fiber Fabr. 2012, 7, 17–22. [Google Scholar] [CrossRef]
- Tang, S.; Zeng, Y.; Wang, X. Splashing Needleless Electrospinning of Nanofibers. Polym. Eng. Sci. 2010, 50, 2252–2257. [Google Scholar] [CrossRef]
- Wang, X.; Niu, H.; Lin, T.; Wang, X. Needleless Electrospinning of Nanofibers with a Conical Wire Coil. Polym. Eng. Sci. 2009, 49, 1582–1586. [Google Scholar] [CrossRef]
- Niu, H.; Lin, T.; Wang, X. Needleless Electrospinning. I. A Comparison of Cylinder and Disk Nozzles. J. Appl. Polym. Sci. 2009, 114, 3524–3530. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Y.; Liu, Y.; Duan, H.; Zhou, J.; Zhang, Z.; Wang, Y.; Li, X.; Wang, W.; Lan, W.; et al. Superhigh-Throughput Needleless Electrospinning Using a Rotary Cone as Spinneret. Small 2010, 6, 1612–1616. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Liu, L.Q.; Zhang, Z. Needleless Electro-Spun Nanofibers Used for Filtration of Small Particles. Express Polym. Lett. 2013, 7, 683–689. [Google Scholar] [CrossRef]
- Molnar, K.; Nagy, Z.K. Corona-Electrospinning: Needleless Method for High-Throughput Continuous Nanofiber Production. Eur. Polym. J. 2016, 74, 279–286. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Sun, J.; Liu, C.; Yang, B. Effective Method for High-Throughput Manufacturing of Ultrafine Fibres via Needleless Centrifugal Spinning. Micro Nano Lett. 2015, 10, 81–84. [Google Scholar] [CrossRef]
- Yu, D.-G.; Li, J.-J.; Williams, G.R.; Zhao, M. Electrospun Amorphous Solid Dispersions of Poorly Water-Soluble Drugs: A Review. J. Control Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Atıcı, B.; Ünlü, C.H.; Yanilmaz, M. A Review on Centrifugally Spun Fibers and Their Applications. Polym. Rev. 2022, 62, 1–64. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T. Rotary Jet Spinning Review—A Potential High Yield Future for Polymer Nanofibers. Nanocomposites 2017, 3, 97–121. [Google Scholar] [CrossRef]
- Ngoc Doan, H.; Kim Nguyen, D.; Phong Vo, P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Phu Huynh, D. Facile and Scalable Fabrication of Porous Polystyrene Fibers for Oil Removal by Centrifugal Spinning. ACS Publ. 2019, 4, 15992–16000. [Google Scholar] [CrossRef]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental Study of Nanofiber Production through Forcespinning. J. App. Phys. 2013, 113, 24318. [Google Scholar] [CrossRef]
- Kántor, J.; Gergely, A.; Farmos, R.L.; Hodgyai, N. Poly (Styrene-b-Isobutylene-b-Styrene) Triblock Copolymer Fiber Generation with Centrifugal Spinning, and Its Potential Application in Oil Collection. In Proceedings of the IEEE Joint 22nd International Symposium on Computational Intelligence and Informatics and 8th International Conference on Recent Achievements in Mechatronics, Automation, Computer Science and Robotics (CINTI-MACRo 2022), Budapest, Hungary, 21–22 November 2022. [Google Scholar]
- Legrand, E. Aceclofenac in the Management of Inflammatory Pain. Expert. Opin. Pharmacother. 2004, 5, 1347–1357. [Google Scholar] [CrossRef]
- Iolascon, G.; Giménez, S.; Mogyorósi, D. A Review of Aceclofenac: Analgesic and Anti-Inflammatory Effects on Musculoskeletal Disorders. J. Pain Res. 2021, 14, 3651–3663. [Google Scholar] [CrossRef]
- Dooley, M.; Spencer, C.M.; Dunn, C.J. Aceclofenac: A Reappraisal of Its Use in the Management of Pain and Rheumatic Disease. Drugs 2001, 61, 1351–1378. [Google Scholar] [CrossRef]
- Noh, K.; Shin, B.S.; Kwon, K.; Yun, H.Y.; Kim, E.; Jeong, T.C.; Kang, W. Absolute Bioavailability and Metabolism of Aceclofenac in Rats. Arch. Pharm. Res. 2015, 38, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical Particle Technologies: An Approach to Improve Drug Solubility, Dissolution and Bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Bitay, E.; Gergely, A.; Balint, I.; Molnar, K.; Fülöp, I.; Fogarasi, E.; Szabó, Z.I. Preparation and Characterization of Lapatinib-Loaded PVP Nanofiber Amorphous Solid Dispersion by Electrospinning. Express Polym. Lett. 2021, 15, 1041–1050. [Google Scholar] [CrossRef]
- Mutalik, S.; Anju, P.; Manoj, K.; Usha, A.N. Enhancement of Dissolution Rate and Bioavailability of Aceclofenac: A Chitosan-Based Solvent Change Approach. Int. J. Pharm. 2008, 350, 279–290. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Naik, J.B. Aceclofenac Microspheres: Quality by Design Approach. Mater. Sci. Eng. C 2014, 36, 320–328. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Manaswini, P.; Divyavani, E.; Rao, J.V.; Talla, V.; Subudhi, S.K. Fabrication of Aceclofenac Nanocrystals for Improved Dissolution: Process Optimization and Physicochemical Characterization. J. Drug Deliv. Sci. Technol. 2015, 29, 199–209. [Google Scholar] [CrossRef]
- Jana, S.; Sen, K.K. Chitosan—Locust Bean Gum Interpenetrating Polymeric Network Nanocomposites for Delivery of Aceclofenac. Int. J. Biol. Macromol. 2017, 102, 878–884. [Google Scholar] [CrossRef]
- Sipos, E.; Kósa, N.; Kazsoki, A.; Szabó, Z.I.; Zelkó, R. Formulation and Characterization of Aceclofenac-Loaded Nanofiber Based Orally Dissolving Webs. Pharmaceutics 2019, 11, 417. [Google Scholar] [CrossRef]
- Sebe, I.; Kállai-Szabó, B.; Oldal, I.; Zsidai, L.; Zelkó, R. Development of Laboratory-Scale High-Speed Rotary Devices for a Potential Pharmaceutical Microfibre Drug Delivery Platform. Int. J. Pharm. 2020, 588, 119740. [Google Scholar] [CrossRef]
- Sipos, E.; Szabó, Z.I.; Rédai, E.; Szabó, P.; Sebe, I.; Zelkó, R. Preparation and Characterization of Nanofibrous Sheets for Enhanced Oral Dissolution of Nebivolol Hydrochloride. J. Pharm. Biomed. Anal. 2016, 129, 224–228. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Bitay, E.; Gergely, A.L.; Kántor, J.; Szabó, Z.-I. Evaluation of Lapatinib-Loaded Microfibers Prepared by Centrifugal Spinning. Polymers 2022, 14, 5557. [Google Scholar] [CrossRef] [PubMed]
- Step ICH Guideline Q3C (R8) on Impurities: Guideline for Residual Solvents. Available online: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf (accessed on 1 March 2023).
- Vadher, A.H.; Parikh, J.R.; Parikh, R.H.; Solanki, A.B. Preparation and Characterization of Co-Grinded Mixtures of Aceclofenac and Neusilin US2 for Dissolution Enhancement of Aceclofenac. AAPS PharmSciTech 2009, 10, 606–614. [Google Scholar] [CrossRef] [PubMed]


| d (μm) | ||||||
|---|---|---|---|---|---|---|
| Concentration (w/w) | ||||||
| Rpm | 20% | Beads | 25% | Beads | 30% | Beads |
| 3500 | 3.6 ± 1.1 | Yes | -- | -- | -- | -- |
| 4500 | 3.7 ± 1.1 | Yes | -- | -- | -- | -- |
| 5500 | 4.8 ± 1.8 | Yes | 3.8 ± 2 | Yes | -- | -- |
| 6500 | 3.2 ± 1.6 | Yes | 4.3 ± 1.5 | No | -- | -- |
| 7500 | 4 ± 1.8 | Yes | 4.6 ± 1.6 | Yes | 6.8 ± 2.4 | Yes |
| Concentration (w/w%) | Concentration (% of Theoretical Concentration) | |||||
|---|---|---|---|---|---|---|
| Nr. | 1. | 2. | 3. | 1. | 2. | 3. |
| Value | 15.49 | 15.83 | 15.59 | 98.40 | 100.58 | 99.06 |
| Avg. ± st. dev. | 15.6 ± 0.2 | 99 ± 1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitay, E.; Gergely, A.L.; Szabó, Z.-I. Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning. Pharmaceutics 2023, 15, 2256. https://doi.org/10.3390/pharmaceutics15092256
Bitay E, Gergely AL, Szabó Z-I. Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning. Pharmaceutics. 2023; 15(9):2256. https://doi.org/10.3390/pharmaceutics15092256
Chicago/Turabian StyleBitay, Enikő, Attila Levente Gergely, and Zoltán-István Szabó. 2023. "Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning" Pharmaceutics 15, no. 9: 2256. https://doi.org/10.3390/pharmaceutics15092256
APA StyleBitay, E., Gergely, A. L., & Szabó, Z.-I. (2023). Optimization and Production of Aceclofenac-Loaded Microfiber Solid Dispersion by Centrifugal Spinning. Pharmaceutics, 15(9), 2256. https://doi.org/10.3390/pharmaceutics15092256








